Abstract
IMPORTANCE
Current guidelines recommend an intravenous bolus dose of a proton pump inhibitor (PPI) followed by continuous PPI infusion after endoscopic therapy in patients with high-risk bleeding ulcers. Substitution of intermittent PPI therapy, if similarly effective as bolus plus continuous-infusion PPI therapy, would decrease the PPI dose, costs, and resource use.
OBJECTIVE
To compare intermittent PPI therapy with the currently recommended bolus plus continuous-infusion PPI regimen for reduction of ulcer rebleeding.
DATA SOURCES
Searches included MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials databases through December 2013; US and European gastroenterology meeting abstracts from 2009 to 2013; and bibliographies of systematic reviews.
STUDY SELECTION
Randomized trials of patients with endoscopically treated high-risk bleeding ulcers (active bleeding, nonbleeding visible vessels, and adherent clots) comparing intermittent doses of PPIs and the currently recommended regimen (80-mg intravenous bolus dose of a PPI followed by an infusion of 8 mg/h for 72 hours).
DATA EXTRACTION AND SYNTHESIS
Duplicate independent data extraction and risk-of-bias assessment were performed. Data were pooled using a fixed-effects model or a random effects model if statistical heterogeneity was present.
MAIN OUTCOMES AND MEASURES
The primary outcome was rebleeding within 7 days; additional predefined outcomes included rebleeding within 3 and 30 days, need for urgent intervention, mortality, red blood cell transfusion, and length of hospital stay. The primary hypothesis, defined before initiation of the literature review, was that intermittent use of PPIs was noninferior to bolus plus continuous infusion of PPIs, with the noninferiority margin predefined as an absolute risk difference of 3%.
RESULTS
The risk ratio of rebleeding within 7 days for intermittent vs bolus plus continuous infusion of PPIs was 0.72 (upper boundary of 1-sided 95% CI, 0.97) and the absolute risk difference was −2.64% (upper boundary of 1-sided 95% CI, −0.28%, which is well below the predefined noninferiority margin of 3%). Risk ratios for rebleeding within 30 days and 3 days, mortality, and urgent interventions were less than 1 and mean differences for blood transfusion and hospital length of stay were less than 0, indicating that no summary estimate showed an increased risk with intermittent therapy. The upper boundaries of 95% CIs for absolute risk differences were less than 1.50% for all predefined rebleeding outcomes.
CONCLUSIONS AND RELEVANCE
Intermittent PPI therapy is comparable to the current guideline-recommended regimen of intravenous bolus plus a continuous infusion of PPIs in patients with endoscopically treated high-risk bleeding ulcers. Guidelines should be revised to recommend intermittent PPI therapy.
Ulcers are the most common cause of upper gastrointestinal bleeding.1 Current guidelines2,3 recommend that patients with bleeding ulcers who have high-risk endoscopic findings (active bleeding, nonbleeding visible vessels, and adherent clots) receive an intravenous bolus dose followed by a continuous infusion of a proton pump inhibitor (PPI) after endoscopic treatment. Specifically, an 80-mg intravenous bolus dose of a PPI followed by a continuous infusion at 8 mg/h for 72 hours is recommended.2
In vitro data suggest that an intragastric pH above 6 may be required to promote clot formation and stability.4,5 High-dose, continuous-infusion PPI therapy was consequently studied6 in an attempt to maintain an intragastric pH above 6. Elimination half-lives of PPIs are short (approximately 1 hour). Thus, after clearance of a PPI administered as a bolus, whether intravenous or oral, new proton pumps may produce acid. It was therefore hypothesized7 that a constant infusion would be required to maintain an intragastric pH above 6, with a PPI present continuously to inhibit newly activated proton pumps. A meta-analysis7 of randomized trials compared high-dose continuous-infusion PPI therapy with placebo or no therapy and showed a significant decrease in further bleeding, as well as surgery and mortality, among patients with high-risk bleeding ulcers after endoscopic therapy. However, a meta-analysis7 of randomized trials of intermittent PPI therapy vs placebo or no therapy also showed a significant reduction in further bleeding in patients with high-risk bleeding ulcers after endoscopic therapy.
An important issue in clinical practice is whether intermittent PPI therapy can be substituted for the currently recommended bolus plus continuous-infusion PPI therapy. If intermittent PPI treatment achieves comparable clinical efficacy, it would be the preferred regimen given the decrease in cost and resources (eg, infusion pump, nursing and pharmacy personnel time, and requirement for monitored setting), the decrease in the PPI dose, and the greater ease of administration.
Although randomized trials comparing intermittent boluses with continuous infusion of PPIs have been performed, the absence of a significant difference in a trial cannot be interpreted as documenting that the 2 treatments are comparable. These trials were not designed or adequately powered to assess noninferiority of intermittent PPI therapy.
Meta-analyses of PPI therapy have been performed,7–11 but they have not addressed the clinically relevant question in this population: Is intermittent PPI therapy noninferior to the guideline-recommended intravenous bolus plus continuous-infusion regimen? Prior meta-analyses have included patients without high-risk stigmata8–11 or patients with high-risk stigmata who did not undergo endoscopic therapy8–11 and have compared high-vs low-dose PPIs rather than continuous vs intermittent administration.9,11 Furthermore, several randomized trials have been published since the literature searches of the prior meta-analyses were performed.
We therefore performed a systematic review and meta-analysis to assess the clinical efficacy of intermittent PPI regimens vs the standard bolus plus continuous-infusion regimen after successful endoscopic therapy in patients with bleeding ulcers. We hypothesized that the risk of recurrent bleeding with this regimen was noninferior to (ie, not unacceptably greater than) the risk with the currently recommended bolus plus continuous-infusion PPI therapy.
