Abstract
Immunotherapy and vaccine development for hepatitis C virus (HCV) will depend on broadly reactive neutralizing antibodies. However, studies in infectious JFH1-based culture systems expressing patient derived Core-NS2 proteins have suggested neutralization resistance for specific HCV strains, in particular of genotype 2. To further examine this phenomenon, we developed a panel of HCV genotype 2 recombinants for testing of sensitivity to neutralization by chronic-phase patient sera and lead human monoclonal antibodies. The novel Core-NS2 recombinants, with patient derived genotype 2a (strain T9), 2b (strains DH8 and DH10), and 2c (strain S83) consensus sequences, were viable in Huh7.5 hepatoma cells without requirement for adaptive mutations, reaching HCV infectivity titers of 3.9–4.5 log10 focus-forming units per mL. In in vitro neutralization assays, we demonstrated that the novel genotype 2 viruses as well as prototype strains J6(2a) and J8(2b), all with authentic envelope proteins, were resistant to neutralization by genotype 2a, 2b, 2c, 2j, 2i, and 2q patient sera. These patient sera, however, had high titers of HCV-specific neutralizing antibodies, since they efficiently reduced the infectivity of J6(2a) and J8(2b) with deleted hypervariable region 1. The genotype 2a, 2b, and 2c viruses, found resistant to polyclonal patient sera neutralization, were efficiently neutralized by two lead human monoclonal antibodies, AR4A and HC84.26. Conclusion: Using novel 2a, 2b, and 2c cell culture systems, expressing authentic envelope proteins, we demonstrated resistance of HCV to patient-derived polyclonal high-titer neutralizing antibodies. However, the same genotype 2 culture viruses were all sensitive to human monoclonal HCV antibodies recognizing conformational epitopes, indicating that neutralization resistance of HCV can be overcome by applying recombinant antibodies. These findings have important implications for HCV immunotherapy and vaccine development.
Keywords: HCV, genotype 2 recombinants, neutralizing antibodies, vaccine, therapeutic antibodies
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease worldwide1. The acute phase-infection is often sub-clinical, with clearance in only 20–30% of the cases. Furthermore, vigorous cellular immune responses are essential for viral clearance2, whereas the role of neutralizing antibodies (NAbs) remains controversial3–6. During chronic HCV infection the virus persists despite HCV-specific CD8+ T-cell responses2,7, and continuous pressure from NAbs apparently drives viral evolution and reduces viral load5. A recent study showed that clearance of a chronic HCV infection was induced after an initial strong NAb response had reduced viral load, facilitating effective cellular immune responses8. This supports the importance of NAbs in controlling HCV, thus strengthening the case for their therapeutic relevance. Several promising human monoclonal antibodies (HMAbs) were developed with neutralizing effect in vitro and in vivo9,10. These antibodies could be of great importance as potential therapeutics and as tools to study the function of HCV envelope proteins revealing potential targets for vaccine design.
A major challenge in developing prophylactic and therapeutic HCV antibodies is its great diversity, with 6 epidemiologically important major genotypes and numerous subtypes11. In an infected individual the virus replicates rapidly, generating closely related quasispecies of importance for immune evasion2. Since the discovery of HCV genotype 2a strain JFH112, recombinant cell-culture systems expressing strain specific Core-NS2 proteins (Core, E1, E2, p7, and NS2) have been developed for all major HCV genotypes13–20, including a genotype 1a and 1b panel17. The isolate specific envelope proteins enable detailed cross-genotype and -subtype neutralization studies using HCV patient polyclonal antibodies. Prior studies revealed differential neutralization susceptibility and patterns of neutralization for the major genotypes but differences also occurred between subtypes13,21. Especially genotype 2 viruses showed differences on a subtype-specific level. In one study we found that a 2a isolate was difficult to neutralize, whereas a 2b isolate showed intermediate neutralization susceptibility13. In contrast, genotype 1a and 1b isolates showed intermediate susceptible to neutralization13. In another study, we reported that the genotype 2a virus without hypervariable region 1 (HVR1) did not require adaptive mutations and had significantly increased susceptibility to NAb compared to the wild-type virus21.
Considering that genotypes 1 and 2 are widely distributed worldwide, and are commonly found in Europe, Japan, and USA22, further studies exploring differences in neutralization among genotype 2 viruses would be highly relevant. However, in order to make valid comparisons, several strains of each subtype should be studied. At the outset of this study, genotype 2 was represented by two Core-NS2 systems, J6/JFH1(2a) and J8/JFH1(2b), and by one full-length system, JFH1(2a)12–14. Genotype 2 is diverse with numerous subtypes (2a-2r); six subtypes were confirmed by full-length sequences (2a, 2b, 2c, 2i, 2k, and 2q)12,23–27. Subtypes 2a, 2b, and 2c are the most prevalent, and we therefore sought to develop Core-NS2 recombinants of these subtypes to investigate the neutralization potential of human polyclonal antibodies present in genotype 2 patient sera and to compare it with the neutralizing potential of two lead HMAbs, AR4A9 and HC84.2610.
Materials and Methods
HCV source and plasmid construction
The 2a (T9), 2b (DH8 and DH10), and 2c (S83) strains were recovered from sera of chronic HCV patients from Taiwan, Denmark, and Italy, respectively11. RNA was extracted using High Pure viral nucleic acid kit (Roche) or TRIzolLS (Invitrogen). Reverse transcription was performed with SuperScriptIII (Invitrogen), and reverse-primers 5085JR_J6(5′TGCTTTGTCTGGGAGAGGAA3′) for DH8, DH10, and S83 and 3774R_JFH119 for T9. For PCR, the Advantage 2 System (Clontech) and the same reverse primers were used with forward primers -285S_HCV-MOD or 84S_HCV-MOD11,13. Amplicons were cloned using TopoXL (Invitrogen).
