Abstract
Considerable mechanistic insight into the function of histone posttranslational modifications and the enzymes that install and remove them derives from in vitro experiments with modified histones, often embedded in nucleosomes. We report the first semisyntheses of native-like histone 3 (H3) bearing tri- and di- methyllysines at position 79 and trimethylsine at 36, as well as more facile and traceless semisyntheses of K9 and K27 trimethylated species. These semisyntheses are practical in multi-milligram scale and can also generate H3 with combinations of marks. Each of these modifications has distinct functional consequences, although the pathways by which H3K36me3 and H3K79me2/3 act have not been entirely mapped. To this end, we demonstrate that our semisynthetic histones, when reconstituted into nucleosomes, are valuable affinity reagents for unbiased binding-partner discovery, and compare them to their methyllysine analog (MLA) counterparts at the nucleosome level.
Keywords: Histone methylation, protein engineering, binding partner discovery, bioorganic chemistry, native chemical ligation
Methylated histone lysine residues play critical roles in regulating DNA-templated processes and are associated with either activation or repression of transcription depending on the site and degree of methylation.[1] Five of the six most studied histone methyllysines are on histone 3 (H3). As the nuclesome core particle, the assemblage of two copies of each of the four core histones on DNA, is the fundamental repeating unit of chromatin, it serves an essential recognition unit in epigenetics research. In vitro reconstituted nucleosomes bearing these methyllysines, have proven instrumental in studying their mechanisms of regulation.[2] Because histone methyltransferases often display modest control over the site, degree, and extent of methylation in vitro, the expediently generated thioether analogs of methyllysine created by reaction of 2-aminoethyl alkylating agents with engineered cysteines (known as methyllysine analogs, MLAs) are widely used as surrogates.[3] However, MLAs are distinct from their native counterparts in a number of chemical properties that may render them problematic in biochemical assays.[4] When probed quantitatively for binding affinity with cognate binding proteins, histone peptides bearing native methyllysines typically bind 5–13 fold tighter the corresponding MLAs.[5] Here our data show that MLAs may not be effective reagents in critical applications like pull-down assays.
Genetically encoded methods have been successfully used to incorporate acetyllysine and monomethyllysine into H3,[6] dimethyllysine indirectly after 4 steps of chemical manipulation on whole-protein level.[7] However, the most important methylation state for recruiting binding-partners, trimethyllysine, is not accessible via this methodology.
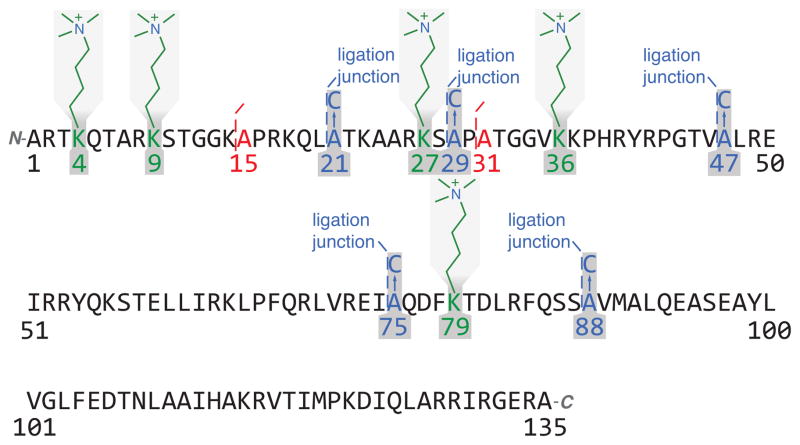
We decided to generate native-like H3 bearing trimethylated lysine 9 (H3K9me3), H3K27me3, H3K4me3K27me3, H3K36me3, and H3K79me2/3 by chemical ligation.[8] To our knowledge, the semisyntheses of H3K4me3K27me3, H3K36me3, and H3K79me2/3 have no precedent, whereas that of H3K9me3 and H3K27me3 has been reported with an alanine or threonine residue mutated to cysteine.[2b, 9] Given that others have found such mutations may alter the structure of nucleosome, and any change in nucleosomal substrate could potentially alter apparent activity in biochemical assays, this is a potential drawback.[10] Histones bearing acetyllysines and other post-translational modifications (PTMs) have been synthesized,[8b, 9a, 9c, 11] providing unique insight into the biological mechanisms of these PTMs.[2b, 2c, 2e, 12] Because H3 commonly used in the reconstitution of nucleosome in vitro does not have cysteine, native chemical ligation (NCL)[13] is performed at the site of an alanine, mutated to cysteine and restored by radical initiated desulfurization afterwards.[14] The sites of ligation are chosen with some precautions in mind (Figure 1). For H3K9me3, alanine 21 (A21) is chosen instead of A15 because lysine at the C-terminus of peptidyl thioester appears to cyclize, forming an unstable lactam that is very susceptible to hydrolysis. For H3K27me3, A29 is preferable to A31 because a peptidyl thioester with proline at the C-terminus reacts very slowly in NCL.[15] H3K4me3K27me3, H3K36me3, and H3K79me2/3, are made by three-piece ligations in one pot,[16] (A21, A29), (A29, A47), and (A75, A88) are chosen as the ligation sites, respectively.
