Abstract
Clinical and preclinical research suggest that activation of the mesolimbic dopamine (DA) system is involved in mediating the rewarding actions of drugs of abuse, as well as promoting drug-seeking behavior. Inhibition of DA D1 receptors in the nucleus accumbens (Acb) can reduce ethanol (EtOH)-seeking behavior of non-selective rats triggered by environmental context. However, to date, there has been no research on the effects D1 receptor agen ts on EtOH-seeking behavior of high alcohol preferring (P) rats following prolonged abstinence. The objective of the present study was to examine the effects of microinjecting the D1 antagonist SCH 23390 or the D1 agonist A-77636 into the Acb shell or Acb core on spontaneous recovery of EtOH-seeking behavior. After 10 weeks of concurrent access to EtOH and water, P rats underwent 7 extinction sessions (EtOH and water withheld), followed by 2 weeks in their home cages without access to EtOH or operant sessions. In the 2nd week of the home cage phase, rats were bilaterally implanted with guide cannula aimed at the Acb shell or Acb core; rats were allowed 7 days to recover before EtOH-seeking was assessed by the Pavlovian spontaneous recovery (PSR) model. Administration of SCH23390 (1 μg/side) into the Acb shell inhibited responding on the EtOH lever, whereas administration of A-77636 (0.125 μg/side) increased responding on the EtOH lever. Microinfusion of D1 receptor agents into the Acb core did not alter responding on the EtOH lever. Responses on the water lever were not altered by any of the treatments. The results suggest that activation of D1 receptors within the Acb shell, but not Acb core, are involved in mediating PSR of EtOH-seeking behavior of P rats.
Keywords: Pavlovian Spontaneous Recovery, alcohol-seeking behavior, dopamine-1 receptors, SCH 23390, A-77636, alcohol-preferring P rat
Introduction
Several studies have provided evidence that ethanol (EtOH)–seeking is associated with increased c-Fos expression in the ventral tegmental area (VTA) (Zhao et al., 2006) and nucleus accumbens (Acb) (Zhao et al., 2006; Dayas et al. 2007; Funk et al., 2006), thus suggesting that neural substrates in the dopamine (DA) system are activated during EtOH-seeking behavior. Hauser et al. (2011) findings indicated that reduction of VTA DA neuronal firing in the posterior VTA, but not the anterior VTA, with local quinpirole (D2 receptor agonist) administration, blocked spontaneous recovery of EtOH–seeking behavior of alcohol preferring (P) rats, suggesting that activation of DA neurons in the posterior VTA are required for expression of EtOH-seeking behavior.
D1 receptors within Acb have been implicated in the involvement of drug reward and drug seeking behaviors. The majority of the neurons projecting from the Acb to the VTA express mRNA for D1 receptors (Lu et al., 1998). Local application of the D1 receptor antagonist SCH 23390 into the Acb shell reduced context-induced heroin-seeking and cocaine-seeking behaviors (Anderson et al. 2003; Bachtell et al. 2005). However, antagonism of D1 receptors in the Acb core reduced discrete-cue-induced heroin-seeking (Bossert et al. 2007), but did not alter cocaine-seeking (Anderson et al. 2003). In contrast, activation of D1 receptors via an agonist into the Acb shell and Acb core reinstated cocaine-seeking (Bachtell et al. 2005; Schmidt and Pierce 2006), or was effective in the Acb shell, but not the Acb core (Schmidt et al. 2006). Collectively, these studies provide evidence that the activation of D1 receptors in the Acb shell and possibly the core may be involved in mediating drug-seeking behaviors.
There have been a few studies that examined the involvement of DA D1 receptors in EtOH-seeking behavior. Peripheral administration of SCH 23390 can decrease EtOH-seeking behavior (Liu and Weiss 2002; Hamlin et al. 2007). In addition, Hamlin et al. (2007) demonstrated that peripheral administration of SCH 23390 can abolish c-Fos expression in the ventral Acb shell following context-induced EtOH-seeking (Hamlin et al. 2007). Chaudhri et al. (2009) reported that D1 receptors in the Acb shell and Acb core may be involved in mediating context-induced EtOH-seeking behavior of male Long-Evans rats, as indicated by the microinfusion of a D1 antagonist into these Acb sub-regions. However, there have been no studies examining the involvement of D1 receptors in the Acb shell or Acb core in regulating EtOH-seeking behavior of alcohol-preferring (P) rats. The P rat satisfies the criteria for a suitable animal model of alcoholism (McBride and Li, 1998; Murphy et al., 2002; McBride et al., 2014), and exhibits robust EtOH self-administration and EtOH-seeking behavior (Rodd et al., 2006; Rodd-Henricks et al., 2002a, b). In addition, the P rat exhibits innate differences in the DA system compared to alcohol-non-preferring (NP) rats (reviewed in Murphy et al., 2002), the Acb shell (but not the core) supports the rewarding actions of EtOH in the P rat (Engleman et al., 2009), and there are differences in the sensitivity and response to EtOH of the Acb shell between P and Wistar rats (Engleman et al., 2009). Therefore, it would be important to determine if the Acb is involved in mediating EtOH-seeking behavior of P rats and if there are Acb sub-regional differences.
