Abstract
Aims
Calcitonin gene related peptide (CGRP) receptor antagonists are effective acute migraine treatments. A capsaicin-induced dermal vasodilatation (CIDV) model has been developed to provide target-engagement information in healthy volunteers. In the model, CGRP release is provoked after dermal capsaicin application, by activating transient receptor potential vanilloid-type-1 (TRPV1) receptors at peripheral sensory nerves. Laser Doppler imaging is used to quantify CIDV and subsequent inhibition by CGRP receptor antagonists. We sought to evaluate a CGRP receptor antagonist, MK-3207, in the biomarker model and to assess the predictability of the CIDV response to migraine clinical efficacy.
Methods
An integrated population pharmacokinetic/pharmacodynamic (PK/PD) model was developed to describe the exposure−response relationship for CIDV inhibition by CGRP and TRPV1 receptor antagonists. MK-3207 dose−response predictions were made based on estimated potency from the PK/PD model and mean plasma concentrations observed at the doses investigated.
Results
The results suggested that a 20 mg dose of MK-3207 (EC50 of 1.59 nm) would be required to attain the peripheral CIDV response at a target level that was shown previously to correlate with 2 h clinical efficacy based on phase 3 telcagepant clinical data, and that a plateau of the dose−response would be reached around 40–100 mg. These predictions provided a quantitative rationale for dose selection in a phase 2 clinical trial of MK-3207 and helped with interpretation of the efficacy results from the trial.
Conclusions
The integrated CIDV PK/PD model provides a useful platform for characterization of PK/PD relationships and predictions of dose−response relationships to aid in future development of CGRP and TRPV1 receptor antagonists.
Keywords: capsaicin, CGRP, human, MK-3207, vasodilatation
What is Already Known about this Subject
A previous study with telcagepant suggested that a capsaicin-induced dermal vasodilatation model might provide useful target-engagement information in healthy volunteers for calcitonin gene related peptide (CGRP) receptor antagonists intended to be investigated in the clinic for the treatment of migraine pain.
What this Study Adds
This study confirmed the utility of the capsaicin-induced dermal vasodilatation model for dose selection and interpretation of the efficacy results from a phase 2 trial of the CGRP receptor antagonist MK-3207.
The model provides early stage pharmacological and quantitative insights into dose−response to aid in future development of CGRP receptor antagonists.
Introduction
Calcitonin gene related peptide (CGRP) is a potent vasodilator and neurotransmitter involved in migraine pathophysiology and is a pharmacological target for the treatment of migraine headache 1,2. Blocking CGRP receptors results in effective migraine treatment, as confirmed in clinical trials with the CGRP receptor antagonists, olcegepant and telcagepant (MK-0974) 3,4. Current triptan treatments have direct vasoconstrictor activity through 5-HT1B receptor activation and therefore their use is contra-indicated in patients with cardiovascular disease 5,6. As CGRP receptor antagonists lack direct vasoconstrictor activity, they have the potential to become the next generation of specific antimigraine drugs without the cardiovascular risk of the triptans 6.
In order to facilitate the development of CGRP receptor antagonists, a human pharmacodynamic model for the non-invasive in vivo assessment of CGRP receptor antagonist activity was developed. This capsaicin-induced dermal vasodilatation (CIDV) model was first established and validated in the rhesus monkey 7,8. Subsequently it was translated into humans 9 and proved to be a reproducible pharmacodynamic assay which could easily be incorporated in early phase clinical drug development studies in healthy subjects. The CIDV model is a useful target engagement biomarker for CGRP receptor antagonists and can be used to predict dose−response and support dose selection in early clinical trials for acute treatment of migraine. In the CIDV model, capsaicin is applied topically onto the skin and activates transient receptor potential vanilloid type 1 (TRPV1) receptors at peripheral sensory nerves 10. This activation results in the local release of vasoactive mediators which initiate a process of neurogenic inflammation characterized by local vasodilation. The accompanied increase in dermal blood flow (DBF), which can be measured using laser Doppler imaging, is largely mediated by CGRP and can be almost completely blocked by CGRP receptor antagonists 11,12.
