Abstract
The incidence of Kaposi’s Sarcoma (KS) is high in South Africa but the impact of antiretroviral therapy (ART) is not well defined. We examined incidence and survival of KS in HIV-infected patients enrolled in South African ART programs. We analyzed data of three ART programs: Khayelitsha township and Tygerberg Hospital programs in Cape Town and Themba Lethu program in Johannesburg. We included patients aged >16 years. ART was defined as a regimen of at least three drugs. We estimated incidence rates of KS for patients on ART and not on ART. We calculated Cox models adjusted for age, sex and time-updated CD4 cell counts and HIV-1 RNA. 18,254 patients (median age 34.5 years, 64% female, median CD4 cell count at enrolment 105 cells/μL) were included. During 37,488 person-years follow-up 162 patients developed KS. The incidence was 1,682/100,000 person-years (95% confidence interval [CI] 1,406–2,011) among patients not receiving ART and 138/100,000 person-years (95% CI 102–187) among patients on ART. The adjusted hazard ratio comparing time on ART with time not on ART was 0.19 (95% CI 0.13–0.28). Low CD4 cell counts (time-updated) and male sex were also associated with KS. Estimated survival of KS patients at one year was 72.2% (95% CI 64.9–80.2) and higher in men than in women. The incidence of KS is substantially lower on ART than not on ART. Timely initiation of ART is essential to preventing KS and KS-associated morbidity and mortality in South Africa and other regions in Africa with a high burden of HIV.
Keywords: Kaposi Sarcoma incidence, HIV/AIDS, epidemiology, South Africa, antiretroviral therapy
Introduction
With an estimated 5.4 million HIV-infected people in 2011, South Africa is the country with the largest number of people living with HIV in the world.1 About 18% of the adult general population and 30% of pregnant women in antenatal care clinics are infected with HIV.1 The prevalence of infection with Kaposi’s Sarcoma-associated herpesvirus (human herpesvirus 8, HHV-8) is estimated to be between 30 and 50%.2, 3 Infection with HHV-8 is a necessary, but not sufficient, cause of Kaposi’s Sarcoma (KS).4 Immunodeficiency induced by HIV-infection substantially increases the risk of KS 5, 6 and since the advent of the HIV/AIDS epidemic the incidence of KS has increased substantially in South Africa. KS is now the most common cancer with a high morbidity and mortality in HIV-infected South Africans.7 In addition, treatment for KS requires specialized services.8
In high-income countries a decline in the incidence of new AIDS-defining events became evident soon after the introduction of highly active combination antiretroviral combination therapy (ART), and KS was among a group of diseases showing the most pronounced reductions.9, 10 In the Swiss HIV Cohort Study, for example, after the introduction of potent ART (1997 to 1998) the incidence of KS declined by 92% from before the introduction of combination ART (1992 to 1994).11
Since 2004, ART has been scaled up in sub-Saharan Africa with the support of the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), the Global Fund, the World Health Organization (WHO), non-governmental organizations and other agencies.12, 13 WHO estimates that in low-income and middle-income countries, 47% of adults and children eligible for ART received therapy at the end of 2010, compared with only 2% in 2002.14 In South Africa, the number of people receiving ART reached 1.4 million in 2010, which corresponds to an estimated coverage based on 2010 WHO guidelines of 55%,14 making it the largest national ART program worldwide.15
While a decline in the incidence of KS in the ART era has been well documented in resource-rich settings, data on the incidence and prognosis of KS in the era of ART are scarce for resource-limited settings.16 We examined incidence rates, survival and the impact of ART on the development of KS in a large cohort of HIV-infected patients followed up in South Africa.
Materials and methods
The International epidemiological Databases to Evaluate AIDS
The International epidemiological Databases to Evaluate AIDS (IeDEA) is a research consortium established in 2006 which includes four regional networks in sub-Saharan Africa that aim to inform the scale-up of ART through clinical and epidemiological research. The African regions of IeDEA have been described in detail elsewhere.17 The Southern African region (IeDEA-SA, www.iedea-sa.org) includes ART programs located in seven countries (Botswana, Lesotho, Malawi, Republic of South Africa, Zambia, Mozambique, Zimbabwe). We restricted the current analysis to cohorts from South Africa that prospectively collect information on incident KS in adults and systematically monitor HIV-1 RNA (viral load): i) the Khayelitsha township ART program located in Cape Town;18 ii) the ART program at Tygerberg Hospital, a tertiary hospital located in Parow Cape Town;19 and iii) the Themba Lethu Clinic ART program in Johannesburg.20 All three programs provide care following the guidelines of the South African National Department of Health21 and have received local approval from ethics committees or institutional review boards for use of the data. Data are collected using standardized methods and are transferred to the coordinating centers at the School of Public Health and Family Medicine, University of Cape Town, South Africa and the Institute of Social and Preventive Medicine, University of Bern, Switzerland.
Inclusion criteria and definitions
We included all ART-naïve adult patients (aged >16 years) who enrolled into a cohort in 2004 or thereafter. 2004 was chosen as cut off because it corresponds to the era of national ART rollout in South Africa. All patients had to be under follow-up for at least 30 days. Patients with a follow-up time of less than 30 days were excluded from the analysis. Prevalent cases, i.e. patients who were diagnosed with KS before or within the first 30 days of follow-up, were excluded. Follow-up time was classified as ART exposed or unexposed, see next paragraph. However, starting ART was not a requirement for inclusion into the analysis.
CD4 cell count and plasma HIV-1 RNA at enrolment were defined as the measurement closest to the first visit date, within a window of 180 days before and 30 days after enrolment. The CD4 cell count at start of ART was defined as the count closest to the date of starting ART, within the same time window. WHO clinical stage was recorded at and after the start of ART but not at enrolment into the cohort. We defined ART as a regimen of at least three antiretroviral drugs from at least two drug classes, including nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors and protease inhibitors. The data were merged on February 28th 2011 and the median date of the last follow-up was June 22nd 2009 (IQR November 29th 2007 to March 17th 2010).
