Abstract
Fixed dose combination (FDC) dual bronchodilators that co-administer a long acting β2-adrenoceptor agonist (LABA) and a long acting muscarinic antagonist (LAMA) are a new class of inhaled treatment for chronic obstructive pulmonary disease (COPD). This review focuses on the clinical evidence for the benefit of LABA/LAMA FDCs compared with monocomponent treatments, and also compared with active comparators that are widely used for the treatment of COPD, namely tiotropium and salmeterol-fluticasone. Novel FDC dual bronchodilators include QVA149 and umeclidinium/vilanterol (UMEC/VI). Long term clinical trials show that QVA149 and UMEC/VI are superior to monocomponent therapy in terms of trough forced expiratory volume in 1 s (FEV1), although the FEV1 improvement was limited to approximately 80–90% of the added monocomponent values. This suggests that the effect of combining a LABA and a LAMA is not fully additive. LABA/LAMA FDC were associated with the largest mean changes in symptoms and health status that were above the minimal clinically important difference, in contrast to the monocomponents. Furthermore, these LABA/LAMA FDCs demonstrated superiority over the active comparators tiotropium and salmeterol-fluticasone in terms of trough FEV1 and patient-reported outcomes. LABA/LAMA FDCs offer a simplified means of maximizing bronchodilation for COPD patients, with the improvements in lung function being mirrored by benefits in terms of symptoms and exacerbations. The use of LABA/LAMA FDCs in clinical practice is set to grow and further studies are needed to define their optimal place in treatment guidelines.
Keywords: bronchodilators, COPD, fixed dose combinations, QVA149, umeclidinium, vilanterol
Introduction
Chronic obstructive pulmonary disease (COPD) is caused by the inhalation of noxious particles, such as tobacco smoke, and is characterized by poorly reversible airflow obstruction and a chronic inflammatory response in the lungs 1. The pathological hallmark features of COPD are airway inflammation associated with small airway narrowing, mucus hypersecretion and parenchymal destruction 2. In addition, systemic manifestations of COPD include muscle wasting and an increased incidence of co-morbidities such as cardiovascular disease, diabetes and depression 1.
Bronchodilators are the primary pharmacological intervention for COPD. These drugs improve symptoms and quality of life by improving airflow and hence gaseous exchange, and by reversing air trapping and dynamic lung hyperinflation through dilatation of the distal airways 3. Long acting bronchodilators with up to 24 h bronchodilator activity are used as maintenance therapy for the prevention and reduction of symptoms 3. There are two classes of long acting bronchodilators that act by different mechanisms, long acting muscarinic antagonists (LAMAs) and β2-adrenoceptor agonists (LABAs). LAMAs inhibit the action of acetylcholine at muscarinic receptors, while LABAs enhance cAMP signalling through stimulation of β2-adrenergic receptors 3.
The GOLD management strategy recommends classification of patients into categories A,B,C or D depending on the level of symptoms (patients with greater symptoms are classified as B or D) and risk (patients with FEV1 < 50% predicted or a history of frequent exacerbations are classified as high risk into groups C or D) 3. GOLD recommends either LAMAs or LABAs as maintenance therapy, and that patients with more severe disease may be prescribed a LABA and a LAMA, or a fixed dose combination (FDC) of a LABA with an inhaled corticosteroid (ICS), which is similar to guidelines used in clinical practice such as NICE 4–6. Co-prescribing of LABAs and LAMAs has been impeded by the need to use separate inhalers, with different delivery and often different dosing schedules. A recent development is the availability of FDC dual bronchodilators that provide co-administration of LABAs and LAMAs in a single inhaler (Table 1 shows LABA/LAMA FDC phase III programmes). An important aim of these programmes is to establish that the ‘combination rule’ required by the regulators, namely that the FDC is superior to the individual LABA and LAMA components for FEV1, has been met.
Table 1.
LABA/LAMA FDCs approved or in phase III clinical development
| LABA | LAMA | FDC development phase | Dosing | Inhaler | Company |
|---|---|---|---|---|---|
| Indacaterol | Glycopyrronium | Approved (Ultibro®) in EU, Japan | 110/50 μg | Breezhaler® | Novartis |
| once daily | |||||
| Vilanterol | Umeclidinium | Approved (Anoro®) in USA, EU | 62.5/25 μg | ELLIPTA® | GSK, Theravance |
| once daily | |||||
| Formoterol | Aclidinium | Positive opinion† (Duaklir®) in EU | 400/12 μg | Genuair® | Almirall, Forest |
| twice daily | |||||
| Olodaterol | Tiotropium | Filed* in EU and USA | 5 μg/5 μg | Respimat® Soft Mist® | Boehringer Ingelheim |
| once daily | |||||
| Formoterol | Glycopyrronium | Phase III trials | Twice daily | Pressurized hydrofluoroalkane (HFA MDI) | AstraZeneca |
Filed = submitted to regulatory authorities. †Positive opinion = regulatory have reviewed submission and provided a positive opinion regarding approval.
Effective and sustained bronchodilation is a key strategy for improving dyspnoea and exercise performance in COPD patients 3. A major goal of new LABA/LAMA FDC therapies is therefore to improve lung function effectively and thereby enhance exercise tolerance and improve patient-reported outcomes such as dyspnoea and quality of life. In this review, the current evidence base for benefit of LABA/LAMA FDCs on lung function and on patient-reported outcomes is critically evaluated. Furthermore, the future place of these new combined bronchodilators in the shifting treatment paradigm for COPD is discussed.
This review was based on identification of phase III clinical trials describing FDC dual bronchodilators. Searches of PubMed and Google Scholar and hand-searches of bibliographies of journal articles were performed. European Respiratory Society and the American Thoracic Society abstracts from 2008–2013 were searched. There were no phase III data published on olodaterol/tiotropium or formoterol/glycopyrronium.
Scientific rationale for combining LABAs and LAMAs
The bronchodilator effects of β2-adrenoceptor agonists and anti-muscarinics used alone in laboratory models demonstrate a linear response at low concentrations followed by a flatter curve at higher concentrations 7,8. Inhaled delivery in humans also shows a similar dose–response curve, with a reduced effect at higher doses 9,10. In clinical practice, this means that higher doses may achieve little extra benefit but cause unwanted side effects through systemic absorption.
Clinical development programmes in COPD patients have defined the doses of long acting bronchodilator monotherapies (either LABAs or LAMAs alone) with the optimum therapeutic index 11,12, and discovered differences between drugs in terms of onset of action and dose responsiveness, e.g. formoterol is an example of a β-adrenoceptor agonist with a fast onset of action and a relatively linear dose−response curve 13. Additional bronchodilation can be achieved by combining LABAs and LAMAs due to the distinct and complementary mechanisms of action 13. This concept is well accepted for the short acting β2-adrenoceptor agonist and anti-muscarinic combination inhaler containing ipratropium bromide/albuterol (Combivent®) 14. Studies combining long acting bronchodilators administered using separate devices have also demonstrated an additive benefit 15–25. The magnitude of lung function additive benefit achieved has varied greatly between studies, due to differences in the timing of lung function, the lack of placebo control in some studies and relatively small sample sizes. Nevertheless, these studies support the clinical rationale for dual long acting bronchodilator therapy. Furthermore, it has been demonstrated that increasing the dose of the LABA, indacaterol, from 300 to 600 μg day–1 was less effective than using indacaterol 300 μg with the LAMA, glycopyrronium, delivered in the same inhaler 26.