Methods
Data Sources and Searches
The search strategy, study inclusion and exclusion criteria, primary and secondary outcomes, and analyses were defined a priori and are described below. The protocol was not registered on an online registry site.
We searched 3 bibliographic databases—MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials—from inception (MEDLINE, 1946; EMBASE, 1974; and Cochrane Central Register of Controlled Trials, 1898) to December 31, 2013, without language restriction. MEDLINE and EMBASE were searched using the Ovid interface. An extensive search strategy using a combination of subject headings and text words was constructed to find articles that relate to the treatment of upper gastrointestinal bleeding from ulcers with PPIs (eFigure 1 in the Supplement).
In addition, we searched for relevant abstracts from major gastroenterology scientific meetings (Digestive Disease Week, United European Gastroenterology Week, and American College of Gastroenterology) from 2009 to 2013. Bibliographies of prior systematic reviews were also evaluated.8–11
Two of us (H.S. and K.V.) independently reviewed titles and abstracts produced by the search. Studies deemed potentially relevant by either author were independently retrieved and reviewed in full by both authors to determine eligibility. Disagreements regarding the inclusion of a study were resolved by discussion; if a consensus could not be reached, the senior author (L.L.) served as the final arbiter.
Study Selection
Study Design and Population
Only randomized clinical trials were included. Studies were included if patients presented with upper gastrointestinal bleeding; were found to have a gastric or duodenal ulcer with active bleeding, a nonbleeding visible vessel, or an adherent clot; and had received successful endoscopic hemostatic therapy, with randomization to intermittent or continuous PPI treatment, after endoscopic therapy. We excluded patients who had ulcers with flat spots and clean bases because such patients have a very low rate of clinically significant rebleeding, and current guidelines2 do not recommend endoscopic therapy or PPI infusion for these patients.
Intervention
The study therapy was defined as PPIs administered in intermittent boluses. Because the degree of acid suppression required to reduce rebleeding is not known, no restrictions were applied to the frequency of boluses (they could be once daily or more often), the doses of boluses, or the route of administration (oral vs intravenous). Because an oral bolus of a PPI provides a pharmacodynamic effect comparable to that of the equivalent intravenous dose of a PPI,6 equal oral and intravenous doses would be postulated to have comparable efficacy.
Comparison
The control regimen was the standard PPI bolus plus continuous-infusion that has been documented to be effective in this population in a meta-analysis of randomized trials7 and is recommended by current guidelines2: an 80-mg intravenous bolus followed by a continuous 8-mg/h intravenous infusion for 72 hours.
Outcomes
Studies reporting 1 or more of the following outcomes were eligible for inclusion: recurrent bleeding, mortality, need for urgent intervention (subsequent endoscopic therapy, surgery, or radiologic intervention), red blood cell transfusions, and length of hospitalization.
The primary outcome was defined as recurrent bleeding within 7 days as recommended by an international consensus conference12 on the methods used in trials for nonvariceal upper gastrointestinal bleeding. Recurrent bleeding within 3 days (because this is the duration of treatment studied and recommended for continuous-infusion PPI therapy) and 30 days were assessed as secondary outcomes. Other predefined outcomes were mortality, need for surgery and radiologic intervention, need for urgent intervention, red blood cell transfusions, and length of hospitalization. Blood transfusion results reported in milliliters were converted to units (250 mL = 1 U of packed red blood cells). Studies that did not report the SD or allow calculation of the SD (eg, SEs or CIs not provided13) for continuous outcomes were excluded for analysis of that outcome.
Data Extraction and Risk-of-Bias Assessment
A data extraction sheet was constructed to record information on study characteristics, patient characteristics, and predefined outcomes. Risk of bias was assessed using the Cochrane Collaboration’s13 risk-of-bias tool and criteria for judging risk of bias. The domains assessed were random sequence generation and allocation concealment (selection bias), blinding of study participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias.
Two of us (H.S. and K.V.) independently extracted data and assessed the risk of bias for each of the articles. Once this was done, the forms containing the assessments were exchanged and reviewed for comparison. Any disagreements were again resolved by discussion and consensus, with the senior author (L.L.) serving as the final arbiter if consensus could not be reached. If studies had apparent contradictions that could not be resolved during data extraction (eg, bleeding definition varied within the same article) or did not report the results of their predefined outcomes sufficiently to allow inclusion in our meta-analyses (eg, SDs not given), the study’s authors were contacted.
Data Synthesis and Analysis
The dichotomous pooled outcomes were calculated as risk ratios (RRs) using the Mantel-Haenszel statistical method. For the continuous pooled outcomes, mean difference was calculated. Results for our primary analysis, which was a noninferiority analysis, are presented with the upper boundary of the 1-sided 95% CI. We chose 1-sided testing because we know of no physiologic, pharmacologic, or clinical basis on which to postulate that intermittent PPI therapy might be more effective than high-dose bolus plus continuous-infusion PPI therapy. We calculated the absolute risk difference and upper boundary for the 1-sided 95% CI for proportional outcomes, such as rebleeding, by multiplying the incidence of the outcome in the control group by the relative risk increase (or reduction) with the intermittent regimen compared with the control regimen.
The primary objective of the study was to demonstrate that the incidence of recurrent bleeding within 7 days of starting an intermittent PPI regimen is noninferior to the incidence of rebleeding with the standard bolus plus continuous-infusion PPI regimen. We defined the margin of noninferiority as an absolute risk difference of 3%. A prior meta-analysis7 reported an absolute risk difference of 8% in rebleeding with bolus plus continuous PPI infusion vs placebo or no treatment in the same population as analyzed in our systematic review. The margin of 3% represents 50% of the lower boundary of the 95% CI (ie, 6%) from that analysis.