Statistical analyses
Incidence rates of KS were calculated by dividing the number of patients developing KS by the number of person-years at risk. In patients not on ART we measured time from the date of enrolment plus 30 days to the earliest of: date of diagnosis, start of ART or the last follow-up visit. In patients on ART we measured time from the start of ART plus 30 days to the date of the KS diagnosis or the last follow-up visit. For the purpose of a sensitivity analysis we right censored follow-up time at 2.5 years. In patients who were ART naïve at enrolment and started ART during observation we split the observation time at start of ART plus 30 days so that these patients contributed observation time both to the not on ART and the on ART analysis. We used an intent-to-continue-treatment approach and ignored subsequent changes to ART regimens, including treatment interruptions and terminations. In analyses of survival in patients with KS we measured time from the date of diagnosis to the date of death or the date the patient was last known to be alive. The vital status of KS patients with civil identification numbers was checked with the most recent data available in the civil registry of the Department of Home Affairs in November 2011, details for this procedure are reported elsewhere.12, 22 Previous studies have estimated that about 90% of all deaths in adults in South Africa are captured in the civil registry of the Department of Home Affairs.23, 24
We calculated crude incidence rate ratios, Kaplan-Meier survival curves and examined risk factors for incident KS in Cox proportional hazard models and Poisson regression stratified by cohort. Cox models for the KS incidence analyses were adjusted for time-updated ART (model 1) and additionally for age and sex (model 2), and age, sex and time-updated CD4 cell counts (model 3). We conducted similar models including HIV-1 RNA viral loads. In sensitivity analyses we accounted for heterogeneity between cohorts using frailty models.25 Age and CD4 cell counts at baseline refer to start of observation, i.e. enrolment into cohort, plus 30 days in patients not on ART and start of ART plus 30 days in patients on ART. In Kaplan-Meier curves for incidence analyses time zero was set at start of observation, i.e. enrolment into cohort, plus 30 days in patients not on ART and start of ART plus 30 days in patients on ART. Cox models for the survival analysis of patients with KS were adjusted for gender, age and CD4 cell counts at time of KS diagnosis, calendar year of KS diagnosis (2004 to 2006 versus 2007 to 2009), and ART status at the time of KS diagnosis (defined as ART treatment duration of more of less than 30 days before KS diagnosis). The effect of current ART on KS survival was not assessed because all patients who had been ART naïve at the time of KS diagnosis started ART shortly after the KS diagnosis. Patients with missing covariate information were excluded from all Cox models.
Results are presented as incidence rates per 100,000 person-years, incidence rate ratios, Kaplan-Meier estimates of the cumulative incidence of KS and of death in patients with KS and crude and adjusted hazard ratios (HRs), with 95% confidence intervals (95% CI). Statistical analyses were done in R (version 2.13.1, The R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of cohorts and patients
The three cohorts included 27,975 patients of whom 18,254 patients were eligible for this analysis: 9,721 patients were excluded for the reasons detailed in supplementary Figure S1. Compared to excluded patients those included were more likely to present with CD4 counts below 50 cells/μL at enrolment (27.3% versus 19.6%) and at start of ART (29.3% versus 18.7%) but were similar in terms of age and gender. Of all KS patients excluded 48% (122/255) were considered prevalent cases. Included patients were mostly female (64%) and the median age at enrolment was 34.5 years (Table 1). The median CD4 cell count among included patients at enrolment was 105 cells/μL; in patients developing KS not on ART the median CD4 count at enrolment was 123 cells/μL; whereas in patients developing KS after starting ART the median CD4 cell count was 52 cells/μL at start ART. The CD4 count at enrolment was missing in 3,655 of 18,254 patients (20%) and missing at start of ART in 1,407 of 17,516 patients (8%). There were a total of 75,237 CD4 and 60,397 HIV-1 RNA measurements, on average 4.1 CD4 and 3.3 HIV-1 RNA measurements per patient. In patients developing KS there were 234 CD4 count and 72 viral load measurements before the diagnosis of KS for an average of 1.4 and 0.4 measurements respectively. Median follow-up time was 660 days (IQR 304 to 1,168 days).
Table 1.
Characteristics of HIV-infected patients developing and not developing Kaposi sarcoma in South Africa.