Context has been shown to influence extinction learning and reinstatement of previously learned behaviors (Bouton, 2002). In a spontaneous recovery paradigm, subjects are allowed to self-administer in a specific environment, and the behavior is extinguished in the same environment; the subjects are withheld from that environment for a prolonged period, and behavior is recorded when the animals are returned to the original environment. Since spontaneous recovery is defined in the alcohol clinical literature as the cessation of alcohol consumption in human alcoholics without treatment intervention, we have altered the term to Pavlovian Spontaneous Recovery (PSR). PSR is a unique phenomenon in that it is time-dependent (Bouton, 1988) and is directly correlated to reward saliency (Robbins, 1990). In addition, contextual cues in the PSR procedure are associated with first-learned signals, and the amount of first- and second-learned associations (Brooks, 2000).
The objective of the present study was to determine the involvement of D1 receptors within the Acb shell and core in mediating expression of PSR of EtOH-seeking behavior of P rats, following a 2-week EtOH-free period. In addition to using an animal model that shows robust EtOH-seeking behavior, the current study used both a D1 receptor antagonist (SCH 23390) and an agonist (A-77636), which should provide more supportive evidence for the involvement D1 receptors than the use of an antagonist alone.
Experimental Procedures
Animals
Adult female P rats from the 70th generations, weighing 250-325 g at the start of the experiment, were used. Female P rats were used in the present study because female rats maintain their body and head size better than male P rats for more accurate and reliable stereotaxic placements. Rats were maintained on a 12-hr reversed light dark cycle (lights off at 0900 hr). Food and water were available in the home cage ad libitum throughout the experiment. All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Research Institute for Laboratory Animal Research, 2011).
Chemical Agents and Vehicle
Ethyl alcohol (190 proof; McCormick Distilling Co., Weston, MO) was diluted to 15% with distilled water for operant oral self-administration sessions. SCH 23390, a D1 receptor antagonist, (Sigma, St. Louis, MO) and A-77636, D1 receptor agonist, (Tocris Bioscience, Ellisville, MO) were dissolved in artificial cerebral spinal fluid (aCSF), and the pH adjusted to 6.5 ± 0.1 for microinjections. The aCSF consisted of: 120.0 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM Mg SO4, 25.0 mM NaHCO3, 2.5 mM CaCl2, and 10.0 mM D-glucose.
Operant Training
EtOH self-administration procedures were conducted in standard two-lever experimental chambers (Coulbourn Instruments, Allentown, PA), as previously described (Rodd et al., 2006). Two operant levers were located on the same wall and were placed 15 cm above the grid floor and 13 cm apart. Directly beneath each lever was a trough through which a dipper cup (0.1 ml) was raised to deliver response-contingent fluid. Upon a reinforced response, a small light cue was illuminated in the drinking trough during the 4-sec dipper access.
Rats were placed into the operant chamber to self-train. Operant sessions were 60-min in duration and occurred daily for 10 weeks (Rodd et al. 2006; Hauser et al., 2011). The EtOH concentration used for operant administration was 15% (vol/vol). During the initial 4 weeks of daily operant access, both solutions (water and EtOH) were reinforced on an FR-1 schedule. At the end of this time, the response requirement for EtOH was increased to an FR-3 schedule for 3 weeks, and then to FR-5 schedule for 3 weeks. Water remained at FR-1 throughout.
After the P rats had established stable levels of responding on the FR5 schedule for EtOH and FR1 for water, they underwent 7 days of extinction (60 min/day), when neither water nor EtOH was available (Rodd et al. 2006; Hauser et al., 2011). With the exception of no fluid being present, the delivery system operated exactly as the preceding EtOH self-administration sessions, i.e., rats still received the auditory stimulus of the dipper raising and the visual cue of the small light being illuminated above the dipper trough. After extinction training, all rats were maintained in the home cages for 14 days. Previous research has shown that two weeks of home cage produce a robust expression of EtOH-seeking (Rodd –Henricks et al. 2002a, b; Rodd et al. 2006).
