Abstract
There have been observational reports that maternal vitamin D status at baseline and not closest to delivery is a better predictor of pregnancy outcomes, suggesting that a cascade of events is set into motion that is not modifiable by vitamin D supplementation during later pregnancy. To address this issue, in this exploratory post-hoc analysis using correlation and logistic regression, we sought to measure the strength of the association between serum 25(OH)D concentrations at 3 timepoints during pregnancy: baseline, 1st trimester (<16 wks); 2nd trimester (16-26 wks); and 3rd trimester (≥27 wks) and preterm birth. It was hypothesized that the 25(OH)D value closest to delivery would be most significantly associated with preterm birth. To accomplish this objective, the datasets from NICHD (n=333) and Thrasher Research Fund (n=154) vitamin D supplementation pregnancy studies were combined.
The results of this analysis were that 25(OH)D values closer to delivery were more strongly correlated with gestational age at delivery than earlier values: 1st trimester: r=0.11 (p=0.02); 2nd trimester: r=0.08 (p=0.09); and 3rd trimester: r=0.15 (p=0.001). When logistic regression was performed with preterm birth (<37 weeks) as the outcome and 25(OH)D quartiles as the predictor variable, adjusting for study and participant race/ethnicity, as with the correlation analysis, the measurements closer to delivery were more significantly associated and had a higher magnitude of effect. That is, at baseline, those who had serum concentrations <50 nmol/L (20 ng/mL) had 3.3 times of odds of a preterm birth compared to those with serum concentrations ≥100 nmol/L (40 ng/mL; p=0.27). At 2nd trimester, the odds were 2.0 fold (p=0.21) and at the end of pregnancy, the odds were 3.8 fold (p=0.01). The major findings from this exploratory analysis were: (1) maternal vitamin D status closest to delivery date was more significantly associated with preterm birth, suggesting that later intervention as a rescue treatment may positively impact the risk of preterm delivery, and (2) a serum concentration of 100 nmol/L (40 ng/mL) in the 3rd trimester was associated with a 47% reduction in preterm births.
Keywords: Cholecalciferol, Pregnancy, Preterm birth, Vitamin D
Introduction
There have been observational reports that maternal vitamin D status at baseline and not closest to delivery is a better predictor of pregnancy outcomes (1), suggesting that a cascade of events is set into motion that may not be modifiable by vitamin D supplementation. Others suggest that pregnancy outcomes are modifiable through vitamin D repletion as late as the second trimester and even into the third trimester (2-8), suggesting that vitamin D supplementation given as a rescue could impact later outcome.
Utilizing a preexisting pregnancy database of close to 500 pregnant women followed from 12-16 weeks of gestation until delivery, the objective of this exploratory post-hoc analysis was to determine the relationship between the timing of total circulating 25(OH)D concentrations during pregnancy and the pregnancy outcome of preterm birth. It was hypothesized that the total circulating 25(OH)D concentration closest to delivery would be most significantly associated with preterm birth as opposed to vitamin D status earlier in pregnancy.
Methods
Study Design
In this post-hoc analysis, the datasets from two randomized controlled vitamin D supplementation studies—NICHD (n=333) and Thrasher Research Fund (n=154) were combined. Details about both clinical trials have been published previously (9-11) and are summarized below. The studies administered identical questionnaires to produce comparable sociodemographic and clinical characteristics using the same criteria. Outcome measures for both clinical trials included the following: [1] maternal baseline and delivery 25(OH)D; [2] neonatal 25(OH)D concentration; and [3] gestational age at delivery in weeks. Of note, the subjects and study team were blinded to treatment throughout both study periods. In addition, during the conduct of these studies it was not known that there was a relationship between vitamin D status and preterm birth, and therefore, women were not counseled regarding this potential risk factor.
