Summary
PlsX is an acyl-acyl carrier protein (ACP):phosphate transacylase that interconverts the two acyl donors in Gram-positive bacterial phospholipid synthesis. The deletion of plsX in Staphylococcus aureus results in a requirement for both exogenous fatty acids and de novo type II fatty acid biosynthesis. Deletion of plsX (SP0037) in Streptococcus pneumoniae did not result in an auxotrophic phenotype. The ΔplsX S. pneumoniae strain was refractory to myristic acid-dependent growth arrest, and unlike the wild-type strain, was susceptible to fatty acid synthesis inhibitors in the presence of exogenous oleate. The ΔplsX strain contained longer-chain saturated fatty acids imparting a distinctly altered phospholipid molecular species profile. An elevated pool of 18- and 20-carbon saturated fatty acids was detected in the ΔplsX strain. A S. pneumoniae thioesterase (TesS, SP1408) hydrolyzed acyl-ACP in vitro, and the ΔtesS ΔplsX double knockout strain was a fatty acid auxotroph. Thus, the TesS thioesterase hydrolyzed the accumulating acyl-ACP in the ΔplsX strain to liberate fatty acids that were activated by fatty acid kinase to bypass a requirement for extracellular fatty acid. This work identifies tesS as the gene responsible for the difference in exogenous fatty acid growth requirement of the ΔplsX strains of S. aureus and S. pneumoniae.
Keywords: fatty acid synthesis, PlsX, PlsY, acyl carrier protein, acyl-phosphate, S. pneumoniae
Introduction
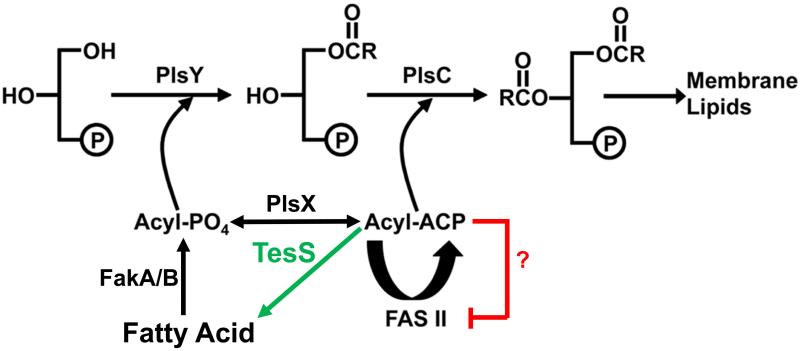
Phosphatidic acid is a universal intermediate in the biosynthesis of membrane phospholipids in eubacteria (Yao and Rock, 2013). In Gram-positive pathogens, such as Streptococcus pneumoniae and Staphylococcus aureus, phosphatidic acid is generated by the PlsX/PlsY/PlsC pathway (Fig. 1). PlsX is a soluble acyl-acyl carrier protein (ACP):PO4 transacylase that converts the acyl-ACP end-products of de novo fatty acid synthesis (FASII) to their acyl-PO4 derivatives (Lu et al., 2006). These activated fatty acids are then used by the integral membrane protein PlsY to acylate glycerol-3-phosphate (Lu et al., 2006; Lu et al., 2007). The second acyltransferase, PlsC (Coleman, 1992), acylates the 2-position and is specific for acyl-ACP thioesters as acyl donors (Lu et al., 2006). Exogenous fatty acids can access these acyltransferase systems after their uptake by the cell and activation by fatty acid kinase (Parsons et al., 2014a). Fatty acid kinase is composed of two subunits: a kinase domain protein (FakA) and a fatty acid binding protein (FakB) that work together to produce acyl-PO4. The acyl-PO4 arising from extracellular fatty acids is either incorporated into the 1-position by PlsY or converted to acyl-ACP by PlsX for either elongation by FASII or utilization by PlsC (Fig. 1) (Parsons et al., 2011). Thus, PlsX is a key enzyme that interconverts the two acyltransferase acyl donors.
Fig. 1.
Proposed thioesterase (TesS) bypass of PlsX in S. pneumoniae.
The known proteins in fatty acid metabolism in Gram-positive bacteria are shown in black. PlsX plays a key role in fatty acid metabolism by interconverting acyl-ACP and acyl-PO4. However, S. pneumoniae strains lacking PlsX are not fatty acid auxotrophs. Acyl-PO4 is postulated to be supplied by the action of a S. pneumoniae thioesterase (TesS, green) on acyl-ACP from type II fatty acid synthesis (FASII) that would accumulate in strains lacking PlsX. Acyl-ACP is proposed as a feedback regulator of FASII (red), but the regulation of specific enzymes steps has not been demonstrated in vitro. The liberated free fatty acids are converted to acyl-PO4 by fatty acid kinase (FakA/B) and incorporated into the 1-position of phospholipids by the sn-glycerol-3-phosphate acyltransferase PlsY. The second acyltransferase (PlsC) uses acyl-ACP from FASII as the acyl donor.
The deletion of the plsX gene in S. aureus results in a strain that is a fatty acid auxotroph (Parsons et al., 2014b). The extracellular fatty acids are used for the acylation of the 1-position following fatty acid kinase activation to acyl-PO4. However, they cannot be converted to acyl-ACP and elongated due to the absence of PlsX. This means that FASII is also required in ΔplsX S. aureus strains to provide acyl-ACP for the acylation of the 2-position (Fig. 1). Insertions in the plsX gene were identified in a recent transposon mutagenesis screen to identify genes that are important for S. pneumoniae survival in saliva (Verhagen et al., 2014). This deletion did not have a discernible growth phenotype suggesting a distinct difference in lipid metabolism between S. aureus and S. pneumoniae that is not apparent from previous research.
The goal of this study was to determine the difference in lipid metabolism that accounts for the distinctive behavior of the plsX deletions in these two strains. Although the S. pneumoniae ΔplsX strain grew normally, it had an altered fatty acid and phospholipid molecular species profile and an elevated free fatty acid pool. We postulated that the unique behavior of the S. pneumoniae ΔplsX strain was due to the expression of a thioesterase (TesS, SP1408) that produces intracellular fatty acids from the acyl-ACP that accumulates arising from the PlsX block. The fatty acids are activated by fatty acid kinase (FakA/B) to bypass a requirement for extracellular fatty acids (Fig. 1). The phenotype of the ΔtesS ΔplsX double knockout strain verifies that the key difference between the two organisms is the expression of the TesS thioesterase.
Results
Growth phenotypes of the S. pneumoniae ΔplsX knockout
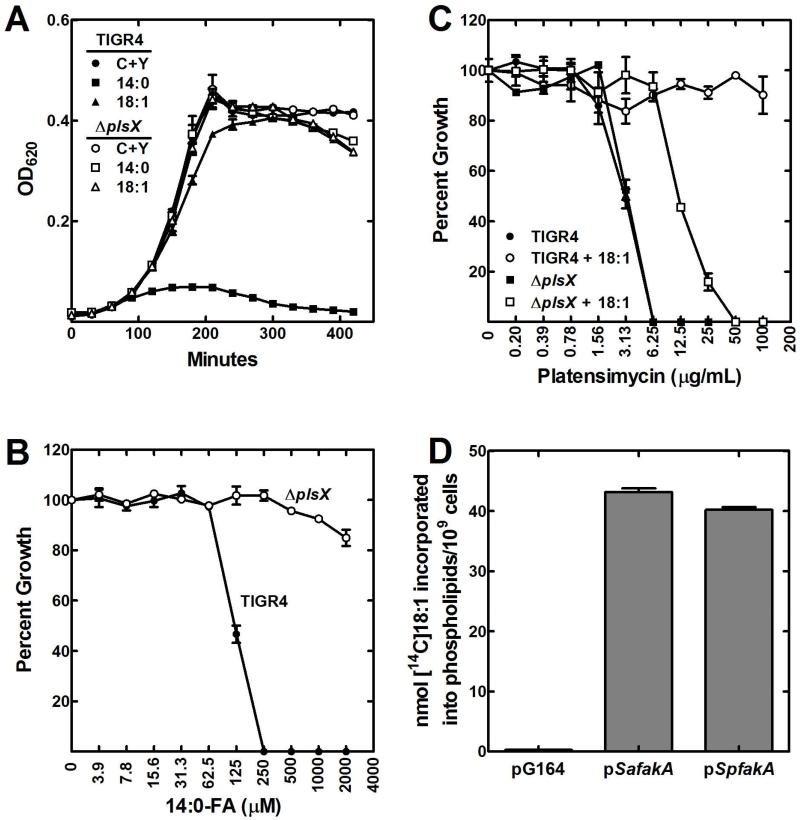
First, a plsX knockout was constructed in strain TIGR4 by allelic exchange of plsX with a spectinomycin cassette using a megaprimer PCR method. Strain MDJ01 (ΔplsX) was not a fatty acid auxotroph and grew normally on CY/BSA medium (Fig. 2A). S. pneumoniae strains shut off FASII when exposed to extracellular fatty acids and their membranes become composed entirely of the supplied fatty acid (Parsons et al., 2011). Growth on oleate (18:1Δ9) resulted in >90% of the phospholipid fatty acid being 18:1Δ9 (Table 1). A similar compositional change occurs in cells grown with exogenous 14:0, although in this case there was also significant elongation to longer-chain saturated fatty acids (Table 1). However, supplementation with this fatty acid causes the membrane phospholipids to become highly saturated leading to growth arrest (Fig. 2A). A characteristic phenotype of the ΔplsX strain was that it was refractory to growth inhibition by exogenous 14:0 (Fig. 2A). This observation was corroborated by determining the apparent 14:0 MIC for wild-type strain TIGR4 (250 μM), whereas strain MDJ01 (ΔplsX) was refractory to growth arrest by 14:0 at all concentrations examined (Fig. 2B). The fatty acid composition of MDJ01 grown with 14:0 showed that the phospholipids were not composed entirely of saturated fatty acids. Rather, about 50% of the fatty acids remained unsaturated and they were paired with 14:0 to produce a fatty acid composition that was compatible with growth (Table 1). The inability of 14:0 supplementation to replace the unsaturated fatty acids in the membrane phospholipids of the ΔplsX strain explained why it was refractory to 14:0 growth inhibition.
