Abstract
Diabetes is a metabolic disorder, and it is considered as a major risk factor for Alzheimer’s disease (AD). In the present study, we evaluated whether treadmill exercise ameliorates progression of AD in relation with glycogen synthase kinase-3β (GSK-3β) activity using streptozotocin (STZ)-induced diabetic rats. For this study, step-down avoidance task, immunohistochemistry for glycogen synthase kinase-3β (GSK-3β) and tau, and western blot for phosphor-phosphoinositide 3 kinase (p-PI3K)/PI3K and phosphor-Akt (p-Akt)/Akt were performed. Diabetes mellitus was induced by intraperitoneal injection of STZ. The rats in the exercise groups were made to run on the treadmill for 30 min per one day, five times a week, during 12 weeks. The present results showed that short-term and long-term latencies in the step-down avoidance task were decreased by induction of diabetes, and treadmill exercise inhibited these latencies in the diabetic rats. Induction of diabetes suppressed the ratio of p-PI3K to PI3K and the ratio of p-Akt to Akt, and treadmill exercise increased these ratios in the diabetic rats. The numbers of GSK-3β-positive and tau-positive cells in the hippocampal dentate gyrus was higher in the diabetes-induction group than that in the control group, and treadmill exercise inhibited these numbers in the diabetic rats. In the present study, treadmill exercise suppressed hyperphosphorylation of tau in the hippocampus by decreased GSK-3β activity through PI3K/Akt pathway activation in the diabetic rats. Based on the present results, treadmill exercise may helpful to prevent diabetes-associated AD occurrence.
Keywords: Diabetes mellitus, Alzheimer’s disease, Treadmill exercise, Glycogen synthase kinase-3β, Tau
INTRODUCTION
Alzheimer’s disease (AD) is a multifactorial neurodegenerative disease, and its hallmark symptom is memory impairment. AD is characterized by co-existence of neuritic plaques and neurofibrillary tangles, and mostly constituted by hyperphosphorylated tau protein in the brain (Carvalho et al., 2009). Diabetes mellitus is a major risk factor for AD. Epidemiological studies have shown that diabetic patients have a higher risk to be developed AD, which is independent of the risk for vascular dementia (Maher and Schubert, 2009). Cognitive impairment, the main characteristic of AD, is more common in diabetic patients than in nondiabetic subjects (Strachan et al., 2011). Effective treatment for the diabetes may have effectiveness on the prevention and reduction of AD (Jolivalt et al., 2008).
Under the normal conditions, tau protein is implicated in the stabilization of axonal microtubules to maintain cellular morphology and axonal transport (Sergeant et al., 2008). However, hyperphosphorylated tau reduces ability stabilizing microtubules and induces self-aggregation into the infraneuronal neurofibrillary tangles. Presence of neurofibrillary tangles, composed of aggregated and hyperphosphorylated forms of tau protein, is one of the pathological hallmarks of AD (Hartig et al., 2007).
The molecular mechanisms causing abnormal hyperphosphorylation of tau protein in AD are not fully understood. Glycogen synthase kinase-3β (GSK-3β) phosphorylates tau protein (Li et al., 2006), and hyperactivation of GSK-3β induces abnormal tau phosphorylation and aggregation in AD (Martin et al., 2011). Phosphoinositide 3 kinase/Akt (PI3K/Akt) pathway inhibits GSK-3β, thereby promotes cell survival (Hashomoto et al., 2002). Suppression on PI3K/Akt pathway activates GSK-3β activity, and then facilitates cell death. In the early-onset familial AD, PI3K activity was suppressed (Vanhaesbroeck and Alessi, 2000). Suppressed PI3K activity increased GSK-3β expression, over-expression of GSK-3β may lead to hyperphosphorylated tau protein and to memory impairment (Jolivalt et al., 2008).