The patterns of response to a single bronchodilator monotherapy can vary between patients. Some patients may show much greater responsiveness to one class of bronchodilator compared with the other. Furthermore, it is well known that the magnitude of bronchodilation with the same drug can vary from day to day 27. Combination treatment with a LABA plus a LAMA has the potential to maximize bronchodilation and so overcome such variations in response that occur with monotherapy. Laboratory studies suggest that synergistic effects may occur between LABAs and LAMAs by a variety of mechanisms. For example, β2-adrenoceptor agonists can activate pre-junctional β2-adrenoceptors to reduce acetylcholine release 13, and inhibitory crosstalk may exist between M3 receptors and β2-adrenoceptors in airway smooth muscle 28. It could be hypothesized that the use of LAMA/LABA FDCs will enhance the possibility of synergistic interactions by co-deposition of LABAs and LAMAs in the airways.
Indacaterol/glycopyrronium (QVA149)
The Indacaterol GlycopyrroNium bromide clInical sTudiEs (IGNITE) phase III programme is a series of 11 clinical trials assessing the efficacy and safety of QVA149 once daily in more than 10 000 patients with COPD. This FDC comprises indacaterol and glycopyrronium (110/50 μg once daily) administered by the Breezhaler® device, which is a single dose dry powder inhaler. A double-blind trial (BEACON) demonstrated no difference in lung function or symptoms between QVA149 and the concurrent administration of its monocomponents, thus demonstrating that the FDC provides similar clinical improvements to separate components 29.
Assessing superiority of QVA149 over monocomponents
The 26 week, placebo- and active-controlled (open label tiotropium 18 μg once daily administered via the HandiHaler® device) SHINE study compared QVA149 with indacaterol and glycopyrronium 30. Indacaterol and glycopyrronium monotherapy improved trough FEV1 (24 h after dosing) at week 26 by 130 ml and 120 ml, respectively. The effect of QVA149 was significantly greater (P < 0.001) than the monocomponents (indacaterol, Δ = 70 ml; glycopyrronium, Δ = 90 ml) and tiotropium (Δ = 80 ml), thus fulfilling the combination rule criteria (Figure 1A). Interestingly, the effect of QVA149 on trough FEV1 (Δ = 200 ml) was less than the sum of the monocomponent improvements (130 ml + 120 ml = 250 ml). A subgroup analysis showed that the effects of QVA149 compared with placebo on trough FEV1 appeared to be greater in patients with moderate (Δ = 240 ml) compared with severe COPD (Δ = 120 ml).
Figure 1.
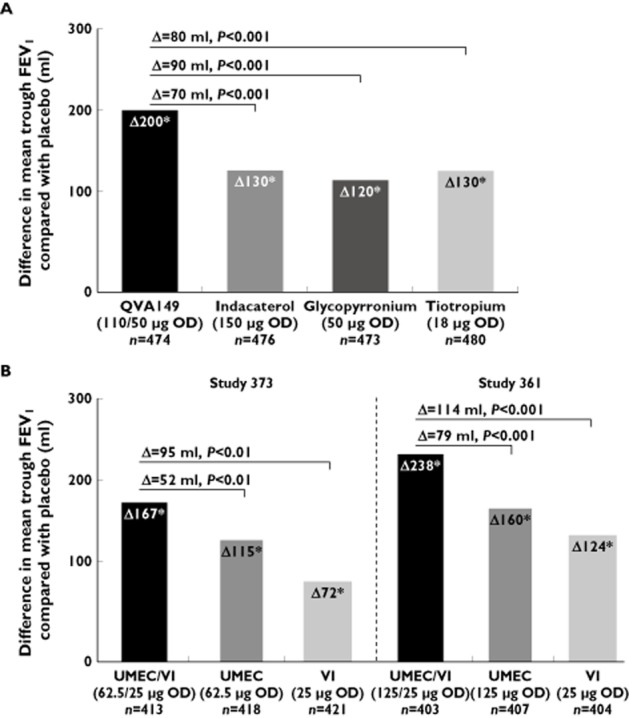
Effect of QVA149, its monocomponents and tiotropium (A) and UMEC/VI and its monocomponents (B) on mean trough FEV1 at week 26. (A) SHINE trial data; (B) studies 373 and 361 data.*P < 0.001 for comparisons with placebo. The horizontal lines are the comparisons of QVA149 with indacaterol, glycopyrronium (primary end point) and tiotropium in (A) and of UMEC/VI with UMEC and VI alone (primary end point) in (B)
An improvement in breathlessness can be measured using the transition dyspnoea index (TDI) 31. A one point increase compared with placebo is considered the minimal clinically important difference (MCID) 32. At week 26, there were mean TDI improvements with indacaterol (Δ = 0.84, P < 0.001), glycopyrronium (Δ = 0.89, P < 0.001) and tiotropium (Δ = 0.58, P = 0.017) compared with placebo that did not reach the MCID threshold (Figure 2A) 30. QVA149 achieved a TDI treatment difference of 1.09 (P < 0.001) vs. placebo. However, this improvement was not significantly different compared with the monocomponents, although QVA149 was superior to tiotropium (Δ = 0.51, P = 0.007). TDI responder analysis showed the same pattern, with more responders with QVA149 compared with tiotropium and placebo, but not the monocomponents (Table 2).
Figure 2.
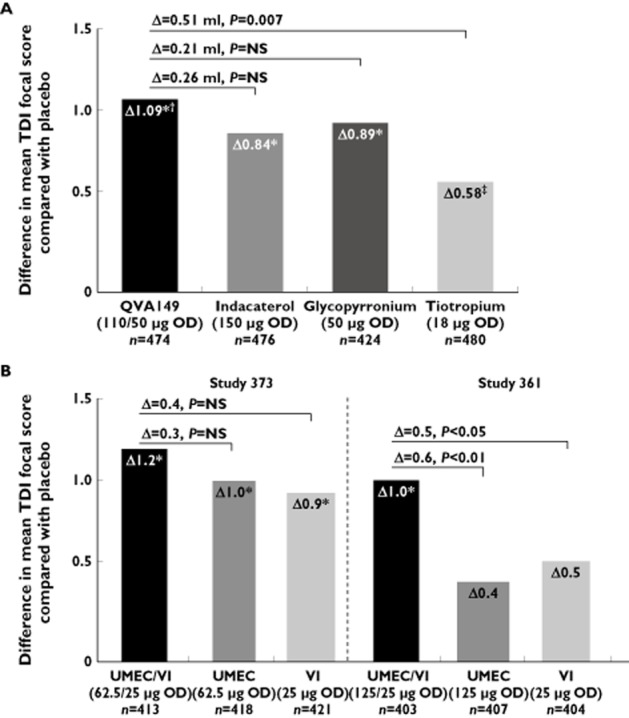
Effect of QVA149, its monocomponents and tiotropium (A) and UMEC/VI and its monocomponent (B) on mean TDI focal score at week 26. (A) SHINE trial data; (B) studies 373 and 361 data. *P < 0.001 for comparisons with placebo; †P = 0.007 for comparison with tiotropium; ‡P = 0.017 for comparison with placebo. The horizontal lines are the comparisons of QVA149 with indacaterol, glycopyrronium and tiotropium in (A) and of UMEC/VI with UMEC and VI alone in (B)
Table 2.