Fig. 2.
Growth phenotypes of S. pneumoniae strain MDJ01 ΔplsX).
A. Growth of S. pneumoniae strains TIGR4 (wild-type) and MDJ01 (ΔplsX) in CY/BSA medium supplemented with either 1 mM 18:1Δ9 or 500 μM 14:0 fatty acids.
B. Determination of the minimum inhibitory concentration of 14:0 fatty acid for wild-type strain TIGR4 and MDJ01 (ΔplsX) grown in CY/BSA (C+Y) medium.
C. Determination of minimum inhibitory concentration of platensimycin in wild-type strain TIGR4 and MDJ01 (ΔplsX) grown in CY/BSA medium with and without a 1 mM 18:1Δ9 supplement. Platensimycin is a potent inhibitor of the elongation condensing enzyme (FabF) of FASII.
D. The S. pneumoniae fakA (SP0443) gene encodes a functional fatty acid kinase. Unlike in S. aureus, S. pneumoniae fakA is an essential gene. Therefore its function as a fatty acid kinase in fatty acid uptake was evaluated by expressing SpFakA in a S. aureus ΔfakA strain. Like its S. aureus counterpart, SpFakA expression restored [14C]oleate (18:1Δ9) incorporation into phospholipid when expressed in a ΔfakA background.
Table 1.
Fatty acid composition (weight %) of strains TIGR4, MDJ01 (ΔplsX) and MDJ02 (ΔtesS) grown in CY/BSA media without or with a fatty acid supplement. (18:1Δ9, 17:1Δ10, or 14:0)
| TIGR4 |
Δ
plsX
|
Δ
tesS
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid |
None | +18:1a | +17:1Δ10 | +14:0 | None | +18:1 | +17:1Δ10 | +14:0 | None |
| 14:0 | — b | — | — | 82.7 | — | — | — | 18.9 | 1.9 |
| 16:0 | 24.8 | 4.4 | 5.6 | 12.0 | 8.1 | 6.3 | 5.4 | 2.7 | 22.6 |
| 16:1 | 9.4 | 0.8 | 1.2 | 1.4 | 0.9 | — | 0.4 | 0.8 | 14.4 |
| 17:1 | — | — | 81.3 | — | — | — | 38.6 | — | — |
| 18:0 | 10.6 | 4.6 | 7.2 | 1.8 | 30.0 | 20.4 | 18.5 | 18.3 | 10.8 |
| 18:1 | 55.2 | 90.2 | 4.7 | 2.1 | 51.8 | 64.7 | 25.5 | 50.1 | 49.7 |
| 20:0 | — | — | — | — | 4.6 | 4.1 | 7.2 | 4.6 | — |
| 20:1 | — | — | — | — | 4.7 | 4.5 | 4.4 | 4.6 | — |
Fatty acid concentrations were: 18:1Δ9, 1 mM; 17:1Δ10, 1 mM; and 14:0, 0.5 mM.
— means not detected (<0.2%). The reported values are rounded to the nearest 0.1%.
Platensimycin is a potent inhibitor of the FabF elongation condensing enzyme of bacterial FASII (Wang et al., 2006). Growth of wild-type TIGR4 was arrested by this antibiotic, but the strain was refractory to platensimycin inhibition in the presence of 18:1Δ9 (Fig. 2C) (Parsons et al., 2011). Strain MDJ01 (ΔplsX) was as sensitive to the antibiotic as wild-type when grown in the absence of an exogenous fatty acid supplement (Fig. 2C). Although the MIC increased, the ΔplsX strain remained sensitive to platensimycin growth inhibition even when 18:1 was present (Fig. 2C). These data showed that the ΔplsX strain required de novo FASII even when provided with sufficient extracellular fatty acids to support growth in the absence of pathway activity. Exogenous fatty acids are activated by fatty acid kinase in S. aureus (Parsons et al., 2014a). Fatty acid kinase is composed of two proteins, FakA, a kinase domain protein, and FakB, a fatty acid binding protein. In S. aureus fakA is not essential, but in S. pneumoniae it is (van Opijnen T. and Camilli, 2012). We therefore assessed the function of FakA in S. pneumoniae by expressing SpFakA in a S. aureus ΔfakA strain. Expression of SpFakA restored fatty acid activation and incorporation into the S. aureus ΔfakA strain (Fig. 2D) illustrating that fatty acid uptake via fatty acid kinase was also operating in S. pneumoniae.
The lipid composition phenotype of strain MDJ01 (ΔplsX)
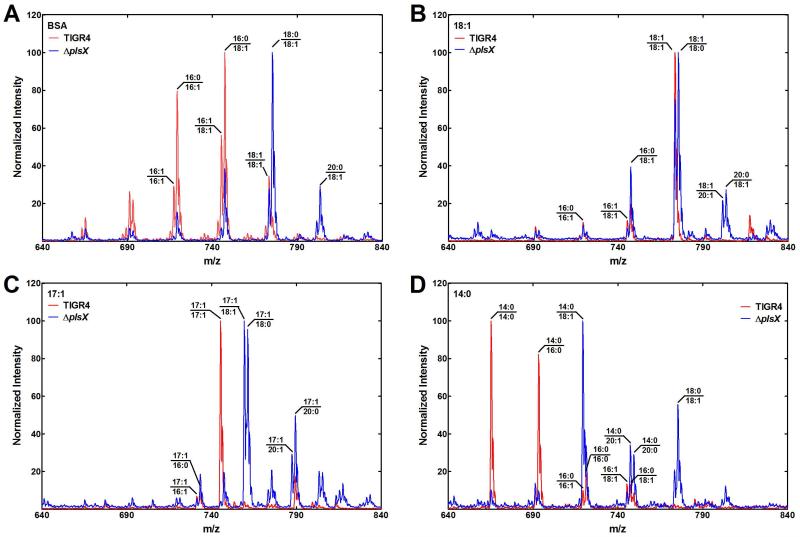
A lipidomic analysis of the ΔplsX strain grown under different conditions was carried out to determine the impact of PlsX inactivation on the membrane lipid composition. Although there was no discernible growth phenotype in the ΔplsX mutant, there was a pronounced fatty acid compositional change (Table 1). Overall the fatty acid chain-lengths are longer resulting in the appearance of 20-carbon fatty acids that were not detected in the wild-type parent. This effect is clearly illustrated by the changes in the phosphatidylglycerol (PtdGro) molecular species in the two different strains (Fig. 3A). The predominant molecular species in the wild-type strain containing 16-carbon fatty acids were shifted to species containing 18- and 20-carbon fatty acids. Growth of wild-type TIGR4 with exogenous 18:1 converted the diversity of molecular species seen on minimal medium to mostly the di-18:1 variety (Fig. 3B). This did not occur in strain MDJ01 (ΔplsX) where 18:1/18:0 was the major molecular species (Fig. 3B). Long-chain saturated fatty acids (18 and 20) appear in the lipids of the ΔplsX strain whether it is supplemented with 18:1 (Fig. 3B) or not (Fig. 3A). Because one cannot distinguish the endogenously produced 18:1Δ11 and the exogenously supplied 18:1Δ9 by mass spectrometry, we also grew the strains in the presence of 17:1Δ10 and analyzed the molecular species (Fig. 3C). As anticipated, the wild-type strain consisted of mostly di-17:1 molecular species when grown with 17:1Δ10. However, in the ΔplsX strain, the exogenous fatty acid was not elongated and was paired with a saturated or unsaturated fatty acid derived from FASII. This experiment showed that 17:1 was not converted to acyl-ACP in the ΔplsX strain and flowed into the 1-position to be paired with an endogenously produced saturated or unsaturated fatty acid. Finally, growth of strain TIGR4 on 14:0 suppressed de novo FASII leading to the production of only saturated PtdGro molecular species (Fig. 3D). In these experiments, a proportion of 14:0 was also elongated to 16:0. The conversion of the membrane phospholipids to primarily di-saturated molecular species explains the growth arrest of strain TIGR4 when grown in the presence of 14:0. In contrast, 14:0 was not elongated by the ΔplsX strain and was most often paired with an unsaturated fatty acid creating a molecular species profile compatible with growth (Fig. 3D).
