Abstract
Aims
Although there are reports that β-adrenoceptor antagonists (beta-blockers) and diuretics can affect glycaemic control in people with diabetes mellitus, there is no clear information on how blood glucose concentrations may change and by how much. We report results from a systematic review to quantify the effects of these antihypertensive drugs on glycaemic control in adults with established diabetes.
Methods
We systematically reviewed the literature to identify randomized controlled trials in which glycaemic control was studied in adults with diabetes taking either beta-blockers or diuretics. We combined data on HbA1c and fasting blood glucose using fixed effects meta-analysis.
Results
From 3864 papers retrieved, we found 10 studies of beta-blockers and 12 studies of diuretics to include in the meta-analysis. One study included both comparisons, totalling 21 included reports. Beta-blockers increased fasting blood glucose concentrations by 0.64 mmol l−1 (95% CI 0.24, 1.03) and diuretics by 0.77 mmol l−1 (95% CI 0.14, 1.39) compared with placebo. Effect sizes were largest in trials of non-selective beta-blockers (1.33, 95% CI 0.72, 1.95) and thiazide diuretics (1.69, 95% CI 0.60, 2.69). Beta-blockers increased HbA1c concentrations by 0.75% (95% CI 0.30, 1.20) and diuretics by 0.24% (95% CI −0.17, 0.65) compared with placebo. There was no significant difference in the number of hypoglycaemic events between beta-blockers and placebo in three trials.
Conclusions
Randomized trials suggest that thiazide diuretics and non-selective beta-blockers increase fasting blood glucose and HbA1c concentrations in patients with diabetes by moderate amounts. These data will inform prescribing and monitoring of beta-blockers and diuretics in patients with diabetes.
Keywords: beta-blockers, diabetes, diuretics, glycaemic control, meta-analysis
What Is Already Known about the Subject
Antihypertensive medications are commonly used in people with diabetes mellitus.
β-adrenoceptor blockers and diuretics may alter blood glucose control but it is not clear how large the effects are.
What This Study Adds
This is the first systematic review of studies of the effects of β-adrenoceptor blockers and diuretics on glycaemic control in diabetes mellitus.
The analysis confirms previous views that non-selective β-adrenoceptor blockers and thiazide diuretics increase fasting blood glucose concentrations in diabetes.
Closer monitoring of glycaemic control for a short time after initiating one of these medications, and adjustment of glucose-lowering therapy if required, would be appropriate.
Introduction
Around 85% of people with diabetes mellitus have co-morbidities that may require them to take other medications 1,2. While it is important that co-existent risk factors are treated effectively, it is also important for blood glucose control to be maintained. However, many medications are reported to affect blood glucose concentrations or the required dose of insulin. Extensive lists of medications that may adversely affect blood glucose control in people with diabetes are available from both regulatory agencies, such as the European Medicines Agency (EMA 3) and internet-based information resources (e.g. Diabetes in Control 4 and dLife 5). Despite evidence that certain drugs affect glycaemic control, the available lists contain neither references to the sources of information nor information about the magnitude of the effect that can be expected when a medication is used. If patients and clinicians had access to information about how medications can affect glycaemic control, they would be able to make informed decisions regarding HbA1c monitoring and the type of medication or dosage to use. This could help clinicians to avoid prescribing certain medications that pose higher risks of hypoglycaemia or hyperglycaemia. Alternatively, some drugs that were previously avoided may be found to have minimal effects on blood glucose control and thus be safer to use than originally thought.
We chose to study β-adrenoceptor antagonists (beta-blockers) and diuretics because both are commonly prescribed in diabetes and they have been associated with adverse effects on carbohydrate metabolism 6. Beta-blockers have several different effects on blood glucose control through mechanisms that can oppose each other. For example, they can reduce blood glucose concentrations by blocking the actions of catecholamines in promoting glycogenolysis and gluconeogenesis 7. However, they can also increase blood glucose concentrations by inhibiting the release of insulin from pancreatic β-cells 8, which is mediated by β2-adrenoceptors. Furthermore, beta-blockade also increases growth hormone release in response to growth hormone releasing hormone 9 which would tend to cause hyperglycaemia. In children the balance of these actions may result in hypoglycaemia 10 and in adults with heart failure, hyperglycaemia 8.
Several trials have reported that some non-selective vasodilating beta-blockers may have favourable effects on insulin sensitivity and glycaemic control compared with selective beta-blockers 11–13. These trials suggest that some beta-blockers can be used safely in people with diabetes, but at present the available information is conflicting. A meta-analysis comparing the rates of cardiovascular events for people with diabetes taking atenolol compared with other antihypertensive drugs showed an increased risk ratio of 1.12 (95% CI 1.00, 1.25, P = 0.06) 14.
Randomized trials have shown that low dose diuretic treatment prevents major cardiovascular events in people with and without diabetes 15,16. However, thiazide diuretics have been linked to adverse metabolic effects, glucose intolerance and hyperglycaemia 17, as well as incident diabetes 18. Some studies have suggested that the use of diuretics in diabetes may be dangerous. For example, a cohort study from 1991 reported that using diuretics to reduce hypertension in diabetes was associated with an increased risk of mortality 19. Diuretics can also cause hypokalaemia 20, which can cause reduced insulin secretion and an increased risk of diabetes 17,21.
Information on whether these medications have adverse effects on glucose control in people with diabetes is hard to find. Despite its importance in monitoring and care, this information has not to date been systematically assessed, making it difficult for clinicians to make informed decisions about how these medications should be used. We have carried out a systematic review and meta-analysis to quantify the effects of beta-blockers and diuretics on glycaemic control and the incidence of adverse events in people with type 1 and type 2 diabetes.