Patients achieving a minimal clinically important difference in transition dyspnoea index (≥1 point improvement); data from placebo-controlled pivotal phase III clinical trials of QVA149 and UMEC/VI

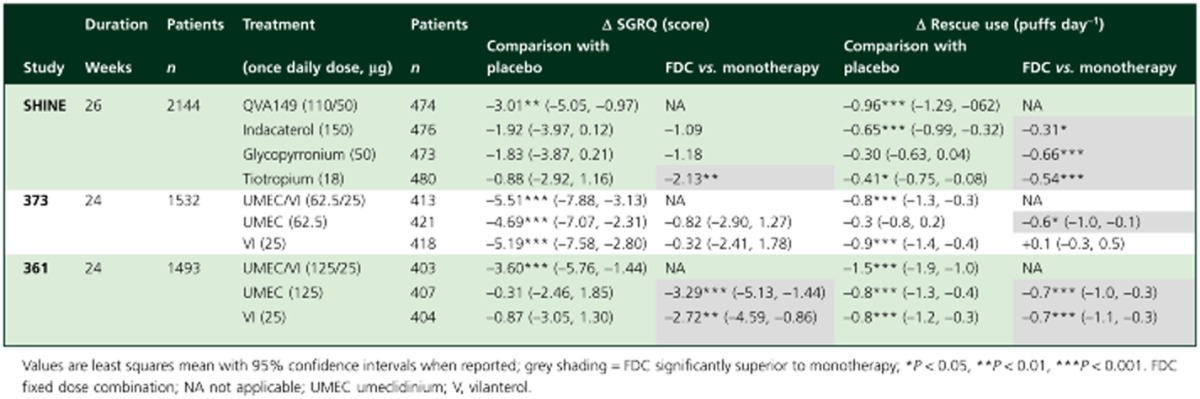
Changes in health status were assessed using the St Georges Respiratory Questionnaire (SGRQ), which has a MCID of a 4 point decrease. QVA149 resulted in significant improvements in SGRQ total score at week 26 compared with placebo (Δ = –3.01; P = 0.002) and tiotropium (Δ = –2.13; P = 0.009), with no significant difference vs. indacaterol (Δ = –1.09) and glycopyrronium (Δ = –1.18) (Table 3). The use of rescue medication was significantly less with QVA149 than monotherapy (Table 3).
Table 3.
Treatment differences in SGRQ scores and rescue medication use in the placebo-controlled pivotal phase III clinical trials of QVA149 and UMEC/VI
The SPARK study assessed the effect of QVA149 compared with LAMA monotherapy on exacerbation frequency in COPD patients with FEV1 < 50% predicted 33. The primary end point of SPARK was the comparison of the rate of moderate to severe exacerbations (defined as worsenings needing treatment with systemic corticosteroids or antibiotics or both, or hospitalization) between QVA149 and glycopyrronium. There was a 12% reduction in events with QVA149 (annualized rate of exacerbations, 0·84 vs. 0·95; P = 0·038) (Figure 3). QVA149 reduced exacerbations by 10% compared to tiotropium (annualized rate of 0.93), but this did not reach statistical significance (P = 0.096). There were significant improvements from baseline in SGRQ total score with QVA149 compared with glycopyrronium and tiotropium up to 64 weeks (Table 3), with treatment differences generally between −2 to −3 points at the different time points. The use of rescue salbutamol was decreased to a greater extent with QVA149 compared with glycopyrronium and tiotropium (Table 3).
Figure 3.
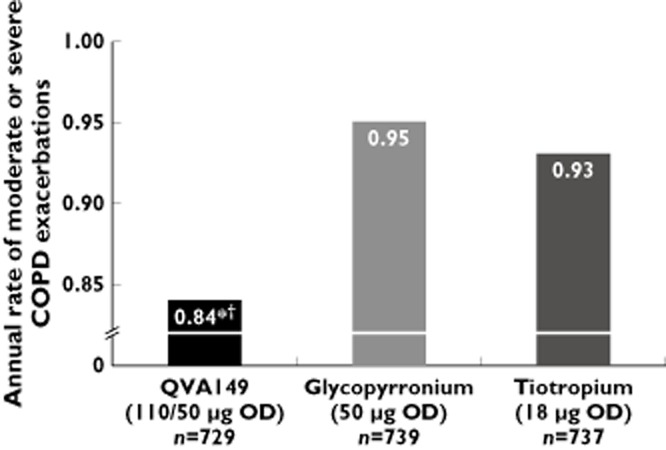
Annual rate of moderate or severe COPD exacerbations according to treatment group (SPARK trial). *P = 0.038 for comparison with glycopyrronium. †P = 0.096 for comparison with tiotropium. OD once daily
Comparison of QVA149 with tiotropium
In addition to the SHINE study, there have been two other studies comparing QVA149 with tiotropium. In the 6 week placebo-controlled, three period, crossover study (BLAZE), COPD patients were randomized to QVA149, tiotropium 18 μg once daily (administered via the HandiHaler® device) or placebo 34. A novel self-administered computerized reporting system to measure the TDI was used to reduce the potential bias with the interviewer-based version. QVA149 significantly improved TDI score compared with both placebo (Δ = 1.37, 95% CI 0.95, 1.79; P < 0.001) and blinded tiotropium (Δ = 0.49, 95% CI 0.07, 0.91; P = 0.021). QVA149 also significantly improved FEV1 compared with tiotropium up to 4 h post-dose after the first dose (P < 0.001), and at pre-dose and up to 4 h post-dose at 6 weeks (P < 0.001).
BRIGHT was a blinded, three period, crossover study for 3 weeks that investigated exercise tolerance and hyperinflation in patients with moderate to severe COPD 35. At day 21, QVA149 significantly improved inspiratory capacity (IC) during exercise at isotime compared with both placebo (Δ = 320 ml; P < 0.001) and tiotropium (Δ = 140 ml; P < 0.01), and with significant improvements in trough IC of 190 ml (P < 0.01) and 150 ml (P < 0.01), respectively. QVA149 and tiotropium caused similar improvements in exercise endurance time compared with placebo (59.5 s and 66.3 s respectively; both P < 0.01). The advantage of QVA149 over tiotropium for IC may not have translated into superior exercise endurance time because of other factors such as muscle deconditioning meaning that only limited exercise improvements were possible, and perhaps concurrent pulmonary rehabilitation is necessary to show the full benefit of improved IC with QVA149.
Comparison of QVA149 with salmeterol-fluticasone
The ILLUMINATE study compared QVA149 with twice daily salmeterol-fluticasone (SFC) 50/500 μg (administered via the Accuhaler® device) in moderate to severe COPD patients without an exacerbation in the previous year 36. Inhaled corticosteroids, including combination treatments such as SFC, are licensed for use in COPD patients with a history of exacerbations, although these drugs are often prescribed to patients without a history of exacerbations 37. The purpose of ILLUMINATE was to compare QVA149 with SFC in patients who should not be taking ICS according to the license indication 36. The trough FEV1 was significantly higher at week 26 with QVA (treatment difference 103 ml, P < 0.001). Phase 3 clinical trials often focus on trough FEV1, which is measured in the morning prior to the next planned dose. However, bronchodilation during the day is also important, and measuring FEV1 AUC(0–12 h) provides a good estimate of the average post-dose effect during the day. FEV1 AUC(0–12 h) was significantly higher with QVA149 compared with SFC at week 26, with a significant treatment difference of 138 ml (P < 0·0001), with similar results after the first dose and week 12.