The strain specific cloned Core-NS2 consensus sequence fused with JFH1 was inserted into FL-J6/JFH114 with EcoRI and SpeI (NEB) for DH8 and T9, and AgeI and SpeI for DH10 and S83, using T4 Rapid Ligation kit (Roche). The HCV sequences of final maxipreps (Qiagen) were confirmed (Macrogen).
Cell culture
Culturing of Huh7.5 hepatoma cells was as described19. 24h before transfection or infection, 4×105 cells per well were plated in 6-well plates (Nunc). Plasmids were linearized with XbaI followed by in vitro transcription with T7 RNA polymerase (Promega) for 2h at 37°C. For transfections, 2.5μg RNA was incubated with 5μL Lipofectamine 2000 in 500μL OptiMEM (Invitrogen) for 20min. Cells were incubated with RNA-lipofectamine complexes for 16–24h. For infections, cells were inoculated with filtered virus containing culture supernatant for 16–24h.
Cultures were evaluated by immunostaining with NS5A antibody 9E1019. HCV RNA titers were determined by TaqMan19. HCV infectivity titers were determined by adding 10-fold dilutions (starting at 1:2) of supernatants, in triplicate, into 6×103 Huh7.5 cells/well of poly-D-lysine coated 96-well plates (Nunc). After 48h incubation, cells were fixed and immunostained with 9E10 antibody. The number of focus forming units (FFU) was determined using an ImmunoSpot series 5 UV analyzer (CTL Europe GmbH)17,21,28.
Procedures to generate amplicons for direct sequencing of the complete open reading frame (ORF) and primers for the JFH1 portion were reported19; Core-NS2 specific primers are shown in supplementary table 1. Sequences were analyzed using Sequencher (Gene Codes) and Vector NTI (Invitrogen). Phylogenetic trees were generated using the Jukes-Cantor model and the Neighbor-joining algorithm implemented in the by Molecular Evolutionary Genetics Analysis (MEGA) software.
Subtype determination of HCV
We analyzed two panels of chronic-phase sera from HCV genotype 2 patients originating from Hospital Clinic, Spain, and National Institutes of Health, USA. All patients were presumable HCV mono-exposed, according to clinical records. The genotype and subtype of the infecting HCV was determined by direct sequencing of Core-E1 amplicons29; analysis of sample K1118 required cloning of the amplicon. For phylogenetic analysis we used MEGA.
Neutralization assay
Heat-inactivated (56°C for 30min) patient sera were tested in 2-fold dilutions against J6/JFH1, T9/JFH1, DH8/JFH1, DH10/JFH1, J8/JFH1, and S83/JFH1, and in 5-fold dilutions against J6/JFH1ΔHVR1 and J8/JFH1ΔHVR116. Polyclonal IgG was purified from 100μL of serum from four selected samples using a Protein G HP SpinTrap/Ab Spin Trap system (GE Healthcare), and tested against J6/JFH1 and J6/JFH1ΔHVR1 in 5-fold dilutions starting at 100 μg/mL.
Between 20-150 FFU of recombinant viruses were incubated 1h with serum, IgG, or HMAbs, followed by 3 hours incubation on 6×103 naïve Huh7.5 cells in poly-D-lysine-coated-96 well plates. The AR4A batch had previously been tested9 while a new HC84.26 batch was used. After washing and 48h incubation, NS5A antigen staining was performed with 9E10 antibody and FFU counts were determined as above. The mean background level of 6 negative wells was below 15 in all experiments; the negative mean was subtracted from FFU counts in experimental wells.
As controls, previously tested HCV-negative sera were tested against the J6/JFH1ΔHVR1 and J8/JFH1ΔHVR1 viruses21, and HCV-positive IgG-depleted serum was tested against J6/JFH1 and J6/JFH1ΔHVR1. The unmodified viruses were tested against b6, a AR4A control, and against R04, a HC84.26 control9,10.
Percent neutralization was calculated by relating the mean FFU of the experimental wells in three replicates for the serum and four replicates for the HMAb samples to the mean of six replicate cultures inoculated with virus only16. The serum dilution and IgG concentration against the HVR1-deleted culture viruses and the HMAb-concentration against the unmodified culture viruses causing 50% reductions in FFU (IC50) were determined by Best-fit Sigmoidal dose-response curves with variable slope and Bottom constraint of 0 (Y=Bottom+((Top-Bottom)/(1+10(log10IC50-X)*Hill slope) (GraphPad Prism). Due to limited neutralization of the unmodified recombinant viruses by patient serum and IgG, IC50 values were instead reported as the highest serum dilution or the lowest concentration of IgG where neutralization ≥50% was observed.
Results
Development of HCV recombinant genotype 2a (strain T9), 2b (strains DH8 and DH10), and 2c (strain S83) viruses with authentic Core-NS2
For development of JFH1-based recombinants we determined the Core-NS2 consensus sequence deduced from 5 to 7 molecular clones from each patient’s viral population (supplementary table 2). The variation between the T9 Core-NS2 consensus and the 5 clonal sequences was <0.6% at the nucleotide (nt) and amino acid (aa) level. For DH8 and S83, 6 of 7 clones analyzed diverged <1% from the respective consensus sequences; for each isolate there was a single clone deviating by 2–3.5%. The DH10 quasispecies consisted of two subpopulations separated in 5 and 2 clones, respectively. The DH10 consensus was developed from the most prevalent subpopulation, deviating from the consensus by <0.2%. As for prototype strains J6(2a) and J8(2b), the Core-NS2 of T9(2a), DH8(2b), DH10(2b), and S83(2c) consisted of 3090 nts encoding 1030 aa. At the aa level, the Core-NS2 of T9(2a) differed from J6(2a) by 9.5%, while DH8(2b) and DH10(2b) differed from J8(2b) by 8.2% and 8.7%, respectively. S83(2c) differed from J6(2a) and J8(2b) by 18.5% and 20.5%, respectively. Thus, Core-NS2 sequences of the novel genotype 2 isolates deviated significantly from those of the previously developed genotype 2 recombinants (Fig. 1).