Methods
Our review and protocol were registered, in advance of searching the literature, on the Prospero database (registration number CRD42013004261). We searched Medline and EMBASE databases and the Cochrane database of registered controlled trials from 1946 to the end of March 2013 with no language restrictions. In addition, we searched the ClinicalTrials.gov clinical trials registry and scanned reference lists of reviews and relevant papers for eligible trials. The Medline search strategy is shown in Supporting Information. All identified studies were screened independently by two reviewers (JH and BF) for eligibility. We included placebo-controlled randomized trials of any duration in which the effects of either beta-blockers or diuretics on measures of glycaemic control in people with diabetes were assessed. We also included trials in which a diuretic or beta-blocker was added to another medication, provided that the other medication was the same in both the intervention and comparator arms. Two reviewers extracted data on study characteristics (intervention and comparator medications and doses, length of follow-up), patient characteristics (mean age, gender, BMI and diabetes duration), study quality (randomization and blinding 22), and patient outcomes (measures of glycaemic control) from included trials. The primary outcome was glycaemic control, measured as HbA1c, fasting blood glucose or hypoglycaemic episodes between intervention and control groups. Secondary outcomes were systolic blood pressure and adverse events. We wrote to the authors of trials published in the past 10 years to request unpublished data.
The definitions of episodes of symptomatic hypoglycaemia reported in the methods of each paper were accepted as the criteria for our analysis (including tremor, sweating, tachycardia, palpitation, and piloerection). We also extracted data on end point systolic blood pressure when it was reported. The quality of included studies was assessed, and studies in which randomization or double blinding were not stated were excluded in a sensitivity analysis to see whether this affected the results. We assessed the potential risk of publication bias using Egger's test 22.
Statistical methods
All analyses were carried out using Stata 12.1SE (StataCorp, Tx, USA). Fasting blood glucose concentrations that were reported as mg dl−1 were converted to mmol l−1. We pooled data on the mean difference between intervention and comparator groups in fasting blood glucose, HbA1c concentrations and systolic blood pressure reported at the end of the trial using a fixed effects inverse variance weighted meta-analysis. HbA1c was only pooled in trials that lasted 8 weeks or longer. Numbers of hypoglycaemic events or other adverse events were pooled using the Mantel Haenszel method to calculate the risk ratio 22. When total or mean numbers of adverse events per patient were reported, we calculated the number of events per patient-week in the trial, to enable pooling of the results. Standard deviations were imputed in one trial in which they were not reported 23 by averaging standard deviations from all the included trials in which they were reported, as recommended in the Cochrane Handbook 22, and the geometric mean was approximated to the mean. Trials in which approximations were made were excluded in a sensitivity analysis. Prespecified sub-group analysis and meta-regression was used to assess whether selective and non-selective beta-blockers 24,25 gave significantly different results from each other, and whether thiazide diuretics gave significantly different results from other diuretics.
Results
We identified 3864 papers, 188 of which were duplicate references resulting from searching multiple databases, leaving 3676 papers for review (Figure 1). After review of titles and abstracts, 3587 papers were excluded, leaving 89 papers to be included for full text examination (55 using beta-blockers, 30 using diuretics and four using both). After examining the full texts, we included 21 randomized controlled trials, 10 of beta-blockers (15 comparisons) involving 1889 participants and 12 of diuretics (13 comparisons) with a total of 366 participants. One RCT included both interventions 26. The ClinicalTrials.gov registry yielded a further 157 possible trials, from which no additional trials were identified for inclusion. Several eligible trials were excluded from the analysis because no measure of glycaemic control was reported and data could not be obtained from the authors 23,27–32. One comparison of two doses of cyclopenthiazide was also excluded 33. Included trials are shown in Table 1. All but three of the included trials were of 3 months duration or shorter. The mean trial duration in the 10 beta-blocker trials was 17 weeks, all trial participants were adults, and most had type 2 diabetes, were hypertensive and were not using insulin. The mean trial duration in the 11 diuretic trials was 7.5 weeks with only one trial of longer than 12 weeks, all trial participants were adults, and most had type 2 diabetes, were hypertensive, and were not using insulin.
Figure 1.
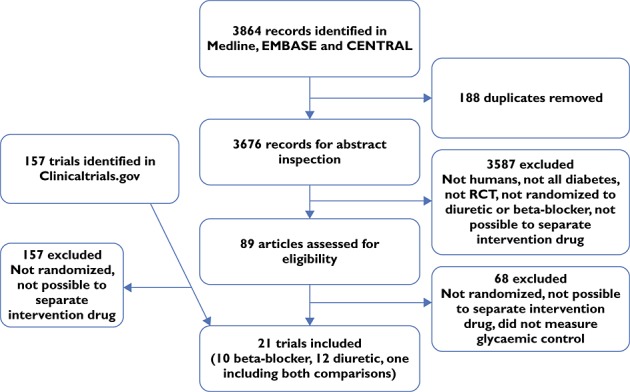
Flow chart of searches
Table 1.