Memory enhancing effect of exercise has been reported (Hillman et al., 2008; Kim et al., 2010; Radak et al., 2006) Physical activity prevented cognitive decline in the elderly and inhibited the occurrence of dementia in AD patients (Teri et al., 2003). Individuals who had physically activity accrued inhibited benefit on dementia in AD (Scarmeas et al., 2009). Treadmill exercise improved memory function and motor symptoms in AD rats (Kim et al., 2014; Lee et al., 2014).
The effects of physical exercise are closely associated with PI3K/Akt pathway (Bruel-Jungerman et al., 2009), and exercise enhances PI3K/Akt expression (Jung et al., 2013). Akt is a major upstream modulator of GSK-3β and directly inhibits GSK-3β by phosphorylation of GSK-3β, thereby inactivates its kinase activity. Devising strategies interfering with the abnormal activation of GSK-3β might be new therapeutic methods to inhibit AD occurrence. In the present study, we evaluated whether treadmill exercise ameliorates progression of AD in relation with GSK-3β activity using streptozotocin (STZ)-induced diabetic rats.
MATERIALS AND METHODS
Animals and treatment
The experimental procedures were performed in accordance with the animal care guidelines of the National Institute of Health (NIH) and the Korean Academy of Medical Sciences. Thirty-six male Sprague-Dawley rats weighing 200±10 g (7 weeks in age) were used in this experiment. Rats were housed under controlled temperature (20±2°C) and lighting conditions (07:00 to 19:00), with food and water made available ad libitum. Animals were randomly divided into four groups (n=9 in each group): control group, exercise group, diabetes-induction group, and diabetes-induction and exercise group.
Induction of diabetes
To induce diabetes in the experimental animals, a single intraperitioneal injection of STZ (50 mg/kg, dissolved in 0.01 M citrate buffer at pH 4.5; Sigma Chemical Co., St. Louis, MO, USA) was given to each animal, as the previously described method (Lee et al., 2006). Blood glucose levels were determined 2 days after STZ injection using a blood glucose tester (Arkray, Kyoto, Japan). Only the animals with blood glucose levels of 300 mg/dL or higher were used in the diabetes groups.
Exercise protocol
The rats in the exercise groups were made to run on the treadmill for 30 min per one day, five times a week, during 12 weeks. The workload of the exercise consisted of running at a speed of 3 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 20 min, with 0% grade of inclination.
Step-down avoidance task
In order to evaluate the memory function of the rats, the latency of the step-down avoidance task was conducted, as the previously described method (Kim et al., 2014). The rats were trained in a step-down avoidance task in which they were positioned on a 7×25 cm platform with a height of 2.5 cm, and then allowed to rest on the platform for 1 min. The platform faced a 42×25 cm grid of parallel 0.1 cm-caliber stainless steel bars, which were spaced 1 cm apart. In the training session, the animals received a 0.5 mA scramble foot shock for 2 sec immediately upon stepping of off the platform. Latency was assessed at 2 h and 48 h after training session. The interval of rats stepping down and placing all 4 paws on the grid was defined as the latency in the step-down avoidance task. Any latency over 300 sec was counted as 300 sec.
Tissue preparation
The animals were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). The brains were removed and postfixed in the same fixative overnight and then transferred into a 30% sucrose solution for cryoprotection. Serial coronal sections of 40 μm thickness were obtained using a freezing microtome (Leica, Nussloch, Germany).
Immunohistochemistry for GSK-3β and Tau
Immunohistochemistry was conducted todetermine the GSK-3β-positive and the tau-positive cells in the hippocampus, as the previously described method (Jung et al., 2014; Sung et al., 2010). To begin the procedure, the sections were incubated in PBS for 10 min and then washed 3 times in the same buffer. Free-floating sections were then incubated in 3% H2O2 for 30 min. Next, the sections were incubated in blocking solution (1% BSA and 10% goat serum for GSK-3β and tau in 0.05 M PBS) for 90 min at room temperature. The sections were then incubated overnight with rabbit polyclonal anti-GSK-3β antibody (1:200; Cell Signaling Technology Inc., Beverly, MS, USA) and anti-tau antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sections were next incubated for 90 min with biotinylated rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Bound secondary antibody was then amplified with Vector Elite ABC kit® (1:100; Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.03%-diaminobenzidine (DAB) and the sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific, Fairlawn, NJ, USA).