Subgroups were predefined and used to assess the influence of specific factors on the results. Factors related to the intermittent PPI regimen were route of administration (oral vs intravenous), frequency (once-daily vs greater than once-daily dosing), total dose (≤240 mg vs >240 mg and ≤120 mg vs >120 mg), and use of a higher-dose bolus at initiation of intermittent PPI therapy. In addition, the effect of dividing studies according to the risk of bias (lower risk [no high-risk domains and ≥5 low-risk domains] vs higher risk) was assessed, as was the effect of including abstracts in the analysis. Studies were also divided based on their geographic location (Asian vs non-Asian).
Our primary population for analysis was per protocol, as is commonly recommended for noninferiority analyses.12 However, a sensitivity analysis for our primary analysis was planned using the intention-to-treat population. In addition, traditional forest plots and pooled analyses with 2-sided 95% CIs for our predefined rebleeding outcomes were constructed using the intention-to-treat population. A post hoc sensitivity analysis using trial sequential meta-analysis was performed because of relatively sparse rebleeding events. For studies that did not specifically conduct a per-protocol analysis, we selectively extracted and analyzed data on patients who had no protocol violations. A protocol violation was considered to have occurred if a patient met the predefined exclusion criteria after randomization (eg, gastric ulcer was malignant) or failed to follow-up within the period predefined for the assessment of the primary outcome (eg, 7 days).
Heterogeneity was assessed for the individual meta-analyses using the χ2 test and the I2 statistic. Significant heterogeneity was defined as P < .10 using the χ2 test or I2 greater than 50%. A fixed-effects model was used when heterogeneity was not significant, and a random-effects model was used when statistical heterogeneity was present. Treatment-by-subgroup interaction was assessed by calculating the heterogeneity between subgroups using the χ2 test. Significant heterogeneity was defined as P < .05. A funnel plot was created to assess for publication and other reporting biases; the funnel plot was examined visually for asymmetry, and an Egger test for asymmetry of a funnel plot was conducted. Analyses were done using RevMan, version 5.2, software (Nordic Cochrane Centre, The Cochrane Collaboration) and Trial Sequential Analysis software (Copenhagen Trial Unit).
Results
Search and Study Selection
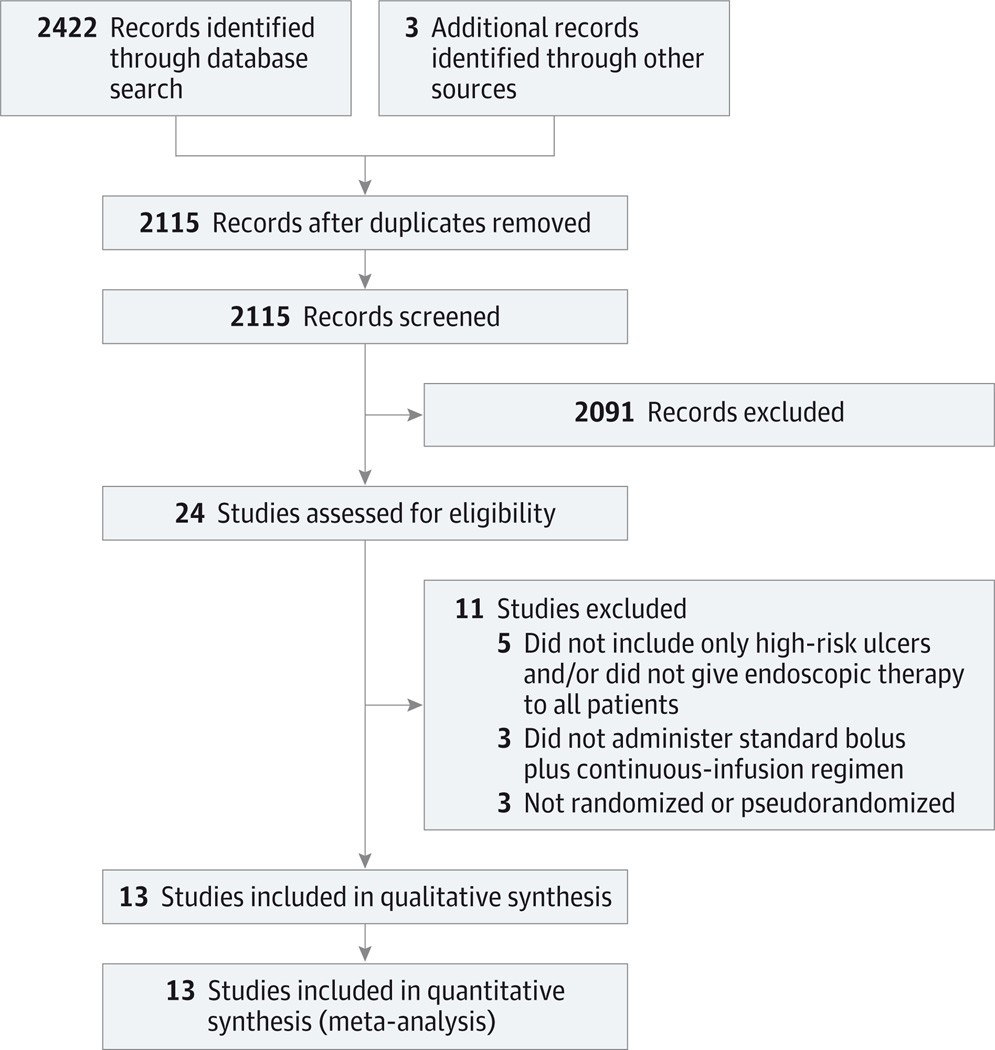
We identified 2115 citations after removal of duplicates, including 2112 from our bibliographic database searches and 3 from other sources (Figure 1). Review of these titles and abstracts resulted in 21 full-text articles from the database searches being retrieved and assessed for eligibility. Eleven studies were excluded for reasons detailed in Figure 1, leaving 10 full-text articles from the database searches.14–23 In addition, 1 relevant full-text article was identified from the reference list of a systematic review24 and 2 meeting abstracts that met eligibility criteria were identified,25,26 resulting in a total of 13 studies for inclusion in the analysis.