Stereotaxic Surgeries
Stereotaxic implantation was performed after the animals had been in the home cages for 7 days. While under isoflurane anesthesia, rats were prepared for bilateral stereotaxic implantation of 22-gauge guide cannula (Plastic One, Roanoke, VA) into the Acb shell or Acb core; the guide cannula was aimed 1.0 mm above the target region. Coordinates for placements to target the Acb shell were +1.7 mm AP, + 2.4 mm lateral to the midline, and -7.5 mm ventral from the surface of the skull at a 10° angle to the vertical (Paxinos and Watson, 1998). Coordinates for placements to target Acb core were +1.7 mm AP, + 2.7 mm lateral to the midline, and -6.5 mm ventral from the surface of the skull at a 10° angle to the vertical. A 28-gauge stylet was placed into the guide cannula and extended 0.5 mm beyond the tip of the guide. After surgery, rats were individually housed and allowed to recover for 7 days in their home cage. Animals were handled for at least 5-min daily beginning on the fourth recovery day and were habituated for 2 consecutive days to the handling procedures necessary for microinjections.
Pavlovian Spontaneous Recovery (PSR) testing for expression of EtOH-seeking behavior
The PSR sessions were identical to the extinction protocol conditions. The FR5-FR1 schedule, lever contingencies and dipper functioning were maintained but EtOH and water were absent. There are 4 consecutive PSR session because previous studies have shown that exposure to EtOH odor cues or EtOH priming (Rodd-Henricks et al., 2002a, b) and some drugs (Dhaher et al., 2010) may enhance responding on the ethanol-associated lever for more than one session.
Microinjection of SCH 23390 or A-77636 into the Acb shell or Acb core on responding on the EtOH and water levers during PSR testing
Rats were microinjected bilaterally with aCSF, SCH 23390, a D1 receptor antagonist, (0.25 or 1 μg), or A-77636 (0.125 or 0.25 μg), a D1 receptor agonist. SCH 23390, A-77636 or aCSF was administered into Acb shell or Acb core through 28-gauge injectors inserted to a depth of 1 mm beyond the end of the guide cannulae; the injector was connected to a Hamilton 25-μl syringe driven by a microinfusion syringe pump (Harvard Apparatus, MA, USA). A total volume of 0.5 μl was administered over a 30-sec period per side; the injector tip was left in place for an additional 30 sec per side. SCH 23390, A-77636 and aCSF were given 4 min prior to only the first 60-min PSR session. The doses of SCH 23390 administered in the current study were based on previous research from our lab that demonstrated microinjections of 0.25-2μg of SCH 23390 into Acb (Levy et al., 1991) or ventral pallidum (Melendez et al., 2005) reduced EtOH intake of P rats, but did not alter their motor activity.
Histology
At the termination of the experiment, 1% bromophenol blue (0.5 μl) was injected into the infusion site. Subsequently, the animals were given a fatal dose of Nembutal and then decapitated. Brains were removed and immediately frozen at -70°C. Frozen brains were subsequently equilibrated at -15°C in a cryostat microtome and 40-μm sections prepared. Sections then were stained with cresyl violet and examined under a light microscope for location of the injection site using the rat brain atlas of Paxinos and Watson (1998).
Statistical Analysis
Overall operant responding (60 min) data were analyzed with a mixed factorial ANOVA with a between subject factor of dose and a repeated measure of ‘session’. For the PSR experiments, the baseline measure for the factor of ‘session’ was the average number of responses on the EtOH lever for the last three extinction sessions. Post hoc Tukey's b was performed to determine individual differences. All analyses that were p ≤ 0.05 was considered significant.
Results
As seen in Fig. 1, the Acb shell and Acb core injector placements were at +1.7 to +1.0 mm relative to bregma. The success rate for correct Acb shell and Acb core dual placements was 95%. The incorrect injections sites were located ventral to the Acb. Only animals that had correct injector placements were used in the data analysis.
Fig. 1.
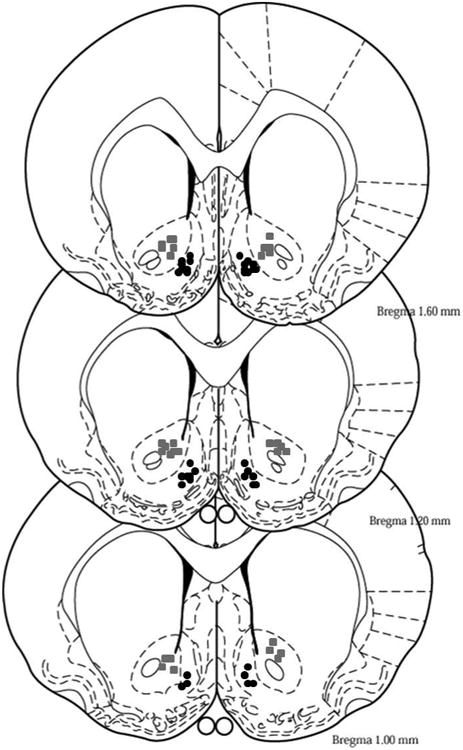
Representative placements for the micro-infusion of aCSF, SCH 23390, or A-77636 into the Acb shell or Acb core of adult female P rats are shown. Black circles represent placements of injection sites within the Acb shell (defined as +1.7 to + 1.0 mm bregma) and grey squares represent placements of injection sites within the Acb core (defined as +1.7 to + 1.0 mm bregma).