Figure 1.
The sequence of human H3.2 C110A, with each methyllysine in green, alanines chosen as ligation sites in blue, ligation sites not chosen in red.
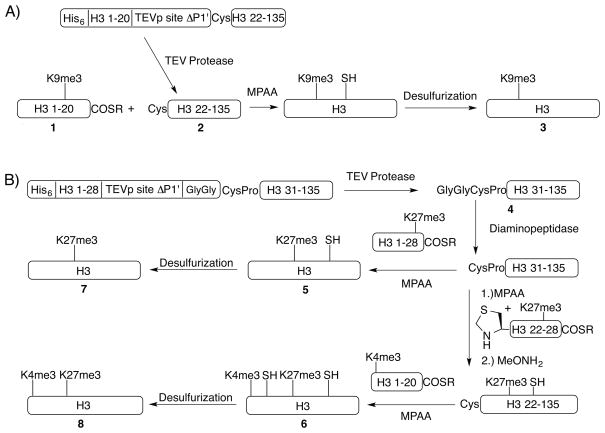
To construct H3K9me3 (3) (Scheme 1A), peptidyl thioester H3(1-20)K9me3-SR (1) is synthesized on the solid support via Boc chemistry.[17] The requisite trimethyllysine monomer Boc-Lys(me3)-OH is prepared by treating Boc-Lys-OH with 10 equivalents of MeI and KHCO3,[18] purified by preparative HPLC instead of organic solvent extraction described in the original report, and thus is free of excessive inorganic salts which inhibit coupling during SPPS. The desired truncated histone H3Δ20-A21C (2, H3 with the first 20 N-terminal residues deleted and alanine 21 mutated to cysteine) is produced recombinantly with a His6-tag, subsequently removed by tobacco etch virus (TEV) protease.[19] The TEV protease site consists of seven residues ENLYFQX; TEV protease cleaves between Q and X, the P1′ site (either a cysteine or a glycine in the present work). Retention of the H3 tail markedly facilitates TEV digestion (Figure S1A and B), most probably due to the basic tail solubilizing the truncated histone construct in the pH 7.5–8.0 range at which TEV protease has optimal activity. The N-terminal cysteine of 2 and other recombinant truncated histones produced in this study form adducts mostly with pyruvate and its derivatives,[20] which is efficiently resolved by methoxyamine treatment in one pot after the TEV protease digestion.[11] NCL proceeds to near completion after 24 hours with the the peptidyl thioester and H3Δ20-A21C (2) at or above 2 mM concentrations. With 2-mercaptoethanol supplanting t-BuSH as the radical scavenger,[14a] the desulfurization furnishes H3K9me3 (3) with 92% yield.