Details of included studies
| Authors (ref) | Year | Number of participants | Type of diabetes | Insulin use | Setting | Intervention | n | Dose (mg day−1) | Comparator | n | Mean age | Duration of diabetes (years) | Length of trial (weeks) | Diabetes medication adjusted? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-blockers | ||||||||||||||
| Chalon et al. [34] | 1999 | 14 | ns | yes | hypoglycaemia | propranolol | 9 | 60 | placebo | 5 | 40 | 15.0 | 4 | ns |
| Chellingsworth et al. [35] | 1989 | 19 | 2 | no | hypertension | propranolol | 19 | 160 | placebo | 19 | ns | ns | 4 | no |
| Dornhorst et al. [26] | 1985 | 15 | 2 | 47% | hypertension | propranolol | 15 | 160 | no treatment | 15 | ns | 6.8 | 3 | ns |
| Lewis et al. [47] | 1991 | 22 | 2 | no | – | propranolol | 22 | 160 | placebo | 22 | 59 | ns | 4 | ns |
| Whitcroft et al. [45] | 1990 | 27 | 2 | no | hypertension | propranolol | 27 | 160 | placebo | 7 | ns | ns | 13 | no |
| Dornhorst et al. [26] | 1985 | 15 | 2 | 47% | hypertension | propranolol + hydrocholrothiazide | 15 | 160 | hydro-chlorothiazide | 15 | ns | 6.8 | 3 | ns |
| Deedwania et al. [48] | 2005 | 985 | ns | ns | heart failure | metoprolol | 495 | 160 | placebo | 490 | 65 | ns | 52 | ns |
| Profozic et al. [49] | 1997 | 30 | 2 | no | hypertension | atenolol | 20 | 50 | placebo | 10 | 61 | 6.8 | 6 | no |
| Whitcroft et al. [45] | 1990 | 27 | 2 | no | hypertension | atenolol | 27 | 100 | placebo | 7 | ns | ns | 13 | no |
| Whitcroft et al. [45] | 1990 | 10 | 2 | no | hypertension | atenolol | 10 | 100 | placebo | 7 | ns | ns | 13 | no |
| Whitcroft et al. [45] | 1990 | 15 | 2 | no | hypertension | atenolol+ prazosin | 14 | 100 | prazosin | 15 | ns | ns | 13 | ns |
| de Boer et al. [50] | 2010 | 555 | ns | ns | heart failure | nebivolol | 287 | 10 | placebo | 268 | 76 | ns | 84 | ns |
| Gundersen & Kjekshus [28], [51], Rodda [52] | 1983–1985 | 99 | ns | ns | acute myocardial infarction | timolol | 53 | 20 | placebo | 46 | ns | ns | 74 | ns |
| Larijani et al. [53] | 2006 | 40 | 2 | ns | – | carvedilol | 20 | 18.75 | placebo | 20 | 50 | 6.4 | 2 | ns |
| Whitcroft et al. [45] | 1990 | 16 | 2 | no | hypertension | nadolol | 16 | 80 | placebo | 6 | ns | ns | 13 | ns |
| Diuretics | ||||||||||||||
| Davies et al. [54] | 2004 | 42 | 2 | no | – | spironolactone | 42 | 47.5 | placebo | 42 | 60 | ns | 4 | ns |
| Rossing et al. [55] | 2005 | 20 | 2 | ns | diabetic nephropathy | spironolactone | 20 | 25 | placebo | 20 | 58 | 12.0 | 8 | ns |
| Mehdi et al. [23] | 2009 | 54 | 15% type 1 | ns | diabetic nephropathy | spironolactone | 27 | 25 | placebo | 27 | 51 | 16 | 48 | ns |
| Schjoedt et al. [56] | 2006 | 20 | 45% type 1 | ns | diabetic nephropathy | spironolactone | 20 | 25 | placebo | 20 | 49 | 21 | 8 | ns |
| Swaminathan et al. [57] | 2008 | 38 | 2 | no | hypertension | spironolactone | 38 | 25 | placebo | 38 | 63 | ns | 4 | ns |
| Dornhorst et al. [26] | 1985 | 15 | 2 | 47% | hypertension | hydrochlorothiazide | 15 | 100 | no treatment | 15 | ns | 6.8 | 3 | ns |
| Klauser et al. [36] | 1991 | 6 | 2 | no | – | hydrochlorothiazide | 6 | 50 | placebo | 6 | 57 | ns | 4 | no |
| Dornhorst et al. [26] | 1985 | 15 | 2 | 47% | hypertension | hydrochlorothiazide + propranolol | 15 | 100 | propranolol | 15 | ns | 6.8 | 3 | ns |
| Pacy et al. [37] | 1984 | 50 | ns | 20% | hypertension | bendroflumethiazide | 25 | 10 | diet | 25 | 55 | 6.5 | 12 | ns |
| Hunter et al. [46] | 1999 | 11 | 2 | no | hypertension | bendroflumethiazide + captopril | 11 | 2.5 | placebo + captopril | 11 | 58 | ns | 12 | no |
| McLaughlin et al. [58] | 2008 | 15 | 2 | no | hypertension | bendroflumethiazide | 15 | 1.25 | placebo | 15 | 53 | ns | 12 | ns |
| Kuo et al. [38] | 2003 | 56 | 2 | no | hypertension | indapamide | 28 | 1.5 | placebo | 28 | 60 | ns | 12 | ns |
| Passmore et al. [33] | 1991 | 24 | 2 | no | hypertension | cyclopenthiazide | 24 | 0.125 | cyclopenthiazide | 24 | 59 | ns | 12 | no |
ns, not stated.
The methodological quality of the included trials was high and most reported obtaining informed consent from participants. Only one beta-blocker trial reported the method of randomzation 34 and all but two trials 26,35 reported double blinding. Two trials of diuretics did not clearly report randomization 36,37 and three did not report double blinding 26,37,38.