Responses on the EtOH and water levers during self-administration and extinction
Average responses on the EtOH lever during the last 5 sessions of the EtOH self-administration part of the procedure ranged from 193 to 222 responses/session across the 4 experiments (Table 1). Average responses on the water lever during these sessions were usually less than 20 responses/session. Average EtOH intakes during these 5 sessions ranged from 1.4 to 1.5 g/kg/session. Responses on the EtOH lever were markedly reduced across extinction sessions, whereas the low responses on the water lever were not significantly altered across extinction sessions (Table 1).
Table 1.
Representation of EtOH and water lever responses (means ± SEM) during the last 5 days of maintenance, the 1st day of extinction, and last 3 days extinction that were used for baseline prior to 1st PSR session.
| EtOH Lever (FR5) | Water Lever (FR1) | EtOH (g/kg) | |
|---|---|---|---|
|
|
|||
| Experiment 1 (SCH 23390 ; AcbShell) | |||
|
| |||
| EtOH Maintenance | 222 ± 22 | 13 ± 3 | 1.5 ± 0.2 |
| Extinction (Day 1) | 150 ± 13 | 12 ± 2 | |
| Base Extinction (last 3 days) | 32 ± 3 | 9 ± 1 | |
|
| |||
| Experiment 2 (SCH 23390 ; AcbCore) | |||
|
| |||
| EtOH Maintenance | 217 ± 21 | 12 ± 3 | 1.5 ± 0.2 |
| Extinction (Day 1) | 221 ± 22 | 17 ± 5 | |
| Base Extinction (last 3 days) | 22 ± 9 | 10 ± 2 | |
|
| |||
| Experiment 3 (A-77636; AcbShell) | |||
|
| |||
| EtOH Maintenance | 193 ± 15 | 10 ± 1 | 1.4 ± 0.3 |
| Extinction (Day 1) | 178 ± 15 | 15 ± 2 | |
| Base Extinction (last 3 days) | 32 ± 2 | 14 ± 3 | |
|
| |||
| Experiment 4 (A-77636; AcbCore) | |||
|
| |||
| EtOH Maintenance | 202 ± 22 | 10 ± 2 | 1.4 ± 0.2 |
| Extinction (Day 1) | 149 ± 14 | 24 ± 10 | |
| Base Extinction (last 3 days) | 30 ± 3 | 10 ± 1 | |
|
| |||
Effects of SCH 23390 into the Acb shell on responses on the EtOH and water levers
Examining the number of responses on the lever previously associated with the delivery of EtOH (Fig. 2A) indicated a significant effect of ‘session’ (F4, 10 = 3.53; p < 0.048) and ‘session’ by ‘treatment’ interaction (F4, 11 = 13.23; p = 0.001). Comparisons with extinction baseline indicated that P rats administered aCSF or 0.25 μg SCH 23390 directly into the Acb shell responded more on the EtOH lever during the 1st PSR test session, whereas the rats given 1.0 μg responded less (pairwise t-test, p < 0.01). Individual ANOVAs performed for each session indicated a significant effect of ‘dose’ during the 1st PSR test session (F2,13 = 15.53; p < 0.01). Post-hoc comparisons indicated that administration of 0.25 and 1 μg SCH 23390 directly into the Acb shell decreased the number of EtOH lever responses during the 1st PSR test session compared to aCSF, and 1 μg significantly decreased EtOH responding compared to 0.25 μg SCH 23390 (p values < 0.01).
Fig. 2.
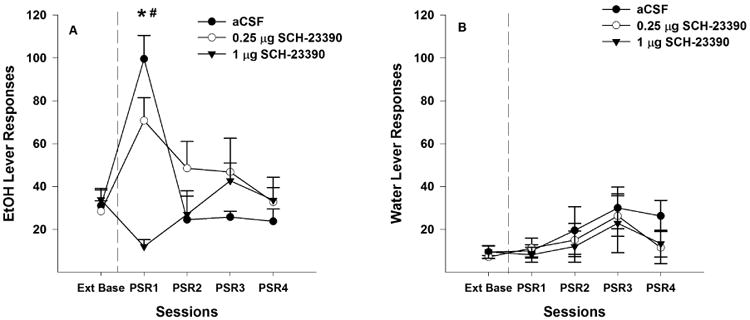
Mean (± S.E.M.) responses per session on the lever previously associated with the delivery of EtOH (A) or water (B) by P rats given aCSF (n = 4), or 0.25 (n=6) or 1 μg/0.5 μl (n = 6) of SCH 23390 into the Acb shell prior to only the 1st PSR session. Asterisk (*) indicates that rats administered aCSF responded significantly (p < 0.05) more on the EtOH lever during the 1st PSR session compared to extinction baseline levels, whereas rats administered 1 μg/0.5 μl of SCH 23390 responded significantly less than extinction baseline. Pound (#) indicates that both doses of SCH 23390 reduced EtOH responding during the 1st PSR session compared to the aCSF group (p<0.05). There were no significant differences with regard to responses on the water lever among the 3 groups.