Fig. 1.
Phylogenetic analysis of the Core-NS2 sequence of isolates used for intergenotypic JFH1-based cell-culture viruses. Genotype 1-7 isolates used for HCV Core-NS2 recombinants were previously described13–20. Novel genotype 2a, 2b, and 2c isolates used in this study are indicated with diamonds. Alignment was made using ClustalW in MEGA software. The phylogenetic tree was generated using the Jukes Cantor model and Neighbor-joining algorithm. The percentages (≥80%) of 1000 replicates in which the associated sequences clustered together in the bootstrap test are shown; 80% was considered significant. The unit is the number of nucleotide substitutions per site.
Compared with the respective consensus, the final sequences cloned into the JFH1 backbone contained one silent mutation for DH8 (A1121G) and two silent mutations for DH10 (T2924G and T2960C) (nt positions according to H77, GenBank accession number AF009606). The cloned T9 and S83 sequences did not differ from the respective consensus sequences.
The novel JFH1-based 2a, 2b, and 2c recombinants with isolate-specific Core-NS2 were in vitro transcribed and transfected into Huh7.5 cells along with J6/JFH1(2a) and J8/JFH1(2b) (Fig. 2); within 10 days, the number of NS5A-antigen positive cells increased to >80% for all recombinants. T9(2a) and S83(2c) had peak infectivity titers of 4.3 Log10 FFU/mL, while DH8(2b) and DH10(2b) had peak infectivity titers of 4.0 and 3.2 Log10 FFU/mL, respectively. After passage of culture supernatant to naive Huh7.5 cells (MOI 0.001–0.016), the number of NS5A-antigen positive cells increased to >80% within 13 days. The 1st passage 2a and 2c recombinants had the highest peak infectivity titers of >4.1 Log10 FFU/mL compared to 3.2 and 3.9 Log10 FFU/mL for the 2b recombinants. The HCV infectivity and RNA titers of various cultures are listed in Table 1.
Fig. 2.

HCV infectivity titers in cell cultures after transfection with the novel genotype 2 Core-NS2 recombinants. Huh7.5 cells were transfected with RNA transcripts from T9/JFH1(2a), DH8/JFH1(2b), DH10/JFH1(2b), and S83/JFH1(2c) recombinants, and the previously developed J6/JFH1(2a) and J8/JFH1(2b) recombinants. The HCV infectivity titers are shown in bars for each recombinant virus at indicated time points. Data from different recombinants are from different experiments. J6/JFH1 was included as a common positive control; data from one representative experiment are shown. Error bars indicate standard errors of the mean (SEM) from at least triplicate determinations. The lower limit of detection in the experiments was 102.3 FFU/mL.
Table 1.
Infectivity titers determined for the four novel HCV genotype 2 recombinants after transfection and infection of Huh7.5 cells.
| Peak infectivity titer transfection Log10 FFU/mL |
Peak infectivity titer 1st passage Log10 FFU/mL |
HCV RNA 1st passage Log10 IU/mL |
Infectivity titer of virus pools Log10 FFU/mL |
|
|---|---|---|---|---|
| T9/JFH1 (2a) | 4.3 (day 10) | 4.5 (day 8) | 7.2 | 3.9 (1st)* |
| DH8/JFH1 (2b) | 4.0 (day 6) | 3.9 (day 8) | 7.3 | 4.4 (2nd) |
| DH10/JFH1 (2b) | 3.2 (day 8) | 3.2 (day 13) | 7.1 | 3.9 (2nd) |
| S83/JFH1 (2c) | 4.3 (day 3) | 4.1 (day 10) | 7.6 | 3.9 (2nd) |
|
| ||||
| J6/JFH1 (2a) | 4.1 (day 10) | 4.8 (3rd) | ||
| J8/JFH1 (2b) | 3.5 (day 6) | 4.4 (1st) | ||
| J6/JFH1ΔHVR1 (2a) | 5.2 (3rd) | |||
| J8/JFH1ΔHVR1 (2b) | 3.5 (2nd) | |||
Peak FFU titers per ml of supernatants from transfection and the 1st viral passage infection experiments are listed (day in parentheses). These are the highest representative titers. J6/JFH1 and J8/JFH1 were included as controls in the transfections. 1st passage experiments were inoculated with the following multiplicity of infection (MOI) dose: T9/JFH1 MOI = 0.01, DH8/JFH1 MOI = 0.01, DH10/JFH1 MOI = 0.001 and S83/JFH1 MOI = 0.016. HCV RNA titers were measured on the 1st passage supernatants with peak infectivity titers.
The column to the far right shows the HCV infectivity titers of the virus pools used for neutralization experiments. Virus stocks were generated by inoculating naive Huh7.5 cells with MOI’s of approximately 0.003. Supernatants were harvested at peak infection (>80% HCV positive cells, day 9–12). The number of viral passage is shown in parentheses. Core, E1, and E2 sequences of the pools were verified by direct sequencing, thus confirming the lack of mutations in the structural proteins.
From the 1st passage experiment, day 10 post infection.
Sequencing of the virus genomes recovered from the 1st passage cultures demonstrated that the novel recombinant genotype 2 viruses did not require amino acid changes for efficient spread in cells. Similar findings were reported previously for J6/JFH1 and J8/JFH113,14. Direct sequencing of the entire ORF from 1st passage viruses of T9/JFH1 and S83/JFH1 did not reveal any nt changes, whereas the recovered DH8/JFH1 showed the 50/50 coding mutation T7021T/C(V2227V/A). DH10/JFH1 had the noncoding mutation C6410T.