Beta-blockers
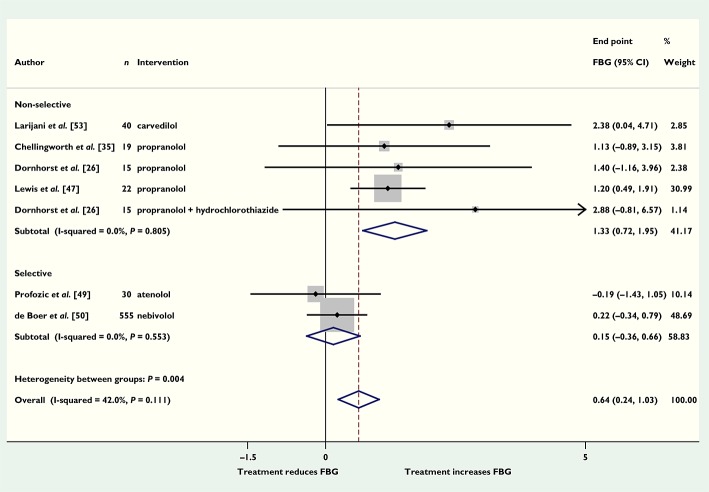
Of the trials of beta-blockers, six (seven comparisons) reported fasting blood glucose concentrations (696 participants). Beta-blockers increased pooled end point fasting blood glucose by 0.64 mmol l−1 (95% CI 0.24, 1.03) compared with placebo (Figure 2). Four trials (five comparisons) of non-selective beta-blockers (propranolol and celiprolol) had a significantly larger pooled effect size than two trials of selective beta-blockers (atenolol and nebivolol) (1.33 mmol l−1, 95% CI 0.72, 1.95 compared with 0.15 mmol l−1, 95% CI −0.36, 0.66) (P = 0.034). Pooling data from five comparison arms of one trial that reported HbA1c showed that beta-blockers increased HbA1c by 0.75% (95% CI 0.30, 1.20), corresponding to 8.2 mmol mol−1 (95% CI 3.3, 13.1) compared with placebo (Figure S1), with no difference between selective and non-selective beta-blockers (results not shown). Four trials (nine comparisons) reported blood pressure; pooling end point data showed that systolic blood pressure was 8 mmHg (95% CI 4, 13) lower in patients who had taken beta-blockers compared with placebo. Sensitivity analyses excluding trials that were not double-blind did not substantially change the results. Three trials reported the numbers of hypoglycaemic events. The pooled data showed that there was no significant difference in the numbers of events between those who took beta-blockers and those who did not, risk ratio 0.80 (95% CI 0.31, 2.06). Treatment with beta-blockers resulted in fewer cardiovascular events in five trials, RR 0.78 (95% CI 0.68, 0.90, P < 0.001), and lower mortality in four trials, RR 0.77 (95% CI 0.63, 0.96, P = 0.019) compared with control groups. There was no significant difference in the numbers of other adverse events between beta-blockers and the comparator group (Figure S2).
Figure 2.
Mean difference in end point fasting blood glucose (FBG) (mmol l−1) with beta-blockers vs. placebo (boxes) and pooled estimates (diamond) calculated by the inverse variance fixed effects model. Horizontal bars and diamond widths represent 95% CIs and box sizes indicate relative weights in the analysis
Diuretics
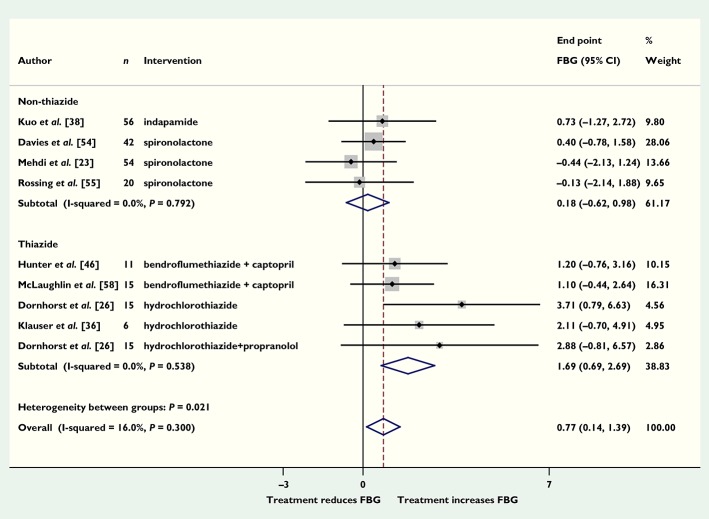
Eight of the diuretics trials (nine comparisons) reported fasting blood glucose concentrations. Pooling the end point data showed that patients randomized to diuretics had fasting blood glucose concentrations 0.77 mmol l−1 (95% CI 0.14, 1.39) higher than those randomized to placebo (Figure 3). The four trials (five comparisons) that used thiazide diuretics had a larger effect size than those that used non-thiazide diuretics (1.69, 95% CI 0.69, 2.69 and 0.18, 95% CI −0.62, 0.98, respectively), which was of borderline significance (P = 0.054). Six trials of 8 weeks or longer reported HbA1c concentrations; pooling end point data showed that patients taking diuretics had HbA1c concentrations 0.24% higher (95% CI −0.17, 0.65), corresponding to 2.6 (95% CI −1.9, 7.1) mmol mol−1 compared with placebo, but this was not significant (P = 0.58; Figure S3). Trials of thiazide diuretics showed a slightly greater increase in HbA1c than trials of non-thiazide diuretics, but neither result was significant (results not shown). When data from the potassium-sparing diuretic spironolactone were examined separately, the pooled fasting blood glucose was 0.08 mmol l−1 (95% CI −0.79, 0.95) higher in three trials and HbA1c was 0.24% (95% CI −0.39, 0.88) higher in two trials compared with placebo. However, we were unable to assess the extent to which the effect of the thiazides was related to potassium depletion 8, since electrolyte concentrations were not reported in the included studies. Eleven trials (12 comparisons) reported blood pressures; pooling end point data showed that in patients who took diuretics systolic blood pressure was 12 mmHg (95% CI 10, 14; P < 0.0001) lower than with placebo. Sensitivity analyses to exclude trials in which estimations were made and trials that did not clearly report randomization or double blinding did not substantially change the results for HbA1c (data not shown), but did reduce the effect size of diuretics on fasting blood glucose to 0.62 (95% CI −0.15, 1.40) mmol l−1 (P = 0.11). None of the included diuretic trials reported numbers of adverse events. The result from Egger's test (P = 0.045) was consistent with possible publication bias for trials reporting fasting blood glucose.