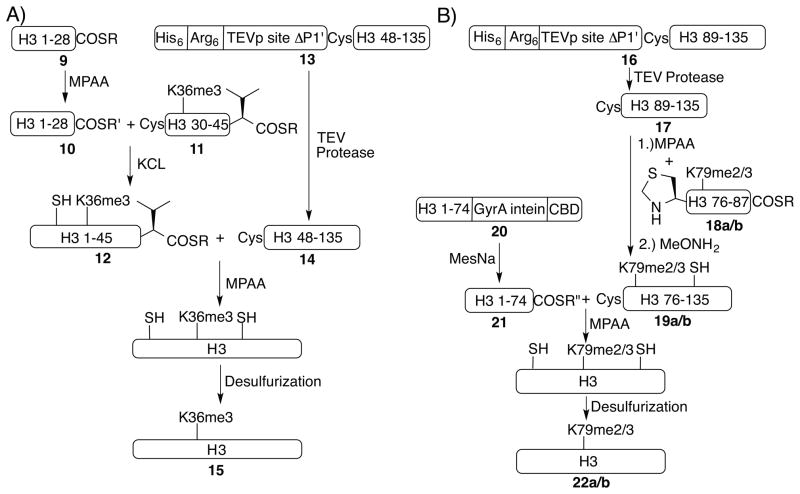
Scheme 1.
Semisynthesis of A) H3K4me3 and H3K9me3; B) H3K27me3 and H3K4me3K27me3. Refer to supporting information for the detailed methods and characterization data.
Next we constructed H3K27me3 (7) and H3K4me3K27me3 (8) (Scheme 1B) for our ongoing study of bivalency – the co-existence of H3K4me3 and H3K27me3, seemingly within the same nucleosome.[21] To generate the requisite H3Δ28-A29C fragment, a GlyGly dipeptide is inserted after the ENLYFQ sequence of the His6-tagged H3Δ28-A29C construct because TEV protease does not tolerate proline at the P2′ position (the second residue C-terminal to the cleavage site).[22] With the basic tail of H3 retained, the His6-tag is removed quantitatively by TEV protease to furnish 4 (Figure S1C). Then the residual GlyGly dipeptide is efficiently excised with diaminopeptidase (TAGzyme, Qiagen), which cleaves 2 amino acids off the N-terminus at a time but cannot cut after proline. H3K27me3 A29C (5) is prepared by one-step ligation with 80% yield and H3K4me3K27me3 A21C A29C (6) two sequential ligations in one pot (see Figure S2 for efficiency of these ligations) with 64% overall yield.[16] Radical initiated desulfurization furnishes 7 and 8 with 90% yield. The previous 3 semisyntheses can produce around 10 mg of final purified product easily, attesting to the high efficiency of the semisynthetic approach.
With methyllysines situated in the central part of the protein, both H3K36me3 (15) and H3K79me2/3 (22a/b) are made by three-piece ligations in one pot. In the preparation of H3K36me3 (Scheme 2A), the N-terminal piece (10) and the central piece (11) are first ligated by kinetically controlled ligation (KCL).[23] Crude synthetic peptidyl thioester H3(1-28)-SR (9) is treated with MPAA (4-mercaptophenylacetic acid)[24] and purified to yield a highly reactive thioester H3(1-28)-SR′ (10), which ligates quantitatively with the central piece H3(29-46)K36me3-A29C-SR (11). Then the H3Δ46-A47C fragment (14) is added to the mixture together with 150 mM MPAA and 100 mM of TCEP. The second ligation goes to ~80% completion after 72 hours (Figure S3) due to the steric demand of a β-branched valine at the C-terminus of the thioester (12). Both high concentration of the catalyst MPAA and long reaction time are conducive to the completion of the second ligation (Figure S3). We anticipated the sluggishness of this ligation and staged it last to reduce side products such as 10 ligating with 14.
Scheme 2.
Semisynthesis of A) H3K36me3, and B) H3K79me2/3. SR = 3-mercapto-propionamide thioester; SR′ = MPAA thioester; SR″ = 2-Mercaptoethanesulfonate (MesNa) thioester. Refer to supporting information for the detailed methods and characterization data.