Figure 1. Flow Diagram.
Search and selection process used for studies included in the systematic review.
No study was excluded from our review because of a continuous-infusion dosage regimen that did not match our predefined criterion of an 80-mg bolus and 8-mg/h infusion. One of the 2 continuous-infusion arms in one study17 was excluded from analysis because the regimen (40-mg bolus and 4-mg/h infusion) in this arm was half our predefined dosage. One of 3 arms in another study19 was excluded because no PPI treatment was administered in that group. Two authors were contacted and provided clarification of contradictory statements20,25; a third author was contacted to provide SDs for transfusion and hospital length of stay outcomes,19 but these data were not obtained. Details of the included studies are displayed in Table 1,14–26 and the risk-of-bias assessment is shown in eFigure 2 in the Supplement.
Table 1.
Characteristics of Studies Included in the Meta-analysis
| Source | PPI | Dose, Route, and Frequency of Intermittent PPI |
Cumulative Dose of Intermittent PPI, mg |
Type of Study | Stigmata of Recent Hemorrhage |
Endoscopic Therapy |
|---|---|---|---|---|---|---|
| Andriulli et al,14 2008 | Omeprazole (n = 330); pantoprazole (n = 144) | 40 mg/d IV | 120 | Superiority | Spurting, 50; oozing, 155; NBVV, 166; clot, 103 | Epinephrine; epinephrine with bipolar/argon plasma coagulation; epinephrine with clips |
| Chan et al,15 2011 | Omeprazole | 40 mg/d IV | 120 | Equivalence | Spurting, 8; oozing, 46; NBVV, 39; clot, 29 | Epinephrine; epinephrine with heater probe; epinephrine with clips |
| Chen et al,16 2012 | Omeprazole | 40 mg/d IV | 120 | Superiority | Spurting,12; oozing, 71; NBVV, 117; clot, 0 | Epinephrine with heater probe |
| Choi et al,17 2009 | Pantoprazole | 40 mg/d IV | 120 | Superiority for pH difference | Spurting, NS; oozing, NS; NBVV, NS; clot, NS | Epinephrine with argon plasma coagulation with or without clips |
| Hsu et al,18 2010 | Pantoprazole | Bolus: 80 mg IV once, then 40 mg IV every 6 h | 560 | Superiority | Spurting,12; oozing, 40; NBVV, 52; clot, 16 | Epinephrine with bipolar; bipolar |
| Hung et al,19 2007 | Pantoprazole | Bolus: 80 mg IV once, then 40 mg IV every 12 h | 320 | Superiority of PPI infusion to no treatment | Spurting, 11; oozing, 52; NBVV, 26; clot, 13 | Epinephrine; epinephrine with heater probe |
| Jang et al,24 2006 | Pantoprazole | 40 mg PO every 12 h | 400 | Uncertain | Spurting,2; oozing, 4; NBVV, 13; clot, 0 | Epinephrine; argon plasma coagulation; clips |
| Javid et al,20 2009 | Omeprazole (n = 36); pantoprazole (n = 35); rabeprazole (n = 35) | Bolus: 80 mg PO once, then 40 mg PO every 12 h; bolus: 80 mg PO once, then 80 mg PO every 12 h; bolus: 80 mg PO once, then 40 mg PO every 12 h | 320, 520, 320 | Noninferiority for pH difference | Spurting, 17; oozing, 20; NBVV, 53; clot, 0 | Epinephrine with heater probe |
| Kim et al,21 2012 | Rabeprazole | 20 mg PO every 12 h | 120 | Noninferiority | Spurting, 10; oozing, 29; NBVV, 44; clot, 23 | Epinephrine; epinephrine with monopolar; epinephrine with clips; epinephrine with monopolar and clips |
| Sung et al,25 2012 | Esomeprazole | 40 mg PO every 12 h | 240 | Superiority | Spurting, NS; oozing, NS; NBVV, NS; clot, NS | NS |
| Ucbilek et al,26 2013 | Pantoprazole | Bolus: 80 mg IV once, then 40 mg IV every 12 h | 320 | Uncertain | Spurting, NS; oozing, NS; NBVV, NS; clot, NS | Epinephrine with sclerotherapy |
| Yamada et al,22 2012 | Pantoprazole | Bolus: 80 mg IV once, then 40 mg IV every 12 h | 240 | Superiority | Spurting, 13; oozing, 3; NBVV, 6; clot, 5 | Epinephrine; epinephrine with bipolar; epinephrine with clips |
| Yüksel et al,23 2008 | Pantoprazole | 40 mg IV every 12 h | 240 | Uncertain | Spurting, 7; oozing, 60; NBVV, 30; clot, 0 | Epinephrine with heater probe |
Abbreviations: IV, intravenous; NBVV, nonbleeding visible vessel; NS, not stated; PO, orally; PPI, proton pump inhibitor.