MK-3207 is a structurally novel, potent, highly selective and orally bioavailable CGRP receptor antagonist which has shown clinical efficacy for acute migraine in a phase 2 trial 8,13. In healthy humans, MK-3207 is rapidly absorbed (median tmax 1–2 h) and exhibits a biexponential decay post-Cmax, with an apparent half-life of about 3–6 h for the α phase, and 9–18 h for the β phase. The primary objective of the present study was to evaluate the effect of MK-3207 on CIDV in healthy subjects in order to better understand the pharmacokinetic/pharmacodynamic (PK/PD) relationship and further validate the CIDV model. The secondary objective was to predict the MK-3207 dose−response relationship for CIDV inhibition and compare with clinical efficacy in order to establish a biomarker-efficacy correlation for future development of compounds targeting the CGRP pathway. Although MK-3207 is no longer in clinical development due to the finding that some subjects experienced liver test abnormalities 13, the methodological approach and results are potentially useful for those developing other treatments targeting CGRP such as CGRP antibodies 14.
Methods
Ethics
The protocol for the MK-3207 CIDV study (Merck Protocol 002) was reviewed and approved by the Independent Ethics Committee of the University Hospitals of Leuven, Belgium. Before enrolment, all participants gave informed consent in writing after a full verbal and written explanation of the study. The study was conducted in accordance with local law, the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Design of MK-3207 CIDV study
Two groups (part I and part II), consisting of 16 healthy male subjects each, with an age range of 18–42 years, participated in a randomized, double-blind, placebo-controlled, four-period, crossover study to evaluate the effects of orally administered MK-3207 on dermal vasodilatation induced by topical capsaicin.
As part of the screening, subjects underwent a baseline laser Doppler scan to measure baseline dermal blood flow. Subsequently, a topical dose of 1000 μg 20 μl−1 capsaicin solution was applied within a 10 mm rubber ‘O’ ring placed on the volar surface of one forearm. Thirty minutes post-capsaicin application, subjects underwent a second laser Doppler scan at the site of interest to measure CIDV. Subjects were considered eligible for participation in the study if they demonstrated an increase of at least 100% in dermal flow 30 min after capsaicin application (two subjects did not show a response and were excluded). The details of the laser Doppler skin perfusion measurement methodology have been previously reported 9,15.
In part I of the study, periods 1 to 4, 16 subjects received single oral doses of 100, 40 or 20 mg of MK-3207 or matching placebo. Based on the results from part I, three additional dose levels were selected to be tested in part II to enable adequate characterization of the dose−response curve for inhibition of CIDV. In part II, periods 1 to 4, 16 subjects received single oral doses of 20, 2 or 0.25 mg MK-3207 or matching placebo.
In both part I and part II, the sequence in which these treatments was administered over the course of four periods was randomly allocated. There was at least a 7 day washout between each treatment period for all subjects. After dosing with MK-3207 or matching placebo, subjects received two single topical doses (300 μg 20 μl−1 and 1000 μg 20 μl−1) of capsaicin solution (in ethanol : polysorbate 20 : water [3:3:4]) at two time points in 10 mm rubber ‘O’ rings on the volar surface of each forearm (a total of four capsaicin applications). Capsaicin applications were randomized by arm and timed so that the blood flow response (i.e. 30 min post-capsaicin application) could be evaluated over the time interval relevant to anticipated migraine analgesic activity (0–4 h) and the tmax (1–2 h) of MK-3207. Capsaicin was applied at 0.5 and 3.5 h post-MK-3207/matching placebo intake. For each evaluation of capsaicin-induced vasodilatation, a baseline laser Doppler scan was performed pre-dose MK-3207/matching placebo administration and just prior to each capsaicin application. Laser Doppler scans were then performed again post-capsaicin application at 1 and 4 h post-MK-3207/matching placebo intake.
For all treatment periods in part I and part II, blood was collected for pharmacokinetic evaluation at pre-dose, and at 0.5, 1, 1.5, 2 and 4 h post-dose.