Fig. 3.
Impact of fatty acid supplementation on phosphatidylglycerol (PtdGro) molecular species.
The wild-type strain TIGR4 (red traces) and MDJ01 (ΔplsX) (blue traces) were grown in the presence of the indicated fatty acid in CY/BSA medium and the PtdGro molecular species, determined by mass spectrometry as described under Experimental Procedures.
A. Strains were grown in CY/BSA medium without a fatty acid supplement.
B. Strains were grown in CY/BSA medium supplemented with 18:1Δ9 (1 mM).
C. Strains were grown in CY/BSA medium supplemented with 17:1Δ10 (1 mM).
D. Strains were grown in CY/BSA medium supplemented with 14:0 (0.5 mM).
Positional placement of endogenous fatty acids
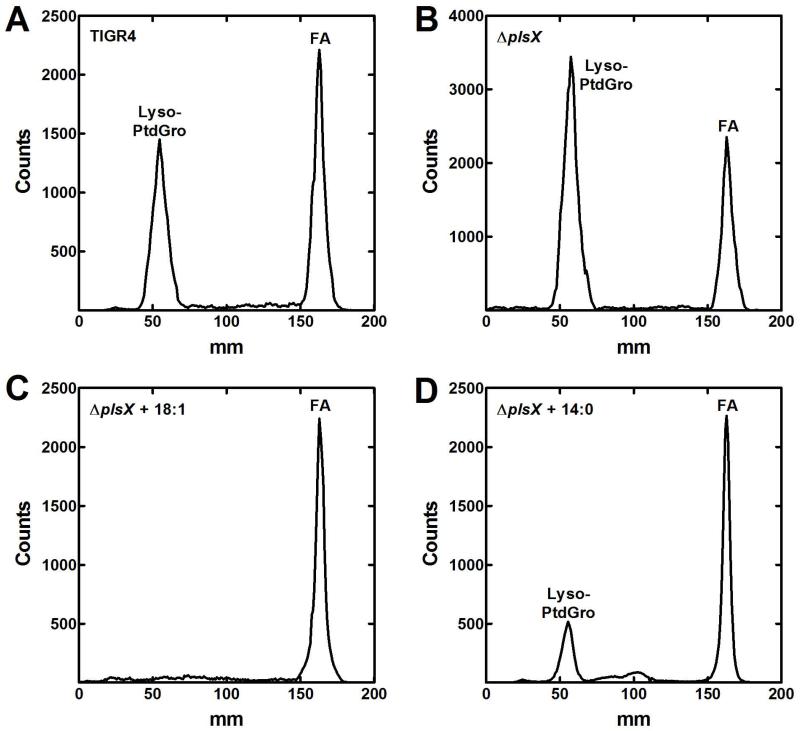
The positional distribution of exogenous fatty acids incorporated into phospholipids was determined to verify the biochemical pathway illustrated in Fig. 1. S. pneumoniae strains TIGR4 and MDJ01 (ΔplsX) were grown in CY/BSA medium containing [14C]acetate supplemented with or without fatty acids to determine how FASII-derived fatty acids were incorporated into the sn-1 and sn-2 positions of PtdGro in the wild-type and mutant strains (Fig. 4). As expected, TIGR4 grown in the absence of a fatty acid supplement showed that the de novo synthesized fatty acids were distributed between the 1- and 2-positions (Fig. 4A). The ΔplsX strain also showed an even distribution, reflecting a PlsX-independent pathway for acyl-ACP from FASII to be converted to acyl-PO4 (Fig. 4B). The analysis of the ΔplsX strain grown with 18:1Δ9 demonstrated that all the endogenous fatty acids were in the sn-2 position (Fig. 4C), consistent with acyl-ACPs supplying PlsC to acylate the sn-2 position of lyso-phosphatidic acid, and exogenous fatty acids acylating the sn-1 position of the glycerol backbone. The result was similar when strain MDJ01 (ΔplsX) was grown with 14:0 (Fig. 4D), although there was a small amount of endogenous fatty acids in the sn-1 position also. This observation was consistent with the presence of an endogenously synthesized 18:0/18:1 lipid observed in the mass spectrometry analysis of the ΔplsX strain in Fig. 3D. Strain TIGR4 grown with 18:1 could not be analyzed due to the lack of acetate incorporation via FASII when wild-type S. pneumoniae was grown with a fatty acid supplement (Parsons et al., 2011). These data were consistent with the biochemical pathway illustrated in Fig. 1, showed that suppression of FASII by exogenous fatty acids required a functional PlsX, and supported the existence of a PlsX-independent pathway for the synthesis of acyl-PO4.
Fig. 4.
Positional distribution of [14C]acetate incorporation in the presence of endogenous fatty acids.
Wild-type strain TIGR4 and MDJ01 (ΔplsX) were grown without or with different fatty acid supplements and labeled with [14C]acetate (5 μCi/ml) for 3 hours in mid-log phase in CY/BSA medium. Lipids were extracted and PtdGro isolated by thin-layer chromatography and subjected to digestion with Naja naja phospholipase A2. The digested samples were separated on Silica Gel H thin-layers developed with chloroform/methanol/acetic acid (55/20/5, v/v/v). The distribution of label between the 1- and 2-positions was determined using a Bioscan Imaging detector. The lyso-PtdGro (1-position) peak and fatty acid (FA, 2-position) peaks are labeled.
A. Strain TIGR4 labeled with [14C]acetate without a fatty acid supplement.
B. Strain MDJ01 (ΔplsX) labeled with [14C]acetate in the absence of a fatty acid supplement.
C. Strain MDJ01 (ΔplsX) labeled with [14C]acetate in the presence of 1 mM 18:1Δ9.
D. Strain MDJ01 (ΔplsX) labeled with [14C]acetate in the presence of 0.5 mM 14:0.
A fatty acid pool in strain MDJ01 (ΔplsX)
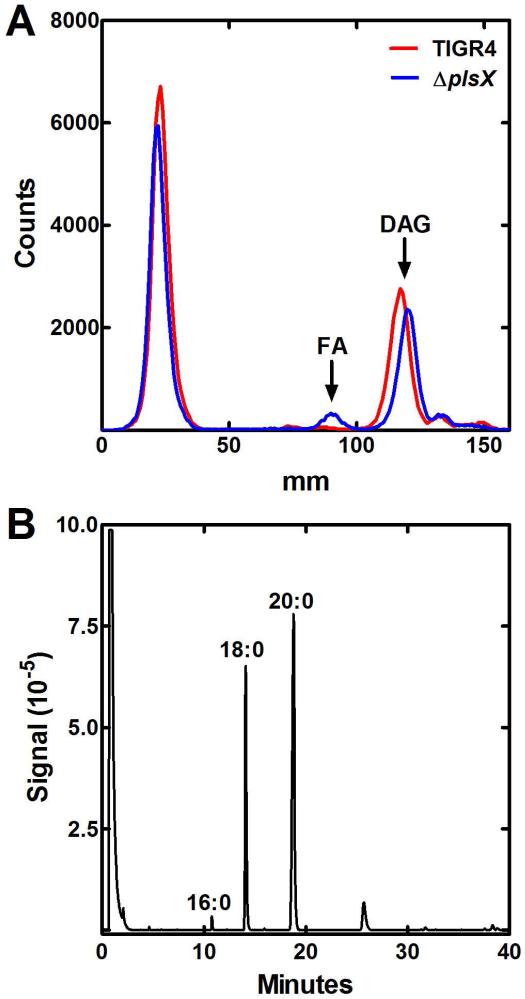
If free fatty acids are being generated to bypass PlsX using fatty acid kinase (Fig. 1), then a fatty acid pool should be present in the ΔplsX strain that is absent in the parent. When the S. aureus ΔplsX mutant was deprived of an exogenous fatty acid supplement, acyl-ACPs accumulated due to the absence of acyl-PO4 needed for PlsY to initiate phospholipid biosynthesis (Parsons et al., 2014b). A potential pathway for the bypass of PlsX in strain MDJ01 (ΔplsX) was through the production of free fatty acid from FASII derived acyl-ACPs that were postulated to accumulate in the plsX knockout. The liberated free fatty acid could be phosphorylated by fatty acid kinase, and the acyl-PO4 utilized by PlsY (Fig. 1). Strains TIGR4 and its ΔplsX derivative were grown in CY/BSA medium supplemented with [14C]acetate. The labeled neutral lipid pool was analyzed by thin-layer chromatography. Diacylglycerol (DAG) was the major neutral lipid in both S. pneumoniae strains (Fig. 5A). A peak that co-migrated with the fatty acid standard was detected in ΔplsX strain but not in strain TIGR4 (Fig. 5A). The fatty acids were isolated by preparative thin-layer chromatography, and the methyl esters analyzed by gas-liquid chromatography to identify and quantify the fatty acid species (Fig. 5B). The fatty acid pool in the ΔplsX strain consisted of a series of saturated fatty acids predominately 18:0 and 20:0. These abnormally long chain lengths arose because the utilization of acyl-ACP end products by the acyltransferases was blocked in the ΔplsX strain allowing additional elongation of the acyl-ACP by FASII. This phenomenon was first described in E. coli plsB mutants (Cronan, et al., 1975), and many further experiments have led to the conclusion that competition between elongation and acyltransfer is the primary determinant of fatty acid chain length in bacteria (for review, see Parsons and Rock, 2013). The presence of these unusually long-chain fatty acids reflected the shift in PtdGro molecular species composition characteristic of the ΔplsX strain (Table 1; Fig. 3A). These data suggested the existence of an acyl-ACP thioesterase that cleaved acyl-ACP arising from FASII that would otherwise accumulate in cells lacking a PlsX enzyme.