| Patients developing KS
|
Patients not developing KS
|
|||
|---|---|---|---|---|
| Not on ART | On ART | |||
| All patients | 120 | 42 | 18,092 | |
|
| ||||
| Cohort | Khayelitsha | 25 (20.8%) | 18 (42.9%) | 5,609 (31.0%) |
| Themba Lethu | 88 (73.3%) | 24 (57.1%) | 10,272 (56.8%) | |
| Tygerberg | 7 (5.8%) | 0 (0.0%) | 2,211 (12.2%) | |
|
| ||||
| Gender | Female | 56 (46.7%) | 21 (50.0%) | 11,675 (64.5%) |
| Male | 64 (53.3%) | 21 (50.0%) | 6,417 (35.5%) | |
|
| ||||
| Age at baseline [years] | Median, IQR | 34.8 (29.7 – 41.4) | 37.1 (34.1 – 42.6) | 34.4 (29.4 – 40.9) |
| 16 – 29 | 32 (26.7%) | 5 (11.9%) | 5,073 (28.0%) | |
| 30 – 39 | 54 (45.0%) | 23 (54.8%) | 7,985 (44.1%) | |
| 40 – 49 | 22 (18.3%) | 9 (21.4%) | 3,721 (20.6%) | |
| ≥ 50 | 12 (10.0%) | 5 (11.9%) | 1,313 (7.3%) | |
|
| ||||
| Calendar year of enrolment | 2004 – 2006 | 77 (64.2%) | 23 (54.8%) | 10,652 (58.9%) |
| 2007 – 2010 | 43 (35.8%) | 19 (45.2%) | 7,440 (41.1%) | |
|
| ||||
| WHO stage at start of ART | I/II | - | 11 (26.2%) | 7,508 (42.9%) |
| III/IV | - | 31 (73.8%) | 9,800 (55.9%) | |
| Missing | - | 0 | 208 (1.2%) | |
|
| ||||
| CD4 count at baseline [cells/μL] | Median, IQR | 123 (49 – 197) | 52 (18 – 93) | 105 (45 – 173) |
| < 50 | 20 (16.7%) | 16 (38.1%) | 3,949 (21.8%) | |
| 50 – 99 | 14 (11.7%) | 12 (28.6%) | 2,977 (16.5%) | |
| 100 – 349 | 35 (29.2%) | 9 (21.4%) | 7,180 (39.7%) | |
| ≥ 350 | 8 (6.7%) | 0 (0.0%) | 379 (2.1%) | |
| Missing | 43 (35.8%) | 5 (11.9%) | 3,607 (19.9%) | |
ART, antiretroviral therapy; KS, Kaposi Sarcoma
Baseline refers to enrolment into the cohort plus 30 days in patients not on ART and to start of ART plus 30 days in patients on ART
Incidence of KS
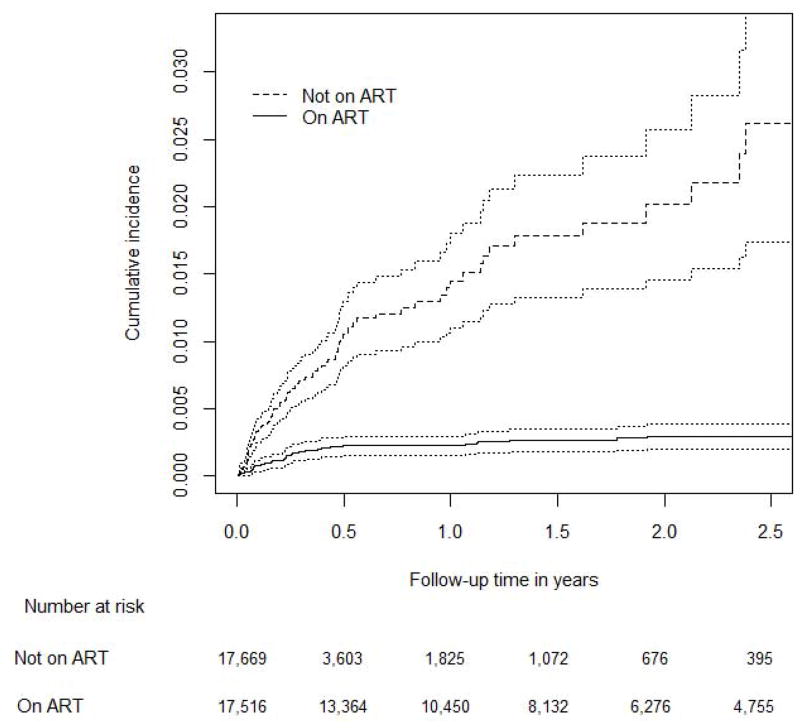
Of the 18,254 persons included in the analysis 17,516 (96%) patients started ART either at enrolment or within a median of 66 days (IQR 35 to 147) days after enrolment. The observation time was 7,136 person-years while not on ART and 30,352 person-years on ART. The longest follow-up time was 5.4 years in patients not on ART and 6.4 years in patients on ART. Forty-two cases of KS were diagnosed while on ART and 120 cases while not on ART (Table 2). The incidence rate per 100,000 person-years was 138 (95% CI 102 to 187) on ART and 1,682 (95% CI 1,406 to 2,011) not on ART. When we censored follow-up time at 2.5 years, the incidence rate of KS in patients not on ART remained similar and increased in patients on ART (171 per 100,000 person-years; 95% CI 126 to 231). In patients not on ART the cumulative incidence of KS 2.5 years after enrolment was 2.61% (95% CI 1.87% to 3.65%) compared to 0.29% (95% CI 0.21% to 0.40%) 2.5 years after starting ART (Figure 1).
Table 2.
Incidence rates of Kaposi Sarcoma per 100,000 person-years before and after starting antiretroviral therapy in South Africa.
| Not on ART
|
On ART
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Person-years at risk | No. of KS cases | Incidence rate | 95% CI | Person-years at risk | No. of KS cases | Incidence rate | 95% CI | ||
| All patients | 7,136 | 120 | 1,682 | (1,406 – 2,011) | 30,352 | 42 | 138 | (102 – 187) | |
|
| |||||||||
| Gender | Female | 4,612 | 56 | 1,214 | (935 – 1,578) | 20,115 | 21 | 104 | (68 – 160) |
| Male | 2,525 | 64 | 2,535 | (1,984 – 3,239) | 10,237 | 21 | 205 | (134 – 315) | |
|
| |||||||||
| Age at baseline [years] | 16 – 29 | 2,168 | 32 | 1,476 | (1,044 – 2,088) | 7,833 | 5 | 64 | (27 – 153) |
| 30 – 39 | 3,054 | 54 | 1,768 | (1,354 – 2,309) | 13,949 | 23 | 165 | (110 – 248) | |
| 40 – 49 | 1,432 | 22 | 1,536 | (1,012 – 2,333) | 6,423 | 9 | 140 | (73 – 269) | |
| ≥ 50 | 483 | 12 | 2,486 | (1,412 – 4,378) | 2,147 | 5 | 233 | (97 – 559) | |
|
| |||||||||
| Calendar year of enrolment | 2004 – 2006 | 5,095 | 77 | 1,511 | (1,209 – 1,889) | 21,087 | 23 | 109 | (72 – 164) |
| 2007 – 2010 | 2,041 | 43 | 2,107 | (1,562 – 2,841) | 9,265 | 19 | 205 | (131 – 321) | |
|
| |||||||||
| WHO stage at start of ART | I/II | - | - | - | - | 13,280 | 11 | 83 | (46 – 150) |
| III/IV | - | - | - | - | 16,699 | 31 | 186 | (131 – 264) | |
| Missing | - | - | - | - | 373 | 0 | 0 | - | |
|
| |||||||||
| CD4 count at baseline [cells/μL] | < 50 | 610 | 20 | 3,281 | (2,117 – 5,086) | 8,497 | 16 | 188 | (115 – 307) |
| 50 – 99 | 648 | 14 | 2,160 | (1,279 – 3,647) | 6,362 | 12 | 189 | (107 – 332) | |
| 100 – 349 | 2,738 | 35 | 1,278 | (918 – 1,780) | 13,255 | 9 | 68 | (35 – 130) | |
| ≥ 350 | 663 | 8 | 1,206 | (603 – 2,412) | 116 | - | - | - | |
| Missing | 2,477 | 43 | 1,736 | (1,288 – 2,341) | 2,122 | 5 | 236 | (98 – 566) | |
ART, antiretroviral therapy; KS, Kaposi Sarcoma; CI, confidence interval; WHO, World Health Organization
Baseline refers to enrolment into the cohort plus 30 days in patients not on ART and to start of ART plus 30 days in patients on ART.