There were no significant differences (p ≥ 0.49) for responding on the lever previously associated with water during the initial PSR test session for rats given aCSF, 0.25 μg or 1 μg SCH 23390 (Fig. 2B). In addition, there were no significant differences in water lever responses between ‘dose’ groups during PSR session 1.
Effects of SCH 23390 into the Acb core on responses on the EtOH and water levers
P rats administered aCSF or 1 μg SCH 23390 directly into the Acb core responded more on the EtOH lever during the 1st PSR test session (Fig. 3A) than during extinction baseline (pairwise t-test, p ≤ 0.012). Examining the number of responses on the lever previously associated with the delivery of EtOH (Fig. 3A) indicated a significant effect of ‘session’ (F4,4 = 102.99; p < 0.001) and ‘session’ by ‘treatment’ interaction (F4,4 = 3.39; p < 0.01). Individual ANOVAs performed for each session indicated a significant effect of ‘dose’ for extinction base (F1,7 = 7.28; p = 0.031), but there was no significant difference in EtOH lever responses between aCSF and 1 μg SCH 23390 during the 1st PSR test session (F1,7 = 2.00; p = 0.200).
Fig. 3.
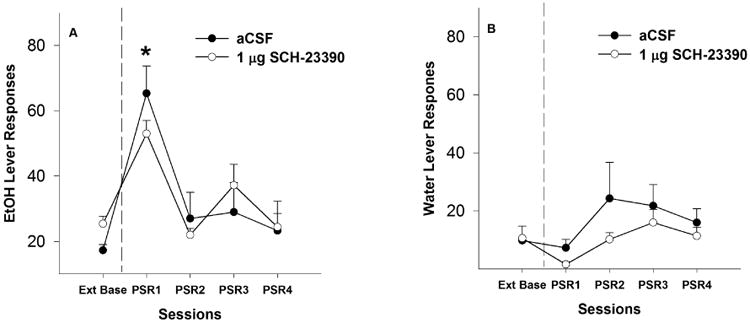
Mean (± S.E.M.) responses per session on the lever previously associated with the delivery of EtOH (A) or water (B) by P rats given aCSF (n = 4) or 1 μg/0.5 μl (n = 5) of SCH 23390 into the Acb core prior to only the first PSR session. Asterisk (*) indicates that rats administered aCSF and 1 μg of SCH 23390 responded significantly (p < 0.05) more on the EtOH lever during the 1st PSR session compared to extinction baseline levels. There were no significant differences between the 2 groups with regard to responses on the EtOH lever. There were no significant differences with regard to responses on the water lever between the 2 groups.
There were no significant differences (p ≥ 0.12) for responding on the lever previously associated with water during the 1st PSR test session for rats given aCSF or 1 μg SCH 23390 (Fig. 3B). In addition, there were no significant differences in water lever responses between ‘dose’ groups during the 1st PSR session.
Effects of A-77636 into the Acb shell on responses on the EtOH and water levers
P rats administered aCSF, or 0.125 or 0.25 μg A-77636 directly into the Acb shell responded more on the EtOH lever during the 1st PSR test session (Fig. 4A) than during the extinction baseline (pairwise t-test, p < 0.01). Examining the number of responses on the lever previously associated with the delivery of EtOH (Fig. 4A) indicated a significant effect of ‘session’ (F4,7 = 48.59; p < 0.001), but there was no significant ‘session’ by ‘treatment’ interaction (F4,8 = 3.18; p = 0.077). Individual ANOVAs performed for each session indicated a significant effect of ‘dose’ during the 1st PSR test session (F2,10 = 5.59; p = 0.023). Post-hoc comparisons indicated that administration of 0.125 and 0.25 μg A-77636 directly into the Acb shell increased the number of EtOH lever responses during the 1st PSR test session compared to aCSF (p values < 0.05).
Fig. 4.