There was a significant increase in the TDI focal score at weeks 12 and 26 with QVA149 compared with SFC; Δ = 0.58 (95% CI 0.07, 1.08; P = 0.025) and Δ = 0.76 (95% CI 0.26, 1.26; P = 0.0031), respectively. QVA149 patients used significantly less rescue medication (0.39 puffs day−1 treatment difference; P = 0.019) compared with SFC 36. SGRQ total scores improved to a similar degree with both treatments.
Umeclidinium/vilanterol
The UMEC/VI clinical development programme was principally comprised of seven phase III studies in approximately 6000 subjects. The UMEC/VI inhalation powder is administered once daily by a novel, single step activation, multidose DPI for oral inhalation (ELLIPTA®), and is licensed at a dose of 62.5/25 μg (this is the metered dose, the delivered dose is 55/22 μg).
Assessing superiority over monocomponents
In two, 24 week, placebo-controlled trials (studies 373 (n = 1532) 38 and 361 (n = 1489) 39; full GSK study numbers were DB2113373 and DB2113361, respectively), the effect of UMEC/VI (62.5/25 μg and 125/25 μg, respectively) was compared with its monocomponents. Figure 1B shows that UMEC and VI monocomponents improved trough FEV1 in both studies. Significantly greater improvements were achieved by UMEC/VI compared with the monocomponents in both studies. Interestingly, the effect of UMEC/VI (62.5/25) compared with placebo on FEV1 (167 ml) was slightly lower than the sum of the components (187 ml), with a similar pattern for the higher UMEC dose (125/25; 238 ml compared with 284 ml).
In study 373, there were statistically significant increases in mean TDI compared with placebo for UMEC (Δ = 1.0), VI (Δ = 0.9) and UMEC/VI (Δ = 1.2), with no differences between active treatments (Figure 2B) 38. In study 361, the mean changes in TDI were not significantly different compared with placebo for the individual components, while UMEC/VI (125/25) caused improvements that reached the MCID (Δ = 1.0) and were significantly greater than placebo and the monocomponents 39. In both studies, the proportion of patients achieving the TDI MCID at the end of the study was greatest with UMEC/VI, and the odds of being a TDI responder were significantly greater with UMEC/VI compared with placebo (Table 2). In study 361, the odds of being a TDI responder were also greater for UMEC/VI compared with both monocomponents, while in study 373 this was true for the comparison of UMEC/VI with VI only.
Table 3 shows that UMEC/VI (62.5/25) was associated with a significant improvement in SGRQ scores compared with placebo, with no significant differences compared with the monocomponents. UMEC/VI (62.5/25) significantly reduced daily rescue medication use over 24 weeks compared with placebo, and compared with umeclidinium, but not vilanterol. For the higher UMEC/VI dose (125/25), there was a significant improvement in SGRQ scores and a significant reduction of daily rescue medication use over placebo and the monocomponents.
Comparison of UMEC/VI with tiotropium
Two active comparator studies comparing both UMEC/VI doses (62.5/25 and 125/25) with tiotropium 18 μg have been reported. In study 360 (full GSK study number; DB2113360), VI 25 μg was also investigated, while UMEC 125 μg was included in study 374 (full GSK study number; DB2113374) 40. Placebo arms were not included in these studies, as the comparisons of interest were between the active treatments. There were no significant differences for lung function, TDI, SGRQ or rescue medication use between the UMEC/VI doses, and so the lower dose has been licensed for clinical use. The effect of both doses of UMEC/VI on trough FEV1 at week 24 was significantly greater than tiotropium in the two studies (effect size ranging from 60–90 ml). All active treatments improved TDI and SGRQ above the MCID thresholds, with no differences between both UMEC/VI doses and tiotropium. However, there were significant reductions in reliever medication use with both UMEC/VI doses compared with tiotropium, ranging from mean 0.6 to 1.1 puff day−1 reduction.
Aclidinium/formoterol
The clinical development programme for aclidinium/formoterol FDC investigated two doses (400/6 μg twice daily and 400/12 μg twice daily) in more than 4000 patients with moderate to severe COPD 41–44. These studies are not yet fully published, and so only data from abstracts have been reviewed here. The programme included two placebo-controlled 24 week efficacy studies (ACLIFORM/COPD and AUGMENT/COPD) comparing the FDC with the monocomponents (formoterol 12 μg and aclidinium 400 μg), and two long term safety studies, LAC-MD-32 which is a 1 year trial and LAC-MD-36 which is a 6 month extension of AUGMENT. The FDC is administered using Genuair®, a multiple dose dry powder inhaler.
The AUGMENT/COPD and ACLIFORM/COPD studies have reported co-primary end points measured at week 24 43,44, trough (pre-dose) morning FEV1 for the comparison of FDC vs. formoterol and 1 h morning post-dose FEV1 for the comparison of FDC vs. aclidinium. These are shown in Table 4. The 1 h post-dose FEV1 was significantly higher with both FDC doses compared with aclidinium in both studies. In AUGMENT, the higher FDC dose significantly improved trough FEV1 compared with formoterol by 45 ml (P = 0.01), but there was no significant difference for the lower dose. A greater effect for the higher FDC on trough FEV1 was also observed in ACLIFORM, indicating that the higher FDC is superior to the monocomponents and the lower FDC dose.
Table 4.
Treatment differences in lung function at week 24 in the phase III clinical trials of aclidinium/formoterol
| Primary outcomes | Study | Aclidinium/formoterol 400/6 μg | Aclidinium/formoterol 400/12 μg |
|---|---|---|---|
| Pre-dose trough FEV1 (ml) (comparison of FDC vs. formoterol) | AUGMENT | 26 | 45* |
| ACLIFORM | 53** | 85¶ | |
| 1 h post-dose FEV1 (ml) (comparison of FDC vs. aclidinium) | AUGMENT | 87¶ | 108¶ |
| ACLIFORM | 69¶ | 125¶ |
P < 0.05
P < 0.01
P < 0.001.
Safety of LABA/LAMA FDCs
The combination of a LABA with a LAMA could potentially result in a greater incidence of adverse events (AE), in particular cardiovascular (CV) events. LABAs can increase stimulation of cardiac β2-adrenoceptors 45,46, while LAMAs can block cardiac M2 receptors thereby predisposing to tachycardias 47.
The ENLIGHTEN study was a placebo-controlled study that evaluated QVA149 safety over 52 weeks. The overall incidence of AEs with QVA149 and placebo was similar (57.8% vs. 56.6%) 48. Cardio-cerebrovascular (CCV) AEs were reported in 5.3% of QVA149-treated patients and 2.7% of placebo-treated patients. The authors noted imbalances in baseline characteristics of the populations that could explain this difference 48, including more severe COPD and more patients with a previous history of myocardial infarction, stroke and diabetes in the group treated with QVA149. A pooled analysis of the SHINE, ILLUMINATE, ENLIGHTEN and ARISE studies showed that CCV AEs occurred in a comparable proportion of patients in the QVA149 (1.8%) and tiotropium groups (1.7%), but importantly there was no increase compared with placebo (2.3%) 49. Meta-analysis has demonstrated no significant differences in SAEs and serious CCVs with QVA149 compared with tiotropium and glycopyrronium 50.
A long term safety study of UMEC/VI 51 showed similar AEs (range 52–58%) and serious AEs (6–7%) to placebo. Similarly, the incidence of treatment-emergent AEs was comparable between aclidinium/formoterol, placebo and other active treatment arms in ACLIFORM 44. A long term safety trial reported that SAEs were similar in patients treated with aclidinium/formoterol (9.7%) or formoterol (10.6%) 52. Overall, these adverse event data do not show any significant safety concerns for LABA/LAMA FDCs compared with monotherapy.