Figure 1.
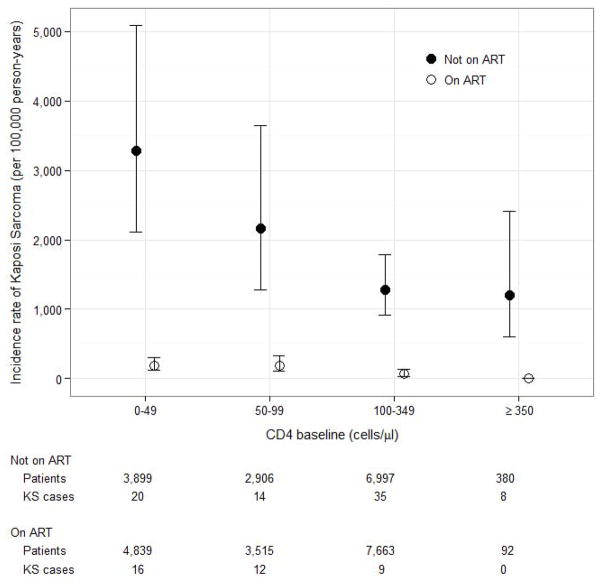
Both on ART and not on ART the incidence of KS was about twice as high in men than in women, increased with age, and was higher in more recent calendar years (Table 2). A lower CD4 cell count at enrolment and an advanced clinical stage were associated with a higher incidence of KS in patients on ART. Figure 2 shows the incidence of KS by CD4 cell counts in patients on ART and not on ART. The risk of developing KS decreases with increasing CD4 cell counts at baseline or start of ART respectively in patients not on ART and on ART. Hazard ratios from univariable Cox models are presented in supplementary Table S1.
Figure 2.
Risk factors for KS
Table 3 presents the results from Cox models treating ART as a time-updated variable. CD4 cell counts were missing for 378 patients and hence these were excluded from the analysis. 17,890 patients (148 with and 17,742 without KS) remained in the analysis set. In univariable analysis (model 1), the HR comparing ART with no ART was 0.22 (95% CI 0.15 to 0.32). This estimate remained unchanged when adding age and gender (model 2) and was similar when adding time-updated CD4 cell count to the model (model 3; HR 0.19, 95% CI 0.13 to 0.28). Compared to patients with CD4 cell counts below 50 cells/μL in patients with CD4 cell counts above 350 cells/μL the risk of developing KS was reduced by 65% (HR 0.35, 95% CI 0.16 to 0.76). There was no evidence for an association of HIV-1 viral loads and the risk of developing KS (supplementary Table S2).
Table 3.
Hazard ratios for developing Kaposi Sarcoma among HIV-infected patients in South Africa from multivariable Cox models.
| Model 1
|
Model 2
|
Model 3
|
||
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Patients included [total/KS cases] | 17,890 / 148 | 17,890 / 148 | 17,890 / 148 | |
|
| ||||
| Time-updated ART | Not on ART | 1 | 1 | 1 |
| On ART | 0.22 (0.15 – 0.32) | 0.22 (0.15 – 0.32) | 0.19 (0.13 – 0.28) | |
|
| ||||
| Gender | Female | - | 1 | 1 |
| Male | - | 1.99 (1.43 – 2.77) | 1.88 (1.35 – 2.62) | |
|
| ||||
| Age at baseline [years] | 16 – 29 | - | 1 | 1 |
| 30 – 39 | - | 1.14 (0.75 – 1.74) | 1.13 (0.75 – 1.73) | |
| 40 – 49 | - | 0.89 (0.53 – 1.49) | 0.89 (0.53 – 1.48) | |
| ≥ 50 | - | 1.13 (0.59 – 2.17) | 1.16 (0.60 – 2.23) | |
|
| ||||
| Time-updated CD4 cell count [cells/μL] | < 50 | - | - | 1 |
| 50 – 99 | - | - | 0.61 (0.37 – 0.99) | |
| 100 – 349 | - | - | 0.46 (0.31 – 0.68) | |
| ≥ 350 | - | - | 0.35 (0.16 – 0.76) | |
ART, antiretroviral therapy; KS, Kaposi Sarcoma. All models were stratified by cohort and include only patients with complete information for ART status, age, gender and CD4 cell counts. Age at baseline refers to age at enrolment into the cohort plus 30 days in patients not on ART and to start of ART plus 30 days in patients on ART.
We adjusted models for time-updated ART (model 1), plus gender and age (model 2), plus time-updated CD4 cell counts (model 3)
Survival
162 patients with incident KS (120 diagnosed before, 42 after starting ART) were included in the survival analysis. Median age at KS diagnosis was 36.2 years (IQR 30.7 to 42.1 years) and about half of the patients were female (48%). Overall, the median CD4 cell count at KS diagnosis was 125 cells/μL (IQR 49 to 206 cells/μL). In patients developing KS before starting ART the median CD4 cell count at diagnosis was 115 cells/μL (IQR 45 to 195 cells/μL) compared to 161 cells/μL (IQR 58 to 260 cells/μL) in patients developing KS on ART (P=0.12). In 107 of 162 (66%) KS patients we obtained additional information on vital status from the civil registry or next of kin. A total of 51 deaths were ascertained. Estimated survival at 1 year was 72.2% (95% CI 64.9 to 80.2). In univariable and multivariable Cox models men were half as likely to die compared to women. Survival was similar in patients receiving or not receiving ART at the time of KS diagnosis (HR 0.83, 95% CI 0.39 to 1.79; adjusted for gender, as well as age, CD4 cell count and calendar year at KS diagnosis). Of note, all patients who were not receiving ART at the time of KS diagnosis started ART within a median of 17 days (IQR 12 to 51 days) after KS diagnosis which precluded an analysis on the effect of current ART status on KS survival. There was little evidence for an influence of age or CD4 cell count at KS diagnosis on the probability of survival (Table S3).