Primary Outcome
Ten trials reported on recurrent bleeding within 7 days.14,16,17,20–26 Only 1 of the 10 studies26 reported a statistically significant difference, which was in favor of the intermittent PPI bolus regimen. The RR of recurrent rebleeding within 7 days for intermittent vs continuous PPI administration was 0.72 with a 1-sided 95% CI upper boundary of 0.97, without evidence of statistical heterogeneity. The upper boundary of the 1-sided 95% CI of the absolute risk difference was −0.28% (Table 2), which was well below the predefined noninferiority margin of 3%.
Table 2.
Meta-analysis of Intermittent PPI vs Bolus With Continuous-Infusion PPIa
| Outcome | No. of Studies | No. of Patients |
(95% CI, Upper Boundary) | |
|---|---|---|---|---|
| Risk Ratio | Absolute Risk Difference, % | |||
| Recurrent bleeding | ||||
| Within 7 d | 1014,16,17,20–26 | 1346 | 0.72 (0.97) | −2.64 (−0.28) |
| Within 30 d | 1314–26 | 1691 | 0.89 (1.17) | −0.97 (1.49) |
| Within 3 d | 914,16,17,20–24,26 | 1146 | 0.73 (1.02) | −2.36 (0.17) |
| Mortality | 1114–16,18–24,26 | 1453 | 0.64 (1.21) | −0.74 (0.43) |
| Surgery/RI | 1214–24,26 | 1491 | 0.87 (1.49) | −0.30 (1.12) |
| Urgent interventions | 914–20,22,23 | 1283 | 0.95 (1.27) | −0.45 (2.43) |
| Length of hospital stay, d | 814–16,18,21–23,26 | 1204 | −0.26 (0.09)b | |
| Blood transfusion, U | 914–16,18,21–24,26 | 1242 | −0.22 (−0.02)b | |
Abbreviations: PPI, proton pump inhibitor; RI, radiologic intervention.
Statistical heterogeneity was not noted in any analysis.
Data represent the mean difference.
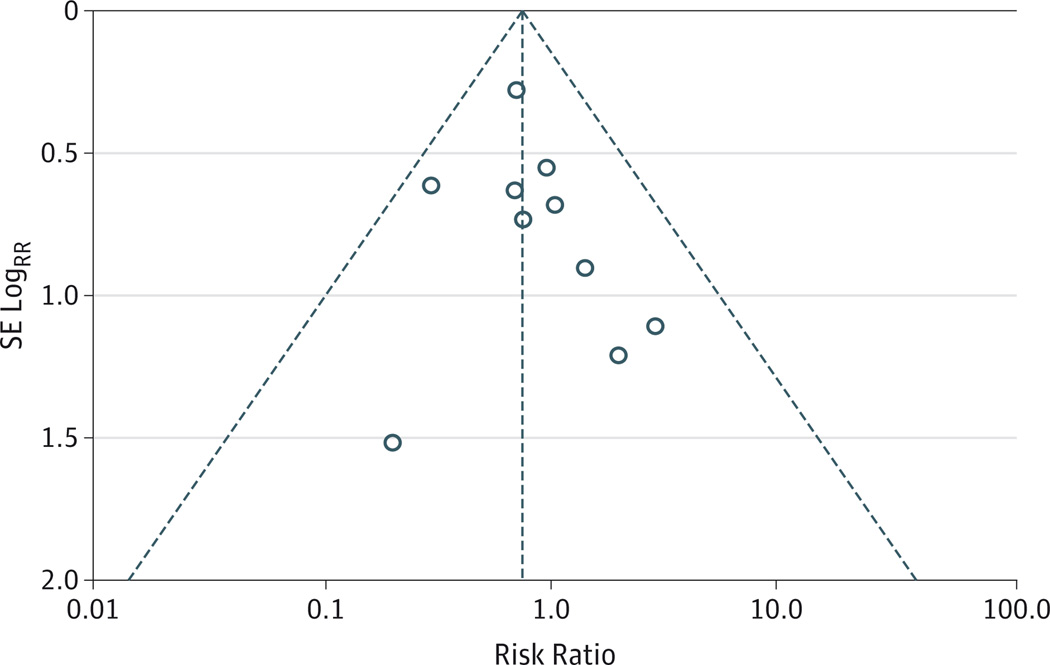
A funnel plot is presented in Figure 2. Visual inspection shows no suggestion of publication bias favoring intermittent therapy. The Egger test indicated no statistically significant reporting bias (P = .49). No significant interaction effect was seen in any of the predefined subgroup analyses of the primary outcome (eTable in the Supplement).
Figure 2. Funnel Plot of Rebleeding Within 7 Days From Individual Studies in the Meta-analysis.
SE indicates standard error.