Definition of Preterm Birth: Information on gestational age was based on the mother's report of the first day of her last menstrual period generating the obstetrical Expected Date of Confinement or Delivery (EDC or EDD) with ultrasound confirmation at the time of mother's first obstetrical visit. All women were enrolled in either the NICHD trial or the Thrasher Research Fund trial <16 weeks of gestation and thus ultrasound confirmation of dating was within the first trimester/early second trimester. Preterm birth was defined as delivery of a liveborn infant at <37 completed weeks of gestation.
Laboratory measurements
Maternal and Cord Blood/Neonatal Total Circulating 25(OH)D Concentrations: A rapid, direct RIA developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN) was used to measure total circulating 25(OH)D concentration in serum samples as previously described (9). The laboratory participated in an independent quality assessment/assurance program (DEQAS)(12) using National Institute of Standards and Technology (NIST) standards in place throughout both clinical trials. The inter- and intra-assay coefficient of variation is ≤10%.
Statistical Analyses
Baseline demographic characteristics were summarized for the NICHD and Thrasher cohorts and compared using chi-square tests for categorical variables (race, insurance, education, season at enrollment, and preterm birth), Mann-Whitney tests for continuous variables (age and baseline maternal 25(OH)D), and Poisson regression for count data (parity). Pearson's correlation was used to measure the association between maternal serum 25(OH)D at 3 timepoints during pregnancy: baseline, 1st trimester (<16 wks); 2nd trimester (16-26 wks); and 3rd trimester (≥27 wks to delivery) and gestation week at birth. If a woman had more than one measurement during any of the three time periods, the mean of the values was used. Logistic regression was used to determine the association between 25(OH)D serum levels and the risk of having a preterm birth at each timepoint, adjusting for study and participant race/ethnicity. Participants with a race of “other” were excluded due to small cell sizes. Correlation and logistic analyses were also conducted separately for each race/ethnic group to identify differences in trend by race/ethnicity, adjusting for study. Statistical analyses were performed using SPSS statistics version 22 (IBM, Armonk, NY).
Results
There were a total of 487 women who had participated in the previous pregnancy vitamin D supplementation studies: 333 in the NICHD trial and 154 in the Thrasher Research Fund trial on whom all study variables were available. There were an additional 16 women in the NICHD trial and 9 women in the Thrasher Research Fund study on whom key variables—25(OH)D measurements at all three timepoints were not available, and so those women were excluded from the analysis. Of the 25 that were excluded, 9 were preterm births, of which 4 were born <32 weeks gestation. Focusing on the 25 who were excluded: 12 did not have measurements at <16 weeks; 8 did not have measurements at 16-26 weeks and 5 did not have measurements ≥27 weeks to delivery. Of the 9 women who delivered preterm and who were excluded: 2 did not have measurements at <16 weeks; 3 did not have measurements at 16-26 weeks; and 4 did not have measurements ≥27 weeks to delivery.
The sociodemographic characteristics of the cohort are found in Table 1. In these diverse groups of women, there were significant differences between the NICHD and Thrasher Research Fund cohorts by race/ethnicity, maternal age, parity, insurance status, education level, and season of enrollment but not baseline 25(OH)D or proportion of preterm births. Forty-one (8.4%) of women in the combined cohort had a preterm birth (<37 weeks). The median gestational age at delivery was 39 weeks with the earliest delivery being at 27 weeks of gestation, which corresponds to the beginning of the 3rd timepoint.
Table 1.