Discussion
In South Africa, where up to 48% of HIV-infected adults are tested positive for HHV-8,2 ART was associated with reductions in the risk of developing KS of 70% to 80%. Other risk factors for KS included male sex and more advanced clinical stage. In time-updated analyses CD4 cell counts increasing above 350 cells /μL provided protection against KS. Survival at one year was 72.2% and similar in patients who had started ART before or shortly after KS diagnosis.
This is one of the first large-scale studies examining the incidence of KS and survival in HIV-infected patients on ART and not on ART in sub-Saharan Africa. We included almost 20,000 patients attending ART programs in three different treatment programs in South Africa. Patients were seen in the clinics and CD4 cell counts and HIV-1 viral loads were measured in regular intervals. The ascertainment of deaths in the cohorts was improved through linking of the clinical records with the national vital registration system. Our study nevertheless has several limitations. Data on the clinical presentation, histology and stage of KS, the incidence of immune reconstitution inflammatory syndromes following ART and details on chemotherapy or radiotherapy were not collected. Patients were not followed from a uniform point in time, such as time of HIV-infection, which limits our ability to directly compare pre-ART (starting at enrolment) and ART (starting at ART) time periods. KS diagnoses were made by the treating physicians. Physicians were not blinded, i.e. they knew the ART treatment status of their patients. This information may have influenced their diagnostic work-up of clinical lesions suspicious of KS. Only three cohorts from South Africa participating in the IeDEA network could be included in the analyses. The other IeDEA cohorts in South Africa were excluded because they did not systematically ascertain KS diagnoses. Similarly, cohorts from other countries participating in IeDEA were excluded because they do not regularly measure HIV-1 viral load. In the IeDEA collaboration, only the ART programs in South Africa and Botswana have access to routine viral load monitoring.17
Semeere et al 16 recently reviewed the impact of ART on the incidence of KS in studies from resource-rich and resource-limited settings published up to 2011. We updated their review by adding studies published since 2012 which reported incidence rates for patients on ART and not on ART (Table 4). Studies reporting KS incidence rates per ART calendar periods, i.e. before or during ART era, were not included in this table. Our results are in line with several previous studies that showed a reduction in the incidence of KS of at least 70%.6, 9, 26, 27 Some cohorts reported higher incidence rates in patients on ART which might be explained by inclusion of KS patients who were diagnosed during the first month of ART.28 Including patients developing KS soon after starting ART may have led to selection bias: patients starting ART will have more advanced disease and are therefore more likely to develop KS, and some may have early stages of KS which remain unnoticed.28 As a result the reduction in incidence associated with ART will be under estimated.28 Other studies included patients from before ART was available 29 or were restricted to the early years of the combination ART era when patients received less effective therapy.30 Conversely, the effect of ART may be overestimated if ascertainment of KS in patients not receiving ART was less complete than in the more closely monitored patients on ART or person time under observation was shortened before starting ART.26, 28, 31
Table 4.
Kaposi Sarcoma incidence rates in patients on antiretroviral therapy and patients not on therapy reported for resource rich and resource limited settings
| Authors | Cohort | Country | Calendar years | No. of patients included | KS incidence per 100,000 person-years
|
% Reduction in KS incidence* | |
|---|---|---|---|---|---|---|---|
| Not on ART | On ART | ||||||
|
Resource rich countries
| |||||||
| Carrieri 200326 | DMI-2 | France | 1996 – 2001 | 3,510 | 640 | 122 | 78% |
| Clifford 2005 49 | SHCS | Switzerland | 1985 – 2002 | 7,304 | 1,229 | 109 | 91% |
| Franceschi 200827 | SHCS | Switzerland | 1984 – 2006 | 12,638 | 1,500 | 130 | 88% |
| Ledergerber 19999 | SHCS | Switzerland | 1995 – 1999 | 2,410 | 2,020 | 140 | 93% |
| Lodi 201028 ** | CASCADE | Europe, Australia, Canada | 1986 – 2006 | 9,473 | 822 | 358 | 57% |
| Mocroft 200030 | EuroSIDA | Europe, Israel | 1994 – 1999 | >7,300 | 1,800 | 700 | 61% |
| Guiguet 20096 | FHDH | France | 1992 – 2006 | 52,278 | NR | NR | 70% |
| Pipkin 201129 *** | SFAR | USA | 1990 – 2000 | 14,183 | NR | NR | 19% |
| Lacombe 201350 | FHDH | France | 1996 – 2009 | 53,871 | 408 | 237 | 42% |
|
| |||||||
|
Resource limited countries
| |||||||
| Martin 201231 | IeDEA | Uganda | 2008 – 2011 | 7,360 | 1,876 | 201 | 88% |
| IeDEA | Kenya | 2008 – 2011 | 90,664 | 596 | 270 | 50% | |
| Current analysis | IeDEA | South Africa | 2004 – 2010 | 18,254 | 1,682 | 138 | 72% |
Adapted from Semeere et al 2012 16. SHCS, Swiss HIV Cohort Study; DMI-2, longitudinal database of HIV-infected individuals followed at Nice University Hospital, France; CASCADE, Concerted Action of Seroconversion to AIDS and Death in Europe; EuroSIDA, Collection of European cohort studies; FHDH; French Hospital Database on HIV; SFAR, San Francisco AIDS Registry; IeDEA, International Epidemiological Databases to Evaluate AIDS; NA, not available.
Estimates from different studies are adjusted for different variables;
Includes only men who have sex with men;
includes only patients with AIDS.