For preparation of H3K79me2/3 (22a/b, Scheme 2B), the requisite H3Δ87-A88C (17) species is first ligated quantitatively with peptidyl thioester Thz-H3(75-87)K79me2/3-A75-SR (18a and 18b see Figure S2D, Thz, 1,3 thiazolidine-4-carboxylic acid, used to protect cysteine). The thiazolidine is then unmasked by methoxyamine treatment and ligates directly with peptidyl thioester H3(1-74)-SR″ (21), generated recombinantly by the intein cleavage method.[25] The H3(1-74)-intein-CBD (20) construct expresses well and is largely pure out of inclusion bodies. It is then extracted with buffer containing 6 M Guanidinium chloride (GdmCl), refolded, and cleaved with MesNa (Scheme 2B). We find 20 refolds and cleaves most efficiently in the presence of 0.8–1 M GdmCl (Figure S4). For 22a and 22b, the isolated yield for the final ligated product is 35% based on 17, and the yield for the desulfurization is 89%.
To prepare H3Δ46-A47C (14) and H3Δ87-A88C (17), we first attempted expression of these fragments as N-fMet fusions (with a start codon and no tag): there was no detectable expression in inclusion bodies, even when we codon-optimized one of the constructs. Next we attempted TEV-digestion of full-length H3 constructs with the ENLYFQC sequence inserted at the respective cleavage sites. However, both constructs were refractory to proteolysis even when 1:1 mass ratio of TEV protease is used, despite no visible precipitation. We speculate that H3 forms soluble aggregates under the digestion conditions, with the N-terminal tail protruding out of the aggregate and accessible to TEV protease and the more hydrophobic core occluded in the aggregate. In order to disrupt the aggregate, we replaced the H3 sequence before the TEV site in these two H3 constructs with 6 arginines (13 and 16, respectively). This strategy renders both constructs digestible by TEV protease (Figure S1D and S1E) and increases their expression level. For both H3K36me3 (15) and H3K79me2/3 (22a/b), 1–2 mg of final products can be easily prepared.
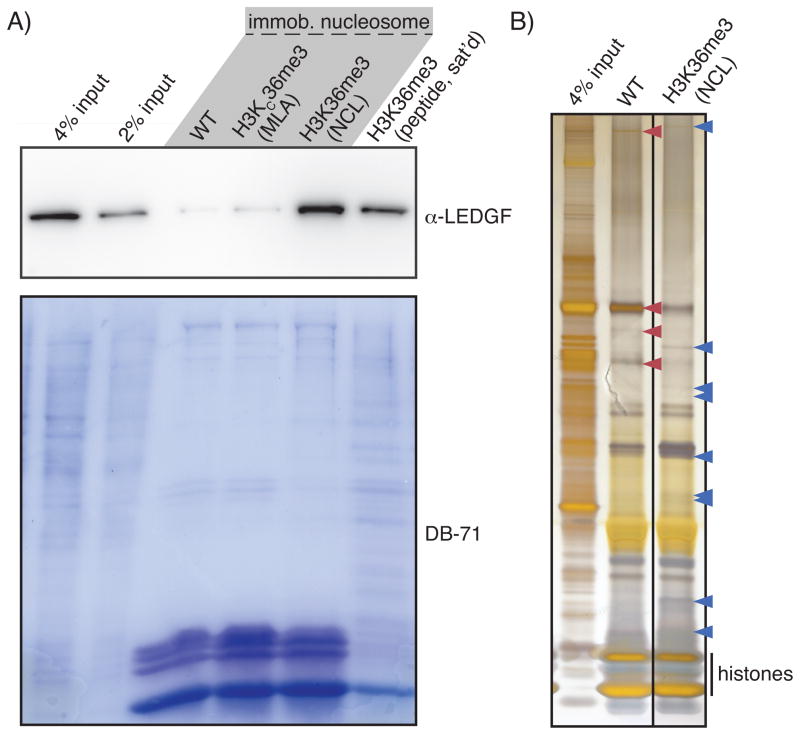
Although both H3K36me3 and H3K79me2/3 have important functions,[26] validated binding partners reported for them remain limited (four reported for H3K36me3[27] and none for H3K79me2/3 to date). Because these modifications are in the structured part of the nucleosome, we reason that the nucleosomal context is very important for potential binding partners, thus nucleosomes bearing these modifications are better substrates for capturing these potential readers from nuclear extract[2b] than the corresponding peptides. In fact, most reported binders of H3K36me3 show low binding affinity (Kd ~ 1mM) for the modified peptides,[27b, 27d, 28] yet presentation of this mark in a nucleosomal context enhances the binding affinity by ~10,000 fold for LEDGF, the only such binding module examined at the nucleosome level.[28] To validate our approach, we tried pulling down LEDGF with equivalent amount of streptavidin-coated magnetic beads saturated with biotinylated nucleosomes bearing wild type H3, H3KC36me3 (the MLA), H3K36me3 made by NCL in this study or biotinylated peptide bearing H3K36me3. By western blot, the H3K36me3 nucleosome pulls down LEDGF most effectively while MLA nucleosome yields almost background level of signal (Figure 2). The peptide affords some signal too, probably because of much higher resin loading level due to smaller size than the nucleosomes (2.4 kDa and 220 kDa, respectively). However, the peptide pulls down copious other proteins as well, whereas the nucleosomes give much cleaner background (Figure 2)-- what matters most in pull-down assays is the signal-to-noise ratio.[29] As LEDGF is crucial for guiding HIV integrase to sites of integration,[30] this reagent may be useful for in vitro study of HIV integrase on chromatinized templates.