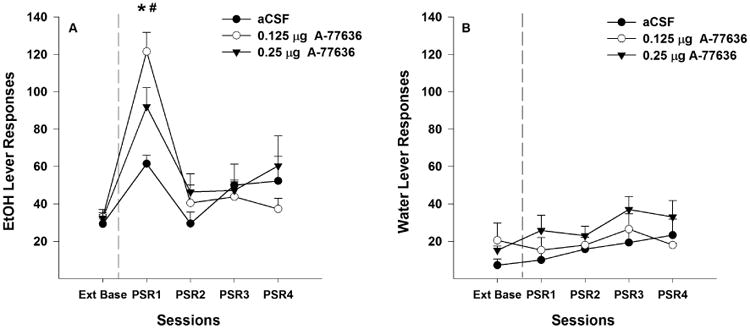
Mean (± S.E.M.) responses per session on the lever previously associated with the delivery of EtOH (A) or water (B) by P rats given aCSF (n = 4), or 0.125 (n = 4) or 0.25 μg/0.5 μl (n = 5) of A-77636 into the Acb shell prior to only the first PSR session. Asterisk (*) indicates that rats given aCSF, or 0.125 or 0.25 μg/0.5μl responded significantly (p < 0.05) more on the EtOH lever during the 1st PSR session compared to extinction baseline levels. Pound (#) indicates that rats given 0.125 or 0.25 μg/0.5 μl of A-77636 responded significantly more during the 1st PSR session than rats given aCSF (p < 0.05). There were no significant differences with regard to responses on the water lever among the 3 groups.
Compared to extinction baseline values, there were no significant differences (p ≥ 0.18) for responding on the lever previously associated with water during the initial PSR test session for rats given aCSF, 0.125 μg or 0.25 μg A-77636 (Fig. 4B). In addition, there were no significant differences in water lever responses between ‘dose’ groups during the 1st PSR session.
Effects of A-77636 into the Acb core on responses on the EtOH and water levers
P rats administered aCSF, or 0.125 or 0.25 μg A-77636 directly into the Acb core responded more on the EtOH lever during the 1st PSR test session (Fig. 5A) than during the extinction baseline (pairwise t-test, p ≤ 0.05). Examining the number of responses on the lever previously associated with the delivery of EtOH (Fig. 5A) indicated a significant effect of ‘session’ (F4,7 = 38.93; p < 0.01), but there was no significant ‘session’ by ‘treatment’ interaction (F4,8 = 2.47; p = 0.128). Individual ANOVAs performed for each session indicated there was no significant effect of ‘dose’ during the 1st PSR test session on responses on the EtOH lever (F2,10 = 0.633; p = 0.551).
Fig. 5.
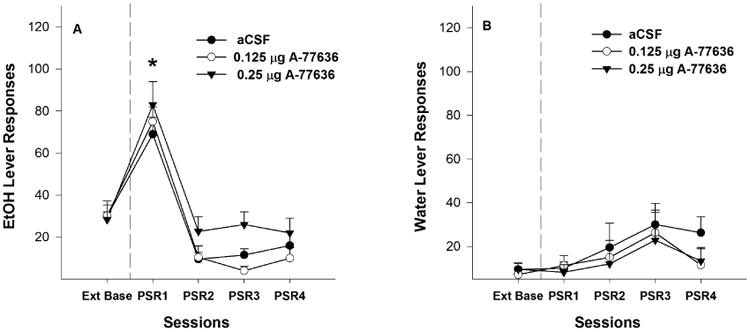
Mean (± S.E.M.) responses per session on the lever previously associated with the delivery of EtOH (A) or water (B) by P rats given aCSF (n = 4), or 0.125 (n = 5) or 0.25 μg/0.5 μl (n =4) of A-77636 into the Acb core prior to only the 1st PSR session. Asterisk (*) indicates that rats given aCSF, or 0.125 or 0.25 μg/0.5 μl of A-77636 responded significantly (p < 0.05) more on the EtOH lever during the 1st PSR session compared to extinction baseline levels; there were no significant group differences. There were no significant differences in responses on the water lever among the 3 groups.
There were no significant differences for responding on the lever previously associated with water during the initial PSR test session (Fig. 5B) for rats given aCSF, or 0.125 or 0.25 μg A-77636 (pairwise t-test, p ≥ 0.24). In addition, there were no significant differences between ‘dose’ groups during the 1st PSR session.
Discussion
The major finding of this study is that the activation of local D1 receptors in the Acb shell (Figs. 2A and 4A), but not in the Acb core (Figs. 3A and 5A), is involved in mediating spontaneous recovery of EtOH-seeking behavior of P rats. The current findings support previous reports indicating that D1 receptors in the Acb shell are involved in mediating EtOH-seeking behavior triggered by environmental context (Chaudhri et al. 2009). However, unlike the findings indicating the involvement of D1 receptors within the Acb core in mediating EtOH-seeking behavior (Chaudhri et al., 2009), the present results did not support a role for D1 receptors within the Acb core in regulating EtOH-seeking.