Our study confirms previous reports from high and low income countries showing an increased risk of developing KS at lower CD4 cell counts.6, 27–29, 31 This was evident in the association with the CD4 count at enrolment in patients not on ART, at start of cART in patients on ART, and in models including time-updated CD4 cell counts which showed a decreased risk of developing KS in patients reaching CD4 cell counts above 350 cells /μL. The CD4 cell count is a time-dependent mediator of the effect of ART and inclusion of time-updated CD4 cell counts into the model would be expected to reduce the effect of ART. Surprisingly, this was not the case in our analysis. Ideally, the effect of ART on the incidence of KS would be investigated using causal modeling; however, the limited number of CD4 cell counts available in our study did not permit this type of analysis. Our study could not confirm an association between HIV-1 viral load and the risk of developing KS observed in previous studies.6, 28 Again, this might be due to the relatively few HIV-1 viral load measurements in patients developing KS. Despite a wealth of viral load data on the entire patient population, in patients developing KS there were only 72 HIV-1 RNA measurements from 35 KS patients.
In our study men were at higher risk of developing KS but at lower risk of death from KS compared to women. These results are in line with previous studies from sub-Saharan Africa which have also shown higher incidence rates in HIV-infected and uninfected men compared to women.32, 33
This gender difference in the KS incidence rate in sub-Saharan Africa is not well understood. In contrast to populations in the US,34 in sub-Saharan Africa the HHV-8 prevalence appears to be similar in HIV-infected men and women. For example, in HIV-infected men and women under care at Themba Lethu clinic in Johannesburg, about 46% of men and 48% of women tested positive for HHV-8.3 It therefore seems unlikely that HHV-8 infection explains the gender difference observed in our study although in the absence of data on HHV-8 infection we cannot exclude it. Survival of KS patients has increased substantially since the advent of ART both in resource-rich and resource-limited countries.18, 28, 35, 36 This might be explained by both wider access to effective ART and earlier diagnosis of KS in less advanced stages among patients under regular follow-up in ART programs. The results of our analysis compare well with survival data in KS patients receiving ART in Khayelitsha, Cape Town18 and Durban36 but also with survival estimates from studies conducted in resource rich countries in the ART era.28, 35 Survival was similar in patients on and not on ART at the time of KS diagnosis. However, all patients who were ART naïve at the time of KS diagnosis started ART within short time thereafter. This may explain why we did not see an impact of ART status at KS diagnosis on survival. Interestingly, our and previous observational studies from Italy37 and South Africa36 have shown that survival in women with KS is lower than in men. There is some evidence that women are more likely to present with systemic symptoms which may point to more aggressive KS or unidentified co-infections.37, 38
In settings with limited access to cancer treatment the prevention of cancer is key to reducing the burden of disease and mortality.39 Our study indicates that ART may prevent a substantial proportion of incident KS cases in HIV-infected patients in low resource settings. Even starting ART at CD4 cell counts well below the currently recommended threshold of 350 CD4 cells/μL (South African ART guidelines) does prevent the development of new KS cases.40 Several mechanisms may contribute to the prevention of KS in patients on ART. ART restores the immune system which in turn improves control over malignant transformed cells.41 Our and other prospective cohort studies have shown that the risk of developing KS decreases with increasing CD4 cell counts.6, 27–29, 31 The preventative effect of ART would probably be even greater when started at higher CD4 counts. Our ability to examine this issue was limited: during the study period the South African guidelines for ART recommended initiation of ART at or below a CD4 cell count of 200 cells/μL (or below 350 CD4 cells/μL in patients with TB and pregnant women) and regardless of the CD4 cell count in patients with WHO stage IV disease and pregnant women with WHO stage III or IV.21 The patients starting ART at higher CD4 counts in our study thus were a small and selected group. Besides immune restoration control of HIV-replication may contribute to the preventive effect of ART. In vitro studies have demonstrated that HIV-1 activates HHV-8 replication and induces tumor genesis.42 Epidemiological studies found no evidence for an association between HIV RNA viral load and HHV-8 seropositivity3, 43 or HHV-8 viremia.44 However, cohort studies in HIV-infected patients have shown that the risk of KS increases with increasing HIV-1 viral load.6, 28 There was some debate on specific antiangiogenic effects of protease inhibitors 45 but this was not confirmed in prospective cohort studies.28, 46
In conclusion, KS among HIV-1 infected patients continues to be of concern in African settings where large numbers of patients who are eligible for ART do not receive it. The incidence of KS in patients on ART observed in our study (138 new cases per 100,000 person-years) is still comparatively high and similar to the incidence of frequent cancers diagnosed in HIV-uninfected men and women in the USA, for example prostate cancer (138 per 100,000 person-years) or breast cancer (123 per 100,000 person-years).47 We conclude that in the absence of effective and affordable vaccines against HHV-848 and HIV, initiation of ART at CD4 ≤ 350 cells/μL irrespective of WHO clinical stage as recommended in the latest South African guidelines,40 is the most promising approach to preventing KS in African settings.
Supplementary Material
Novelty and impact.
We analyzed the data of prospective cohorts treating HIV-infected adult patients in South Africa. Our study shows that antiretroviral therapy (ART) reduces the risk of developing Kaposi Sarcoma (KS) by 80%. We conclude that timely initiation of ART is essential to preventing KS in South Africa and other regions in Africa with a high burden of HIV. This is one of the first studies to confirm the beneficial effects of ART in an African setting.
Acknowledgments
This study was funded by NIAID (grant number U01AI069924) and also supported by NCI (grant number 3U01AI069924-05S2) and the President’s Emergency Plan For AIDS Relief (PEPFAR), the Swiss Bridge Foundation and the Swiss National Foundation (Ambizione-PROSPER PZ00P3_136620_3), and USAID Cooperative Agreement AID 674-A-12-00029. Matthew Fox was supported by the National Institute of Allergy and Infectious Diseases [K01AI083097].