Figure 3.
Mean difference in end point fasting blood glucose (FBG) (mmol l−1) with diuretics vs. placebo (boxes) and pooled estimates (diamond) calculated by the inverse variance fixed effects model. Horizontal bars and diamond widths represent 95% CIs and box sizes indicate relative weights in the analysis
Discussion
We have found that both beta-blockers and diuretics, in doses that are highly effective in lowering blood pressure, significantly increase fasting blood glucose in adults with diabetes mellitus. The effect of beta-blockers was most clearly seen in studies of non-selective beta-blockers (propranolol and celiprolol). The selective beta-blockers atenolol and nebivolol had little effect, although this result was based on only two studies. The effect of diuretics was most clearly seen with thiazide diuretics, which is consistent with reports from studies in individuals without diabetes, in whom thiazide diuretics have been associated with hyperglycaemia 39. There was only one included study of beta-blockers and HbA1c. Across six studies of diuretics there was a modest and non-significant increase in HbA1c.
Although antihypertensive medications are widely used in diabetes, this is the first systematic review of the literature with meta-analysis to quantify the extent to which beta-blockers or diuretics affect glycaemic control. Many medications have been reported to affect blood glucose concentrations, but there is very little information to guide clinicians and patients on which are safe to use and which should be avoided in people with diabetes.
We have found that beta-blockers increase fasting blood glucose by around 0.6 mmol l−1 (1.3 mmol l−1 for non-selective beta-blockers) and diuretics by around 0.8 mmol l−1 (1.7 mmol l−1 for thiazide diuretics). Trials of non-selective beta-blockers increased fasting blood glucose significantly more than those of selective beta-blockers. This is incongruent with some previous reports 11–13,40. However, non-selective beta-blockers have opposing mechanisms of action on insulin secretion and glucose utilization 41,42, and the results of this study suggest that in people with diabetes the mechanisms by which beta-blockers cause an overall increase in blood glucose concentration predominate over those that would cause a reduction, and that this effect is primarily mediated via β2-adrenoceptors.
Diuretics on the other hand, probably only affect insulin secretion 17. It is possible that the combined use of diuretics and beta-blockers results in even greater increases in blood glucose concentrations; as evidenced from a single trial in our review which included both agents 26. Since only one of the included trials of diuretics reported any adverse events we are unable to report pooled results for diuretics. We found no evidence of an increase in adverse events with beta-blockers. However, the trials included in our analysis were relatively short (maximum duration 20 months), and it is possible that in the longer term an increased risk of the microvascular and macrovascular complications of diabetes may result from the deterioration in glycaemic control 43. In a minor deviation from the protocol, we included trials of 8 weeks or longer in the meta-analysis of HbA1c, since previous studies have reported that most of the change in HbA1c takes place within the first 8 weeks of a medication change 44.
Our systematic review has some limitations. Most importantly, several large published trials of beta-blockers or diuretics had to be excluded from the analysis because they did not report outcome data for either HbA1c or fasting blood glucose and we were unable to obtain the data from the authors. If these trials had reported no significant differences in glycaemic control between groups, then our meta-analysis could be overestimating the effect sizes. We have been unable to examine the effect of diuretics on adverse events, as insufficient trials reported these outcomes. Most of the trials we included did not report the method of randomization, which is a potential source of bias. The impact of this could not be assessed because there were too few trials. We found a significant risk of publication bias in the beta-blocker studies. However we only had eight and nine comparisons for beta-blockers and diuretics, respectively and Egger's test for publication bias is reported to be unreliable when fewer than 10 trials are compared 22. The results of this analysis should therefore be interpreted with caution. Moreover, all the trials were small: only one trial included in the analysis of beta-blockers had more than 40 participants and none of the diuretics trials had more than 56 participants. Most of the trials included in our review were of 3 months duration or less. We were therefore unable to assess the longer term impact of these medications on glycaemic control. Additionally, many of the included trials were old and therefore the generalizability of the findings to present day practice may be limited. Most of our included studies were carried out in hypertensive patients. There were too few studies in patients with heart failure to enable comparison with patients with hypertension. Of the 21 included trials only five reported that doses of blood glucose lowering medications were unchanged for the duration of the trial, two beta-blocker trials 35,45 and three diuretic trials 33,36,46. We were therefore unable to compare effect sizes in individuals with and without changes to their blood glucose lowering medications. However, the majority of trial participants were taking oral medications, and trial durations were short, which may have limited the opportunities for medication changes. Our analysis of the effects of beta-blockers on HbA1c was based on one trial with several arms comparing different beta-blockers. The results from this analysis should therefore be interpreted with caution. There were too few studies to enable comparisons between individual beta-blockers or diuretics or to compare different doses. However, we were able to compare trials of selective beta-blockers (two trials) with trials of non-selective beta-blockers (five trials) and trials of thiazide diuretics (five trials) with trials of non-thiazide diuretics (four trials) and spironolactone (three trials). Although these sub-group analyses were pre-specified, they were indirect comparisons and the results should therefore be interpreted with caution. The results of this review could guide clinicians who are considering prescribing blood pressure lowering medications for people with diabetes.