Discussion
This discussion of the clinical benefits of LABA/LAMA FDCs will now focus mainly on QVA149 and UMEC/VI, as these drugs have more clinical trial results published.
Benefits of LABA/LAMA FDCs over monotherapies
LABA/LAMA FDCs demonstrated significantly greater treatment effects on trough FEV1 compared with placebo and monocomponent therapy 30,33,38,39. The MCID for improvement in trough FEV1 in COPD clinical trials is estimated at 100 ml 53. The monocomponents of QVA149 and UMEC/VI generally reached this threshold compared with placebo, but significantly larger increases were caused by the FDC compared with the monocomponents 30,38,39. It is interesting that these FDC LABA/LAMA trough FEV1 improvements were less than the sum of the monocomponent improvements. The use of a second long acting bronchodilator drug appears to cause an additive response that is not linear, possibly because the second drug causes bronchodilation near the top of the dose−response curve where the response is flatter. This lack of a full addition may be due to a ‘ceiling effect’, whereby the magnitude of bronchodilation that is achievable in poorly reversible COPD patients is limited, and so the linear response part of the bronchodilation dose−response curve is relatively short. An alternative explanation is that LABAs and LAMAs act initially on different cell signalling mechanisms but ultimately exert bronchodilation through the same downstream mechanisms. In vitro studies suggest that LABA/LAMA combinations may allow beneficial synergistic interactions 13,28. However, clinical trials using LABA/LAMA FDCs show that a full additive effect is not seen, let alone the prospect of synergistic benefits.
A key question is whether the benefit of LABA/LAMA FDCs in terms of FEV1 leads to a clinically meaningful improvement in symptoms. The TDI is often used to measure dyspnoea in clinical trials, with the 1 point MCID established based on the difference between active treatment and placebo 54. Neither QVA149 nor UMEC/VI reached a 1 point improvement compared with the monocomponents, and it appears that applying the same MCID to studies that compare active drugs is not appropriate. Furthermore, most studies were not statistically powered specifically to detect a 1 point difference in TDI between active treatments. Indeed, other patient reported outcomes and measurements related to symptoms, such as reliever medication use, have also been secondary end points, which again were not specifically powered for. However, a combined view of the TDI data with rescue medication use allows a proper understanding of changes in dyspnoea. Both QVA149 and UMEC/VI FDCs caused numerically greater mean TDI improvements compared with the monocomponents, with statistical significance reached for the higher UMEC/VI dose compared with the monocomponents. Responder analysis is recognized as an alternative and appropriate way to evaluate the effects of two active treatments compared with one 53, and showed a significant advantage of UMEC/VI over the monocomponents for TDI. Rescue medication use also showed that UMEC/VI and QVA149 reduced symptoms to a significantly greater extent than the monocomponents. Taken together, these TDI responder data and rescue medication use provide strong evidence of clinically significant improvements in dyspnoea with LABA/LAMA FDCs compared with monocomponent therapy.
Further evidence for the potential benefits on symptoms of these LABA/LAMA FDCs over bronchodilator monotherapy comes from comparisons with tiotropium. The SHINE study showed a significant benefit for QVA149 compared with tiotropium on mean TDI (Δ = 0.51), which was confirmed by TDI responder analysis and was consistent with less rescue medication use with QVA149. The BLAZE study showed a similar superiority for QVA149 on mean TDI score (Δ = 0.49). A recent meta-analysis has shown that QVA149 provided superior bronchodilation compared with tiotropium (Δ = 70 ml, P < 0.0001), accompanied by reductions in rescue medication use (−0.63 puffs day−1, P < 0.0001) and a 19% greater chance of achieving the TDI MCID compared with treatment with tiotropium 50. For UMEC/VI, the strongest evidence of symptom benefit compared with tiotropium monotherapy was the reduction in rescue usage ranging from mean 0.6 to 1.1 puff day−1 reduction across the studies. Tiotropium is widely used as bronchodilator monotherapy. It appears that increased benefits in terms of lung function and dyspnoea can be achieved with LABA/LAMA FDCs.
The SPARK study demonstrated that QVA149 had a greater effect on moderate to severe exacerbations compared with glycopyrronium 33. It is known that long acting bronchodilators used as monotherapy reduce exacerbation rates 55,56 and the SPARK study showed for the first time that two long acting bronchodilators are superior to one with regard to this end point 33. The 10% difference between QVA149 and tiotropium for moderate to severe exacerbations was similar to the 14% benefit observed for tiotropium added to standard of care in UPLIFT 56. This suggests that the benefit of adding an additional long acting bronchodilator to existing therapy, including long acting bronchodilator monotherapy, is an approximately 12–14% reduction in exacerbations. It would be valuable to see more clinical trials evaluating exacerbation reduction caused by dual bronchodilators to confirm this. Furthermore, SPARK was conducted in patients with FEV1 <50% predicted. Investigating the effects of dual bronchodilators in frequent exacerbators with FEV1 >50% would be of interest.
A pooled analysis of 3313 patients in indacaterol studies showed that increasing FEV1 was associated with significant improvements in TDI, SGRQ and a decline in exacerbation rate 57. This indicates that larger improvements in FEV1 are likely to be associated with larger, patient-reported benefits across a range of clinical outcomes. In general, the outcomes from the QVA149 and UMEC/VI studies support this concept.
Comparison of LABA/LAMA FDCs with ICS/LABA FDCs
ICSs are licensed for use in COPD patients with a history of exacerbations, but in real life these drugs are commonly also prescribed to patients without a history of exacerbations 37. ILLUMINATE suggests that LABA/LAMA FDCs are a more effective treatment option than ICS/LABA FDCs in COPD patients without a history of exacerbations 36. Furthermore, there are also potential corticosteroid-sparing advantages in terms of side effects.
Let us consider what result would be observed if LABA/LAMA FDCs were compared with ICS/LABA FDCs in patients with a history of exacerbations (GOLD category C and D). The INSPIRE study showed no difference between tiotropium and salmeterol-fluticasone for the overall rate of moderate to severe exacerbations, although there appeared to be a difference in the nature of the exacerbations with more oral corticosteroid courses required in patients taking tiotropium but more pneumonia events in patients taking salmeterol-fluticasone 58. SPARK showed significant superiority of QVA149 over glycopyrronium for the rate of moderate to severe exacerbations, and there was a numerical advantage over tiotropium 33. INSPIRE showed that tiotropium was similar to ICS/LABA in terms of exacerbation reduction, so one could speculate that QVA149 would also not be inferior to salmeterol-fluticasone for exacerbation reduction in patients with a history of exacerbations. Future clinical trials that compare LABA/LAMA FDCs with ICS/LABA FDCs hopefully will inform us of the relative merits of these treatments for GOLD category C and D patients.
Differences between LABA/LAMA FDCs
There are insufficient clinical data to determine whether there are clinically important differences in efficacy between the various LABA/LAMA FDCs reviewed here. It is not wise to compare effect sizes between studies to draw definitive conclusions on whether one drug is better than the other, as differences in the characteristics of the patients and the design of the studies can impact on the effect sizes observed. Head to head studies would be needed to compare properly LABA/LAMA FDCs. Nevertheless, the efficacy results in terms of lung function for QVA149 and UMEC/VI are broadly similar and one could speculate that any difference in lung function between these two treatments is likely to be small in magnitude.