Microinfusion of SCH 23390 into the Acb shell inhibited responding on the EtOH lever in the PSR test, with the 1 μg dose of SCH 23390 having the most robust effect (Fig. 2A), essentially reducing responding on the EtOH lever below extinction baseline levels. This effect on EtOH lever responding is not likely due to a general effect on motor activity impairment because (a) doses in this range and higher, when administered into the Acb, did not impair responding for sucrose (Bossert et al., 2007) or heroin (Gerrits et al., 1994), (b) the 1-μg dose did not alter responding when administered into the core (which is involved in regulating motor activity), and (c) the 1- μg dose did not reduce responding on the water lever (Fig. 2B).
The reasons for the difference between the results of the current study and the findings of Chaudhri et al. (2009), with regard to the involvement of D1 receptors in the Acb core, may be due to a combination of factors. Most notably would be differences in the behavioral paradigm (PSR after 2 weeks of EtOH abstinence following extinction training vs. environmental context-induced renewal EtOH-seeking within the same day after completion of extinction training), strain of rat (P rats vs. Long Evans rats), and amount of EtOH consumed (P rats averaged 1.4-1.5 g/kg EtOH vs. 0.5-0.6 g/kg EtOH for Long-Evans rats) could account for the differences in the involvement of D1 receptors within the Acb core in mediating EtOH-seeking behavior between the two studies. In addition, differences in the sex between the two studies and possible differences in the DA system between the P and Long-Evans rats could also be factors contributing to the differences observed in the Acb core between the two studies.
The finding of a differential involvement between the Acb shell and core, in mediating EtOH-seeking behavior of P rats (Fig 3A), is compatible with other studies demonstrating differences between the shell and core in supporting the rewarding actions of EtOH (Engleman et al., 2009), and a recent report indicating that activation of D1 receptors within Acb shell, but not the Acb core, is required to maintain the rewarding actions of EtOH within posterior VTA (Ding et al., 2014). Activation of posterior VTA DA neurons is needed for expression of spontaneous recovery of EtOH-seeking behavior (Hauser et al., 2011). Taken together, these results suggest the EtOH rewarding and EtOH-seeking behaviors of P rats may share common neuronal circuits, and that activation of D1 receptors in the Acb shell is likely part of the feed forward pathway involved in processing information from the posterior VTA DA neurons to brain regions mediating the expression of EtOH-seeking behavior.
However, the present results do not rule out the involvement of the Acb core in mediating EtOH-seeking behavior of P rats. The Acb shell has been implicated in spatial/contextual drug-seeking behavior (Bossert et al. 2007; Fuchs et al. 2008; Chaudhri et al. 2009), whereas the Acb core appears to be more involved in mediating cue- induced drug-seeking behavior (Fuchs et al. 2004; Bossert et al. 2007; Hollander and Carelli, 2007; Chaudhri et al. 2008) and mediating the incentive value of reward-conditioned stimuli (Ito et al. 2000, 2004). The Acb shell and core have been shown to have distinct functional circuits as well some overlap in mediating drug-seeking behaviors. The inactivation of the Acb core, but not the Acb shell, is involved in mediating cue-induced EtOH-seeking (Chaudhri et al., 2010). In addition, cue-induced EtOH-seeking can increase extracellular glutamate levels in the Acb core, thus providing evidence that Acb core circuits may be involved in discrete –cue induced EtOH-seeking behavior (Gass et al. 2011). It has also been reported that antagonism of metabotropic glutamate 5 (mGlu5) receptors in the Acb core can reduce EtOH- seeking behaviors (Sinclair et al., 2012). Future studies should determine if the Acb core is involved in mediating EtOH-seeking behavior of P rats via discrete-cues, and/or if other DA receptors or neuronal systems projecting to the Acb core may be involved in expression of spontaneous recovery of EtOH-seeking behavior by the P rat.
In order to further determine the involvement of D1 receptors in the Acb in mediating EtOH-seeking behavior, A-77636, a D1 receptor agonist (Kebabian et al. 1992; Domino and Sheng, 1993) was microinfused into the Acb shell or Acb core. Local application of A-77636 into the Acb shell enhanced responding on the EtOH lever (Fig. 4A) without altering water responses (Fig. 4B), whereas A-77636 infused into the Acb core did not alter EtOH- (Fig. 4A) or water-(Fig. 4B) lever responding. These results demonstrated that stimulation of D1 receptors within the Acb shell can enhance EtOH-seeking behavior, and provided further evidence that D1 receptors within the Acb shell, but not the Acb core, are involved in regulating EtOH-seeking behavior of P rats under spontaneous recovery conditions. Several studies have reported effects of A-77636 on cocaine- induced expression of c-Fos in the Acb and striatum (Asin et al., 1994), cocaine-induced locomotor activity (Asin et al., 1994; Chausmer and Katz, 2002), and cocaine drug discrimination tests (Chausmer and Katz, 2002). The current study is the first that examined the effects of A-77636 on EtOH-seeking behavior.