Population PK/PD CIDV modelling and predictions
An integrated population PK/PD model for CIDV was built based on data from the MK-3207 CIDV study as well as three additional studies: (1) a pilot study assessing CIDV 9, (2) a CIDV study with the TRPV1 receptor antagonist MK-2295 16 and (3) a CIDV study with the CGRP receptor antagonist telcagepant (MK-0974) 12. The base model structure is described by equation 1. Blood flow is described as a baseline blood flow plus an incremental blood flow as a result of CIDV. The incremental blood flow is described by a competitive Emax model (between capsaicin and MK-2295) which is inhibited by CGRP receptor antagonists (MK-3207 or MK-0974) by an Emax model.
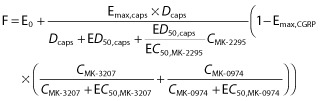 |
1 |
F: flow (arbitrary units); E0: baseline flow; Emax,caps: maximum flow, incremental blood flow from baseline due to capsaicin; Emax,CGRP: maximum inhibition of capsaicin induced flow by a CGRP receptor antagonist; Dcaps: capsaicin dose applied (μg 20 μl−1); ED50,caps: capsaicin dose to achieve 50% vasodilation (μg μl−1); EC50,MK-2295: MK-2295 concentration required to inhibit 50% of the capsaicin effect; EC50,MK-3207: MK-3207 concentration required for 50% of maximum CGRP effect; EC50,MK-0974: MK-0974 concentration required for 50% of maximum CGRP effect; CMK-2295: observed concentration of MK-2295 (nm); CMK-3207: observed concentration of MK-3207 (nm); CMK-0974: observed concentration of MK-0974 (nm). MK-3207 and MK-0974 (telcagepant) are CGRP receptor antagonists. MK-2295 is a TRPV1 receptor antagonist.
Inter-individual variability (IIV) terms (η) were selected using forward substitution/backward elimination with significance levels of 0.05 and 0.001, respectively. Covariate assessments focused on study-to-study differences in the PK/PD parameters. The model was fitted using nonmem® VI (ICON Development Solutions, Dublin, Ireland) using a first order conditional model with interaction.
The population mean estimates of EC50,MK-3207 and Emax,CGRP from the PK/PD model were used to simulate the exposure−response curve of MK-3207 for % inhibition of CIDV. The geometric mean values of observed plasma concentrations of MK-3207 at 2 h post-dose were used as input of the population PK/PD model in order to predict mean response of MK-3207 for inhibition of CIDV at clinically relevant time points of 2 h post-dose (the primary time point used to assess acute efficacy in many migraine trials). The prediction was conducted at five dose levels (0.25, 2, 20, 40 and 100 mg) evaluated in the present study in order to construct the dose−response relationship.
Results
Population PK/PD CIDV modelling
The final parameter estimates of the PK/PD model and associated standard errors are summarized in Table 1. Inter-individual variability terms were included in the final model for E0, Emax,caps, ED50,caps, and Emax,CGRP assuming log normal distributions. Two additive covariates of the pilot study were found to be significant on the baseline blood flow (E0) and maximum blood flow (Emax,caps). The estimated EC50 and EC90 values for MK-3207 for inhibition of CIDV were ∼1.6 nm and ∼14 nm, respectively. The Emax for inhibition of CIDV by CGRP receptor antagonists was 92.4% though notably not 100%.
Table 1.
Parameter estimates for the final PK/PD model for blockade of CIDV response
| Parameters | Parameter estimate | RSE† for parameter | ω estimate‡ | % RSE for ω estimate |
|---|---|---|---|---|
| E0 (arb) | 0.388 | 3.89% | 0.0755 | 17.20% |
| Emax,caps (arb) | 1.88 | 12.00% | 0.533 | 23.10% |
| ED50,caps (μg 20 μl−1) | 363 | 18.50% | 5.45 | 32.80% |
| EC50,MK-3207 (nm) | 1.59 | 31.40% | NA | NA |
| Emax,CGRP (fractional) | 0.924 | 1.59% | 0.00489 | 40.10% |
| EC50,MK-2295 (nm) | 49.7 | 19.30% | NA | NA |
| EC50,MK-0974 (nm) | 102 | 38.00% | NA | NA |
| ΔE0,pilot study (arb) | 0.565 | 7.29% | – | – |
| ΔEmax,pilot study (arb) | 1.07 | 40.50% | – | – |
| Proportional residual error | 0.0692 | 9.83% | – | – |
%RSE is percent relative standard error (100% × SE/EST).