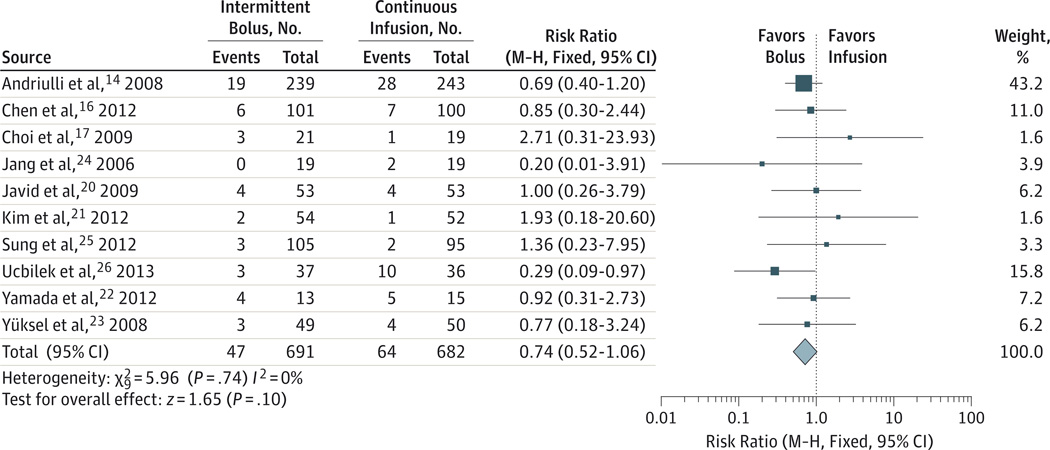
The sensitivity analysis of the primary outcome in the intention-to-treat population showed results that were similar to the primary per-protocol analysis for recurrent bleeding within 7 days: RR, 0.74; 1-sided 95% CI upper boundary, 1.00; and upper boundary of the 1-sided 95% CI of absolute risk difference, 0%, which is well below the noninferiority margin of 3%. A forest plot of the individual studies and pooled analysis with 2-sided 95% CIs for rebleeding within 7 days in the intention-to-treat population is shown in Figure 3.14,16,17,20–26 The results of this standard meta-analysis (RR,0.74; 95% CI,0.52–1.06) and the post hoc sensitivity analysis using trial sequential analysis (RR, 0.74; 95% CI, 0.51–1.07) were virtually identical.
Figure 3. Forest Plot of Studies Comparing Intermittent With Bolus Plus Continuous-Infusion Proton Pump Inhibitors in Patients With High-Risk Bleeding Ulcers.
The outcome examined was rebleeding within 7 days in the intention-to-treat population. M-H indicates Mantel-Haenszel.
Additional Outcomes
Nine trials14,16,17,20–24,26 reported recurrent bleeding at 3 days. One26 of the 9 studies showed a significant difference in favor of the intermittent regimen. Noninferiority for intermittent vs bolus plus continuous-infusion PPI therapy was also seen for rebleeding at 3 days: RR, 0.73; 1-sided 95% CI upper boundary, 1.02; and upper boundary of the 1-sided 95% CI of the absolute risk difference, 0.17% (Table 2). Thirteen trials reported recurrent bleeding within 30 days. Two of the 13 studies reported a significant difference (1 in favor of the bolus plus continuous-infusion PPI regimen15 and the other in favor of the intermittent PPI regimen26), and again noninferiority was demonstrated for intermittent administration of PPIs: RR,0.89; 1-sided 95% CI upper boundary, 1.17; and upper boundary of the 1-sided 95% CI of the absolute risk difference, 1.49% (Table 2). Similar findings were seen for mortality, surgery/radiologic intervention, urgent intervention, red blood cell transfusions, and hospital length of stay (Table 2). All RRs were less than 1 and mean differences were less than 0, indicating that no summary estimate showed an increased risk with intermittent therapy. Forest plots of individual studies and meta-analysis with 2-sided 95% CIs for rebleeding within 3 and 30 days in the intention-to-treat population are shown in eFigure 3 and eFigure 4 in the Supplement.
Discussion
Our systematic review and meta-analysis in patients with bleeding ulcers and high-risk endoscopic findings establish that intermittent PPI therapy is noninferior to the currently recommended regimen of intravenous bolus plus continuous infusion of an intravenous PPI for 3 days. The upper boundary of the 95% CI for the absolute risk difference between intermittent and continuous PPI therapy was −0.28% for our primary outcome of rebleeding within 7 days, indicating that there is no increase in recurrent bleeding with intermittent vs continuous PPI therapy.
The upper boundaries of the 95% CI for the absolute risk differences for secondary outcomes of rebleeding within 3 days (0.17%) and 30 days (1.49%) also were well below our predefined noninferiority margin of 3%. We believe that these very small potential differences would be widely accepted by clinicians as indicative of noninferiority. Furthermore, the treatment effects were consistent across a variety of predefined subgroup analyses and for the predefined sensitivity analysis of the primary outcome, as well as across other predefined clinical outcomes, such as red cell transfusion, hospital stay, need for urgent intervention, and mortality.
The primary objective of our meta-analysis was to assess noninferiority of intermittent PPI therapy vs bolus plus continuous-infusion PPI therapy. The finding that the upper boundary of the 1-sided 95% CI of RR was less than 1.0 in the primary analysis might be taken by some to suggest that intermittent PPI therapy is perhaps marginally superior to the bolus plus continuous PPI infusion therapy. However, this is not a correct interpretation of our findings and is not supported by the results of the standard analysis used to assess for superiority, which was determination of RR with 2-sided 95% CIs in the intention-to-treat population (RR, 0.74; 95% CI, 0.52–1.06).