Abbreviations used
- ART
Antiretroviral therapy
- CI
confidence interval
- HR
hazard ratio
- IeDEA
International epidemiological Databases to Evaluate AIDS
- IQR
interquartile range
- KS
Kaposi Sarcoma
- WHO
World Health Organization
References
- 1.Anonymous. Global Aids Response Progress Report 2012. Republic of South Africa. 2012 [Google Scholar]
- 2.Malope BI, MacPhail P, Mbisa G, MacPhail C, Stein L, Ratshikhopha EM, Ndhlovu L, Sitas F, Whitby D. No evidence of sexual transmission of Kaposi’s sarcoma herpes virus in a heterosexual South African population. Aids. 2008;22:519–26. doi: 10.1097/QAD.0b013e3282f46582. [DOI] [PubMed] [Google Scholar]
- 3.Maskew M, Macphail AP, Whitby D, Egger M, Wallis CL, Fox MP. Prevalence and predictors of kaposi sarcoma herpes virus seropositivity: a cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect Agent Cancer. 2011;6:22. doi: 10.1186/1750-9378-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. The New England journal of medicine. 1998;338:948–54. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 5.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ. Cancer risk in people infected with human immunodeficiency virus in the United States. International journal of cancer Journal international du cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 6.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D Clinical Epidemiology Group of the F-ACOc. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. The lancet oncology. 2009;10:1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. The lancet oncology. 2008;9:683–92. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 8.Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, Di Trolio R, De Placido S, Dezube BJ. Management of AIDS-related Kaposi’s sarcoma. The lancet oncology. 2007;8:167–76. doi: 10.1016/S1470-2045(07)70036-0. [DOI] [PubMed] [Google Scholar]
- 9.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, Battegay M, Vernazza P, Bernasconi E, Opravil M, Kaufmann D, Sudre P, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA : the journal of the American Medical Association. 1999;282:2220–6. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 10.International Collaboration on HIV Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92:1823–30. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 11.Ledergerber B, Telenti A, Egger M. Risk of HIV related Kaposi’s sarcoma and non-Hodgkin’s lymphoma with potent antiretroviral therapy: prospective cohort study. Swiss HIV Cohort Study. Bmj. 1999;319:23–4. doi: 10.1136/bmj.319.7201.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, Ford N, Knight L, Osler M, Myers J, Goemaere E, Coetzee D, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. Aids. 2010;24:563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 13.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, Sutherland D, Vitoria M, Guerma T, De Cock K. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organziation, UNAIDS, UNICEF. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. WHO; Geneva: 2011. [Google Scholar]
- 15. [Accessed April 28th 2014];Republic of South Africa Country Progress Report on the Declaration of Commitment on HIV/AIDS 2010 Report. Reporting period January 2008–December 2009. 2010 available at: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2010countriessouthafrica_2010_country_progress_report_en.pdf.
- 16.Semeere AS, Busakhala N, Martin JN. Impact of antiretroviral therapy on the incidence of Kaposi’s sarcoma in resource-rich and resource-limited settings. Curr Opin Oncol. 2012;24:522–30. doi: 10.1097/CCO.0b013e328355e14b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, Hartwell T, Graber C, Chi BH, Boulle A, Dabis F, Wools-Kaloustian K. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. International journal of epidemiology. 2012;41:1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu KM, Mahlangeni G, Swannet S, Ford NP, Boulle A, Van Cutsem G. AIDS-associated Kaposi’s sarcoma is linked to advanced disease and high mortality in a primary care HIV programme in South Africa. J Int AIDS Soc. 2010;13:23. doi: 10.1186/1758-2652-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eshun-Wilson I, Plas HV, Prozesky HW, Zeier MD, Nachega J, Taljaard JJ. Combined antiretroviral treatment initiation during hospitalization: outcomes in South African adults. Journal of acquired immune deficiency syndromes. 2009;51:105–6. doi: 10.1097/QAI.0b013e3181963cd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MP, Maskew M, Macphail AP, Long L, Brennan AT, Westreich D, Macleod WB, Majuba P, Sanne IM. Cohort Profile: The Themba Lethu Clinical Cohort, Johannesburg, South Africa. International journal of epidemiology. 2012 doi: 10.1093/ije/dys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department of Health, Republic of South Africa. [Accessed April 28th 2014];Clinical guidelines for the management of HIV and AIDS in adults and adolescents. National Department of Health. 2010 Available at: http://www.sahivsoc.org/upload/documents/Clinical_Guidelines_for_the_Management_of_HIV_AIDS_in_Adults_Adolescents_2010.pdf.