We have confirmed the existence of glycaemic effects of beta-blockers and diuretics. Although the mean effects appear small, we cannot rule out the possibility of a larger effect in some individuals. However, we cannot investigate this further because of the parallel group design of the studies included in this review. Until further studies better identify prescriptive and predictive explanations for these variations, the current recommendation, to use other classes of anti-hypertensive agents in diabetes whenever possible, appears well supported by underpinning evidence. Furthermore, closer monitoring of glycaemic control for a short time after initiating one of these medications, and adjustment of glucose-lowering therapy if required, would be appropriate.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare JH had support from the UK's National Institute for Health Research (NIHR) School for Primary Care Research (SPCR) for the submitted work. This article/paper/report presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. There were no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. AF receives support from the NIHR Oxford Biomedical Research Centre and is an NIHR Senior Investigator.
Thank you to Dr Kasper Rossing for providing us with unpublished data and to Dr Clare Bankhead who assisted in the funding application.
Contributors
JH designed the study, performed literature searches, data extraction, and statistical analyses and drafted the manuscript, AF contributed to study design, interpretation of results, and discussion, JKA contributed to study design, interpretation of results and discussion, BF contributed to data extraction and interpretation of results, RS contributed to study design, interpretation of results, and discussion and provided statistical support. All authors reviewed and edited the manuscript and approved the final version.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Mean difference in end point HbA1c of beta-blockers vs. placebo (boxes) and pooled estimates (diamond) calculated by the inverse variance fixed effects model. Horizontal bars and diamond widths represent 95% CIs and box sizes indicate relative weights in the analysis
Figure S2 Beta-blockers – Adverse events
Figure S3 Mean difference in end point HbA1c (%) with diuretics vs. placebo (boxes) and pooled estimates (diamond) calculated by the inverse variance fixed effects model. Horizontal bars and diamond widths represent 95% CIs and box sizes indicate relative weights in the analysis
References
- Litwak L, Goh SY, Hussein Z, Malek R, Prusty V, Khamseh ME. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol Metab Syndr. 2013;5:57. doi: 10.1186/1758-5996-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijks H, Schermer T, Bor H, van Weel C, Lagro-Janssen T, Biermans M, de Grauw W. Prevalence and incidence density rates of chronic comorbidity in type 2 diabetes patients: an exploratory cohort study. BMC Med. 2012;10:128. doi: 10.1186/1741-7015-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novo Nordisk A/S. Annex 1. Summary of product characteristics. Section 4.5 Interaction with other medicinal products and other forms of interaction. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000441/WC500033307.pdf (last accessed 27 November 2014)
- 2011. Diabetes-in-Control. Drugs that can affect blood glucose levels. Available at http://www.diabetesincontrol.com/images/tools/druglistaffectingbloodglucose.pdf (last accessed 27 November 2014)
- dLife. Drugs that affect blood glucose. Available at http://www.dlife.com/diabetes/type-2/diabetes-treatment/drugs-that-raise-blood-glucose (last accessed 27 November 2014)
- Chan JC, Cockram CS, Critchley JA. Drug-induced disorders of glucose metabolism. Mechanisms and management. Drug Saf. 1996;15:135–157. doi: 10.2165/00002018-199615020-00005. [DOI] [PubMed] [Google Scholar]
- Popp DA, Shah SD, Cryer PE. Role of epinephrine-mediated beta-adrenergic mechanisms in hypoglycemic glucose counterregulation and posthypoglycemic hyperglycemia in insulin-dependent diabetes mellitus. J Clin Invest. 1982;69:315–326. doi: 10.1172/JCI110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AJ, Zaman N, Cole GD, Wensel R, Okonko DO, Francis DP. Systematic review of genuine versus spurious side-effects of beta-blockers in heart failure using placebo control: recommendations for patient information. Int J Cardiol. 2013;168:3572–3579. doi: 10.1016/j.ijcard.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Yang I, Woo J, Kim S, Kim J, Kim Y, Park S. Acute hyperglycemia and activation of the beta-adrenergic system exhibit synergistic inhibitory actions on growth hormone (GH) releasing hormone-induced GH release. Eur J Endocrinol. 2003;148:635–640. doi: 10.1530/eje.0.1480635. [DOI] [PubMed] [Google Scholar]
- Hussain T, Greenhalgh K, McLeod KA. Hypoglycaemic syncope in children secondary to beta-blockers. Arch Dis Child. 2009;94:968–969. doi: 10.1136/adc.2008.145052. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT, Jr, Oakes R, Lukas MA, Anderson KM, Bell DS. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292:2227–2236. doi: 10.1001/jama.292.18.2227. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Acampora R, Marfella R, De Rosa N, Ziccardi P, Ragone R, De Angelis L, D'Onofrio F. Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann Intern Med. 1997;126:955–959. doi: 10.7326/0003-4819-126-12-199706150-00004. [DOI] [PubMed] [Google Scholar]
- Jacob S, Rett K, Wicklmayr M, Agrawal B, Augustin HJ, Dietze GJ. Differential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: the carvedilol-metoprolol study. J Hypertens. 1996;14:489–494. [PubMed] [Google Scholar]
- Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta-blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: a systematic review and meta-analysis. Am J Ther. 2009;16:133–142. doi: 10.1097/MJT.0b013e31817fd87e. [DOI] [PubMed] [Google Scholar]
- Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, Camel G, Davis BR, Frost PH, Gonzalez N, Guthrie G, Oberman A, Rutan GH, Stamler J. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276:1886–1892. [PubMed] [Google Scholar]
- Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM, Pressel SL, Davis BR. Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005;95:29–35. doi: 10.1016/j.amjcard.2004.08.059. [DOI] [PubMed] [Google Scholar]
- Pandit MK, Burke J, Gustafson AB, Minocha A, Peiris AN. Drug-induced disorders of glucose tolerance. Ann Intern Med. 1993;118:529–539. doi: 10.7326/0003-4819-118-7-199304010-00008. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2006;166:2191–2201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- Warram JH, Laffel LM, Valsania P, Christlieb AR, Krolewski AS. Excess mortality associated with diuretic therapy in diabetes mellitus. Arch Intern Med. 1991;151:1350–1356. [PubMed] [Google Scholar]
- Sander GE, Giles TD. Thiazide diuretics and beta-blockers in the treatment of hypertension in diabetes mellitus. J Clin Hypertens (Greenwich) 2011;13:296–300. doi: 10.1111/j.1751-7176.2011.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi T, Appel LJ, Miller ER, 3rd, Klag MJ, Parekh RS. Changes in serum potassium mediate thiazide-induced diabetes. Hypertension. 2008;52:1022–1029. doi: 10.1161/HYPERTENSIONAHA.108.119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011 Available at http://www.cochrane-handbook.org (last accessed 27 November 2014)
- Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–2650. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009;54:123–128. doi: 10.1097/FJC.0b013e3181ad207b. [DOI] [PubMed] [Google Scholar]
- Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc Drugs Ther. 1999;13:123–126. doi: 10.1023/a:1007784109255. [DOI] [PubMed] [Google Scholar]
- Dornhorst A, Powell SH, Pensky J. Aggravation by propranolol of hyperglycaemic effect of hydrochlorothiazide in type II diabetics without alteration of insulin secretion. Lancet. 1985;1:123–126. doi: 10.1016/s0140-6736(85)91900-2. [DOI] [PubMed] [Google Scholar]
- Domanski M, Krause-Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, Haffner S, Katz R, Lindenfeld J, Lowes BD, Martin W, McGrew F, Bristow MR BEST Investigators. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol. 2003;42:914–922. doi: 10.1016/s0735-1097(03)00856-8. [DOI] [PubMed] [Google Scholar]
- Gundersen T, Kjekshus J. Timolol-related reduction in mortality and reinfarction in diabetic patients surviving acute myocardial infarction. Horm Metab Res Suppl. 1985;15:58–60. [PubMed] [Google Scholar]
- Harper R, Ennis CN, Heaney AP, Sheridan B, Gormley M, Atkinson AB, Johnston GD, Bell PM. A comparison of the effects of low- and conventional-dose thiazide diuretic on insulin action in hypertensive patients with NIDDM. Diabetologia. 1995;38:853–859. doi: 10.1007/s001250050363. [DOI] [PubMed] [Google Scholar]
- Janka HU, Ziegler AG, Disselhoff G, Mehnert H. Influence of bisoprolol on blood glucose, glucosuria, and haemoglobin A1 in noninsulin-dependent diabetics. J Cardiovasc Pharmacol. 1986;8(Suppl. 11):S96–99. doi: 10.1097/00005344-198511001-00018. [DOI] [PubMed] [Google Scholar]
- Malmberg K, Herlitz J, Hjalmarson A, Ryden L. Effects of metoprolol on mortality and late infarction in diabetics with suspected acute myocardial infarction. Retrospective data from two large studies. Eur Heart J. 1989;10:423–428. doi: 10.1093/oxfordjournals.eurheartj.a059505. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Gottlieb S, Bourque JM, Krause-Steinrauf H, Anand I, Anderson JL, Plehn JF, Silver MA, White M, Carson P, Investigators B. Impact of nonfatal myocardial infarction on outcomes in patients with advanced heart failure and the effect of bucindolol therapy. Am J Cardiol. 2005;95:558–564. doi: 10.1016/j.amjcard.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Passmore AP, Whitehead EM, Crawford V, McVeigh GE, Johnston GD. The antihypertensive and metabolic effects of low and conventional dose cyclopenthiazide in type II diabetics with hypertension. Q J Med. 1991;81:919–928. [PubMed] [Google Scholar]
- Chalon S, Berlin I, Sachon C, Bosquet F, Grimaldi A. Propranolol in hypoglycaemia unawareness. Diabetes Metab. 1999;25:23–26. [PubMed] [Google Scholar]
- Chellingsworth MC, Kendall MJ, Wright AD, Singh BM, Pasi J. The effects of verapamil, diltiazem, nifedipine and propranolol on metabolic control in hypertensives with non-insulin dependent diabetes mellitus. J Hum Hypertens. 1989;3:35–39. [PubMed] [Google Scholar]
- Klauser R, Prager R, Gaube S, Gisinger C, Schnack C, Küenburg E, Schernthaner G. Metabolic effects of isradipine versus hydrochlorothiazide in diabetes mellitus. Hypertension. 1991;17:15–21. doi: 10.1161/01.hyp.17.1.15. [DOI] [PubMed] [Google Scholar]
- Pacy PJ, Dodson PM, Kubicki AJ. Comparison of the hypotensive and metabolic effects of bendrofluazide therapy and a high fibre, low fat, low sodium diet in diabetic subjects with mild hypertension. J Hypertens. 1984;2:215–220. doi: 10.1097/00004872-198404000-00015. [DOI] [PubMed] [Google Scholar]
- Kuo SW, Pei D, Hung YJ, Hsieh AT, Wu LY, Hsieh CH, He CT, Yang TC, Lian WC. Effect of indapamide SR in the treatment of hypertensive patients with type 2 diabetes. Am J Hypertens. 2003;16:623–628. doi: 10.1016/s0895-7061(03)00896-3. [DOI] [PubMed] [Google Scholar]