Neutralizing potential of genotype 2 chronic phase sera
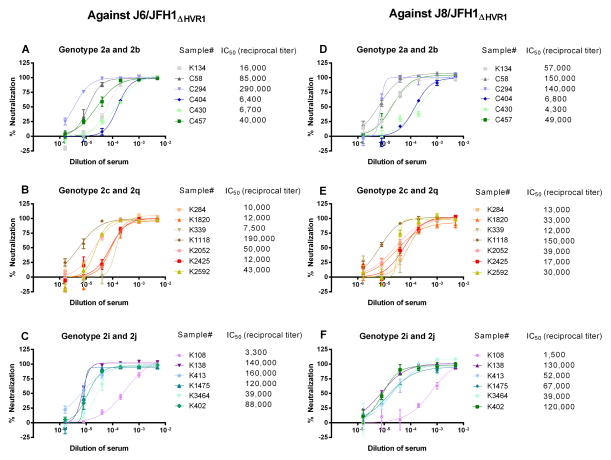
Two panels of chronic phase sera from HCV genotype 2 infected patients from Spain and USA were analyzed. Since the subtype had not been determined, we sequenced Core-E1 of HCV from all patient sera and performed phylogenetic analysis (Fig. 3). A variety of genotype 2 subtypes were found among the 17 patients from Spain; one 2a, five 2c, eight 2j, one 2i, and two 2q. Ten of 11 patients from the USA had genotype 2b; a single patient had 2c. Subtype representative samples were randomly selected for neutralization studies (highlighted in Fig. 3). These genotype 2 sera were all initially tested against two HVR1 deleted viruses, J6/JFH1ΔHVR1(2a) and J8/JFH1ΔHVR1(2b). HVR1-deleted recombinants have previously been shown to be more susceptible to HCV-specific NAbs from chronic phase sera compared to unmodified recombinants21,30. All sera efficiently reduced the number of FFU of the HVR1-deleted viruses with reciprocal serum dilution IC50-titers of 3,300–290,000 for J6/JFH1ΔHVR1 (Fig 4A–C) and 1,500–150,000 for J8/JFH1ΔHVR1 (Fig. 4D–F). HCV-negative serum did not reduce the number of FFU ≥50% for either HVR1-deleted virus in 1:200 or higher dilutions. These findings indicate the presence of NAbs, which were able to target neutralizing epitopes in the viral particle in the absence of HVR1.
Fig. 3.
Phylogenetic analysis of the HCV Core-E1 sequence of 28 genotype 2 patient samples. The genetic relatedness of the Core-E1 sequences (nts 868-1288) is shown. The corresponding Core-E1 sequences previously published for genotype 2a, 2b, 2c, 2i, 2j, and 2q isolates are included and marked with a blue box. The genotype 2 isolates used for development of novel recombinants, T9(2a), DH8(2b), DH10(2b), and S83(2c), are marked with a red box and the isolates used in previously developed recombinants, J6(2a) and J8(2b), are marked with a star. The sera selected for neutralization studies are marked with triangles. H77 is included as an out-group. The alignment was made using ClustalW in MEGA software and manually rearranged to obtain a codon-based nucleotide alignment. The phylogenetic tree was generated using the Jukes Cantor model and Neighbor-joining algorithm. The percentages (≥80%) of 1000 replicates in which the associated clustered together in the bootstrap test are shown. The unit is the number of nucleotide substitutions per site.
Fig. 4.
Genotype 2 Core-NS2 HVR1-deleted HCV recombinant viruses tested against sera from 19 patients chronically infected with HCV genotype 2. Sera were used in neutralization assays in 5-fold dilution series (1:200 to 1:625,000). The graphs show the doses-response curves of genotype 2 Core-NS2 HVR1-deleted recombinant viruses J6/JFH1ΔHVR1 (2a) and J8/JFH1ΔHVR1 (2b) against 19 serum samples from patients with chronic HCV genotype 2; curves were fitted as described in Materials and Methods. The reciprocal serum dilution with a 50% reduction in FFU (IC50-values) is indicated with two significant digits. Graph A-C show the result against J6/JFH1ΔHVR1, while graphs D-F show the results against J8/JFH1ΔHVR1. The genotype 2 subtype of the patient derived viruses is listed above the graphs and divided accordingly. The IC50-values were determined by nonlinear regression (Graph-Pad Prism Software) as described in Materials and Methods. Error bars indicate standard errors of the mean (SEM) from three determinations.
Next, 1/100, 1/200, and 1/400 dilutions of the same panel of genotype 2 sera were tested against the six genotype 2 Core-NS2 recombinant viruses. Despite the significant ability to reduce the number of FFU against HVR1 deleted viruses, the sera had limited or no neutralization capacity against the wild-type genotype 2 viruses. Only 5 sera showed neutralizing potential. C58(2b), K1118(2c), K2592(2c), and K1475(2j) neutralized J6/JFH1(2a) by ≥50% in 1/100 and/or 1/200 dilutions. In addition, K1118(2c) and C294(2b) neutralized S83/JFH1(2c) and DH8/JFH1(2b), respectively, in 1/200 dilutions. The remaining 14 sera were not able to neutralize any of the studied genotype 2 recombinants ≥50% at 1/100 or higher dilutions. The percentage of FFU reduction at 1:200 dilutions of patient serum samples for HVR1-deleted viruses and the unmodified culture viruses are shown in Table 2.
Table 2.
The percentage of focus forming units reduction against HVR1-deleted and unmodified genotype 2 viruses in a 1:200 dilution of serum from HCV infected genotype 2 patients.