Conclusion
The current findings suggest that activation of D1 receptors within the Acb shell, but not the Acb core, is involved in mediating the PSR of EtOH-seeking behavior of P rats.
Highlights.
SCH 23390, a D1 receptor antagonist, into nucleus accumbens shell reduced EtOH-seeking in alcohol preferring (P) rats.
A-77636, a D1 receptors agonist, into nucleus accumbens shell enhanced EtOH-seeking in alcohol preferring (P) rats.
D1 receptor agents into the nucleus accumbens core did not alter EtOH-seeking in alcohol preferring (P) rats.
D1 receptors within nucleus accumbens shell, but not the core, are involved in mediating EtOH-seeking behavior of P rats.
Acknowledgments
The skillful technical assistance of Tylene Pommer and Victoria McQueen is gratefully acknowledged. This research was supported in part by NIAAA grants AA007611, AA022287, AA0200908, AA007462 and AA019366.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Sources of support: This project was supported by research grants AA007611, AA022287, AA020908, AA007462 and AA019366 from NIAAA. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
None of the authors has a conflict of interest associated with this research
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Asin KE, Nikkel AL, Wirtshafter D. Repeated D1 receptor agonist treatment blocks cocaine-induced locomotor activity and c-fos expression. Brain Res. 1994;637:342–344. doi: 10.1016/0006-8993(94)91258-0. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, Ambiguity, and unlearning: Sources of relapse after behavioral extinction. Bio Psych. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and ambiguity in the extinction of emotional learning:implications for exposure therapy. Behav Res Ther. 1988;26:137–49. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC. Recent and remote extinction cues reduce spontaneous recovery. Q J Exper Psycho. 2000;153:25–58. doi: 10.1080/027249900392986. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in contextual- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental contextual is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental contextual and requires the nucleus accumbens core. Eur J Neurosci. 2008;28:2288–2298. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausmer AL, Katz JL. Comparison of interactions of D1-like agonists, SKF 81297, SKF 82958 and A-77636, with cocaine: locomotor activity and drug discrimination studies in rodents. Psychopharmacology (Berl) 2002;159:145–153. doi: 10.1007/s002130100896. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The orexin-1 receptor antagonist SB-334867 reduces alcohol relapse drinking, but not alcohol-seeking, in Alcohol-Preferring (P) rats. J Addict Med. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Ingraham CM, Rodd ZA, McBride WJ. The reinforcing effects of ethanol within the posterior ventral tegmental area depend on dopamine neurotransmission to forebrain cortico-limbic systems. Addict Biol. 2014 doi: 10.1111/adb.12138. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Sheng J. Relative potency and efficacy of some dopamine agonists with varying selectivities for D1 and D2 receptors in MPTP-induced hemiparkinsonian monkeys. J Pharmacol Exp Ther. 1993;265:1387–1391. [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–56. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Lê AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16:215–28. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MA, Ramsey NF, Wolterink G, van Ree JM. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology. 1994;114:486–494. doi: 10.1007/BF02249340. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–36. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, Rodd ZA. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336:857–65. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–9. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–97. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–95. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian JW, Britton DR, DeNinno MP, Perner R, Smith L, Jenner P, Schoenleber R, Williams M. A-77636: a potent and selective dopamine D1 receptor agonist with antiparkinsonian activity in marmosets. Eur J Pharmacol. 1992;229:203–209. doi: 10.1016/0014-2999(92)90556-j. [DOI] [PubMed] [Google Scholar]
- Levy AD, Murphy JM, McBride WJ, Lumeng L, Li TK. Microinjection of sulpiride into the nucleus accumbens increases ethanol drinking in alcohol-preferring (P) rats. Alcohol Alcohol Suppl. 1991;1:417–420. [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–9. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats – animal models of alcoholism. Alcohol. 2014;48:209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Dopamine receptor regulation of ethanol intake and extracellular dopamine levels in the ventral pallidum of alcohol preferring (P) rats. Drug Alcohol Depend. 2005;77:293–301. doi: 10.1016/j.drugalcdep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Research Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Robbins SJ. Mechanisms underlying spontaneous recovery in authoshaping. J Exper Psychol Anim Behav Processes. 1990;16:235–249. [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–15. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav. 2012;101:329–35. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–74. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