Western blot analysis
Western blot was conducted according to the previous method (Kim et al., 2010). Protein extracts from brain tissue were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein separation was performed using 10% polyacrylamide with 0.05% bis-acrylamide. Proteins were then transferred to nitrocellulose and the blots were probed with anti-PI3K mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-p-PI3K rabbit polyclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-Akt rabbit polyclonal antibody (1:1,000, Cell Signaling Technology), and anti-p-Akt rabbit polyclonal antibody (1:1,000, Cell Signaling Technology). Peroxidase anti-rabbit IgG (1:5,000, Vector Laboratories), and peroxidase anti-mouse IgG (1:1,000, Vector Laboratories) were used as the secondary antibodies. Immunoreactivity was detected by enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology). Film auto-radiograms were exposed from 5 to 15 min.
Data analysis
Differences among the groups were evaluated using SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, USA). For the comparison among the groups, one-way analysis of variance (ANOVA) was performed followed by Duncan’s post-hoc test. All values are expressed as the mean±standard error of the mean (SEM). Statistically significant differences were established at P<0.05.
RESULTS
Effect of treadmill exercise on the latency in the step-down avoidance task
The latency in the step-down avoidance task is presented in Fig. 1. On the 2 h after training, the latency (short-term latency) was 300.00±0.00 sec in the control group, 280.00±20.00 sec in the exercise group, 169.00±18.42 sec in the diabetes-induction group, and 270.00±30.00 sec in the diabetes-induction and exercise group (Fig 1, left). On the 48 h after training, the latency (long-term latency) was 226.83±33.38 sec in the control group, 268.83±31.16 sec in the exercise group, 93.60±17.06 sec in the diabetes-induction group, and 282.60±17.40 sec in the diabetes-induction and exercise group (Fig 1, right). The present results showed that short-term and long-term latencies in the step-down avoidance task were decreased by induction of diabetes (P<0.05), and treadmill exercise inhibited short-term and long-term latencies in the diabetic rats (P<0.05). Treadmill exercise exerted no significant effect on the short-term and long-term latencies in the normal rats.
Fig. 1.
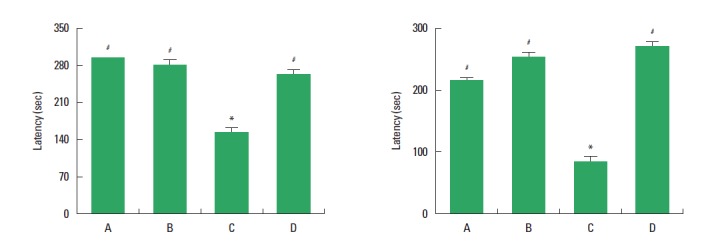
Effect of treadmill exercise on the latency in the step-down avoidance task. Left: Short-term latency. Right: Long-term latency. (A) Control group, (B) exercise group, (C) diabetes-induction group, and (D) diabetes-induction and exercise group. The data are presented as the mean± standard error of the mean (SEM). *P< 0.05 compared to the control group. #P< 0.05 compared to the diabetes-induction group.
Effect of exercise on phospho-PI3K (p-PI3K) and PI3K expressions in the hippocampus
Expressions of p-PI3K and PI3K in the hippocampus are presented in Fig. 2. In the present study, the expression of PI3K (85 kDa) in the control group was set at 1.00. The expression of PI3K was 1.20±0.04 in the exercise group, 1.12±0.02 in the diabetes-induction group, and 1.53±0.11 in the diabetes-induction and exercise group (Fig. 2, left). These results showed that induction of diabetes exerted no significant effect on the PI3K expression in the hippocampus, and treadmill exercise enhanced PI3K expression in the diabetic and normal rats (P<0.05).
Fig. 2.