| Patient number | Subtype | Sample origin | J6/JFH1 ΔHVR1 | J8/JFH1 ΔHVR1 | J6/JFH1 | T9/JFH1 | J8/JFH1 | DH8/JFH1 | DH10/JFH1 | S83/JFH1 |
|---|---|---|---|---|---|---|---|---|---|---|
| K134 | a | Spain | 101 | 96 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 |
| C058 | b | USA | 99 | 106 | 47 | 12 | 18 | 35 | ≤0 | 26 |
| C294 | b | USA | 98 | 104 | 47 | ≤0 | 22 | 51 | ≤0 | 21 |
| C404 | b | USA | 100 | 101 | 38 | ≤0 | ≤0 | 12 | ≤0 | ≤0 |
| C430 | b | USA | 101 | 97 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 |
| C457 | b | USA | 99 | 106 | 36 | ≤0 | 25 | 14 | ≤0 | ≤0 |
| K339 | c | Spain | 97 | 100 | 29 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 |
| K1118 | c | Spain | 99 | 102 | 53 | ≤0 | 36 | 49 | ≤0 | 50 |
| K2052 | c | Spain | 96 | 99 | 20 | ≤0 | 6 | ≤0 | ≤0 | ≤0 |
| K2425 | c | Spain | 96 | 103 | 25 | ≤0 | 17 | ≤0 | ≤0 | ≤0 |
| K2592 | c | Spain | 98 | 99 | 55 | ≤0 | ≤0 | 17 | ≤0 | 10 |
| K402 | i | Spain | 98 | 99 | 48 | ≤0 | 9 | 9 | ≤0 | 5 |
| K108 | j | Spain | 104 | 98 | 9 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 |
| K138 | j | Spain | 104 | 95 | 27 | ≤0 | 22 | ≤0 | ≤0 | ≤0 |
| K413 | j | Spain | 100 | 99 | 33 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 |
| K1475 | j | Spain | 94 | 96 | 51 | ≤0 | ≤0 | 7 | ≤0 | ≤0 |
| K3464 | j | Spain | 98 | 109 | 37 | ≤0 | 4 | 9 | ≤0 | ≤0 |
| K284 | q | Spain | 103 | 98 | 17 | ≤0 | ≤0 | 7 | ≤0 | ≤0 |
| K1820 | q | Spain | 97 | 90 | 21 | ≤0 | ≤0 | ≤0 | ≤0 | ≤0 |
The ability of sera from patients infected with different HCV genotype 2 subtypes, as indicated, to reduce the number of FFU’s of J6/JFH1ΔHVR1 (2a) and J8/JFH1ΔHVR1 (2b) compared to the ability to neutralize J6/JFH1 (2a), T9/JFH1 (2a), J8/JFH1 (2b), DH8/JFH1 (2b), DH10/JFH1 (2b), and S83/JFH1 (2c) is shown. The result is presented as percentage reduction in FFU at a 1:200 dilution. Each patient had a random sample number assigned unrelated to the person, and the HCV subtype was determined by Core-E1 sequencing (see Fig. 3).
To confirm that the reduction in FFU of HVR1-deleted viruses was IgG-dependent, we performed a neutralization assay of J6/JFH1 and J6/JFH1ΔHVR1 with purified IgG and the IgG-depleted serum from sample C294(2b), K2052(2c), K413(2j), and K1475(2j). IgG from these four sera was able to reduce the number of FFU of J6/JFH1ΔHVR1 in a dose dependent manner with IC50-values of 0.1–0.5 μg/mL. In contrast, IgG neutralized J6/JFH1 ≥50% at only the highest concentration of 100 μg/mL for C294, K2052 and K1475; K413 neutralized J6/JFH1 50% at ~20 μg/mL. The IgG-depleted serum was not able to affect the infectivity for J6/JFH1 or J6/JFH1ΔHVR1. Thus, FFU reduction against the HVR1-deleted virus was apparently IgG-dependent. The lack of neutralization of the wild type virus could not be explained by infectivity enhancing factors in the human sera.
Genotype 2 viruses were efficiently neutralized by human monoclonal antibodies
Recently, it was demonstrated that two unique HMAbs, AR4A and HC84.26, recognizing conformational epitopes, had broad neutralizing potential against several HCV genotypes9,10. To study these HMAbs against the genotype 2 panel, each recombinant virus was tested in a concentration-response assay with antibody concentrations ranging from 0.008–25 μg/mL. AR4A neutralized J6(2a), T9(2a), J8(2b), DH8(2b), and S83(2c) with IC50-values of 1.8–8.7 μg/mL; only DH10(2b) had IC50 >25 μg/mL (Fig. 5A). HC84.26 neutralized the recombinant viruses with IC50 of 0.1–8.2 μg/mL; in contrast to ARA4, DH10(2b) was efficiently neutralized by HC84.26 (Fig. 5B). A comparison with the amount of polyclonal IgG purified from selected patients able to neutralize 50% of J6/JFH1 is shown in Table 3. Thus, the genotype 2-virus panel found resistant to NAbs in genotype 2 chronic phase sera could be neutralized efficiently by HMAbs AR4A and HC84.26.
Fig. 5.
Genotype 2 Core-NS2 HCV recombinant viruses tested against two human monoclonal antibodies, AR4A and HC84.26. The dose-response neutralization of the genotype 2 Core-NS2 recombinant viruses J6/JFH1(2a), T9/JFH1(2a), J8/JFH1(2b), DH8/JFH1(2b), DH10/JFH1(2b), and S83/JFH1(2c) using HMAb AR4A (A) and HC84.26 (B) was determined in FFU reduction assays; curves were fitted as described in Materials and Methods. Each recombinant was tested against the two HMAbs at concentrations ranging from 0.008 to 25 μg/mL. The concentration with a 50% reduction in FFU (IC50-values) is indicated. An isotype-matched control was included for both HMAbs at 25 μg/mL and tested against all recombinant viruses (shown as color coded open symbols). Error bars indicate standard errors of the mean (SEM) from four determinations.
Table 3.
Comparing the neutralization efficiency of purified patient IgG and human monoclonal antibodies.
| Sample | Subtype | Neutralization of J6/JFH1 μg/mL |
|---|---|---|
| C294 | 2b | 100* |
| K2052 | 2c | 100* |
| K413 | 2j | 20* |
| K1475 | 2j | 100* |
|
| ||
| AR4A | 1a | 2.9** |
| HC84.26 | 2b | 0.6** |
IgG was extracted from four selected patient samples (Table 2) and tested against the wildtype genotype 2a, J6/JFH1. The result is given as the concentration (μg/mL) able to neutralize the virus by 50%. This approach was used due to limited neutralization. Below is the IC50-value of the same construct against the two HMAbs.