QVA149 and UMEC/VI are administered once per day, which is a convenient treatment regime for patients. LABA/LAMA FDC in development such as aclidinium/formoterol and glycopyrronium/formoterol are administered twice per day, which may be less convenient. However, the profile of lung function after twice a day dosing may be better suited to patients with night time or early morning symptoms, as the administration of the evening dose may offer benefits to these patients. The efficiency, ease of use and patient preference for the different inhaler devices should also be considered. Safety is also a key issue and the current safety profile of these drugs is encouraging.
Place of LABA/LAMA FDCs in COPD treatment
The emerging clinical trial data provide valuable information to help us understand the relative merits of LABA/LAMA FDCs compared with other drug therapies. COPD patients are usually treated initially with short acting bronchodilators, followed by the addition of a long acting bronchodilator (either a LAMA or a LABA) monotherapy 3. The QVA149 and UMEC/VI clinical trials were mostly conducted in COPD patients without frequent exacerbations, and demonstrate that a simple stepwise approach of adding a second bronchodilator drug in these patients is clinically effective, as shown in Figure 4. The UMEC/VI clinical trials were performed in COPD patients with FEV1 ≤ 70% predicted and significant dyspnoea (mMRC ≥ 2). In this population of GOLD B and D patients it appears that LABA/LAMA FDCs are superior to monotherapy, and it would be reasonable to start such patients on LABA/LAMA FDCs without first trying monotherapy, as the therapeutic benefit as a first line therapy is likely to be greater. However, for patients who are less symptomatic or who have better lung function the evidence for this approach is lacking, and the stepwise approach shown in Figure 4 could be advocated. It should be noted that there are no clinical trial or real life data to guide us on whether to start with bronchodilator monotherapy or dual treatment.
Figure 4.
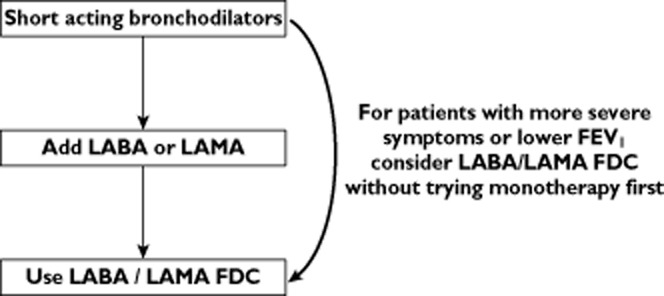
Proposed treatment pathway for COPD patients without frequent exacerbations. LABA long acting β2-adrenoceptor agonist; LAMA long acting muscarinic antagonist; FDC fixed dose combination; FEV1 forced expiratory volume in 1 s.
For COPD patients without a history of exacerbations, ILLUMINATE clearly shows lung function and symptom benefits for QVA149 over seretide. ICS/LABA combinations are not licensed for use in COPD patients without a history of exacerbations, and ILLUMINATE shows that such patients are better treated by using a FDC dual bronchodilator. With disease progression, patients who then suffer with exacerbations could then progress to ‘triple therapy’ with the addition of an inhaled corticosteroid or other anti-inflammatory drug.
Gaps in knowledge
There are important gaps in our knowledge regarding the optimum use of LABA/LAMA FDCs in COPD patients. SHINE suggests that the effect on FEV1 may be reduced in more severe patients 30, which is compatible with other data using monotherapies showing that bronchodilator effects are greater in patients with less severe airflow obstruction 59. Nevertheless, the question of whether a subgroup of patients benefit more from LABA/LAMA FDCs remains largely unanswered. Bronchodilators decrease hyperinflation, and perhaps patients with more hyperinflation will be responders to LABA/LAMA FDCs. BRIGHT showed superiority for QVA149 over tiotropium on resting and dynamic hyperinflation 35. Disappointingly, these physiological advantages for QVA149 did not translate into improved exercise endurance compared with tiotropium. Perhaps the true advantages of LABA/LAMA FDCs on exercise performance will only be seen in the context of pulmonary rehabilitation which can provide concurrent improvements in muscle strength. More studies evaluating the effects of dual and monobronchodilators on lung volumes and exercise performance would be informative.
Table 5 outlines important clinical trials in COPD concerning dual bronchodilator therapies that have either been recently completed or are ongoing. The full publications of these studies are likely to increase further our understanding of the merits of these drugs. There are further comparisons of dual bronchodilators with ICS/LABA. Although the GOLD guidelines include LABA plus LAMA therapy as a treatment option for GOLD C and D patients, the only clinical trial of LABA/LAMA FDCs in GOLD C and D patients with frequent exacerbations is SPARK 33. The important comparison of LABA/LAMA FDCs vs. ICS/LABA FDCs in these patients will be very informative for treatment guidelines.
Table 5.
Important ongoing or recently completed clinical trials assessing fixed dose combination dual bronchodilators
| Fixed dose combination | Identifier and study name | Comparator | Patients | n | Evaluation |
|---|---|---|---|---|---|
| QVA149 | NCT01709903* LANTERN | Salmeterol/fluticasone 500/50 μg twice daily | Moderate to severe COPD | 679 | QVA149 vs. SFC for lung function and rate of exacerbations |
| NCT01574651† QUANTIFY | Tiotropium 18 μg once daily plus formoterol 12 mg twice daily | Moderate to severe COPD | 934 | QVA149 vs. LABA and LAMA co-prescribing for lung function and dyspnoea | |
| UMEC/VI | NCT01822899‡ | Salmeterol/fluticasone twice daily | Moderate to severe COPD without recent history of exacerbation | 716 | UMEC/VI vs. SFC for lung function |
| NCT02014480¶ | Crossover study, | COPD | 389 | Effect of UMEC/VI vs. mono-components in responders/non-responders | |
| and | UMEC 62.5 μg once daily, | ||||
| NCT01716520 | VI 25 μg once daily | ||||
| Tiotropium/olodaterol | NCT01525615§ TORRACTO | Placebo | Moderate to severe COPD | 404 | Impact on exercise tolerance vs. placebo |
| NCT01431274** (TONADO 1) | O 5 μg, T 2.5 μg, T 5 μg, T + O 2.5/5 μg or T + O 5/5 μg | Moderate to very severe COPD | 5162 | T + O 5/5 μg vs. monotherapy for lung function and symptoms | |
| and | |||||
| NCT01431287†† (TONADO 2) | |||||
| FF/UMEC/VI | NCT02164513‡‡ IMPACT | FF/UMEC/VI 100/62.5/25 μg, FF/VI 100/25 μg or UMEC/VI 62.5/25 μg | COPD with CAT score >10 | 10,000 | Superiority of triple therapy over dual therapy for moderate/severe exacerbations |
Clinicaltrials.gov. A 26 week treatment randomized, double-blind, double dummy study to assess the efficacy and safety of QVA149 (LANTERN). NCT01709903. http://clinicaltrials.gov/ct2/show/NCT01709903 [Accessed 23 Sept 2014].
ClinicalTrials.gov. The Effect of QVA149 on Health Related Quality of Life in Patients With Chronic Obstructive Pulmonary Disease (COPD) (QUANTIFY). http://clinicaltrials.gov/ct2/show/NCT01574651 [Accessed 22 Sept 2014].
ClinicalTrials.gov. A Study to Evaluate the Efficacy and Safety of Umeclidinium Bromide/Vilanterol Compared With Fluticasone Propionate/Salmeterol Over 12 Weeks in Subjects With Chronic Obstructive Pulmonary Disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01822899 [Accessed 22 Sept 2014].