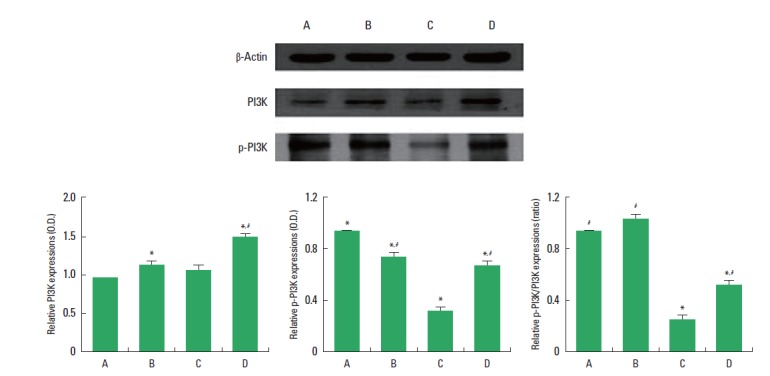
Effect of treadmill exercise on the phospho-phosphoinositide 3 kinase (p-PI3K) and PI3K expressions in the hippocampus. Upper: Representative expressions of p-PI3K, PI3K, and β-actin in the hippocampus. Lower left: Relative PI3K expression in the hippocampus. Lower middle: Relative p-PI3K expression in the hippocampus. Lower right: Relative ratio of p-PI3K/PI3K in the hippocampus. (A) Control group, (B) exercise group, (C) diabetes-induction group, and (D) diabetes-induction and exercise group. The data are presented as the mean± standard error of the mean (SEM). *P< 0.05 compared to the control group. #P< 0.05 compared to the diabetes-induction group.
The expression of p-PI3K (84 kDa) protein was in the control group set at 1.00. The expression of p-PI3K was 0.76±0.02 in the exercise group, 0.30±0.01 in the diabetes-induction group, and 0.70±0.02 in the diabetes-induction and exercise group (Fig. 2, middle). These results showed that induction of diabetes suppressed p-PI3K expression in the hippocampus (P<0.05), and treadmill exercise enhanced p-PI3K expression in the diabetic rats. Treadmill exercise suppressed p-PI3K expression in the normal rats (P<0.05).
The ratio of p-PI3K to PI3K was calculated. The ratio of p-PI3K/PI3K in the control group was set at 1.00. The ratio of p-PI3K/PI3K was 1.10±0.06 in the exercise group, 0.23±0.01 in the diabetes-induction group, and 0.60±0.03 in the diabetes-induction and exercise group (Fig. 2, right). These results showed that induction of diabetes suppressed the ratio of p-PI3K to PI3K (P<0.05), and treadmill exercise increased the ratio of p-PI3K to PI3K in the diabetic and normal rats (P<0.05).
Effect of exercise on phospho-Akt (p-Akt) and Akt expressions in the hippocampus
Expressions of p-Akt and Akt in the hippocampus are presented in Fig. 3. In the present study, the expression of Akt (60 kDa) in the control group was set at 1.00. The expression of Akt was 0.79±0.01 in the exercise group, 0.80±0.01 in the diabetes-induction group, and 0.92±0.01 in the diabetes-induction and exercise group (Fig. 3, left). These results showed that induction of diabetes suppressed Akt expression in the hippocampus (P<0.05), and treadmill exercise enhanced Akt expression in the diabetic rats (P<0.05). Treadmill exercise suppressed Akt expression in the normal rats (P<0.05).
Fig. 3.