Concentration able to neutralize 50%
IC50-value
Discussion
To investigate antibody neutralization susceptibility of HCV, we developed HCV genotype 2a, 2b, and 2c Core-NS2 culture viruses. The S83/JFH1 recombinant represents the first culture system for genotype 2c, a subtype frequently found in Southern Europe24. We showed a general lack of neutralization sensitivity of genotype 2-culture viruses using patient sera containing high levels of HCV genotype 2- specific neutralizing polyclonal antibodies. However, neutralization resistance could be overcome by lead HMAbs HC84.26 and AR4A. Interestingly, HC84.26 was isolated from a chronic HCV patient infected with genotype 2b10, indicating that HC84.26-like antibodies are rarely or poorly elicited during chronic infection.
HCV cell-culture systems for genotype 2 isolates consisting of JFH1-based recombinants with isolate specific Core-NS2 (J6/JFH1(2a) and J8/JFH1(2b)) or with isolate specific Core-NS3Protease, NS4A-NS5A (MA(2b)), as well as full-length recombinants (J6cc(2a), J8cc(2b), JFH-1(2a), and JFH-2(2a)), were previously developed12–14,31–33. In our experience, JFH1-based Core-NS2 recombinants containing the consensus HCV isolate sequence can function in vitro13,16–18. The J6/JFH1 and J8/JFH1 viruses effectively spread in culture without adaptive mutations13,14, whereas Core-NS2 recombinants of other major genotypes required adaptive mutations13,17,18. We found that the novel genotype 2a, 2b, and 2c Core-NS2 recombinants were viable in cell-culture without adaptive mutations. This strengthens the argument that a genotype specific relation between Core-NS2 and the remaining genome exists.
To test subtype-specific differences in neutralization susceptibility the JFH1-based Core-NS2 genotype 2 recombinants are valuable tools, because they do not require adaptive mutations in the envelope proteins that could influence neutralization potential. Previous studies showed a general increase in susceptibility for viruses of different genotypes lacking HVR121,30. We thus tested genotype 2 sera against genotype 2 recombinant viruses with and without HVR1, and found that all 19 genotype 2 sera significantly reduced the number of FFU of J6/JFH1ΔHVR1 and J8/JFH1ΔHVR1 compared to no or limited neutralization of unmodified 2a, 2b, and 2c viruses. This finding indicated that chronic phase sera contained high levels of NAbs, and that the lack of neutralization of unmodified viruses cannot be explained by lack of neutralizing epitopes since the only difference between the envelope sequences of J6/JFH1ΔHVR1 and J6/JFH1 is the HVR1 deletion. Previous studies found that neutralizing activities of antibodies from chronic-phase sera are inhibited by the presence of HVR121,30,34. An interplay between human serum components and the HVR1 region has been suggested to cause protection of these viruses from neutralization. HVR1 is of importance for cell entry through its interaction with scavenger receptor BI (SR-BI) and apparently also shields other relevant epitopes located outside the HVR130,34. A recent study showed that three positions in the E2 protein defined a conformational epitope important for E2-CD81 interaction during entry, and suggests a disruption of the conformational epitope might happen in the postbinding step35. The HVR1 region might be involved in such conformational changes being important for attachment of the virion. Our findings suggests that HVR1-dependent shielding could be a likely explanation for why some chronic phase sera display very limited ability to neutralize unmodified HVR1 containing genotype 2 recombinants.
Limited ability of the tested sera to neutralize the genotype 2 recombinant viruses corroborates the findings by Gottwein et al.13 reporting limited neutralization of J6/JFH1(2a) and intermediate neutralization of J8/JFH1(2b) by sera from patients infected with genotype 1a, 4a, and 5a. Compared to recombinants of genotype 1a, 4a, 5a, 6a and 7a, it appears that the genotype 2 Core-NS2 recombinants are generally less susceptible to neutralization by polyclonal serum antibodies regardless of the genotype infecting the patient. However, the difference in neutralization susceptibility between the genotype 2a and 2b recombinants was not confirmed in this study, where none of the nineteen sera samples was able to neutralize J8/JFH1(2b) ≥50% and only four of the samples showed limited ability to neutralize J6/JFH1(2a). Thus, no difference in susceptibility between recombinant genotype 2 subtypes is found, when testing antibodies from patients infected with the same major genotype.
One of the potential mechanisms by which HCV is protected against NAb is through interaction with serum high-density lipoproteins (HDL), which has been shown to facilitate entry and thereby reduce the neutralizing effect of antibodies34. In the present study IgG was extracted from four samples and the neutralization ability was correlated with that of serum. At IgG levels corresponding to the estimated level in serum, purified IgG was able to neutralize J6/JFH1 slightly more efficiently compared to serum neutralization for three samples. One sample had the same level of neutralization. In addition, testing IgG-depleted serum, no enhancement was observed for any of the samples. Taken together these data suggest that HDL might play a role in viral resistance to NAb. However, given that the results were not consistent among examined samples, other mechanisms may be competing. Zhang et al.36 proposed that interfering antibodies targeting aa 434-446 (epitope II) could inhibit neutralizing activity of antibodies targeting aa 412-423 (epitope I). However, studies have shown that polyclonal and monoclonal antibodies, which target epitope II (e.g. HC84.26), are able to neutralize HCV10,37.