- Luna B, Feinglos MN. Drug-induced hyperglycemia. JAMA. 2001;286:1945–1948. doi: 10.1001/jama.286.16.1945. [DOI] [PubMed] [Google Scholar]
- Wai B, Kearney LG, Hare DL, Ord M, Burrell LM, Srivastava PM. Beta blocker use in subjects with type 2 diabetes mellitus and systolic heart failure does not worsen glycaemic control. Cardiovasc Diabetol. 2012;11:14. doi: 10.1186/1475-2840-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafidis PA, Bakris GL. Antihypertensive treatment with beta-blockers and the spectrum of glycaemic control. QJM. 2006;99:431–436. doi: 10.1093/qjmed/hcl059. [DOI] [PubMed] [Google Scholar]
- Ferner RE. Drug-induced diabetes. Baillières Clin Endocrinol Metab. 1992;6:849–866. doi: 10.1016/s0950-351x(05)80170-3. [DOI] [PubMed] [Google Scholar]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst JA, Stevens RJ, Farmer AJ. Changes in HbA1c level over a 12-week follow-up in patients with type 2 diabetes following a medication change. PLoS ONE. 2014;9:e92458. doi: 10.1371/journal.pone.0092458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcroft IA, Thomas JM, Rawsthorne A, Wilkinson N, Thompson H. Effects of alpha and beta adrenoceptor blocking drugs and ACE inhibitors on long term glucose and lipid control in hypertensive non-insulin dependent diabetics. Horm Metab Res Suppl. 1990;22:42–46. [PubMed] [Google Scholar]
- Hunter SJ, Wiggam MI, Ennis CN, Whitehead HM, Sheridan B, Atkinson AB, Bell PM. Comparison of effects of captopril used either alone or in combination with a thiazide diuretic on insulin action in hypertensive Type 2 diabetic patients: a double-blind crossover study. Diabet Med. 1999;16:482–487. doi: 10.1046/j.1464-5491.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- Lewis HM, Kendall MJ, Wright AD, Bratty JR, Maxwell S. A comparison of the metabolic effects of flosequinan and propranolol in patients with non-insulin-dependent diabetes mellitus. J Clin Pharm Ther. 1991;16:161–166. doi: 10.1111/j.1365-2710.1991.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Giles TD, Klibaner M, Ghali JK, Herlitz J, Hildebrandt P, Kjekshus J, Spinar J, Vitovec J, Stanbrook H, Wikstrand J. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT-HF. Am Heart J. 2005;149:159–167. doi: 10.1016/j.ahj.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Profozic V, Coce F, Babic D, Metelko Z, Rao BA, Schinzer S, Reuter M. Metabolic effects of ramipril in non-insulin dependent diabetics with essential hypertension. Diabetol Croat. 1997;26:135–144. [Google Scholar]
- de Boer RA, Doehner W, van der Horst ICC, Anker SD, Babalis D, Roughton M, Coats AJ, Flather MD, van Veldhuisen DJ SENIORS Investigators. Influence of diabetes mellitus and hyperglycemia on prognosis in patients ≥70 years old with heart failure and effects of nebivolol (data from the Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure [SENIORS]) Am J Cardiol. 2010;106:78–86. doi: 10.1016/j.amjcard.2010.02.018. .e1. [DOI] [PubMed] [Google Scholar]
- Gundersen T, Kjekshus J. Timolol treatment after myocardial infarction in diabetic patients. Diabetes Care. 1983;6:285–290. doi: 10.2337/diacare.6.3.285. [DOI] [PubMed] [Google Scholar]
- Rodda BE. The Timolol Myocardial Infarction Study: an evaluation of selected variables. Circulation. 1983;67(6 Pt 2):I101–106. [PubMed] [Google Scholar]
- Larijani B, Afshari M, Astanehi-Ashgari F, Mojtahedi A, Rezaie A, Hosseinnezhad A, Heshman R, Mohammadirad A, Abdollahi M. Effect of short-term carvedilol therapy on salivary and plasma oxidative stress parameters and plasma glucose level in type II diabetes. Therapy. 2006;3:119–123. [Google Scholar]
- Davies JI, Band M, Morris A, Struthers AD. Spironolactone impairs endothelial function and heart rate variability in patients with type 2 diabetes. Diabetologia. 2004;47:1687–1694. doi: 10.1007/s00125-004-1510-8. [DOI] [PubMed] [Google Scholar]
- Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005;28:2106–2112. doi: 10.2337/diacare.28.9.2106. [DOI] [PubMed] [Google Scholar]
- Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Tarnow L, Rossing P, Parving HH. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006;70:536–542. doi: 10.1038/sj.ki.5001580. [DOI] [PubMed] [Google Scholar]
- Swaminathan K, Davies J, George J, Rajendra NS, Morris AD, Struthers AD. Spironolactone for poorly controlled hypertension in type 2 diabetes: conflicting effects on blood pressure, endothelial function, glycaemic control and hormonal profiles. Diabetologia. 2008;51:762–768. doi: 10.1007/s00125-008-0972-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin DM, Atkinson AB, Ennis CN, Browne J, Hunter SJ, Sheridan B, Bell PM. Comparison of effects of combined ACE inhibitor and low-dose thiazide diuretic with ACE inhibitor alone on insulin action in patients with hypertension and Type 2 diabetes: a double-blind crossover study. Diabet Med. 2008;25:631–634. doi: 10.1111/j.1464-5491.2008.02437.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Mean difference in end point HbA1c of beta-blockers vs. placebo (boxes) and pooled estimates (diamond) calculated by the inverse variance fixed effects model. Horizontal bars and diamond widths represent 95% CIs and box sizes indicate relative weights in the analysis
Figure S2 Beta-blockers – Adverse events
Figure S3 Mean difference in end point HbA1c (%) with diuretics vs. placebo (boxes) and pooled estimates (diamond) calculated by the inverse variance fixed effects model. Horizontal bars and diamond widths represent 95% CIs and box sizes indicate relative weights in the analysis