Our review does not allow us to determine the reason that the efficacy of intermittent administration of a PPI is similar to a continuous infusion in patients with bleeding ulcers. One possible explanation is that high-dose intermittent PPI therapy achieves an intragastric pH similar to that attained with continuous infusion of PPIs, falling in the range of 6 to 7. Among the studies in our systematic review that assessed intragastric pH, the proportion of time that the pH was greater than 6 was virtually identical at approximately 100% for intermittent (oral 80-mg bolus and 40–80 mg every 12 hours) and continuous infusion of PPIs in one study,20 not significantly different for intermittent (intravenous 80-mg bolus and 40 mg every 12 hours) vs continuous PPI infusion in another study (49% vs 59%),19 and significantly lower with intermittent (intravenous 40-mg bolus every 24 hours) vs continuous infusion in a third trial (39% vs 71%)17; the generalizability of these studies, all performed in Asia, to patients in Western countries is uncertain. An alternative explanation for the similar efficacy of intermittent PPI administration is that an intragastric pH above 4 to 5 may suffice to prevent clot lysis.5,27
Our ability to determine the most appropriate intermittent PPI regimen is limited by the variation in intermittent PPI regimens used in the studies included in our systematic review. A variety of dosing schedules and total doses were used, different PPIs were given, and both oral and intravenous routes of administration were used. Increasing the frequency of administration or the dose of PPIs increases their antisecretory effect,6 although our subgroup analyses did not document that more frequent or higher doses improved the treatment effect. Furthermore, oral administration provides an antisecretory effect comparable to that of equivalent doses of intravenous PPIs.6 Subgroup analysis did not reveal significant heterogeneity between the results for oral and intravenous intermittent PPI therapy, although the 95% CI for the intermittent oral PPI vs continuous-infusion PPI comparison was wide. Given the pharmacodynamic profile of PPIs, we would favor intermittent, high-dose PPIs given at least twice daily, using oral PPIs in patients able to tolerate oral medications. Nevertheless, oral PPI therapy should be studied for noninferiority to intravenous PPI therapy to provide direct evidence supporting the use of the oral dosage form.
Another potential limitation of our analysis relates to the variability in endoscopic therapies used across studies and often within studies. Differing endoscopic therapies may have achieved different results for the primary outcome of rebleeding and therefore theoretically could confound the results. However, where reported, the proportion of patients who received the same endoscopic therapy was comparable in both study arms of the individual studies, and results for comparisons of rebleeding rates for intermittent vs continuous PPI regimens in patients receiving the same endoscopic therapy were similar to the results in the full population. In addition, we were unable to assess whether the results might vary based on risk factors, such as the stigmata of hemorrhage (eg, active bleeding vs adherent clot).
Studies in this systematic review were of variable quality, and many had potential risks of bias related to allocation concealment and blinding. As is common in many reviews, the included studies frequently failed to provide the detail required to be considered low risk for allocation concealment by Cochrane methods,13 and 12 of the 13 studies were considered to have unclear risk of bias in this domain. Perhaps more important, 8 of the 13 studies were not blinded. However, subgroup analysis showed no evidence of significant heterogeneity related to risk of bias, with no suggestion of lower efficacy for intermittent therapy in higher-quality studies (eTable in the Supplement).
Although a methodologically sound randomized clinical trial would be preferred to definitively test our hypothesis, given the relatively low rate of recurrent bleeding in patients receiving endoscopic therapy and continuous-infusion PPI therapy, such a study would be very large and likely impractical to undertake. For example, assuming that the risk of rebleeding within 7 days with the continuous PPI infusion is 9.4%(based on our pooled data), a sample size of 2342 patients would be required to be 80% certain that the intermittent regimen is not worse than the infusion regimen by 3%.
Conclusions
Intermittent PPI regimens are comparable to continuous PPI infusion regimens in patients with bleeding ulcers and high-risk endoscopic findings. Given the greater ease of use and lower cost and resource utilization, intermittent PPI therapy should be the regimen of choice after endoscopic therapy in such patients. Current national and international guidelines2,3 should be revised to incorporate this new information and recommend intermittent PPI therapy.
Supplementary Material
Acknowledgments
Dr Laine reported serving on the data safety monitoring board for a study of a nongastrointestinal investigational drug made by Eisai, which is a manufacturer of PPIs.
Funding/Support: The study was funded by grant T32 DK007017 from the National Institutes of Health (Dr Sachar).
Role of the Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Sachar and Laine had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sachar, Laine.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Sachar, Laine.
Critical revision of the manuscript for important intellectual content: Sachar, Vaidya.
Statistical analysis: All authors.
Administrative, technical, or material support: Laine.
Study supervision: Laine.
Conflict of Interest Disclosures: No other disclosures are reported.
Previous Presentation: This study was presented as an abstract at Digestive Disease Week; May 4, 2014; Chicago, Illinois.
Contributor Information
Hamita Sachar, Section of Digestive Diseases, Department of Medicine, Yale School of Medicine, Yale University, New Haven, Connecticut.
Keta Vaidya, Section of Digestive Diseases, Department of Medicine, Yale School of Medicine, Yale University, New Haven, Connecticut.
Loren Laine, Section of Digestive Diseases, Department of Medicine, Yale School of Medicine, Yale University, New Haven, Connecticut; Section of Digestive Diseases, Department of Medicine, Veterans, Affairs Connecticut Healthcare, System, West Haven.