In order to establish whether the resistance of the recombinant virus panel could be overcome by therapeutically relevant antibiodies, we tested two lead HMAbs, AR4A9 and HC84.2610. AR4A targets an epitope outside the CD81 binding site including the specific E2 residue D698, while the HC84.26 epitope target includes L441 and F442. The latter two residues are within a region previously proposed to include residues with epitopes targeted by interfering antibodies. In dose-response testing, the six genotype 2 recombinants were all sensitive to these two HMAbs, although showing differential sensitivity to neutralization (Fig. 5). Of residues known to bind to these HMAbs, D698 (AR4A) and L441 (HC84.26) were conserved among the 6 recombinants. However, at position 442, which is included in the HC84.26 epitope, the genotype 2b recombinant DH8/JFH1 encoded leucine while all other recombinants encoded phenylalanine. This could explain why neutralization with HC84.26 was more than 10-fold less efficient for DH8/JFH1 than for the other genotype 2b recombinants, J8/JFH1 and DH10/JFH1 (IC50 8.2 μg/mL versus 0.1 μg/mL). Moreover, these findings suggest that residue 442 is not absolutely required for the binding of this HMAb. For HMAb AR4A, DH10/JFH1 was markedly less sensitive than the remainder of the recombinants. Previously highlighted residues important for binding of this HMAb all appear to be conserved among the six recombinants9. However, other residues not previously described could be important, as could other factors affecting the secondary and tertiary structure of the virus and other components than E2 on the viral particle. Nevertheless, DH10/JFH1 could be neutralized 50% using HMAb HC84.26 at a concentration of 0.1 μg/mL, indicating that the neutralization resistance of this virus could be overcome using an alternative target. Experiments testing more than one HMAb simultaneous would be of relevance from a therapeutic point of view aiming to determine if any additive effect could be gained by pooling HMAb targeting different epitopes. Our results suggest that AR4A and HC84.26 might be considered as part of future therapeutics for patients with chronic HCV. Also, their target residues are highly conserved, an important factor for pan-genotypic vaccine design. The fact that AR4A was also found to be efficient against HCV in a modified animal model further supports a potential in vivo role of HMAbs9.
In conclusion, with the aim to investigate neutralization resistance and subtype specific difference in genotype 2 viruses, we developed four novel genotype 2 Core-NS2 recombinant cell-culture viruses. None of these recombinants exhibited a need for adaptive mutations in order to spread in Huh7.5 cells. Thus, these viruses harbor unmodified E1-E2 patient derived glycoproteins, and constitute, in combination with previously developed recombinants, a valuable tool for the study of genotype and subtype specific differences in HCV cell culture systems, and for the testing of future therapeutics and vaccine induced antibodies. The panel furthermore includes the first genotype 2c culture virus developed. Using chronic-phase genotype 2 sera, we demonstrated unexpectedly low neutralizing activity against the genotype 2-panel of viruses. This was not due to low titers of HCV-specific antibodies as HVR1 deleted viruses were highly susceptible by all sera. However, we showed efficient neutralization of all viruses using two lead HMAbs with therapeutic potential, and thus demonstrated that even viruses resistant to patient NAbs could be efficiently neutralized by recombinant HMAbs with therapeutic potential.
Supplementary Material
Acknowledgments
Supported by the Lundbeck Foundation (JG, JB), the Danish Cancer Society (JG, JB), the Novo Nordisk Foundation (JG, JB), the RegionH Research Fund (JB), The Danish Agency for Science Technology and Innovation (JP, NW), and Ph.D. stipends from the Faculty of Health and Medical Sciences, University of Copenhagen (THRC, JPR, and TBJ). JPR and SR are the recipients of Individual Postdoctoral Stipends from the Danish Council for Independent Research (FSS). ML is supported by the United States National Institutes of Health grant number AI79031.
We thank Department of Clinical Biochemistry, Copenhagen University Hospital, Hvidovre, for quantifying IgG. We thank Anne-Louise Sørensen, Lotte Mikkelsen, and Lubna Ghanem (Copenhagen University Hospital, Hvidovre) for general laboratory support as well as assistance locating samples and reagents, Jens Ole Nielsen and Ove Andersen (Copenhagen University Hospital, Hvidovre) for support of the project, and Charles Rice (Rockerfeller University, NY) and Takaji Wakita (National Institute of Infectious Diseases, Tokyo) for providing reagents.
Abbreviations
- HCV
hepatitis C virus
- HCVcc
cell culture produced hepatitis C virus
- NAb
neutralizing antibodies
- HMAb
human monoclonal antibodies
- MOI
multiplicity of infection
- FFU
focus forming units.
Footnotes
Reference List
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Thimme R, Binder M, Bartenschlager R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol Rev. 2012;36(3):663–683. doi: 10.1111/j.1574-6976.2011.00319.x. [DOI] [PubMed] [Google Scholar]
- 3.Lavillette D, Morice Y, Germanidis G, Donot P, Soulier A, Pagkalos E, et al. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79(10):6023–6034. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A. 2007;104(14):6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukh J, Thimme R, Meunier JC, Faulk K, Spangenberg HC, Chang KM, et al. Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J Virol. 2008;82(16):8183–8195. doi: 10.1128/JVI.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A. 2004;101(27):10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann-Haefelin C, Timm J, Spangenberg HC, Wischniowski N, Nazarova N, Kersting N, et al. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology. 2008;47(6):1824–1836. doi: 10.1002/hep.22242. [DOI] [PubMed] [Google Scholar]