- 22.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Tropical medicine & international health : TM & IH. 2010;15:405–13. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorrington RE, Bourne D, Bradshaw D, Laubscher R, Timaeus IM. The impact of HIV/AIDS on adult mortality in South Africa Tygerberg. Cape Town, South Africa: 2001. Available from: http://www.mrc.ac.za/bod/complete.pdf. [Google Scholar]
- 24.Joubert J, Rao C, Bradshaw D, Dorrington RE, Vos T, Lopez AD. Characteristics, availability and uses of vital registration and other mortality data sources in post-democracy South Africa. Global health action. 2012;5:1–19. doi: 10.3402/gha.v5i0.19263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therneau TMGPM. Modeling Survival Data: Extending the Cox Model. Springer; 2000. [Google Scholar]
- 26.Carrieri MP, Pradier C, Piselli P, Piche M, Rosenthal E, Heudier P, Durant J, Serraino D. Reduced incidence of Kaposi’s sarcoma and of systemic non-hodgkin’s lymphoma in HIV-infected individuals treated with highly active antiretroviral therapy. International journal of cancer Journal international du cancer. 2003;103:142–4. doi: 10.1002/ijc.10790. [DOI] [PubMed] [Google Scholar]
- 27.Franceschi S, Maso LD, Rickenbach M, Polesel J, Hirschel B, Cavassini M, Bordoni A, Elzi L, Ess S, Jundt G, Mueller N, Clifford GM. Kaposi sarcoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. British journal of cancer. 2008;99:800–4. doi: 10.1038/sj.bjc.6604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K, Collaboration C. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst. 2010;102:784–92. doi: 10.1093/jnci/djq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pipkin S, Scheer S, Okeigwe I, Schwarcz S, Harris DH, Hessol NA. The effect of HAART and calendar period on Kaposi’s sarcoma and non-Hodgkin lymphoma: results of a match between an AIDS and cancer registry. Aids. 2011;25:463–71. doi: 10.1097/QAD.0b013e32834344e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, Chiesi A, Phillips AN, Kirk O, Lundgren JD. AIDS across Europe, 1994–98: the EuroSIDA study. Lancet. 2000;356:291–6. doi: 10.1016/s0140-6736(00)02504-6. [DOI] [PubMed] [Google Scholar]
- 31.Martin J, Wenger M, Busakhala N, et al. Prospective evaluation of the impact of potent antiretroviral therapy on the incidence of Kaposi’s Sarcoma in East Africa: findings from the International Epidemiologic Databases to Evaluate AIDS (IeDEA) Consortium. Infectious Agents and Cancer. 2012;7(Suppl 1):O19. [Google Scholar]
- 32.Somdyala NI, Bradshaw D, Gelderblom WC, Parkin DM. Cancer incidence in a rural population of South Africa, 1998–2002. International journal of cancer Journal international du cancer. 2010;127:2420–9. doi: 10.1002/ijc.25246. [DOI] [PubMed] [Google Scholar]
- 33.Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. International journal of cancer Journal international du cancer. 2013;133:721–9. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 34.Mbulaiteye SM, Atkinson JO, Whitby D, Wohl DA, Gallant JE, Royal S, Goedert JJ, Rabkin CS. Risk factors for human herpesvirus 8 seropositivity in the AIDS Cancer Cohort Study. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2006;35:442–9. doi: 10.1016/j.jcv.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Biggar RJ, Engels EA, Ly S, Kahn A, Schymura MJ, Sackoff J, Virgo P, Pfeiffer RM. Survival after cancer diagnosis in persons with AIDS. Journal of acquired immune deficiency syndromes. 2005;39:293–9. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 36.Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, Aboobaker J, Coovadia HM. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. Journal of acquired immune deficiency syndromes. 2012;60:150–7. doi: 10.1097/QAI.0b013e318251aedd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasti G, Serraino D, Ridolfo A, Antinori A, Rizzardini G, Zeroli C, Nigro L, Tavio M, Vaccher E, Tirelli U. AIDS-associated Kaposi’s sarcoma is more aggressive in women: a study of 54 patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:337–41. doi: 10.1097/00042560-199904010-00003. [DOI] [PubMed] [Google Scholar]
- 38.Meditz AL, Borok M, MaWhinney S, Gudza I, Ndemera B, Gwanzura L, Campbell TB. Gender differences in AIDS-associated Kaposi sarcoma in Harare, Zimbabwe. Journal of acquired immune deficiency syndromes. 2007;44:306–8. doi: 10.1097/QAI.0b013e31802c83d9. [DOI] [PubMed] [Google Scholar]
- 39.Maule M, Merletti F. Cancer transition and priorities for cancer control. The lancet oncology. 2012;13:745–6. doi: 10.1016/S1470-2045(12)70268-1. [DOI] [PubMed] [Google Scholar]
- 40.Department of Health, Republic of South Africa. [Accessed April 28th 2014];The South African antiretroviral treatment guidelines 2013. 2013 Available at: http://www.kznhealth.gov.za/medicine/2013_art_guidelines.pdf.
- 41.Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi Sarcoma pathogenesis, and treatment of Kaposi Sarcoma. Cancer letters. 2011;305:150–62. doi: 10.1016/j.canlet.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou F, Xue M, Qin D, Zhu X, Wang C, Zhu J, Hao T, Cheng L, Chen X, Bai Z, Feng N, Gao SJ, et al. HIV-1 Tat promotes Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3beta signaling pathway. PloS one. 2013;8:e53145. doi: 10.1371/journal.pone.0053145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sitas F, Carrara H, Beral V, Newton R, Reeves G, Bull D, Jentsch U, Pacella-Norman R, Bourboulia D, Whitby D, Boshoff C, Weiss R. Antibodies against human herpesvirus 8 in black South African patients with cancer. The New England journal of medicine. 1999;340:1863–71. doi: 10.1056/NEJM199906173402403. [DOI] [PubMed] [Google Scholar]
- 44.Parisi SG, Boldrin C, Andreis S, Ferretto R, Fuser R, Malena M, Manfrin V, Panese S, Scaggiante R, Dori L, Sarmati L, Biasolo MA, et al. KSHV DNA viremia correlates with low CD4+ cell count in Italian males at the time of diagnosis of HIV infection. Journal of medical virology. 2011;83:384–90. doi: 10.1002/jmv.21987. [DOI] [PubMed] [Google Scholar]
- 45.Sgadari C, Monini P, Barillari G, Ensoli B. Use of HIV protease inhibitors to block Kaposi’s sarcoma and tumour growth. The lancet oncology. 2003;4:537–47. doi: 10.1016/s1470-2045(03)01192-6. [DOI] [PubMed] [Google Scholar]
- 46.Grabar S, Abraham B, Mahamat A, Del Giudice P, Rosenthal E, Costagliola D. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3408–14. doi: 10.1200/JCO.2005.05.4072. [DOI] [PubMed] [Google Scholar]
- 47.http://globocan.iarc.fr/
- 48.Wu TT, Qian J, Ang J, Sun R. Vaccine prospect of Kaposi sarcoma-associated herpesvirus. Current opinion in virology. 2012;2:482–8. doi: 10.1016/j.coviro.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, Rapiti E, Levi F, Jundt G, Fisch T, Bordoni A, De Weck D, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–32. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 50.Lacombe JM, Boue F, Grabar S, Viget N, Gazaignes S, Lascaux-Cametz AS, Pacanowski J, Partisani M, Launay O, Matheron S, Rosenthal E, Rouveix E, et al. Risk of Kaposi sarcoma during the first months on combination antiretroviral therapy. Aids. 2013;27:635–43. doi: 10.1097/QAD.0b013e32835cba6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.