REFERENCES
- 1.Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107(8):1190–1196. doi: 10.1038/ajg.2012.168. [DOI] [PubMed] [Google Scholar]
- 2.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–361. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 3.Barkun AN, Bardou M, Kuipers EJ, et al. International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 4.Chaimoff C, Creter D, Djaldetti M. The effect of pH on platelet and coagulation factor activities. Am J Surg. 1978;136(2):257–259. doi: 10.1016/0002-9610(78)90241-6. [DOI] [PubMed] [Google Scholar]
- 5.Green FW, Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation: a possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74(1):38–43. [PubMed] [Google Scholar]
- 6.Freston JW, Pilmer BL, Chiu YL, et al. Evaluation of the pharmacokinetics and pharmacodynamics of intravenous lansoprazole. Aliment Pharmacol Ther. 2004;19(10):1111–1122. doi: 10.1111/j.1365-2036.2004.01942.x. [DOI] [PubMed] [Google Scholar]
- 7.Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7(1):33–47. doi: 10.1016/j.cgh.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Neumann I, Letelier LM, Rada G, et al. Comparison of different regimens of proton pump inhibitors for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2013;6:CD007999. doi: 10.1002/14651858.CD007999.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang CH, Ma MH, Chou HC, et al. High-dose vs non–high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(9):751–758. doi: 10.1001/archinternmed.2010.100. [DOI] [PubMed] [Google Scholar]
- 10.Tsoi KKF, Hirai HW, Sung JJY. Meta-analysis: comparison of oral vs intravenous proton pump inhibitors in patients with peptic ulcer bleeding. Aliment Pharmacol Ther. 2013;38(7):721–728. doi: 10.1111/apt.12441. [DOI] [PubMed] [Google Scholar]
- 11.Wu LC, Cao YF, Huang JH, Liao C, Gao F. High-dose vs low-dose proton pump inhibitors for upper gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(20):2558–2565. doi: 10.3748/wjg.v16.i20.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laine L, Spiegel B, Rostom A, et al. Methodology for randomized trials of patients with nonvariceal upper gastrointestinal bleeding: recommendations from an international consensus conference. Am J Gastroenterol. 2010;105(3):540–550. doi: 10.1038/ajg.2009.702. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S. Cochrane Handbook for systematic reviews of interventions, version 5.1.0. [Accessed July 17, 2014]; (updated March 2011). http://handbook.cochrane.org. [Google Scholar]
- 14.Andriulli A, Loperfido S, Focareta R, et al. High- versus low-dose proton pump inhibitors after endoscopic hemostasis in patients with peptic ulcer bleeding: a multicentre, randomized study. Am J Gastroenterol. 2008;103(12):3011–3018. doi: 10.1111/j.1572-0241.2008.02149.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan WH, Khin LW, Chung YF, Goh YC, Ong HS, Wong WK. Randomized controlled trial of standard versus high-dose intravenous omeprazole after endoscopic therapy in high-risk patients with acute peptic ulcer bleeding. Br J Surg. 2011;98(5):640–644. doi: 10.1002/bjs.7420. [DOI] [PubMed] [Google Scholar]
- 16.Chen CC, Lee JY, Fang YJ, et al. Randomised clinical trial: high-dose vs standard-dose proton pump inhibitors for the prevention of recurrent haemorrhage after combined endoscopic haemostasis of bleeding peptic ulcers. Aliment Pharmacol Ther. 2012;35(8):894–903. doi: 10.1111/j.1365-2036.2012.05047.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi KD, Kim N, Jang IJ, et al. Optimal dose of intravenous pantoprazole in patients with peptic ulcer bleeding requiring endoscopic hemostasis in Korea. J Gastroenterol Hepatol. 2009;24(10):1617–1624. doi: 10.1111/j.1440-1746.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsu YC, Perng CL, Yang TH, et al. A randomized controlled trial comparing two different dosages of infusional pantoprazole in peptic ulcer bleeding. Br J Clin Pharmacol. 2010;69(3):245–251. doi: 10.1111/j.1365-2125.2009.03575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung WK, Li VK, Chung CK, et al. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77(8):677–681. doi: 10.1111/j.1445-2197.2007.04185.x. [DOI] [PubMed] [Google Scholar]
- 20.Javid G, Zargar SA, U-Saif R, et al. Comparison of p.o. or i.v. proton pump inhibitors on 72-h intragastric pH in bleeding peptic ulcer. J Gastroenterol Hepatol. 2009;24(7):1236–1243. doi: 10.1111/j.1440-1746.2009.05900.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim HK, Kim JS, Choi SO, et al. Effect of high-dose oral rabeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. Gastroenterol Res Pract. 2012;2012:317125. doi: 10.1155/2012/317125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada S, Wongwanakul P. Randomized controlled trial of high dose bolus versus continuous intravenous infusion pantoprazole as an adjunct therapy to therapeutic endoscopy in massive bleeding peptic ulcer. J Med Assoc Thai. 2012;95(3):349–357. [PubMed] [Google Scholar]
- 23.Yüksel I, Ataseven H, Köklü S, et al. Intermittent versus continuous pantoprazole infusion in peptic ulcer bleeding: a prospective randomized study. Digestion. 2008;78(1):39–43. doi: 10.1159/000158227. [DOI] [PubMed] [Google Scholar]
- 24.Jang JY, Joo KR, Hwangho Y, et al. A comparison of the effect of high-dose oral and intravenous proton pump inhibitor on the prevention of rebleeding after endoscopic treatment of bleeding peptic ulcers. Korean J Gastrointest Endosc. 2006;33(1):6–11. [Google Scholar]
- 25.Sung JJ, Suen BY, Lau J, et al. Effects of intravenous and oral esomeprazole in prevention of recurrent bleeding from peptic ulcers after endoscopic therapy [abstract] Gastroenterology. 2012;142(suppl 1):S192–S193. doi: 10.1038/ajg.2014.105. [DOI] [PubMed] [Google Scholar]
- 26.Ucbilek E, Sezgin O, Altintas E. Low dose bolus pantoprazole following successful endoscopic treatment for acute peptic ulcer bleeding is effective: a randomized, prospective, double blind, double dummy pilot study [abstract] Gastroenterology. 2013;144(suppl 1):S506. [Google Scholar]
- 27.Patchett SE, Enright H, Afdhal N, O’Connell W, O’Donoghue DP. Clot lysis by gastric juice: an in vitro study. Gut. 1989;30(12):1704–1707. doi: 10.1136/gut.30.12.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.