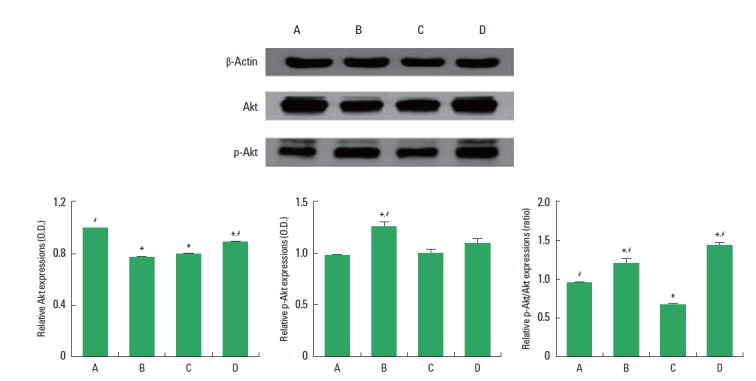
Effect of treadmill exercise on the phosphor-Akt (p-Akt) and Akt expressions in the hippocampus. Upper: Representative expressions of p-Akt, Akt, and β-actin in the hippocampus. Lower left: Relative Akt expression in the hippocampus. Lower middle: Relative p-Akt expression in the hippocampus. Lower right: Relative ratio of p-Akt/Akt in the hippocampus. (A) Control group, (B) exercise group, (C) diabetes-induction group, and (D) diabetes-induction and exercise group. The data are presented as the mean± standard error of the mean (SEM). *P< 0.05 compared to the control group. #P< 0.05 compared to the diabetes-induction group.
The expression of p-Akt (60 kDa) in the control group was set at 1.00. The expression of p-Akt was 1.34±0.11 in the exercise group, 1.01±0.06 in the diabetes-induction group, and 1.21 ±0.10 in the diabetes-induction and exercise group (Fig. 3, middle). These results showed that induction of diabetes did not change p-Akt expression in the hippocampus, and treadmill exercise also did not exert any effects on the p-Akt expression in the diabetes rats. Treadmill exercise enhanced p-Akt expression in the normal rats (P<0.05).
The ratio of p-Akt to Akt was calculated. The ratio of p-Akt/Akt in the control group was set at 1.00. The ratio of p-Akt/Akt was 1.39±0.16 in the exercise group, 0.70±0.02 in the diabetes-induction group, and 1.46±0.06 in the diabetes-induction and exercise group (Fig. 3, right). These results showed that induction of diabetes suppressed the ratio of p-Akt/Akt in the hippocampus (P<0.05), and treadmill exercise enhanced the ratio of p-Akt to Akt in the diabetic and normal rats (P<0.05).
Effect of exercise on the number of GSK-3β-positive cells in the hippocampal dentate gyrus
Photomicrographs of GSK-3β-positive cells in the hippocampal dentate gyrus are shown in Fig. 4. The number of GSK-3β-positive cells in the hippocampal dentate gyrus was 115.09±7.86/mm2 in the control group, 107.27±10.96/mm2 in the exercise group, 373.74±17.25/mm2 in the diabetes-induction group, and 245.81±14.48/mm2 in the diabetes-induction and exercise group. The present results showed that the number of GSK-3β-positive cells in the hippocampal dentate gyrus was higher in the diabetes-induction group than that in the control group (P<0.05), and treadmill exercise inhibited the number of GSK-3β-positive cells in the diabetic rats (P<0.05). Treadmill exercise exerted no significant effect on the number of GSK-3β-positive cells in the normal rats.
Fig. 4.
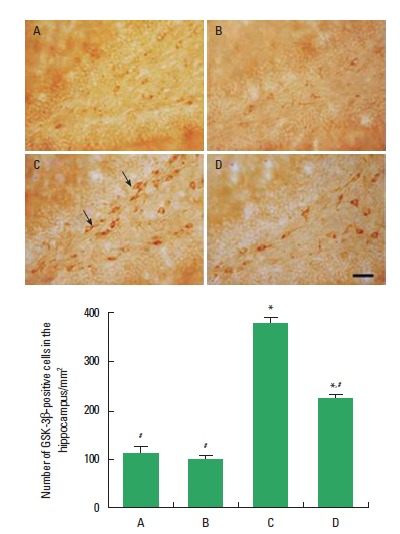
Effects of treadmill exercise on the number of glycogen synthase kinase-3β (GSK-3β)-positive cells in the hippocampal dentate gyrus. Upper: Photomicrographs of GSK-3β-positive cells in the hippocampal dentate gyrus. The scale bar represents 200 µm. Lower: Number of GSK-3β-positive cells in the hippocampal dentate gyrus. (A) Control group, (B) exercise group, (C) diabetes-induction group, and (D) diabetes-induction and exercise group. The data are presented as the mean± standard error of the mean (SEM). *P< 0.05 compared to the control group. #P< 0.05 compared to the diabetes-induction group.