Fig. 5.
A free fatty acid pool in S. pneumoniae MDJ01 (ΔplsX).
A. Strains TIGR4 (red trace) and MDJ01 (ΔplsX) (blue trace) were labeled for 4 hours in CY/BSA medium containing [14C]acetate (5 μCi/ml) during logarithmic growth. Lipids were extracted and analyzed by thin-layer chromatography on Silica Gel G layers developed with chloroform/methanol/acetic acid (90/10/10, v/v/v). Diacylglycerol (DAG) was the major neutral lipid. A new peak of radioactivity corresponding to free fatty acids (FA) was detected in strain MDJ01.
B. The free fatty acid fraction from strain MDJ01 (ΔplsX) was isolated by preparative thin-layer chromatography and the composition was determined by gas-liquid chromatography of the derived fatty acid methyl esters. Only saturated acyl chains were detected and their chain-lengths are indicated on the figure.
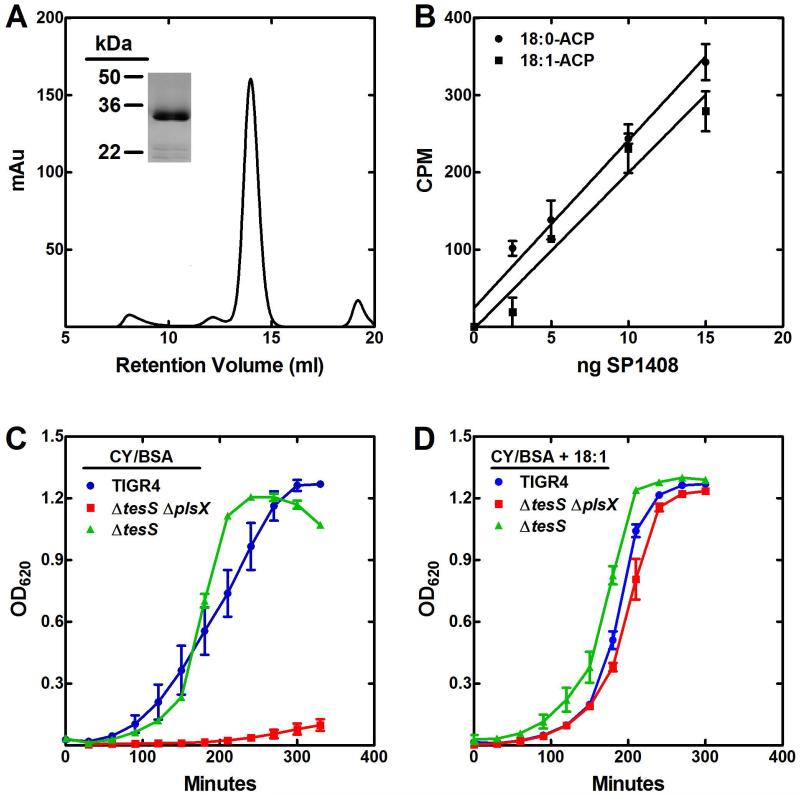
S. pneumoniae thioesterase
The presence of fatty acid suggested the expression of a protein with thioesterase activity that cleaved acyl-ACP to fatty acids. A gene was recently described in Streptococcus pyogenes that was identified as an acyl-ACP thioesterase (Lee et al., 2013). Based on the fatty acids secreted by an E. coli strain expressing the thioesterase, the enzyme hydrolyzed 12:0 and 14:0 saturated acyl-ACP and unsaturated acyl-ACP to a lesser extent. A homologous gene was identified in S. pneumoniae (52% identical) as locus tag SP1408. We named the gene tesS (thioesterase Streptococcus). TesS was expressed and purified from E. coli. The TesS sequence suggests that it belongs to the family of proteins containing a “hot dog” fold, which usually exist as dimers. Analytical gel filtration chromatography was consistent with this interpretation (Fig. 6A), and the purity of the protein preparation is illustrated in Fig. 6A (inset). TesS was tested for its ability to hydrolyze [14C]18:0-ACP and [14C]18:1-ACP in an acyl-ACP thioesterase assay. TesS hydrolyzed both species of acyl-ACP with similar efficiency (approximately 0.66 μmol/min/mg for 18:0-ACP and 0.68 μmol/min/mg for 18:1-ACP) under our reaction conditions (Fig. 6B). One reason to test these two substrates was to determine if TesS was selectively active on saturated acyl-ACP. However, there was no difference between the specific activities of TesS when assayed using E. coli acyl-ACP substrates. The low specific activities indicated that TesS was a poor acyl-ACP thioesterase. We also tested the activity of TesS with [14C]18:1-CoA. The specific activity was estimated to be 4.0 μmol/min/mg. The 6-fold higher activity with acyl-CoA compared to acyl-ACP illustrated the lack of ACP specificity for TesS. TesS activity against acyl-CoA was also not very robust and the idea that TesS is an acyl-CoA thioesterase seems unlikely because there is no known role for long-chain acyl-CoA in either S. aureus or S. pneumoniae metabolism (Parsons et al., 2014b). Quantitative real-time PCR was used to determine the tesS transcript levels relative to gyrA using the ΔCt method. The relative expression of tesS in the wild-type strain was 0.58 ± 0.09, and in the ΔplsX strain the ΔCt was 0.20 ± 0.05. Thus, there was no induction of tesS expression in the plsX knockout. Strain MDJ02 (ΔtesS) was constructed to determine if the absence of TesS alters phospholipid structure. We found no notable differences in the fatty acid composition of strains TIGR4 and its ΔtesS derivative (Table 1). Thus, TesS did not impact the function of FASII or phospholipid synthesis when all the normal components were present. The data suggested that in the absence of PlsX, TesS was able to hydrolyze acyl-ACP derived from FASII to bypass PlsX, but it had no impact on FASII under normal circumstances. TesS could be an esterase, and the true in vivo substrate for TesS remains unknown.
Fig. 6.
TesS (SP1408) is a thioesterase
A. Analysis of purified TesS by gel filtration chromatography. The elution position of TesS was consistent with it existing as a dimer. SDS gel electrophoresis (inset) shows the purity of the TesS preparation.
B. Acyl-ACP thioesterase assay using [14C]18:0-ACP and [14C]18:1-ACP.
C. Growth of strains TIGR4 (blue), MDJ02 (ΔtesS) (green) and MDJ03 (ΔtesS ΔplsX) (red) in CY/BSA medium without a fatty acid supplement.
D. Growth of strains TIGR4 (blue), MDJ02 (ΔtesS) (green) and MDJ03 (ΔtesS ΔplsX) (red) in CY/BSA medium supplemented with 1 mM 18:1Δ9.