Maternal Sociodemographic and Clinical Characteristics of Cohort
| Characteristic | Combined Cohort (N=487) | NICHD Cohort (N=333) | Thrasher Cohort (N=154) | p-value* |
|---|---|---|---|---|
| Race/ethnicity (N, %) | <0.0001 | |||
| Black | 156 (32.0%) | 87 (26.1%) | 69 (44.8%) | |
| White, Non-Hispanic | 127 (26.1%) | 112 (33.6%) | 15 (9.7%) | |
| Hispanic | 201 (41.3%) | 134 (40.2%) | 67 (43.5%) | |
| Other | 3 (0.6%) | 0 (0.0%) | 3 (1.9%) | |
| Age, years (median and range) | 26 (16-44) | 27 (16-44) | 24 (16-39) | <0.0001 |
| Parity, (median and range) | 1 (0-9) | 2 (0-9) | 1 (0-4) | <0.0001 |
| Insurance** | <0.0001 | |||
| None/Medicaid (N, %) | 325 (67.0%) | 203 (61.3%) | 122 (79.2%) | |
| Education** | <0.0001 | |||
| Some college or higher (N, %) | 251 (53.4%) | 202 (63.9%) | 49 (31.8%) | |
| Season at enrollment | 0.005 | |||
| Spring/Summer (N, %) | 258 (53.0%) | 162 (48.6%) | 96 (62.3%) | |
| Baseline Maternal 25(OH)D, nmol/L (median and range) | 58 (5-185) | 58 (5-173) | 55 (15-185) | 0.30 |
| Preterm Birth (<37 weeks) (N, %) | 41 (8.4%) | 25 (7.5%) | 16 (10.4%) | 0.29 |
Race, insurance, education, season at enrollment, and preterm birth compared using chi-square tests. Age and baseline maternal 25(OH)D compared using Mann-Whitney test. Parity compared using Poisson regression.
2 participants missing insurance status data and 17 missing education level data.
When analyzing 25(OH)D as the indicator of vitamin D status of the women during pregnancy, 25(OH)D values closer to delivery were more strongly correlated with gestational age at birth than earlier values as shown in the correlation (see Table 2). While 25(OH)D status at baseline was significantly associated with gestational age at birth, the strength of the association increased at the timepoint ≥27 weeks gestation. There was no correlation between vitamin D status obtained at 16-26 weeks of gestation and gestational age at birth.
Table 2.
Correlation between 25(OH)D Concentration at Three Time Points during Pregnancy and Gestational Age at Delivery
| 25(OH)D at <16 wks | 25(OH)D at 16-26 wks | 25(OH)D at ≥27 wks | |
|---|---|---|---|
| Gestational Age (wks at delivery) | r = 0.108 (p=0.017)* | r = 0.076 (p=0.093) | r = 0.146 (p=0.001)* |
p<0.05
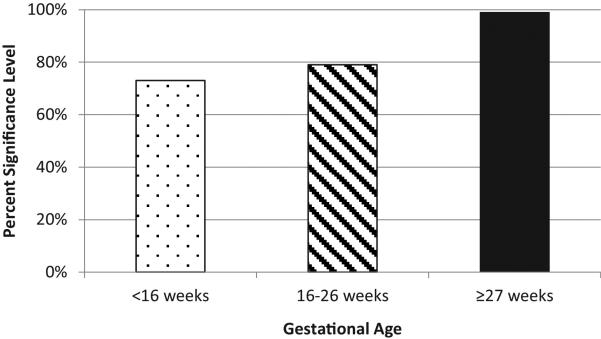
As shown in Table 3 and Figure 1, when logistic regression was performed with preterm birth (<37 weeks) as the outcome and 25(OH)D quartiles as the predictor variable, as with the correlation analysis, the measurements closer to delivery were more significantly associated and had a higher magnitude of effect, adjusting for study and participant race/ethnicity. That is, at baseline serum level, those who had serum levels <50 nmol/L (<20 ng/mL) had 3.3 times the odds of a preterm birth compared to those with serum levels ≥100 nmol/L (≥40 ng/mL) (p=0.27). At 2nd trimester, the odds were 2.0 fold (p=0.21) and at the end of pregnancy, the odds were 3.8 fold (p=0.01). Participants with a serum concentration of ≥100 nmol/L (≥40 ng/mL) in the 3rd trimester had a 47% reduction in preterm births compared to those <100 nmol/L (<40 ng/mL), adjusting for study and race/ethnicity (OR=0.53, p=0.08).