ClinicalTrials.gov. A Cross-Over Study to Evaluate Lung Function Response After Treatment With Umeclidinium (UMEC) 62.5 Micrograms (Mcg), Vilanterol (VI) 25 Mcg, and Umeclidinium/Vilanterol (UMEC/VI) 62.5/25 Mcg Once-Daily in Subjects With Chronic Obstructive Pulmonary Disease (COPD). http://clinicaltrials.gov/ct2/show/NCT02014480 [Accessed 22 Sept 2014].
ClinicalTrials.gov. A Study to Determine the Effect of Tiotropium + Olodaterol Fixed Dose Combination on Exercise Endurance Time During Constant Work Load Cycle Test in COPD. http://clinicaltrials.gov/ct2/show/NCT01525615 [Accessed 22 Sept 2014].
ClinicalTrials.gov. Tiotropium + Olodaterol Fixed Dose Combination (FDC) Versus Tiotropium and Olodaterol in Chronic Obstructive Pulmonary Disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01431274 [Accessed 22 Sept 2014].
ClinicalTrials.gov. Tiotropium + Olodaterol Fixed Dose Combination (FDC) Versus Tiotropium and Olodaterol in Chronic Obstructive Pulmonary Disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01431287 [Accessed 22 Sept 2014].
ClinicalTrials.gov. A Study Comparing the Efficacy, Safety and Tolerability of Fixed Dose Combination (FDC) of FF/UMEC/VI With the FDC of FF/VI and UMEC/VI; Administered Once-daily Via a Dry Powder Inhaler (DPI) in Subjects With Chronic Obstructive Pulmonary Disease (COPD) http://clinicaltrials.gov/ct2/show/NCT02164513 [Accessed 22 Sept 2014].
The majority of clinical trials reviewed here have been parallel group studies. This is the most practical way to conduct long term phase 3 studies. However, crossover designs with shorter treatment periods can be very informative about the potential treatment differences ‘within’ an individual. Table 5 shows that a crossover study with UMEC/VI designed to identify treatment responders to monocomponents and/or dual therapy has been conducted. This study may allow us to understand further the potential benefits to an individual of receiving dual compared with monotherapy.
Clinical trials with LABA/LAMA FDCs have focused on patients with moderate to severe COPD. Patients with FEV1 > 80% predicted have not been studied. The effects of these drugs in this milder population may be different from the published results, and warrants investigation.
Conclusion
LABA/LAMA FDCs have shown greater effects on lung function than monocomponent long acting bronchodilator therapies, and achieved health benefits above those observed with active comparator inhaled therapies, tiotropium and seretide, which have been the standard of care for COPD for many years. These new LABA/LAMA FDCs differ in terms of frequency of administration (one or twice a day) and inhaler device characteristics, which are likely to be key factors in determining their use in clinical practice. The relative clinical efficacy and safety of these drugs can only be properly determined by head-to-head clinical trials.
LABA/LAMA FDCs offer a simplified means of maximizing bronchodilation for COPD patients, with the improvements in lung function being mirrored by benefits in terms of symptoms and exacerbations. The use of LABA/LAMA FDCs in clinical practice is set to grow and further studies are needed to define their optimal place in treatment guidelines.
Competing Interests
The author has completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declares DS had support from Novartis for the submitted work and DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies who may have an interest in the submitted work, or could appear to have influenced this work, including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Forest, Genentech, GlaxoSmithKline, Glenmark, Merck, Napp, Novartis, Pfizer, Takeda and Therevance.
Aidan McManus, PhD CMPP and Ana Martins-Kaczor, PhD, of Edge Medical Communications provided medical writing and editorial support to the authors funded by Novartis UK.
References
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013;143:1436–1443. doi: 10.1378/chest.12-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD. 2014. Global strategy for the diagnosis, management and prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Updated. Available at http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html (last accessed 10 February 2014)
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schunemann H, Wedzicha W, MacDonald R, Shekelle P. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. American College of P American College of Chest P American Thoracic S European Respiratory S. [DOI] [PubMed] [Google Scholar]
- NICE. 2010. Clinical guideline 101. Chronic obstructive pulmonary disease. Management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update issued June 2010) National Institute for Health and Clinical Excellence. Available at http://www.nice.org.uk/guidance/cg101 (last accessed 24 January 2014)
- O'Reilly J, Jones MM, Parnham J, Lovibond K, Rudolf M. Management of stable chronic obstructive pulmonary disease in primary and secondary care: summary of updated NICE guidance. BMJ. 2010;340:c3134. doi: 10.1136/bmj.c3134. Guideline Development G. [DOI] [PubMed] [Google Scholar]
- Ball DI, Brittain RT, Coleman RA, Denyer LH, Jack D, Johnson M, Lunts LH, Nials AT, Sheldrick KE, Skidmore IF. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991;104:665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa P, Bouyssou T, Germeyer S, Schnapp A, Gantner F, Pieper M. Preclinical evaluation of long-acting muscarinic antagonists: comparison of tiotropium and investigational drugs. J Pharmacol Exp Ther. 2009;330:660–668. doi: 10.1124/jpet.109.152470. [DOI] [PubMed] [Google Scholar]
- Borrill ZL, Houghton CM, Woodcock AA, Vestbo J, Singh D. Measuring bronchodilation in COPD clinical trials. Br J Clin Pharmacol. 2005;59:379–384. doi: 10.1111/j.1365-2125.2004.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard D, Looby M, Kramer B, Lawrence D, Morris D, Stanski DR. Characterization of the bronchodilatory dose response to indacaterol in patients with chronic obstructive pulmonary disease using model-based approaches. Respir Res. 2011;12:54. doi: 10.1186/1465-9921-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S, Bantje T, Centanni S, Chanez P, Chuchalin A, D'Urzo A, Kornmann O, Perry S, Jack D, Owen R, Higgins M. A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med. 2008;102:1033–1044. doi: 10.1016/j.rmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Singh D, Magnussen H, Kirsten A, Mindt S, Caracta C, Seoane B, Jarreta D, Garcia Gil E. A randomised, placebo- and active-controlled dose-finding study of aclidinium bromide administered twice a day in COPD patients. Pulm Pharmacol Ther. 2012;25:248–253. doi: 10.1016/j.pupt.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64:450–504. doi: 10.1124/pr.111.004580. [DOI] [PubMed] [Google Scholar]
- 2012. Combivent® (ipratropium bromide and albuterol sulfate) Inhalation Aerosol Prescribing Information (Boehringer Ingelheim, ). Available at http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Combivent+Respimat/CMVTRSPT.pdf (last accessed 31 January 2014)
- Hanania NA, Boota A, Kerwin E, Tomlinson L, Denis-Mize K. Efficacy and safety of nebulized formoterol as add-on therapy in COPD patients receiving maintenance tiotropium bromide: results from a 6-week, randomized, placebo-controlled, clinical trial. Drugs. 2009;69:1205–1216. doi: 10.2165/00003495-200969090-00005. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Littner M, Andrews CP, Tomlinson L, Rinehart M, Denis-Mize K. Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trial. Respir Med. 2008;102:479–487. doi: 10.1016/j.rmed.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6:17–25. doi: 10.1080/15412550902724073. [DOI] [PubMed] [Google Scholar]
- Terzano C, Petroianni A, Conti V, Ceccarelli D, Graziani E, Sanduzzi A, D'Avelli S. Rational timing of combination therapy with tiotropium and formoterol in moderate and severe COPD. Respir Med. 2008;102:1701–1707. doi: 10.1016/j.rmed.2008.07.012. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, Cornelissen PJ. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129:509–517. doi: 10.1378/chest.129.3.509. [DOI] [PubMed] [Google Scholar]