ω is the log scale variance of the inter-individual variability. arb, arbitrary units; caps, capsaicin; E0, baseline flow; Emax,caps, maximum flow, incremental blood flow from baseline due to capsaicin; Emax,CGRP, maximum inhibition of capsaicin induced flow by a CGRP receptor antagonist; EC50,MK-2295, MK-2295 concentration required to inhibit 50% of the capsaicin effect; EC50,MK-3207, MK-3207 concentration required for 50% of maximum CGRP effect; EC50,MK-0974, MK-0974 concentration required for 50% of maximum CGRP effect; ΔE0,pilot study, additive covariate of the pilot study on the baseline blood flow; ΔEmax,pilot study, additive covariate of the pilot study on the maximum flow induced by capsaicin. MK-3207 and MK-0974 (telcagepant) are CGRP receptor antagonists. MK-2295 is a TRPV1 receptor antagonist.
Figure 1 shows the observed blood flow (mean perfusion values) measured by laser Doppler imaging and the predicted blood flow at different plasma concentrations of MK-3207 30 min after administration of 300 and 1000 μg 20 μl−1 of capsaicin. The model describes the observed data reasonably well. The data display a clear exposure−response relationship and the intrinsic variability associated with capsaicin response. Diagnostic plots for goodness of model fit are shown in Figure S1. The ratios between model predicted values and observed values generally centre around the unity line. There are no systematic biases between weighted residual errors across a range of blood flow values and subjects, suggesting adequate model fit.
Figure 1.
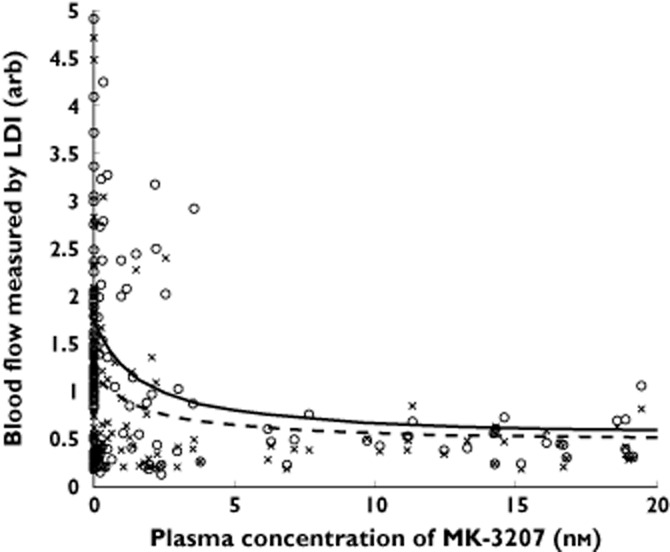
Model predicted vs. observed blood flow by MK-3207 plasma concentration at two capsaicin doses (300 and 1000 μg). Note: Data at MK-3207 concentrations of zero are from subjects who received placebo (instead of MK-3207). x, observed flow at 300 μg 20 μl−1 capsaicin; - - -, population predicted flow at 300 μg 20 μl−1 capsaicin; ○, observed flow at 1000 μg 20 μl−1 capsaicin; —, population predicted flow at 1000 μg 20 μl−1 capsaicin
Prediction of dose−response of MK-3207 for inhibition of CIDV
Figure 2 shows the simulated mean % CIDV inhibition vs. plasma concentration of MK-3207 based on the estimated EC50,MK-3207 and Emax,CGRP from the population PK/PD model. Results suggest that MK-3207 is a potent compound (EC50 of ∼1.6 nm) and that the majority of the exposure−response relationship occurs at low nm concentration range and starts to trend toward a plateau around the estimated EC90 (14 nm) of MK-3207. Table 2 summarizes the geometric mean (geometric %CV) of plasma concentrations of MK-3207 and associated prediction of CIDV response following single oral administrations of 0.25, 2, 20, 40 and 100 mg of MK-3207 at 2 h post-dose. MK-3207 concentration increases more than dose proportionally in this dose range, which is possibly associated with saturation of first pass metabolism by CYP3A4 and P-gp efflux transport with increasing doses. A 20 mg dose is associated with geometric mean plasma concentration of ∼40 nm at 2 h post-dose, and is expected to result in 2 h plasma concentrations above the EC90 (14 nm) level for the majority of subjects. Further increase in dose beyond 20 mg appears to provide limited increase in inhibition of peripheral CIDV response and a plateau appears to be reached between 40 to 100 mg. It is postulated that maintaining EC90 level target engagement during the first 2 h after dosing can be a meaningful metric corresponding to primary clinical efficacy end points (2 h pain relief and 2 h pain freedom) of CGRP receptor antagonists for acute treatment of migraine. A similar correlation was established based on clinical CIDV and clinical efficacy data of telcagepant at a 300 mg dose, which was shown in phase 3 studies to be clinically efficacious for acute treatment of migraine.