Table 3.
Logistic Regression Predicting Preterm Birth (<37 weeks of gestation) with 25(OH)D Quartiles, Adjusted for Study and Race/Ethnicity
| Serum 25(OH)D Concentration | Baseline 25(OH)D levels (<16 weeks) (OR, 95% CI) | Second Trimester 25(OH)D levels (16-26 weeks) (OR, 95% CI) | Third Trimester 25(OH)D levels (≥27 weeks) (OR, 95% CI) |
|---|---|---|---|
| <50 nmol/L (20 ng/mL) | 3.29 (0.39, 27.59) | 1.98 (0.67, 5.86) | 3.81 (1.35, 10.71)* |
| 50-74 nmol/L (20-29 ng/mL) | 1.49 (0.18, 12.27) | 1.07 (0.45, 2.56) | 1.81 (0.70, 4.70) |
| 75-99 nmol/L (30-39 ng/mL) | 2.05 (0.25, 16.81) | 0.51 (0.21, 1.25) | 1.48 (0.63, 3.47) |
| ≥100 nmol/L (40 ng/mL) | 1.00 | 1.00 | 1.00 |
p<0.05
Figure 1.
Significance Level for Odds of Preterm Birth Comparing Participants <50 nmol/L to ≥100 nmol/L, Adjusted for Study and Race/Ethnicity
Additional analyses were conducted using the 25 excluded participants and in each case the relationship between 25(OH)D concentration and preterm birth showed a stronger trend towards maternal 25(OH)D closer to delivery having a larger and more significant magnitude of effect (data not shown). This indicates that excluding participants without data for each timepoint did not influence the results.
Rates of preterm births for the cohort by race/ethnicity were as follows: 13 (6%) of Hispanics, 10 (8%) of whites, and 17 (11%) of black women delivered prematurely (<37 weeks). When looking at timing of 25(OH)D by racial/ethnic group, there were some significant differences. For Hispanic women (n=201), as with the overall cohort, the strongest correlation for gestational age at delivery was seen with 25(OH)D concentration at ≥27 weeks gestation (r=0.14, p=0.05). In the logistic regression predicting preterm birth using 25(OH)D quartiles, Hispanic women with 25(OH)D of <50 nmol/L (<20 ng/mL) at ≥27 weeks were 9.6 times more likely to deliver prematurely compared with those whose 25(OH)D was ≥100 nmol/L (≥40 ng/mL), adjusting for study (95% CI 1.65, 56.37). In black women (n=156), baseline 25(OH)D was more strongly correlated with gestational age at delivery (r=0.20, p=0.01), with a weaker trend at the third timepoint (r=0.15, p=0.07). In the subgroup analyses for white women (n=127), these associations did not reach significance.
Discussion
In this exploratory post hoc analysis of almost five hundred pregnant women, maternal vitamin D status closest to delivery date was most closely associated with preterm birth and gestational age at delivery. In the overall cohort and Hispanic women, the earlier timepoints were more weakly associated with preterm birth. Also, those with lower 25(OH)D concentrations had a higher odds of preterm birth. These findings suggest that while early intervention with vitamin D can impact later risk of preterm delivery, especially in Hispanic women, those who are diagnosed with vitamin D deficiency during pregnancy may also benefit from vitamin D given as “rescue” therapy.