Strain MDJ03 (ΔtesS ΔplsX) was a fatty acid auxotroph
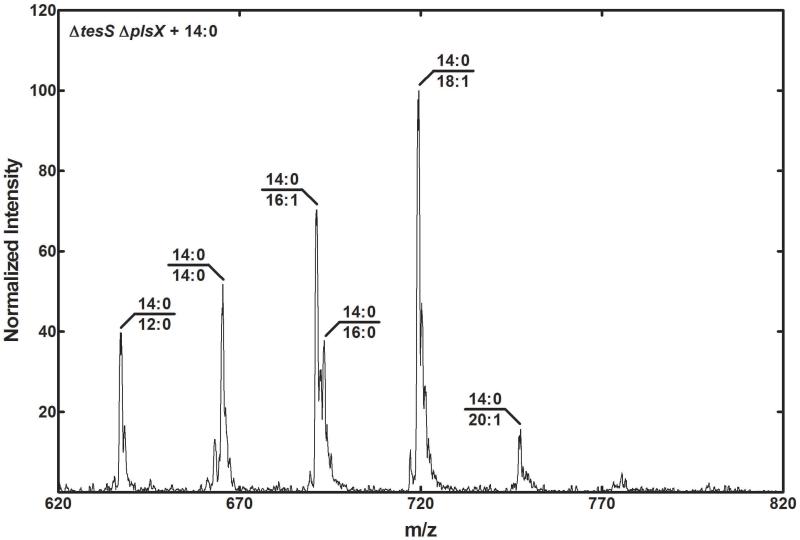
If TesS expression underlies the growth phenotype of the ΔplsX strain, then the double ΔtesS ΔplsX should be a fatty acid auxotroph. Our previous work revealed that a S. aureus ΔplsX strain required exogenous fatty acid for growth and was unable to hydrolyze the acyl-ACP that accumulates from FASII (Parsons, et al., 2014b). There is no homolog for tesS in S. aureus. To determine if the difference in fatty acid auxotrophy between S. aureus and S. pneumoniae was the tesS gene, we generated MDJ02 (ΔtesS) and MDJ03 (ΔtesS ΔplsX) mutants and tested their growth requirements for fatty acids. Strains TIGR4, MDJ02 (ΔtesS) and MDJ03 (ΔtesS ΔplsX) were cultured in 10 ml CY/BSA medium supplemented with 1 mM 18:1 fatty acid. The cells were harvested and washed with 15 ml CY/BSA medium to remove fatty acid and inoculated into fresh CY/BSA medium with and without 1 mM 18:1. The growth of strain MDJ02 (ΔtesS) was unaffected by the removal of fatty acid, whereas the MDJ03 (ΔtesS ΔplsX) strain was unable to grow unless the growth medium contained fatty acids (Fig. 6C). All of the strains grew normally in the presence of 18:1Δ9 (Fig. 6D). The impact of TesS on the ΔplsX lipid phenotype was best illustrated by the molecular species of PtdGro produced by the ΔtesS ΔplsX strain grown with exogenous 14:0 (Fig. 7). The primary PtdGro species in the ΔtesS ΔplsX strain were 14:0/16:1 and 14:0/18:1. In the ΔplsX strain, the 18:0/18:1 molecular species was prominent in cells grown with 14:0 (Fig. 3D). The 18:0/18:1 species was formed completely from endogenous fatty acids via TesS because 14:0 cannot be elongated in the ΔplsX strain. If endogenous fatty acid production by acyl-ACP hydrolysis was absent in the ΔtesS ΔplsX strain, then this molecular species (m/z = 775) should be essentially absent. This is exactly what was observed in ΔtesS ΔplsX double knockout strain grown on 14:0 (Fig. 7). These observations provided strong evidence that the reason strain MDJ01 (ΔplsX) was able to grow in the absence of fatty acids was due to the presence of TesS, which hydrolyzed acyl-ACP produced by FASII to produce free fatty acids that were utilized by fatty acid kinase to generate acyl-PO4 required by PlsY (Fig. 1).
Fig. 7.
PtdGro molecular species in strain MDJ03 (ΔtesS ΔplsX) grown with 14:0. Strain MDJ03 (ΔtesS ΔplsX) was grown in CY/BSA medium containing 1 mM 14:0, and the PtdGro molecular species determined by mass spectrometry as described under Experimental Procedures.
Expression of tesS in S. aureus strain JP121 (ΔplsX)
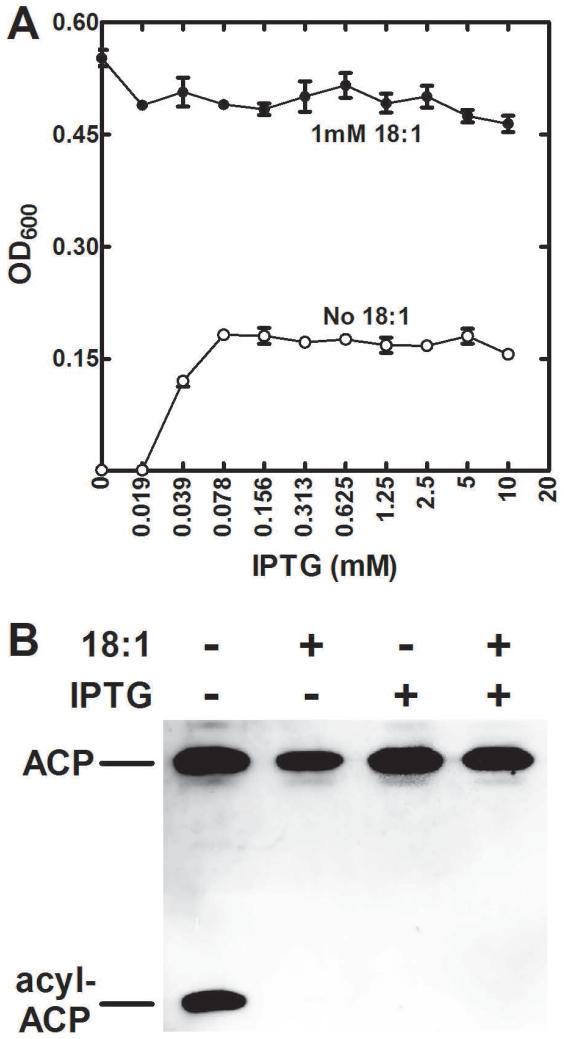
The next experiment was to introduce tesS into a S. aureus ΔplsX strain to determine if acyl-ACP was hydrolyzed by TesS in this heterologous system. The parent strain used for these experiments was S. aureus strain SA178R1 (derived from strain RN4220) that had the T7 polymerase and the LacIq repressor genes integrated into the chromosome under the control of the Pspac promoter/operator allowing tight IPTG-dependent expression from the companion pG164 plasmid (D’Elia et al., 2006). Strain JP121 (ΔplsX) was constructed starting with strain SA178R1 and paired with the pTesS plasmid that expressed a carboxy-terminal Flag-tagged-TesS under control of the IPTG-inducible promoter. Western blotting showed no expression of Flag-tagged TesS in the absence of inducer and robust expression when inducer was present that reached a maximum at 0.125 mM IPTG (not shown). In the absence of inducer, strain JP121 (ΔplsX)/pTesS was a strict fatty acid auxotroph exhibiting no detectable growth (Fig. 8A). However, the induction of TesS expression rescued the growth strain JP121 (ΔplsX)/pTesS (Fig. 8A). Why stationary phase density of the S. aureus ΔplsX/pTesS strain was higher in the presence of a fatty acid supplement than in the presence of IPTG is unknown, and was not investigated. Acyl-ACP accumulated in the ΔplsX S. aureus strain when the fatty acid supplement was removed from the medium in the absence of TesS induction based on immunoblotting with anti-ACP antibodies (Fig. 8B). In the presence of TesS expression, acyl-ACP was not detected in strain JP121 (ΔplsX)/pTesS cell extracts (Fig. 8B). These data provide corroborating evidence that TesS was a hydrolase that degraded acyl-ACP that accumulated in ΔplsX strains deprived of the fatty acid supplement.
Fig. 8.
Complementation of S. aureus ΔplsX mutant by TesS expression.
A) Strain JP121 (ΔplsX) was constructed from strain SA178R1 and complemented by plasmid pG164 containing a Flag-tagged version of tesS under control of an IPTG regulated promoter (pTesS). Growth of this strain in the presence of 18:1 and in different concentrations of IPTG was determined. In the absence of IPTG there was no growth in the absence of the 18:1 supplement, but the addition of IPTG allowed growth to about 40% of the wild-type stationary phase density.
B) Strain JP121/pTesS was grown to mid-log phase in the presence of 18:1. The cultures were split and divided into four cultures that were grown for an additional 2 hours in the presence of the combinations of 18:1 and IPTG indicated in the figure. The cells were harvested and separated by conformationally-sensitive gel electrophoresis, and the ACP visualized by immunoblotting (Parsons et al., 2011).