- Mahler D, D'Urzo A, Peckitt C, Lassen C, Kramer B, Filcek S. Combining once-daily bronchodilators in COPD: indacaterol plus tiotropium versus tiotropium alone. Am J Respir Crit Care Med. 2011;183:A1591. . Presented at the American Thoracic Society 2011 International Conference, Denver, CO. May 13–18, 2011. [Google Scholar]
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, Balter M, O'Donnell D, McIvor A, Sharma S, Bishop G, Anthony J, Cowie R, Field S, Hirsch A, Hernandez P, Rivington R, Road J, Hoffstein V, Hodder R, Marciniuk D, McCormack D, Fox G, Cox G, Prins HB, Ford G, Bleskie D, Doucette S, Mayers I, Chapman K, Zamel N, FitzGerald M Canadian Thoracic Society/Canadian Respiratory Clinical Research C. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Donohue JF, Mahler DA, Huang H, Goodwin E, Schaefer K, Hanrahan JP, Andrews WT. Effects of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPD. Respir Med. 2009;103:516–524. doi: 10.1016/j.rmed.2008.12.014. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Aumann JL, Janssens E, Smeets JJ, Verhaert J, Disse B, Mueller A, Cornelissen PJ. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26:214–222. doi: 10.1183/09031936.05.00140404. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Aumann JL, Janssens E, Smeets JJ, Zaagsma J, Mueller A, Cornelissen PJ. Combining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptoms. Respir Med. 2010;104:995–1004. doi: 10.1016/j.rmed.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir Med. 2008;102:1511–1520. doi: 10.1016/j.rmed.2008.07.020. [DOI] [PubMed] [Google Scholar]
- van Noord JA, Buhl R, Laforce C, Martin C, Jones F, Dolker M, Overend T. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax. 2010;65:1086–1091. doi: 10.1136/thx.2010.139113. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Segreti A, Matera MG. New developments in the combination treatment of COPD: focus on umeclidinium/vilanterol. Drug Des Devel Ther. 2013;7:1201–1208. doi: 10.2147/DDDT.S39449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R, Jadayel D, Alagappan VK, Chen H, Banerji D. Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study. Int J Chron Obstruct Pulmon Dis. 2013;8:501–508. doi: 10.2147/COPD.S49615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, Banerji D. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42:1484–1494. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Witek TJ., Jr The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2:99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, D'Andrea P, Arrasate C, Chen H, Banerji D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Decramer M, D'Urzo A, Worth H, White T, Alagappan VK, Chen H, Gallagher N, Kulich K, Banerji D. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: BLAZE study. Eur Respir J. 2013;43:1599–1609. doi: 10.1183/09031936.00124013. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Horn S, Beier J, Jadayel D, Henley M, Tylek J, Berhane L, D'Andrea P, Banerji D. QVA149 once-daily improves exercise tolerance and lung function in patients with COPD: the BRIGHT study. Thorax. 2012;67(Suppl. 2):A147. . Presented at the British Thoracic Society Winter Meeting 2012, London, December 5–7, 2012. [Google Scholar]
- Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, Banerji D. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1:51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- Roche N, Pribil C, Van Ganse E, Serrier P, Housset B, Poirier D, Texier N, Schück S, Boucot I. Real-life use of fluticasone propionate/salmeterol in patients with chronic obstructive pulmonary disease: a French observational study. BMC Pulm Med. 2014;14:56. doi: 10.1186/1471-2466-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538–1546. doi: 10.1016/j.rmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg CJ, Church A. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145:981–991. doi: 10.1378/chest.13-1579. [DOI] [PubMed] [Google Scholar]
- Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, Tabberer M, Harris S, Church A. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2:472–486. doi: 10.1016/S2213-2600(14)70065-7. [DOI] [PubMed] [Google Scholar]
- Almirall. 2013. Positive Phase III combo results from its 2nd study (aclidinium + formoterol). Press release May 2,. Available at http://investors.almirall.es/phoenix.zhtml?c=209345&p=irol-newsArticle&ID=1814070&highlight (last accessed 28 February 2014)
- D'Urzo A, Mergel V, Leselbaum A, Caracta C. Efficacy and safety of fixed-dose combination aclidinium bromide/formoterol fumarate in patients with COPD: results from the AUGMENT COPD trial. Chest. 2013;144:1025A. . Presented at CHEST, Chicago, IL, October 26–31, 2013. [Google Scholar]
- D'Urzo A, Rennard S, Mergel V, Garcia Gil E, Leselbaum A, Caracta C. The AUGMENT COPD Trial: efficacy and safety of a fixed-dose combination of aclidinium bromide and formoterol fumarate in COPD patients. Chest. 2014;145:426A. . Presented at CHEST, Madrid, March 21–24, 2014. [Google Scholar]
- Singh D, Jones P, Bateman E, Korn S, Serra C, Molins E, Caracta C, Garcia Gil E, Leselbaum A. Evaluation of the efficacy and safety of two doses of aclidinium and formoterol in fixed-dose combination in patients with COPD: the ACLIFORM study. Chest. 2014;145:375A. doi: 10.1186/1471-2466-14-178. . Presented at CHEST, Madrid, March 21–24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–2321. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Matera MG, Donner CF. Inhaled beta2-adrenoceptor agonists: cardiovascular safety in patients with obstructive lung disease. Drugs. 2005;65:1595–1610. doi: 10.2165/00003495-200565120-00001. [DOI] [PubMed] [Google Scholar]
- Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R, Stukel TA. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173:1175–1185. doi: 10.1001/jamainternmed.2013.1016. [DOI] [PubMed] [Google Scholar]
- Dahl R, Chapman KR, Rudolf M, Mehta R, Kho P, Alagappan VK, Chen H, Banerji D. Safety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN study. Respir Med. 2013;107:1558–1567. doi: 10.1016/j.rmed.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Welte T, Vogelmeier C, Dahl R, Chapman K, Rudolf M, Mehta R, D'Andrea P, Chen H, Banerji D. 2013. Once-daily QVA149 has a good safety profile in patients with COPD (P757). Presented at the European Respiratory Society Annual Congress. Barcelona, September 7–11,
- Rodrigo GJ, Plaza V. Efficacy and safety of a fixed-dose combination of indacaterol and glycopyrronium (QVA149) for the treatment of COPD: a systematic review. Chest. 2014;146:309–317. doi: 10.1378/chest.13-2807. [DOI] [PubMed] [Google Scholar]
- Donohue JF, Niewoehner D, Brooks J, O'Dell D, Church A. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res. 2014;15:78. doi: 10.1186/1465-9921-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Make B, Donohue J, Zhong X, Leselbaum A, Caracta C. Long-term safety of a fixed-dose combination of aclidinium bromide/formoterol fumarate in patients with stable moderate to severe COPD. Chest. 2014;145:386A. . Presented at CHEST, Madrid, March 21–24, 2014. [Google Scholar]
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189:250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21:267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J Investigators T. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M, Investigators US. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- Jones PW, Donohue JF, Nedelman J, Pascoe S, Pinault G, Lassen C. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2011;12:161–171. doi: 10.1186/1465-9921-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, Investigators I. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP UPLIFT Investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–1178. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]