- 8.Raghuraman S, Park H, Osburn WO, Winkelstein E, Edlin BR, Rehermann B. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis. 2012;205(5):763–771. doi: 10.1093/infdis/jir835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A. 2012;109(16):6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keck ZY, Xia J, Wang Y, Wang W, Krey T, Prentoe J, et al. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog. 2012;8(4):e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukh J, Purcell RH, Miller RH. At least 12 genotypes of hepatitis C virus predicted by sequence analysis of the putative E1 gene of isolates collected worldwide. Proc Natl Acad Sci U S A. 1993;90(17):8234–8238. doi: 10.1073/pnas.90.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11(7):791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottwein JM, Scheel TK, Jensen TB, Lademann JB, Prentoe JC, Knudsen ML, et al. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology. 2009;49(2):364–377. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 14.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309(5734):623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 15.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A. 2006;103(19):7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheel TK, Gottwein JM, Jensen TB, Prentoe JC, Hoegh AM, Alter HJ, et al. Development of JFH1-based cell culture systems for hepatitis C virus genotype 4a and evidence for cross-genotype neutralization. Proc Natl Acad Sci U S A. 2008;105(3):997–1002. doi: 10.1073/pnas.0711044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheel TK, Gottwein JM, Carlsen TH, Li YP, Jensen TB, Spengler U, et al. Efficient culture adaptation of hepatitis C virus recombinants with genotype-specific core-NS2 by using previously identified mutations. J Virol. 2011;85(6):2891–2906. doi: 10.1128/JVI.01605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen TB, Gottwein JM, Scheel TK, Hoegh AM, Eugen-Olsen J, Bukh J. Highly efficient JFH1-based cell-culture system for hepatitis C virus genotype 5a: failure of homologous neutralizing-antibody treatment to control infection. J Infect Dis. 2008;198(12):1756–1765. doi: 10.1086/593021. [DOI] [PubMed] [Google Scholar]
- 19.Gottwein JM, Scheel TK, Hoegh AM, Lademann JB, Eugen-Olsen J, Lisby G, et al. Robust hepatitis C genotype 3a cell culture releasing adapted intergenotypic 3a/2a (S52/JFH1) viruses. Gastroenterology. 2007;133(5):1614–1626. doi: 10.1053/j.gastro.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Yi M, Ma Y, Yates J, Lemon SM. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J Virol. 2007;81(2):629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentoe J, Jensen TB, Meuleman P, Serre SB, Scheel TK, Leroux-Roels G, et al. Hypervariable region 1 differentially impacts viability of hepatitis C virus strains of genotypes 1 to 6 and impairs virus neutralization. J Virol. 2011;85(5):2224–2234. doi: 10.1128/JVI.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zein NN. Clinical significance of hepatitis C virus genotypes. Clin Microbiol Rev. 2000;13(2):223–235. doi: 10.1128/cmr.13.2.223-235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samokhvalov EI, Hijikata M, Gylka RI, Lvov DK, Mishiro S. Full-genome nucleotide sequence of a hepatitis C virus variant (isolate name VAT96) representing a new subtype within the genotype 2 (arbitrarily 2k) Virus Genes. 2000;20(2):183–187. doi: 10.1023/a:1008182901274. [DOI] [PubMed] [Google Scholar]
- 24.Martro E, Valero A, Jordana-Lluch E, Saludes V, Planas R, Gonzalez-Candelas F, et al. Hepatitis C virus sequences from different patients confirm the existence and transmissibility of subtype 2q, a rare subtype circulating in the metropolitan area of Barcelona, Spain. J Med Virol. 2011;83(5):820–826. doi: 10.1002/jmv.22054. [DOI] [PubMed] [Google Scholar]
- 25.Nakao H, Okamoto H, Tokita H, Inoue T, Iizuka H, Pozzato G, et al. Full-length genomic sequence of a hepatitis C virus genotype 2c isolate (BEBE1) and the 2c-specific PCR primers. Arch Virol. 1996;141(3–4):701–704. doi: 10.1007/BF01718327. [DOI] [PubMed] [Google Scholar]
- 26.Noppornpanth S, Lien TX, Poovorawan Y, Smits SL, Osterhaus AD, Haagmans BL. Identification of a naturally occurring recombinant genotype 2/6 hepatitis C virus. J Virol. 2006;80(15):7569–7577. doi: 10.1128/JVI.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto H, Kurai K, Okada S, Yamamoto K, Lizuka H, Tanaka T, et al. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992;188(1):331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- 28.Gottwein JM, Scheel TK, Callendret B, Li YP, Eccleston HB, Engle RE, et al. Novel infectious cDNA clones of hepatitis C virus genotype 3a (strain S52) and 4a (strain ED43): genetic analyses and in vivo pathogenesis studies. J Virol. 2010;84(10):5277–5293. doi: 10.1128/JVI.02667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbet S, Bukh J, Heinsen A, Fomsgaard A. Hepatitis C virus subtyping by a core-envelope 1-based reverse transcriptase PCR assay with sequencing and its use in determining subtype distribution among Danish patients. J Clin Microbiol. 2003;41(3):1091–1100. doi: 10.1128/JCM.41.3.1091-1100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bankwitz D, Steinmann E, Bitzegeio J, Ciesek S, Friesland M, Herrmann E, et al. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol. 2010;84(11):5751–5763. doi: 10.1128/JVI.02200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Date T, Kato T, Kato J, Takahashi H, Morikawa K, Akazawa D, et al. Novel cell culture-adapted genotype 2a hepatitis C virus infectious clone. J Virol. 2012;86(19):10805–10820. doi: 10.1128/JVI.07235-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li YP, Ramirez S, Gottwein JM, Scheel TK, Mikkelsen L, Purcell RH, et al. Robust full-length hepatitis C virus genotype 2a and 2b infectious cultures using mutations identified by a systematic approach applicable to patient strains. Proc Natl Acad Sci U S A. 2012;109(18):E1101–E1110. doi: 10.1073/pnas.1203829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murayama A, Kato T, Akazawa D, Sugiyama N, Date T, Masaki T, et al. Production of infectious chimeric hepatitis C virus genotype 2b harboring minimal regions of JFH-1. J Virol. 2012;86(4):2143–2152. doi: 10.1128/JVI.05386-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, et al. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79(13):8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fofana I, Fafi-Kremer S, Carolla P, Fauvelle C, Zahid MN, Turek M, et al. Mutations that alter use of hepatitis C virus cell entry factors mediate escape from neutralizing antibodies. Gastroenterology. 2012;143(1):223–233. doi: 10.1053/j.gastro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, et al. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci U S A. 2009;106(18):7537–7541. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarr AW, Urbanowicz RA, Jayaraj D, Brown RJ, McKeating JA, Irving WL, et al. Naturally occurring antibodies that recognize linear epitopes in the amino terminus of the hepatitis C virus E2 protein confer noninterfering, additive neutralization. J Virol. 2012;86(5):2739–2749. doi: 10.1128/JVI.06492-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.