Discussion
This work is consistent with the TesS thioesterase being responsible for the differences in the growth phenotypes of plsX deletions in S. aureus and S. pneumoniae (Fig. 1). During normal growth, acyl-ACP are efficiently used by the PlsX/PlsY/PlsC acyltransferase system and are not available to TesS. The fact that tesS expression was the same in the wild-type and ΔplsX strains is consistent with the idea that it is the acyl-ACP levels rather than TesS levels that increase in the ΔplsX strain. S. aureus lacks a thioesterase and thus its ΔplsX derivatives require exogenous fatty acids in addition to de novo fatty acid synthesis for growth, and when the supplement is removed, acyl-ACP accumulates (Parsons et al., 2014b). In the S. pneumoniae strain MDJ01 (ΔplsX), the TesS thioesterase cleaves accumulating acyl-ACP to free fatty acids that are activated by fatty acid kinase to bypass the requirement for exogenous fatty acids (Fig. 1). When TesS is expressed in an S. aureus ΔplsX strain, growth is restored in the absence of a fatty acid supplement. The absence of a fatty acid compositional phenotype in the ΔtesS strain coupled with the fact that tesS is not found in most Gram-positive bacteria that use the PlsX/PlsY/PlsC pathway suggest that it does not normally function in de novo lipid metabolism. Our ACP antibody does not detect S. pneumoniae ACP in western blots, but acyl-ACP accumulation in the absence of PlsX activity is predicted to occur in the homologous S. pneumoniae PlsX/PlsY/PlsC system. We conclude that the expression of TesS in S. pneumoniae hydrolyzes acyl-ACP when this intermediate rises to supraphysiological levels to liberate fatty acids. These fatty acids are subsequently activated by fatty acid kinase to supply substrate to PlsY and initiate phospholipid synthesis (Fig. 1). One puzzling aspect of the work was the finding of predominantly 18:0 and 20:0 saturated fatty acids in the fatty acid pool of S. pneumoniae strain MDJ01 (ΔplsX) (Fig. 5B). This data is internally consistent with the observed shift in the PtdGro molecular species to longer-chain saturated fatty acids (Fig. 3A). However, purified TesS did not exhibit selectivity for saturated acyl-ACP under our in vitro assay conditions suggesting that TesS substrate specificity does not account for the lack of unsaturated fatty acids in the free fatty acid pool in the ΔplsX strain. Perhaps the saturated fatty acids accumulate because they are poor substrates for fatty acid kinase (FakA/B) and/or PlsY (Fig. 1). The in vivo TesS substrate and the pathway it participates in remains unknown. Our model suggests that TesS only intersects with lipid metabolism when membrane phospholipid formation is seriously compromised by the inactivation of PlsX and does not normally participate in lipid biosynthesis.
The fact that fatty acid synthesis is not completely suppressed in the ΔplsX strain is consistent with acyl-ACP as a key regulator of FASII in S. pneumoniae. The growth of S. pneumoniae with a long-chain fatty acid like 18:1Δ9 leads to the almost complete shutdown of de novo lipogenesis that correlates with a reduction in the levels of malonyl-CoA (Parsons et al., 2011). The resulting membrane phospholipids are composed solely of the exogenous fatty acid and the cells become refractory to FASII inhibitors (Table 1; Fig. 2C). Extracellular fatty acids activated by fatty acid kinase are used by PlsY, and the acyl-ACP for PlsC is provided by the reverse reaction of PlsX (Fig. 1). In the absence of PlsX, the stringent biochemical inhibition of FASII does not occur and the strain remains sensitive to FASII inhibitors (Table 1; Fig. 2C). Thus, either the PlsX product from exogenous fatty acids (acyl-ACP) or PlsX itself is a regulator of acetyl-CoA carboxylase. Acyl-ACP appears a prime candidate for the regulator in light of its regulatory role in feedback inhibition of FabH and acetyl-CoA carboxylase in E. coli (Heath and Rock, 1996; Davis and Cronan, 2001), but additional research is needed to determine if this is correct in S. pneumoniae (Fig. 1). The phenotypic manifestation of this effect is that growth of strain MDJ01 (ΔplsX) is not inhibited by the supplementation with exogenous 14:0 (Fig. 2B). The physiological basis for plsX deletion strains exhibiting significantly reduced survival in saliva (Verhagen et al., 2014) remains unknown. It is tempting to speculate that the membrane compositional changes are responsible, but a function for PlsX in cell signaling cannot be ruled out. Fatty acid kinase formation of acyl-PO4 regulates virulence factor transcription in S. aureus (Parsons et al., 2014a). The contribution of PlsX to the cellular levels of acyl-PO4 in S. pneumoniae is not clear, but the essentiality of fakA in this organism suggests an important role for acyl-PO4 in S. pneumoniae cell physiology.
Our data also provide insight into the mechanism for the antibacterial activity of fatty acids and monoglycerides against S. pneumoniae. It has been known for decades that saturated fatty acids and glycerides have antibacterial activity against this group of organisms (Schlievert et al., 1992; Sun et al., 2002; Ved et al., 1984; Zhang et al., 2009; Brissette et al., 1986). Our work corroborates these data by quantifying the antibacterial effect of 14:0 in strain TIGR4 (Fig. 2B). Exogenous 14:0 suppresses FASII leading to the membrane phospholipids being composed completely of di-saturated molecular species (Fig. 3D). Feeding 10:0 or 12:0 also results in growth arrest and the accumulation of exclusively saturated fatty acids in the membrane phospholipids (not shown). Although bacteria tolerate variations in phospholipid structure, membranes composed of di-saturated phospholipids are established to be too rigid to support growth (Zhang and Rock, 2008). The ΔplsX strain is refractory to growth inhibition by 14:0 because it cannot completely suppress FASII which results in membrane phospholipids having 14:0 paired with an unsaturated fatty acid (Fig. 3D). The lack of a growth inhibitory effect on the ΔplsX strain (Fig. 2B) means that the antibacterial effect of the fatty acid is due solely to its impact on phospholipid structure. The antibacterial mechanism of exogenous fatty acids in S. aureus is not the same as S. pneumoniae because the structure-dependent toxicity toward S. aureus has been traced to their effects on the membrane electrochemical gradient and not their incorporation into phospholipid (Parsons et al., 2012).
Experimental procedures
Strains, materials and growth conditions
S. aureus SA178R1 and pG164 shuttle vector were obtained from Merck (D’Elia et al., 2006). Platensimycin was purchased from BioAustralis. Fatty acids, fatty acid-free bovine serum albumin (BSA) and Phospholipase A2 was purchased from Sigma-Aldrich. [14C]Acetate (55 mCi/mmol), [14C]oleate (56.3 mCi/mmol) and [14C]oleoyl-CoA (60 mCi/mmol) were purchased from Perkin-Elmer. [14C]Stearate (55 mCi/mmol) was purchased from American Radiolabelled Chemicals. C+Y (CY) media or C+Y containing 10 mg/ml BSA (CY/BSA) was used to culture S. pneumoniae (Lacks and Hotchkiss, 1960). Growth measurements were taken using a Biotek Cytation 3 plate reader which incubated the cells at 37°C and CO2 at 5%. The S. aureus SA178R1 ΔfakA (SafakA) mutant was generated by insertion of an intron 733 bp into the fakA gene using the primer design software and pNL9164 plasmid system provided by the Targtetron Gene Knockout Kit (Sigma-Aldrich) (Zhong et al., 2003). Genotyping was performed using a multiplex PCR reaction with internal intron primer SafakA-F1 and gene specific primers SafakA-R1 and SafakA-R2. A modified version of the pG164 E. coli - S. aureus shuttle vector was generated that utilizes an erythromycin resistance cassette instead of chloramphenicol for selection in S. aureus. First, the SalI site in the multiple cloning region of pG164 was destroyed by digesting with SalI and generating blunt ends. A SalI site was then introduced in the 5′ region of the chloramphenicol acetyltransferase gene. The ermC gene conveying resistance to erythromycin was amplified from pNL9164 (Zhong et al., 2003) using primers emrC-F1 and emrC-R1, and ligated into the newly introduced SalI and StuI sites of pG164 to form pGERM64. The fakA gene from S. aureus was amplified from SA178R1 using primers SafakA-F2 and SafakA-R2. It was subsequently ligated into the BamHI and HindIII sites of pGERM64 to form pSaFakA. The gene locus predicted to encode S. pneumoniae fakA was amplified from TIGR4 genomic DNA using primers SpfakA-F3 and SpfakA-R4. The product was ligated into the BamHI and XhoI sites of pGERM64 to form pSpFakA. Strain JP121 (ΔplsX) was constructed in strain SA178R1 as described (Parsons et al., 2014b). A carboxyl terminal Flag-tagged tesS gene was synthesized, and cloned into plasmid pG164 to create pTesS that expressed Flag-tagged-TesS under control of the IPTG-inducible promoter.