Figure 2.
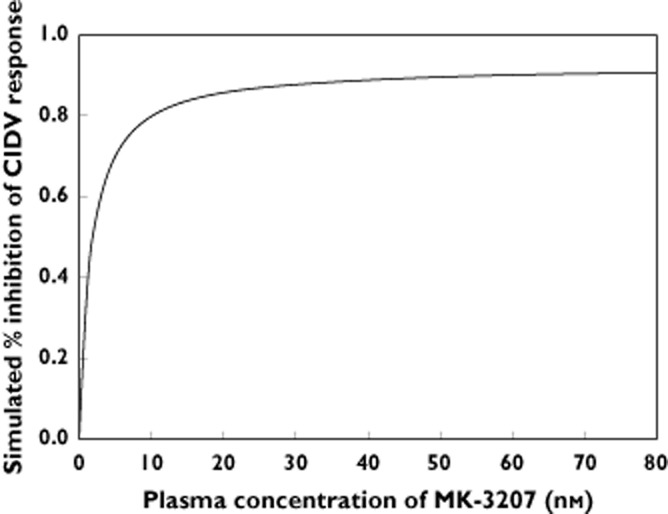
Simulated mean response of inhibition (%) of capsaicin-induced dermal vasodilatation (CIDV) by MK-3207 vs. plasma concentrations of MK-3207
Table 2.
Geometric mean (Geometric %CV) of 2 h plasma concentrations of MK-3207 and model predicted percentage of maximum response for inhibition of capsaicin-induced dermal vasodilatation (CIDV) by CGRP receptor antagonists following single oral administrations of MK-3207
| Dose (mg) | n | Geometric mean of C2h‡ (nm) | Geometric %CV | CIDV inhibition (%)§ | % of maximum response¶ |
|---|---|---|---|---|---|
| 0.25 | 16† | 0.243 | 75.8 | 12 | 13 |
| 2 | 16 | 2.90 | 52.0 | 60 | 65 |
| 20 | 32 | 39.5 | 44.4 | 89 | 96 |
| 40 | 16 | 78.0 | 72.1 | 91 | 98 |
| 100 | 16 | 277 | 57.0 | 92 | 99 |
Four plasma concentration values were below the assay limit of quantification (LOQ is 0.18 nm). These values were imputed as ½ of the assay LOQ.
Observed plasma concentration of MK-3207 at 2 h post-dose.
Predicted mean %CIDV response based on population PK/PD model.
Percentage of predicted maximum blockade of CIDV response by CGRP receptor antagonists.
Discussion
CGRP receptor antagonists have been shown to be clinically efficacious for acute treatment of migraine 3,4,13. In this study, the pharmacodynamic response to a CGRP receptor antagonist, MK-3207, was assessed using the CIDV model as a biomarker for target engagement of receptor antagonism through peripheral mechanisms. By applying topical capsaicin onto the skin, TRPV1 activation leads to dermal vasodilation, which is almost exclusively CGRP dependent. Van der Schueren et al. showed that neither substance P, nitric oxide nor prostaglandins are involved in this vasodilatory response 11. Population PK/PD CIDV modelling in the present study showed that the Emax for inhibition of CIDV by CGRP antagonists is ∼92.4%. This suggests that CGRP is the primary, but perhaps not the only, contributor to CIDV. The other possible and minor mediators of the response are unknown at present. Based on the EC50 values (∼1.6 nm) estimated from the clinical biomarker study, MK-3207 is expected to be ∼63-fold more potent than MK-0974 (EC50 ∼100 nm). This agrees with the in vitro results where MK-3207 is ∼40-fold more potent than MK-0974 (telcagepant) based on inhibition of CGRP binding to human CGRP receptors (Ki = 0.02 and 0.8 nm for MK-3207 and MK-0974, respectively) 8.