Effect of exercise on the number of tau-positive cells in the hippocampal dentate gyrus
Photomicrographs of tau-positive cells in the hippocampal dentate gyrus are shown in Fig. 5. The number of tau-positive cells in the hippocampal dentate gyrus was 51.19±5.42/mm2 in the control group, 47.98±5.03/mm2 in the exercise group, 186.34±18.54/mm2 in the diabetes-induction group, and 147.29±11.32/mm2 in the diabetes-induction and exercise group.
Fig. 5.
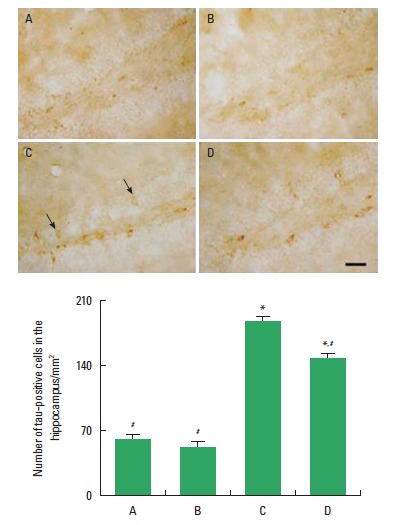
Effects of treadmill exercise on the number of tau-positive cells in the hippocampal dentate gyrus. Upper: Photomicrographs of tau-positive cells in the hippocampal dentate gyrus. The scale bar represents 200 µm. Lower: Number of tau-positive cells in the hippocampal dentate gyrus. (A) Control group, (B) exercise group, (C) diabetes-induction group, and (D) diabetes-induction and exercise group. The data are presented as the mean± standard error of the mean (SEM). *P< 0.05 compared to the control group. #P< 0.05 compared to the diabetes-induction group.
The present results showed that the number of tau-positive cells in the hippocampal dentate gyrus was higher in the diabetes-induction group than that in the control group (P<0.05), and tread-mill exercise inhibited the number of tau-positive cells in the diabetic rats (P<0.05). Treadmill exercise exerted no significant effect on the number of tau-positive cells in the normal rats.
DISCUSSION
Diabetes increases the risk to be developed to AD approximately two-fold (Biessels et al., 2006; Kopf and Frolich, 2009). In the present study, we investigated the possibility that treadmill exercise might prevent developing into AD from diabetes, focusing on the GSK-3β and tau expressions in relation with PI3K/Akt signaling pathway using diabetic rats. Animal models of diabetes showed learning and memory deficits (Kim et al., 2003; Revsin et al., 2009). Our results showed that short-term and long-term latencies were decreased in the diabetic rats. Treadmill exercise increased short-term and long-term latencies in the diabetic rats. Our study suggested that treadmill exercise might possess improving effect on AD-like memory impairment in diabetes.
PI3K/Akt signaling pathway is a classic anti-apoptotic pathway that regulates survival signal transduction. Activation of PI3K/Akt signaling pathway plays a protective role in many neuropathological condition (Chavali et al., 2011). Physical exercise has been reported to facilitate PI3K/Akt signaling pathway (Chen and Russo-Neustadt, 2005; Jung et al., 2014). Activity of PI3K/Akt pathway in the brain of diabetic rats was decreased and physical exercise facilitated levels of PI3K and Akt (Cheng et al., 2013). In present study, the ratio of p-PI3K/PI3K was decreased by induction of diabetes, but treadmill exercise enhanced the ratio of p-PI3K/PI3K by enhancing p-PI3K expression in the diabetic rats. The ratio of p-Akt/Akt was decreased by induction of diabetes, but treadmill exercise enhanced the ratio of p-Akt/Akt in the diabetic rats.