Directed gene deletion mutants of S. pneumoniae strain TIGR4 were generated by allelic exchange of the target gene with a spectinomycin or kanamycin resistance cassette (obtained from pR412T7 or pR410 respectively) using a megaprimer polymerase chain reaction, as described earlier (Verhagen et al., 2014). Strain MDJ02 (ΔtesS) was constructed using extension PCR to join 400-500 bp of the 5′ and 3′ flanking sequences of the target gene (SP1408) with the antibiotic resistance cassette. Subsequently, the amplicon was used for transformation of competent TIGR4. Mutants, selected on blood agar plates containing the relevant antibiotic, were assessed by colony PCR for recombination at the desired location on the chromosome. Chromosomal DNA was isolated from the mutants and used for transformation of competent strain TIGR4. Gene inactivation was confirmed by quantitative real-time PCR (qRT-PCR) gene expression analyses using the primer pairs listed in Table 2. The construction of strain MDJ01 (ΔplsX) was carried out by the same procedure except that we started with amplification of plsX containing the spectinomycin cassette with the flanking regions, using the plsX-L1 and plsX-R1 primers and chromosomal DNA from Spain9v-3 ΔplsX as template (Verhagen et al., 2014). Strain MDJ03 (ΔtesS ΔplsX) was constructed from strain MDJ01 (ΔplsX). Care must be taken when handling strain MDJ03 (ΔtesS ΔplsX). Growth of strain MDJ03 in CY/BSA medium without fatty acids resulted in the outgrowth of a revertant strain in 24-48 h. Sub-culturing this isolate showed that it grew normally with no lag time in the absence of fatty acids. Characterization of the changes in this altered revertant strain was beyond the scope of this study. Primers used were purchased from Biolegio, Nijmegen, The Netherlands, and are listed in Table 2.
Table 2.
Primers used for strain construction and quantitative real-time PCR (qPCR)
| Primer name | Target | Sequence (5′- 3′) |
|---|---|---|
| SafakA-F1 | fakA | CGAAATTAGAAACTTGCGTTCAGTAAAC |
| SafakA-R1 | fakA | TACATTATTGTAAAAGCCAATGAATCAC |
| SafakA-R2 | fakA | CTGATCAAGCTTTTATTCTACTGAAAAGAAATATTGATAAATTG |
| ermC-F1 | emrC | TTAGTCGACGCTGTCTTGGTTCATTGATTGC |
| ermC-R1 | emrC | GAAAGGCCTGTTAAGGGATGCAGTTTATGC |
| SafakA-F2 | fakA | CTACATGGATCCATGATTAGCAAAATTAATGGTAAATTATTTG |
| SpfakA-F3 | fakA | CTTCTTGGATCCATGGTGTCAAAAATTACTACTAGCTTATTT |
| SpfakA-R4 | fakA | CTTCTTCTCGAGTTATTCCACACTAAATAGGTATGGGTA |
| plsX-L1 | plsX | TGGAGTTGATGGAAGCAGGC |
| plsX-L2a | plsX | CCACTAGTTCTAGAGCGGCATGGCCTGAGGTGCGTAATC |
| plsX-R1 | plsX | TCCAAATCTTGAATTGGCATCG |
| plsX-R2a | plsX | GCGTCAATTCGAGGGGTATCCAGACTGCGCGTGAATTTTC |
| 1408-L1 | tesS | CATCATTGCAGAGCTGGAGC |
| 1408-L2 | tesS | TAAGCTTGATATCGAATTCCCCTGAAACTTGCAGTGACAGC |
| 1408-R1 | tesS | GTCGACTTGCCAATCCAGAC |
| 1408-R2 | tesS | GGATGAATTGTTTTAGTACCTGGTTATCAATGCCCAAGCAATC |
| Primers for qPCR | ||
| Q-Sp-plsX-F | plsX | GGAGATGAAGCTAAAATCAAGCAAT |
| Q-Sp-plsX-R | plsX | TCATCATCCGAATCAATCTTCTCA |
| Q-SP 1408-F | tesS | CCTGAGATTGTGGCTCCTTAC |
| Q-SP 1408-R | tesS | TAATCCTTGCTGATCGGTTCTT |
These primers were used to make the ΔplsX construct in S. pneumoniae Spain9v-3 as previously described (Verhagen et al., 2014).
Minimum inhibitory concentrations (MIC)
The MICs for platensimycin and 14:0 fatty acid against S. pneumoniae were determined using a broth microdilution method as described previously (Parsons et al., 2011). Briefly, the strains were grown to an optical density at 600 nm (A600) of 0.6 and diluted 30,000-fold in CY medium. A 10-μl aliquot of diluted cells was added to each well of a U-bottom 96-well plate containing 100 μl of medium with the appropriate concentration of compound. The plate was incubated at 37°C for 20 h and read using a Fusion plate reader at 600 nm. Cells grown in medium containing dimethyl sulfoxide (DMSO; 1%) were used as reference (i.e. 100% growth).
S. aureus ΔfakA complementation
S. aureus strain SA178R1 ΔfakA was transformed with plasmids pG164, pG164-SafakA (pSafakA) and pG164-SpfakA (pSpfakA). Each strain was grown in Luria broth supplemented with 1.25 μg/ml erythromycin and 1 mM IPTG until A600 = 0.5. Then, 1 μCi of [14C]oleic acid was added to each culture and incubated with shaking for 30 minutes. The cells were then harvested by centrifugation and washed with 11 ml broth and once with 11 ml water. The pellets were resuspended in 100 μl distilled water and 360 μl chloroform/methanol/HCl (1/2/0.02, v/v/v) to extract lipids. After vortexing and incubation at room temperature for 20 minutes, 120 μl chloroform and 120 μl 2 M KCl were added to separate phases before centrifugation. The amount of radiolabelled lipids was quantified by scintillation counting.
Lipid mass spectrometry
Mass spectrometry of phospholipids was performed using a Finnigan TSQ Quantum (Thermo Electron, San Jose, CA) triple quadrupole mass spectrometer as described previously (Parsons et al., 2013). Briefly, samples were prepared in 50:50 (v/v) chloroform:methanol. The instrument was operated in the negative ion mode. Ion source parameters were as follows: spray voltage of 3,000 V, capillary temperature of 270°C, capillary offset of 35 V, and the tube lens offset was set by infusion of polytyrosine tuning and calibration solution (Thermo Electron, San Jose, CA) in the electrospray mode. Acquisition parameters were as follows: full scan, scan range 600 – 900 m/z, scan time 0.5 s, peak width Q1 0.7 FWHM. Instrument control and data acquisition was performed with the Finnigan™ Xcalibur™ software (Thermo Electron, San Jose, CA).
Fatty acid composition
Cultures (50 ml) of S. pneumoniae strains were grown in CY/BSA medium and 1 mM of specified fatty acids until A600 = 0.7. The cells were harvested by centrifugation and washed with 50 ml of 10 mg/ml BSA in phosphate-buffered saline. The pellet was resuspended in 1 ml distilled water and 2.4 ml of 2% acetic acid in methanol and 1 ml chloroform was added. The suspension was allowed to incubate for 20 minutes at room temperature before the addition of 1.5 ml chloroform and 1.2 ml distilled water. The cells were vortexed and centrifuged to achieve phase separation. The organic phase was removed and 1 ml chloroform added to the original tube, vortexed and reextracted. The organic phase was dried under nitrogen and lipids dissolved in 1 ml anhydrous methanol containing 1-2 drops of acetyl-chloride before incubation overnight. The reaction was evaporated under nitrogen and brought up in 1 ml of distilled water. The water phase was extracted 3 times with hexanes. The hexane fractions were pooled and evaporated over nitrogen. The fatty acid methyl esters were analyzed by a Hewlett-Packard model 5890 gas chromatograph equipped with a flame ionization detector, and separated on 30 m × 0.536 mm × 0.50 μm DB-225 capillary column. The injector was set at 250°C, and the detector was at 300°C. The temperature program was as followed: initial temp 70°C for 2 min, rate of 20°C/min for 5 min (final 170°C), rate of 2°C/min for 10 min (final 190°C), hold at 190°C for 5 min, rate of 2°C/min for 15 min (final 220°C), hold at 220°C for 5 min. The identity of fatty acid methyl esters were determined by comparing their retention times with identified fatty acid methyl ester standards (Sigma-Aldrich). The compositions were expressed as weight percentages.
Cloning and purification of TesS
The SP1408 (tesS) locus was synthesized with a C-terminal FLAG tag and flanking restriction sites using Invitrogen Gene Art Strings. The gene was ligated into TOPO Zero Blunt (Invitrogen) and verified by sequencing. The gene was subsequently restricted with BamHI and HindIII before ligation into the corresponding sites of pET28b. The resulting vector was transformed into E. coli BL21 (DE3), and 2 L of cells were grown to A600 = 0.7 before induction for 3 hours at 37°C with 1 mM IPTG. The cells were harvested by centrifugation and lysed using a microfluidizer. Due to difficulty isolating the protein using nickel affinity chromatography, a FLAG immunoprecipitation was performed to capture TesS. Anti-FLAG M2 Affinity Gel (Sigma-Aldrich) was equilibrated in 20 mM HEPES pH 7.4 before the E. coli lysate containing the FLAG tagged TesS was applied to the column. The column was washed with 20 column volumes of 20 mM HEPES pH 7.4 and TesS eluted using 100 μg/ml FLAG peptide (Sigma-Aldrich) in 20 mM HEPES pH 7.4. The protein was concentrated and further purified by gel filtration using a Superdex S200 10/300 GL size-exclusion column equilibrated in 20 mM Tris-HCl pH 7.5, 0.2 M NaCl.