Figure 2.
Nucleosome pull-down. A) Pull-down of LEDGF from crude nuclear extract using nucleosomes bearing wide type (WT) H3, MLA (H3KC36me3) or H3K36me3, as well as peptide bearing H3K36me3. The blot is imaged with LEDGF antibody or Direct Blue-71 (DB-71) staining of total protein. B) Silver-stain for the total protein pulled down by H3K36me3-containing and wild-type nucleosomes from partially fractionated HeLa nuclear extract.
To examine the potential of our NCL-produced histones in binding partner discovery, we performed analogous nucleosome pull-downs with HeLa nuclear extract that had been fractionated by heparin-sepharose chromatography, and subject captured material to silver staining. A variety of distinct protein bands appear in the pull-down by H3K36me3-containing nucleosomes that are absent in the pull-down with the wild type nucleosomes (Figure 2B, indicated by blue triangles), highlighting the strength of nucleosome pull-down for unbiased binding partner discovery for internal methyllysines. Interestingly, some proteins that are enriched in the wild type nucleosome pull-down appear to be are repelled by H3K36me3 (Figure 2B, red triangles), indicating some effector proteins might selectively recognize unmodified H3K36 over H3K36me3. Each of these bands represents a potentially novel binding partner, the discovery of which is often the key step in deciphering a new epigenetic pathway, enabled via the toolkit of chemical biology.
In summary, we have developed a practical semisynthetic scheme for H3 bearing specific di- and tri- methyllysines, a combination of methyllysines, and possibly other PTMs and combination thereof. This semisynthetic approach decribed herein is efficient, as all the unmodified large fragments of H3 are derived from optimized recombinant proteins and all three-piece ligations are carried out in one pot. In vitro reconstituted nucleosomes incorporating these semisynthetic histones should serve as valuable reagents for identifying new binding partners for internal methyllysines by pull-down assays and myriad other in vitro applications.
Supplementary Material
Acknowledgments
This work is supported in part by a grant from the National Institute of Health (1R21HG007426 to A.J.R.), the Chicago Biomedical Consortium (A.J.R. is a Jr. Investigator). We want to thank the members of Kent lab at the University of Chicago for helpful discussions and Nhi Kuong for her help in the preparation of this manuscript.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/cbic.20xxxxxxx.