Upregulation of Akt pathway leads to the down-regulation of GSK-3β that plays a major role in learning and memory in the hippocampus (Hu et al., 2013). GSK-3β is main kinase which phosphorylates tau, and GSK-3β activity contributes to amyloid-β production and induces amyloid-β-mediated neuronal death (Hernandez et al., 2013). In the present study, GSK-3β expression in the hippocampus was increased by induction of diabetes, but treadmill exercise decreased GSK-3β expression in the diabetic rats. These results suggest that increased PI3K/Akt signaling pathway by treadmill exercise suppressed GSK-3β expression in the hippocampus of diabetic rats.
Belarbi et al. (2011) showed that physical exercise for 9 months accompanied by a decrement of hippocampal tau pathology, and exercise prevented memory alterations in transgenic model of Alzheimer’s disease mice. In order to confirm whether diabetes increases tau phosphorylation, we measured tau expression in the hippocampus. In the present study, the number of tau-positive cells in the hippocampus was increased by induction of diabetes, but treadmill exercise suppressed the number of tau-positive cells in the diabetic rats.
Reduced insulin signaling was associated with a concomitant increase in tau phosphorylation and amyloid-β level. Treatment with insulin partially restored the phosphorylation of insulin receptor and of GSK3, partially reduced the level of phosphorylated tau in the brain, and partially improved learning ability in insulin-deficient diabetic mice (Jolivalt et al., 2008). Physical exercise reduced the risk for AD and age-related cognition decline (Buchman et al., 2012; Erickson et al., 2012).
In the present study, treadmill exercise suppressed hyperphosphorylation of tau in the hippocampus by decreased GSK-3β activity through PI3K/Akt pathway activation in the diabetic rats. Based on the present results, treadmill exercise may helpful to prevent diabetes-associated AD occurrence.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF- 2012S1 A5A2A01021068).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Belarbi K, Burnouf S, Fernandez-Gomez FJ, Laurent C, Lestavel S, Figeac M, Sultan A, Troquier L, Leboucher A, Caillierez R, Grosjean ME, Demeyer D, Obriot H, Brion I, Barbot B, Galas MC, Staels B, Humez S, Sergeant N, Schraen-Maschke S, Muhr-Tailleux A, Hamdane M, Buée L, Blum D. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol Dis. 2011;43:486–494. doi: 10.1016/j.nbd.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, Davis S. Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS One. 2009;4(11):e7901. doi: 10.1371/journal.pone.0007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C, Correia SC, Santos RX, Cardoso S, Moreira PI, Clark TA, Zhu X, Smith MA, Perry G. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J Bioenerg Biomembr. 2009;41:433–440. doi: 10.1007/s10863-009-9247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali PL, Saini RK, Matsumoto Y, Ågren H, Funa K. Nuclear orphan receptor TLX induces Oct-3/4 for the survival and maintenance of adult hippocampal progenitors upon hypoxia. J Biol Chem. 2011;286:9393–9404. doi: 10.1074/jbc.M110.167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res Mol Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Cheng SM, Ho TJ, Yang AL, Chen IJ, Kao CL, Wu FN, Lin JA, Kuo CH, Ou HC, Huang CY, Lee SD. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int J Cardiol. 2013;67:478–485. doi: 10.1016/j.ijcard.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig W, Stieler J, Boerema AS, Wolf J, Schmidt U, Weissfuss J, Bullmann T, Strijkstra AM, Arendt T. Hibernation model of tau phosphorylation in hamsters: selective vulnerability of cholinergic basal forebrain neurons-implications for Alzheimer’s disease. Eur J Neurosci. 2007;25:69–80. doi: 10.1111/j.1460-9568.2006.05250.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sagara Y, Everall IP, Mallory M, Everson A, Langford D, Masliah E. Fibroblast growth factor 1 regulates signaling via the GSK3β pathway: implications for neuroprotection. J Biol Chem. 2002;277:32985–32991. doi: 10.1074/jbc.M202803200. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Lucas JJ, Avila J. GSK3 and tau: two convergence points in Alzheimer’s disease. J Alzheimers Dis. 2013;33:S141–144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hu YS, Long N, Pigino G, Brady ST, Lazarov O. Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3β, neurotrophin-3 and CREB signaling. PLoS One. 2013;8(5):e64460. doi: 10.1371/journal.pone.0064460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, Masliah E. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res. 2008;86:3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SY, Kim DY, Yune TY, Shin DH, Baek SB, Kim CJ. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med. 2014;7:587–593. doi: 10.3892/etm.2013.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10:2–8. doi: 10.12965/jer.140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Jang MH, Shin MC, Lim BV, Kim YP, Kim KJ, Kim EH, Kim CJ. Treadmill exercise increases cell proliferation in dentate gyrus of rats with streptozotocin-induced diabetes. J Diabet Complications. 2003;17:29–33. doi: 10.1016/s1056-8727(02)00186-1. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kopf D, Frolich L. Risk of incident Alzheimer’s disease in diabetic patients: A systematic review of prospective trials. J Alzheimers Dis. 2009;16:677–685. doi: 10.3233/JAD-2009-1011. [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim H, Lee JW, Kim YS, Yang HY, Chang HK, Lee TH, Shin MC, Lee MH, Shin MS, Park S, Baek S, Kim CJ. Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain Dev. 2006;28:147–154. doi: 10.1016/j.braindev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lee JM, Shin MS, Ji ES, Kim TW, Cho HS, Kim CJ, Jang MS, Kim TW, Kim BK, Kim DH. Treadmill exercise improves motor coordination through ameliorating Purkinje cell loss in amyloid beta23–35-induced Alzheimer’s disease rats. J Exerc Rehabil. 2014;10:258–624. doi: 10.12965/jer.140163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lu F, Tian Q, Yang Y, Wang Q, Wang JZ. Activation of glycogen synthase kinase-3 induces Alzheimer-like tau hyperphosphorylation in rat hippocampus slices in culture. J Neural Transm. 2006;113:93–102. doi: 10.1007/s00702-005-0303-7. [DOI] [PubMed] [Google Scholar]
- Maher PA, Schubert DR. Metabolic links between diabetes and Alzheimer’s disease. Expert Rev Neurother. 2009;9:617–630. doi: 10.1586/ern.09.18. [DOI] [PubMed] [Google Scholar]
- Martin L, Magnaudeix A, Wilson CM, Yardin C, Terro F. The new indirubin derivative inhibitors of glycogen synthase kinase-3, 6-BIDECO and 6-BIMYEO, prevent tau phosphorylation and apoptosis induced by theinhibition of protein phosphatase-2A by okadaic acid in cultured neurons. J Neurosci Res. 2011;89:1802–1811. doi: 10.1002/jnr.22723. [DOI] [PubMed] [Google Scholar]
- Radak Z, Toldy A, Szabo Z, Siamailis S, Nyakas C, Silye G, Jakus J, Goto S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49:387–392. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Revsin Y, Rekers NV, Louwe MC, Saravia FE, Nicola AF, Kloet ER, Oitzl MS. Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin induced type 1 diabetes mice. Neuropsychopharmacology. 2009;34:747–758. doi: 10.1038/npp.2008.136. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant N, Bretteville A, Hamdane M, Caillet-Boudin ML, Grognet P, Bombois S, Blum D, Delacourte A, Pasquier F, Vanmechelen E, Schraen-Maschke S, Buée L. Biochemistry of Tau in Alzheimer’s disease and related neurological disorders. Expert Rev. 2008;5:207–224. doi: 10.1586/14789450.5.2.207. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- Sung YH, Shin MS, Cho S, Baik HH, Jin BK, Chang HK, Lee EK, Kim CJ. Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neurosci Lett. 2010;470:86–90. doi: 10.1016/j.neulet.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, Kukull WA, LaCroix AZ, McCormick W, Larson EB. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K–PDK1 connection: more than just a road to PKB. Biochem J. 2000;3:561–576. [PMC free article] [PubMed] [Google Scholar]