The study results suggest efficacious CGRP receptor antagonism by MK-3207 in an exposure-dependent manner. The PK/PD model predicts that a dose of 20 mg would be required to attain 90% of maximal peripheral CIDV response for 0–2 h post-dose for the majority of the subjects and further increase in dose beyond 20 mg provides minimal gain, with a response plateau reached between 40 and 100 mg. Even though clinical efficacy was demonstrated in the MK-3207 phase 2 clinical trial, there was lack of clarity on the shape of the dose−response relationship due to the high variability of the primary efficacy end point (2 h pain freedom) 13. This was an adaptive design study with a total of seven doses (2.5, 5, 10, 20, 50, 100 and 200 mg) evaluated in migraine patients. Among the tested doses, the pairwise difference vs. placebo for 2 h pain freedom was significant for 200 mg (P < 0.001) and nominally significant for 10 mg and 100 mg (P < 0.05). For 2 h pain relief, the pairwise comparisons vs. placebo were significant for all doses above 10 mg. While there may be an advantage of the 200 mg dose based on a composite measure of efficacy over 24 h, the confidence intervals for efficacy measures for each dose were overlapping, and it is not possible to conclude definitively that the 200 mg dose was more effective than other doses from 10 mg and up. The CIDV predictions of pharmacological dose−response provide a plausible way of interpreting the observed dose−response in the phase 2 trial, in suggesting that MK-3207 may be clinically efficacious at doses of 20 mg or higher, and that a plateau for 2 h efficacy may be achieved around 40–100 mg, based on peripheral blockade. If the phase 2 finding of increased efficacy at the 200 mg dose is valid then it is possible that additional central blockade of CGRP receptors may be a factor in determining efficacy.
The pathophysiology of migraine and the exact site of action of CGRP receptor antagonists, central or peripheral, remain incompletely understood. Migraine is currently conceptualized as a neurovascular headache in which sensitization and activation of the trigeminovascular system results in perivascular release of neuropeptides such as CGRP 17,18. Upon release of CGRP by centrally projecting pain transmission fibres, second order neurons are activated in the brain stem and central pain transmission occurs. Additionally, perivascular release of vasoactive neuropeptides promotes neurogenic inflammation 19 and is thought to cause CGRP mediated vasodilatation of intracranial extracerebral arteries 20. The CGRP receptor is expressed both in the central nervous system and on vascular smooth muscle cells and it remains unclear whether central or peripheral mechanisms are more important determinants of the actions of CGRP receptor antagonists 21,22. Recent PET studies in healthy subjects and migraine patients suggest limited central receptor occupancy of the CGRP receptor antagonist telcagepant at clinically efficacious dose levels, which supports the importance of peripheral mechanisms in the clinical efficacy of CGRP receptor antagonists 22,23. While these findings suggest that central CGRP receptor occupancy is not ‘required’ for clinically meaningful efficacy, they do not exclude the possibility that centrally acting CGRP receptor antagonists might show enhanced efficacy. Phase 2 clinical efficacy results with the CGRP antibody LY2951742 also indicate that a peripheral approach in migraine treatment may be sufficient as antibodies may not easily penetrate the blood−brain barrier 24,25. In the CIDV model, peripheral vasodilation is the primary parameter evaluated and predictions from the CIDV PK/PD analysis suggest a reasonable correlation with clinical efficacy for both telcagepant 12 and MK-3207 (present study).