References
- 1.a) Greer EL, Shi Y. Nature Reviews Genetics. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Martin C, Zhang Y. Nature Reviews Molecular Cell Biology. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 2.a) Allis CD, Muir TW. Chembiochem. 2011;12:264–279. doi: 10.1002/cbic.201000761. [DOI] [PubMed] [Google Scholar]; b) Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. Cell. 2010;143:470–484. doi: 10.1016/j.cell.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]; d) Neumann H, Hancock SM, Buning R, Routh A, Chapman L, Somers J, Owen-Hughes T, van Noort J, Rhodes D, Chin JW. Mol Cell. 2009;36:153–163. doi: 10.1016/j.molcel.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) McGinty RK, Kim J, Chatterjee C, Roeder RG, Muir TW. Nature. 2008;453:812–816. doi: 10.1038/nature06906. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Fierz B, Muir TW. Nat Chem Biol. 2012;8:417–427. doi: 10.1038/nchembio.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Francis NJ, Follmer NE, Simon MD, Aghia G, Butler JD. Cell. 2009;137:110–122. doi: 10.1016/j.cell.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lauberth SM, Nakayama T, Wu X, Ferris AL, Tang Z, Hughes SH, Roeder RG. Cell. 2013;152:1021–1036. doi: 10.1016/j.cell.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Margueron R, Justin N, Ohno K, Sharpe ML, Son J, Drury WJ, 3rd, Voigt P, Martin SR, Taylor WR, De Marco V, Pirrotta V, Reinberg D, Gamblin SJ. Nature. 2009;461:762–767. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Shen X, Kim W, Fujiwara Y, Simon MD, Liu Y, Mysliwiec MR, Yuan GC, Lee Y, Orkin SH. Cell. 2009;139:1303–1314. doi: 10.1016/j.cell.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Simon MD, Chu F, Racki LR, de la Cruz CC, Burlingame AL, Panning B, Narlikar GJ, Shokat KM. Cell. 2007;128:1003–1012. doi: 10.1016/j.cell.2006.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Gloss LM, Kirsch JF. Biochemistry. 1995;34:3990–3998. doi: 10.1021/bi00012a017. [DOI] [PubMed] [Google Scholar]; b) Hopkins CE, Hernandez G, Lee JP, Tolan DR. Arch Biochem Biophys. 2005;443:1–10. doi: 10.1016/j.abb.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 5.a) Munari F, Soeroes S, Zenn HM, Schomburg A, Kost N, Schroder S, Klingberg R, Rezaei-Ghaleh N, Stutzer A, Gelato KA, Walla PJ, Becker S, Schwarzer D, Zimmermann B, Fischle W, Zweckstetter M. J Biol Chem. 2012;287:33756–33765. doi: 10.1074/jbc.M112.390849. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Seeliger D, Soeroes S, Klingberg R, Schwarzer D, Grubmuller H, Fischle W. ACS Chem Biol. 2012;7:150–154. doi: 10.1021/cb200363r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Neumann H, Peak-Chew SY, Chin JW. Nat Chem Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]; b) Nguyen DP, Garcia Alai MM, Kapadnis PB, Neumann H, Chin JW. J Am Chem Soc. 2009;131:14194–14195. doi: 10.1021/ja906603s. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen DP, Garcia Alai MM, Virdee S, Chin JW. Chem Biol. 2010;17:1072–1076. doi: 10.1016/j.chembiol.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 8.a) Muir TW. Annual Review of Biochemistry. 2003;72:249–289. doi: 10.1146/annurev.biochem.72.121801.161900. [DOI] [PubMed] [Google Scholar]; b) Shogren-Knaak MA, Peterson CL. Methods Enzymol. 2004;375:62–76. doi: 10.1016/s0076-6879(03)75004-6. [DOI] [PubMed] [Google Scholar]
- 9.a) He S, Bauman D, Davis JS, Loyola A, Nishioka K, Gronlund JL, Reinberg D, Meng F, Kelleher N, McCafferty DG. Proc Natl Acad Sci U S A. 2003;100:12033–12038. doi: 10.1073/pnas.2035256100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ruthenburg AJ, Li H, Milne TA, Dewell S, McGinty RK, Yuen M, Ueberheide B, Dou Y, Muir TW, Patel DJ, Allis CD. Cell. 2011;145:692–706. doi: 10.1016/j.cell.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shogren-Knaak MA, Fry CJ, Peterson CL. J Biol Chem. 2003;278:15744–15748. doi: 10.1074/jbc.M301445200. [DOI] [PubMed] [Google Scholar]