Thioesterase assay
TesS reactions contained 100 mM Tris-HCl, pH 7.5, 23 μM [14C]18:1-ACP, [14C]18:0-ACP, or [14C]18:1-CoA in a volume of 30 μl. After addition of TesS, reactions were incubated at 37°C for 10 minutes. The incubation was terminated by applying 20 μl of the reaction mixture onto a Silica Gel G layers that were developed in chloroform/methanol/acetic acid (90/10/10, v/v/v) to separate [14C]fatty acid from [14C]acyl-ACP(or CoA), and the percent conversion determined using a Bioscan Imaging detector.
Metabolic labeling
Cultures (10 ml) of S. pneumoniae strains were grown in medium supplemented with 5 μCi/ml [14C]acetate were grown to A600 = 0.6 before harvesting by centrifugation at 4000 × g. Cell pellets were resuspended in 100 μl distilled water and 360 μl chloroform/methanol/HCl (1/2/0.02, v/v/v) and incubated at room temperature for 20 minutes. Following an addition of 120 μl chloroform and 120 μl 2 M KCl, the reactions were vortexed and centrifuged to achieve phase separation. The organic phase was removed and 20μl applied to a Silica Gel G layers and developed in chloroform/methanol/acetic acid (90/10/10, v/v/v) and read using a Bioscan Imaging detector.
The positional distribution was determined as described previously (Parsons et al., 2014b). Briefly, strains were grown in CY/BSA medium with 1 mM of specified fatty acid and 5 μCi/ml [14C]acetate until A600 = 0.7. Lipids were then extracted as described above and [14C]PtdGro isolated by preparative thin-layer chromatography on Silica Gel H layers developed in chloroform/methanol/acetic acid (55/20/5, v/v/v). This [14C]PtdGro was digested with Naja naja snake venom phospholipase A2 (Parsons et al., 2011). After 3 hours, the lipids were extracted and analyzed by thin-layer chromatography on Silica Gel H layers developed in chloroform/methanol/acetic acid (55/20/5, v/v/v) and read using a Bioscan Imaging detector.
Acknowledgements
We thank the Protein Production Shared Resource for protein expression and purification, Hartwell Center for DNA sequencing and Pam Jackson for her technical assistance.
This research was supported by National Institutes of Health Grant GM034496 (C.O.R.), Cancer Center Support Grant CA21765 and the American Lebanese Syrian Associated Charities, GO EFRO, grant I2I 2011-006755, and the Dutch Technology Foundation STW NanoNext NL, grant FES0901: FES HTSM (M.I.d.J). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
References
- Brissette JL, Cabacungan EA, Pieringer RA. Studies on the antibacterial activity of dodecylglycerol. Its limited metabolism and inhibition of glycerolipid and lipoteichoic acid biosynthesis in Streptococcus mutans BHT. J Biol Chem. 1986;261:6338–6345. [PubMed] [Google Scholar]
- Coleman J. Characterization of the Escherichia coli gene for 1-acyl-sn-glycerol-3-phosphate acyltransferase (plsC) Mol Gen Genet. 1992;232:295–303. doi: 10.1007/BF00280009. [DOI] [PubMed] [Google Scholar]
- Cronan JE, Jr., Weisberg LJ, Allen RG. Regulation of membrane lipid synthesis in Escherichia coli. Accumulation of free fatty acids of abnormal length during inhibition of phospholipid synthesis. J Biol Chem. 1975;250:5835–5840. [PubMed] [Google Scholar]
- D’Elia MA, Pereira MP, Chung YS, Zhao W, Chau A, Kenney TJ, et al. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J Bacteriol. 2006;188:4183–4189. doi: 10.1128/JB.00197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MS, Cronan JE., Jr. Inhibition of Escherichia coli acetyl coenzyme A carboxylase by acyl-acyl carrier protein. J Bacteriol. 2001;183:1499–1503. doi: 10.1128/JB.183.4.1499-1503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RJ, Rock CO. Inhibition of β-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:10996–11000. doi: 10.1074/jbc.271.18.10996. [DOI] [PubMed] [Google Scholar]
- Lacks S, Hotchkiss RD. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lee S, Park S, Lee J. Improvement of free fatty acid production in Escherichia coli using codon-optimized Streptococcus pyogenes acyl-ACP thioesterase. Bioprocess Biosyst Eng. 2013;36:1519–1525. doi: 10.1007/s00449-012-0882-2. [DOI] [PubMed] [Google Scholar]
- Lu Y-J, Zhang F, Grimes KD, Lee RE, Rock CO. Topology and active site of PlsY: the bacterial acylphosphate:glycerol-3-phosphate acyltransferase. J Biol Chem. 2007;282:11339–11346. doi: 10.1074/jbc.M700374200. [DOI] [PubMed] [Google Scholar]
- Lu Y-J, Zhang Y-M, Grimes KD, Qi J, Lee RE, Rock CO. Acyl-phosphates initiate membrane phospholipid synthesis in gram-positive pathogens. Molec Cell. 2006;23:765–772. doi: 10.1016/j.molcel.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Parsons JB, Broussard TC, Bose JL, Rosch JW, Jackson P, Subramanian C, Rock CO. Identification of a two-component fatty acid kinase responsible for host fatty acid incorporation by Staphylococcus aureus. Proc Natl Acad Sci U S A. 2014a;111:10532–10537. doi: 10.1073/pnas.1408797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JB, Frank MW, Jackson P, Subramanian C, Rock CO. Incorporation of extracellular fatty acids by a fatty acid kinase-dependent pathway in Staphylococcus aureus. Mol Microbiol. 2014b;92:234–245. doi: 10.1111/mmi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JB, Rock CO. Bacterial lipids: Metabolism and membrane homeostasis. Prog Lipid Res. 2013;52:249–276. doi: 10.1016/j.plipres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JB, Yao J, Frank MW, Jackson P, Rock CO. Membrane disruption by antimicrobial fatty acids releases low molecular weight proteins from Staphylococcus aureus. J Bacteriol. 2012;194:5294–5304. doi: 10.1128/JB.00743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JB, Yao J, Jackson P, Frank M, Rock CO. Phosphatidylglycerol homeostasis in glycerol-phosphate auxotrophs of Staphylococcus aureus. BMC Microbiol. 2013;13:260. doi: 10.1186/1471-2180-13-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JB, Frank MW, Subramanian C, Saenkham P, Rock CO. Metabolic basis for the differential susceptibility of Gram-positive pathogens to fatty acid synthesis inhibitors. Proc Natl Acad Sci U S A. 2011;108:15378–15383. doi: 10.1073/pnas.1109208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert PM, Deringer JR, Kim MH, Projan SJ, Novick RP. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob Agents Chemother. 1992;36:626–631. doi: 10.1128/aac.36.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CQ, O’Connor CJ, Roberton AM. The antimicrobial properties of milkfat after partial hydrolysis by calf pregastric lipase. Chem Biol Interact. 2002;140:185–198. doi: 10.1016/s0009-2797(02)00016-9. [DOI] [PubMed] [Google Scholar]
- van Opijnen T, Camilli A. A fine scale phenotype-genotype virulence map of a bacterial pathogen. Genome Res. 2012;22:2541–2551. doi: 10.1101/gr.137430.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ved HS, Gustow E, Mahadevan V, Pieringer RA. Dodecylglycerol. A new type of antibacterial agent which stimulates autolysin activity in Streptococcus faecium ATCC 9790. J Biol Chem. 1984;259:8115–8121. [PubMed] [Google Scholar]
- Verhagen LM, de Jonge MI, Burghout P, Schraa K, Spagnuolo L, Mennens S, et al. Genome-wide identification of genes essential for the survival of Streptococcus pneumoniae in human saliva. PLoS ONE. 2014;9:e89541. doi: 10.1371/journal.pone.0089541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature (London) 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- Yao J, Rock CO. Phosphatidic acid synthesis in bacteria. Biochim Biophys Acta. 2013;1831:495–502. doi: 10.1016/j.bbalip.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wei H, Cui Y, Zhao G, Feng F. Antibacterial interactions of monolaurin with commonly used antimicrobials and food components. J Food Sci. 2009;74:M418–M421. doi: 10.1111/j.1750-3841.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y-M, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- Zhong J, Karberg M, Lambowitz AM. Targeted and random bacterial gene disruption using a group II intron (targetron) vector containing a retrotransposition-activated selectable marker. Nucleic Acids Res. 2003;31:1656–1664. doi: 10.1093/nar/gkg248. [DOI] [PMC free article] [PubMed] [Google Scholar]