In conclusion, our study demonstrates that the integrated CIDV PK/PD model provides a useful platform for characterization of PK/PD relationships that can be applied to early predictions of the clinical dose to aid in efficient study design and formulation strategy in early clinical development. In addition, given the generally high variability associated with migraine efficacy end points within and between clinical trials, the model predictions can be leveraged to understand the dose−response relationships, which oftentimes can be difficult to extract based on results from phase 2 dose ranging trials alone, given the limited sample sizes. This platform provides early stage pharmacological and quantitative insights into dose-response and can be a useful tool for development of future CGRP/TRPV1-based therapies, such as CGRP antibodies 14, for migraine and other diseases.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare this study was funded by Merck & Co., Inc. C.-C. Li, W.S. Denney, W.P. Kennedy, J. Palcza, A. Gipson, T. H. Han, R. Blanchard, I. De Lepeleire, G. Murphy and K. Van Dyck are current or former employees of Merck & Co., Inc., Whitehouse Station, NJ, or MSD Europe, and own or owned stock/stock options in Merck. S. Vermeersch, M. Depré, and J.N. de Hoon have received research funding from Merck.
We gratefully thank the clinical research team of the Centre for Clinical Pharmacology for their help in performing the experiments and collection of all data. Special thanks also to Christopher Lines from Merck for assistance with drafting and editing this article and Sheila Erespe from Merck for assistance in submitting the article.
This study was funded by Merck & Co., Inc. The funding organization was involved in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review and approval of the manuscript.
Contributors
Participated in research design: C-C. Li, S. Vermeersch, W.S. Denney, W.P. Kennedy, J. Palcza, A. Gipson, R. Blanchard, I. De Lepeleire, J.N. de Hoon.
Conducted experiments: S. Vermeersch, W.P. Kennedy.
Contributed new reagents or analytic tools: S. Vermeersch
Performed data analysis: S. Vermeersch, W.S. Denney, W.P. Kennedy, J. Palcza,
Wrote or contributed to the writing of the manuscript: C-C. Li, S. Vermeersch, W.S. Denney, W.P. Kennedy, J. Palcza, A. Gipson, T. H. Han, R. Blanchard, I. De Lepeleire, M. Depré, M.G. Murphy, K. Van Dyck, J.N. de Hoon.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Diagnostic plots for goodness of fit for the population PK/PD CIDV model for MK-3207
References
- Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol. 2010;9:285–298. doi: 10.1016/S1474-4422(10)70005-3. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM BIBN 4096 BS Clinical Proof of Concept Study Group. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- Papademetriou V. Cardiovascular risk assessment and triptans. Headache. 2004;44(Suppl. 1):S31–39. doi: 10.1111/j.1526-4610.2004.04106.x. [DOI] [PubMed] [Google Scholar]
- Chan KY, Vermeersch S, de Hoon J, Villalón CM, Maassenvandenbrink A. Potential mechanisms of prospective antimigraine drugs: a focus on vascular (side) effects. Pharmacol Ther. 2011;129:332–351. doi: 10.1016/j.pharmthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, Mosser SD, Burgey CS, Paone DV, Shaw AW, Graham SL, Vacca JP, Williams TM, Koblan KS, Kane SA. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther. 2008;324:416–421. doi: 10.1124/jpet.107.130344. [DOI] [PubMed] [Google Scholar]
- Salvatore CA, Moore EL, Calamari A, Cook JJ, Michener MS, O'Malley S, Miller PJ, Sur C, Williams DL, Jr, Zeng Z, Danziger A, Lynch JJ, Regan CP, Fay JF, Tang YS, Li CC, Pudvah NT, White RB, Bell IM, Gallicchio SN, Graham SL, Selnick HG, Vacca JP, Kane SA. Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist. J Pharmacol Exp Ther. 2010;333:152–160. doi: 10.1124/jpet.109.163816. [DOI] [PubMed] [Google Scholar]