- 10.Shimko JC, North JA, Bruns AN, Poirier MG, Ottesen JJ. J Mol Biol. 2011;408:187–204. doi: 10.1016/j.jmb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimko JC, Howard CJ, Poirier MG, Ottesen JJ. Methods Mol Biol. 2013;981:177–192. doi: 10.1007/978-1-62703-305-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon M, North JA, Shimko JC, Forties RA, Ferdinand MB, Manohar M, Zhang M, Fishel R, Ottesen JJ, Poirier MG. Proc Natl Acad Sci U S A. 2011;108:12711–12716. doi: 10.1073/pnas.1106264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 14.a) Wan Q, Danishefsky SJ. Angew Chem Int Ed Engl. 2007;46:9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]; b) Chiang KP, Jensen MS, McGinty RK, Muir TW. Chembiochem. 2009;10:2182–2187. doi: 10.1002/cbic.200900238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Pollock SB, Kent SB. Chem Commun (Camb) 2011;47:2342–2344. doi: 10.1039/c0cc04120c. [DOI] [PubMed] [Google Scholar]; b) Hackeng TM, Griffin JH, Dawson PE. Proc Natl Acad Sci U S A. 1999;96:10068–10073. doi: 10.1073/pnas.96.18.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang D, Kent SB. Angew Chem Int Ed Engl. 2004;43:2534–2538. doi: 10.1002/anie.200353540. [DOI] [PubMed] [Google Scholar]
- 17.Schnolzer M, Alewood P, Jones A, Alewood D, Kent SB. Int J Pept Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen FCM, Benoiton NL. Can J Chem. 1976;54:3310–3314. [Google Scholar]
- 19.Tropea JE, Cherry S, Waugh DS. Methods Mol Biol. 2009;498:297–307. doi: 10.1007/978-1-59745-196-3_19. [DOI] [PubMed] [Google Scholar]
- 20.Virdee S, Kapadnis PB, Elliott T, Lang K, Madrzak J, Nguyen DP, Riechmann L, Chin JW. J Am Chem Soc. 2011;133:10708–10711. doi: 10.1021/ja202799r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Nallamsetty S, Kapust RB, Tözsér J, Cherry S, Tropea JE, Copeland TD, Waugh DS. Protein Expression and Purification. 2004;38:108–115. doi: 10.1016/j.pep.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Bang D, Pentelute BL, Kent SB. Angew Chem Int Ed Engl. 2006;45:3985–3988. doi: 10.1002/anie.200600702. [DOI] [PubMed] [Google Scholar]
- 24.Johnson ECB, Kent SBH. J Am Chem Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]
- 25.Evans TC, Jr, Benner J, Xu MQ. Protein Sci. 1998;7:2256–2264. doi: 10.1002/pro.5560071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.a) Li B, Gogol M, Carey M, Lee D, Seidel C, Workman JL. Science. 2007;316:1050–1054. doi: 10.1126/science.1139004. [DOI] [PubMed] [Google Scholar]; b) Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, Johnston LD, Scott MP, Smith JJ, Xiao Y, Jin L, Kuntz KW, Chesworth R, Moyer MP, Bernt KM, Tseng JC, Kung AL, Armstrong SA, Copeland RA, Richon VM, Pollock RM. Cancer Cell. 2011;20:53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Science. 2010;327:996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. Nature. 2012;489:57–74. [Google Scholar]
- 27.a) Cai L, Rothbart SB, Lu R, Xu B, Chen WY, Tripathy A, Rockowitz S, Zheng D, Patel DJ, Allis CD, Strahl BD, Song J, Wang GG. Mol Cell. 2013;49:571–582. doi: 10.1016/j.molcel.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kumar GS, Chang W, Xie T, Patel A, Zhang Y, Wang GG, David G, Radhakrishnan I. J Mol Biol. 2012;422:519–531. doi: 10.1016/j.jmb.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li F, Mao G, Tong D, Huang J, Gu L, Yang W, Li GM. Cell. 2013;153:590–600. doi: 10.1016/j.cell.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Gottgens B, Bycroft M. Nat Struct Mol Biol. 2010;17:617–619. doi: 10.1038/nsmb.1797. [DOI] [PubMed] [Google Scholar]
- 28.van Nuland R, van Schaik FM, Simonis M, van Heesch S, Cuppen E, Boelens R, Timmers HM, van Ingen H. Epigenetics Chromatin. 2013;6:12. doi: 10.1186/1756-8935-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wysocka J. Methods. 2006;40:339–343. doi: 10.1016/j.ymeth.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P, Ecker JR, Bushman F. Nat Med. 2005;11:1287–1289. doi: 10.1038/nm1329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.