- Van der Schueren BJ, de Hoon JN, Vanmolkot FH, Van Hecken A, Depre M, Kane SA, De Lepeleire I, Sinclair SR. Reproducibility of the capsaicin-induced dermal blood flow response as assessed by laser Doppler perfusion imaging. Br J Clin Pharmacol. 2007;64:580–590. doi: 10.1111/j.1365-2125.2007.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Van der Schueren BJ, Rogiers A, Vanmolkot FH, Van Hecken A, Depré M, Kane SA, De Lepeleire I, Sinclair SR, de Hoon JN. Calcitonin gene-related peptide 8-37 antagonizes capsaicin-induced vasodilation in the skin: evaluation of a human in vivo pharmacodynamic model. J Pharmacol Exp Ther. 2008;325:248–255. doi: 10.1124/jpet.107.133868. [DOI] [PubMed] [Google Scholar]
- Sinclair SR, Kane SA, Van der Schueren BJ, Xiao A, Willson KJ, Boyle J, de Lepeleire I, Xu Y, Hickey L, Denney WS, Li CC, Palcza J, Vanmolkot FH, Depré M, Van Hecken A, Murphy MG, Ho TW, de Hoon JN. Inhibition of capsaicin-induced increase in dermal blood flow by the oral CGRP receptor antagonist, telcagepant (MK-0974) Br J Clin Pharmacol. 2010;69:15–22. doi: 10.1111/j.1365-2125.2009.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–722. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- Vermeersch S, Van Hecken A, Abu-Raddad E, Benschop R, Montieth D, Scherer J, Grayzel D, de Hoon J, Collins E. Translational pharmacodynamics of CGRP monoclonal antibody LY2951742 in capsaicin-induced dermal blood flow model. Cephalagia. 2013;33(Suppl. 8):249–250. [Google Scholar]
- Fullerton A, Stücker M, Wilhelm KP, Wårdell K, Anderson C, Fischer T, Nilsson GE, Serup J European Society of Contact Dermatitis Standardization Group. Guidelines for visualization of cutaneous blood flow by laser Doppler perfusion imaging. A report from the Standardization Group of the European Society of Contact Dermatitis based upon the HIRELADO European community project. Contact Dermat. 2002;46:129–140. doi: 10.1034/j.1600-0536.2002.460301.x. [DOI] [PubMed] [Google Scholar]
- Denney WS, Hang Y, Dockendorf MF, Li C-C, Eid SR, Valesky R, Laethem T, Van Hoydonck P, De Lepeleire I, de Hoon JNJM, Crutchlow M, Blanchard R. Modeling and simulation for determination of the therapeutic window of MK-2295: a TRPV1 antagonist. 2009. Presented at: Annual Meeting of the Population Approach Group in Europe (PAGE) 18; June 23-26 ; St. Petersburg, Russia. Abstract 1507. Available at http://www.page-meeting.org/?abstract=1507 (last accessed 2 October 2009)
- Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46(Suppl. 1):S3–8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Olesen J. Possible site of action of CGRP antagonists in migraine. Cephalalgia. 2011;31:748–750. doi: 10.1177/0333102411398403. [DOI] [PubMed] [Google Scholar]
- Vermeersch SGG, de Hoon J, De Saint-Hubert B, Derdelinckx I, Serdons K, Bormans G, Reynders T, Declercq R, De Lepeleire I, Kennedy W, Blanchard R, Marcantonio E, Hargreaves R, Li CC, Sanabria S, Hostetler E, Joshi A, Evelhoch J, Van Laere K. PET imaging in healthy subjects and migraineurs suggests CGRP receptor antagonists do not have to act centrally to achieve clinical efficacy. J Headache Pain. 2013;1(Suppl. 1):P224. Abstract Book of The European Headache and Migraine Trust International Congress. Available at http://www.thejournalofheadacheandpain.com/content/14/S1/P224. [Google Scholar]
- Hostetler ED, Joshi AD, Sanabria-Bohórquez S, Fan H, Zeng Z, Purcell M, Gantert L, Riffel K, Williams M, O'Malley S, Miller P, Selnick HG, Gallicchio SN, Bell IM, Salvatore CA, Kane SA, Li CC, Hargreaves RJ, de Groot T, Bormans G, Van Hecken A, Derdelinckx I, de Hoon J, Reynders T, Declercq R, De Lepeleire I, Kennedy WP, Blanchard R, Marcantonio EE, Sur C, Cook JJ, Van Laere K, Evelhoch JL. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. J Pharmacol Exp Ther. 2013;347:478–486. doi: 10.1124/jpet.113.206458. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13:885–892. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- Reuter U. Anti-CGRP antibodies: a new approach to migraine prevention. Lancet Neurol. 2014;13:857–859. doi: 10.1016/S1474-4422(14)70126-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Diagnostic plots for goodness of fit for the population PK/PD CIDV model for MK-3207