There are few reported studies that explore the association between vitamin D status and preterm birth. In our earlier Thrasher Research Fund study (10), we found an association between 25(OH)D concentration and preterm birth, but that included those women who had spontaneous preterm birth and women with hypertensive disorders of pregnancy and preeclampsia. The prior health outcomes paper of the Combined Cohort used preterm birth without preeclampsia as the outcome as well hypertensive disorders of pregnancy (11). After controlling for race and study site, preterm birth and labor were inversely associated with predelivery and mean 25(OH)D, but not baseline 25(OH)D. In the NICHD and Thrasher Research Fund Combined Cohort, there was similar association between 25(OH)D and preterm birth, but by treatment group only a trend was seen with fewer preterm births in the higher dose groups (2000 ad 4000 IU vitamin D compared to the control-400 IU/day group) (11). The findings have been explained as due to reverse causality in that there was insufficient time for women to attain higher 25(OH)D if they were deficient at baseline, and thus those women delivering prematurely would have lower 25(OH)D concentrations (13). Yet, the pharmacokinetics of vitamin D during pregnancy shows that women attain steady-state by 8 weeks of supplement intake (9). Further, given the finding in this post-hoc analysis that the 25(OH)D concentration closest to delivery is most predictive of gestational age at delivery (with higher concentrations than at baseline), which persisted across racial/ethnic subgroups, argues against reverse causality and the potential for improved outcome through vitamin D supplementation even if initiated later in pregnancy.
Other literature supports the premise that vitamin D status plays a role in preterm birth. Bodnar et al (14) reported an association between spontaneous preterm birth (sPTB) and circulating 25(OH)D concentrations in their analysis of a random subcohort from the US Collaborative Perinatal Project (1959–1965) (n = 2,629) augmented with all remaining cases of spontaneous preterm birth before 35 weeks’ gestation (n=767). While they found no relationship between 25(OH)D and spontaneous preterm birth among white women, an association was found with nonwhite mothers: serum 25(OH)D concentrations of 30 to <50, 50 to <75, and ≥75 nmol/L were associated with reductions of 1.0–1.6 cases of sPTB per 100 live births and 20%–30% reductions in risk of sPTB compared with 25(OH)D levels less than 30 nmol/L after adjustment for prepregnancy body mass index (weight (kg)/height (m)2), season, and other confounders. In this post-hoc analysis, similar to Bodnar et al, there was no association between 25(OH)D concentrations at the three timepoints and gestational age at delivery and preterm birth in white women, but a reduction in preterm birth with improved vitamin D status most notable in Hispanic women, and to a lesser extent, in black women.
The limitations of this exploratory post-hoc analysis are that this was a post-study analysis that did not include controlling for other covariates related to preterm birth such as body mass index (BMI), placenta previa, hypertension, gestational diabetes, and other sociodemographic factors such as smoking and alcohol use (which were extremely low in this cohort). The second issue is one of generalizability and whether or not the women in the study are representative of the larger population (or other clinic sites). There is also the limitation of sample size, with a larger sample size necessary in order to investigate race/ethnic trends further. Lastly, multiple statistical comparisons were performed. The statistically significant correlation and regression results for the third trimester survive alpha correction; however, the first trimester and race/ethnicity subgroup analysis results do not.
In conclusion, in this exploratory post hoc analysis maternal vitamin D status closest to delivery date was more strongly associated with the risk of preterm birth. Earlier timepoints were only weakly associated with preterm birth and gestational age at delivery. The implications of these findings are that intervention with vitamin D supplementation throughout pregnancy but even given as “rescue therapy” may impact vitamin D status and pregnancy outcome. Further work is needed to assess the impact of early vs. rescue intervention with vitamin D supplementation on preterm birth rates and pregnancy outcomes such as preeclampsia.
Highlights.
Association between serum 25(OH)D & preterm birth during 3 timepoints was examined
25(OH)D value closest to delivery more strongly correlated with preterm birth
Findings most notable in Hispanic women
47% lower preterm birth rate associated with 25(OH)D >100 nmol/L at 27 weeks
Later intervention as rescue therapy may positively impact risk of preterm delivery
Acknowledgements
We wish to acknowledge the hundreds of women who participated in our vitamin D pregnancy supplementation trials. We also would like to thank Dr. Rebecca McNeil for her statistical advice throughout the preparation of this manuscript and Myla Ebeling for her countless hours in toiling over the NICHD data. Lastly, we would like to thank our study coordinators without whom this work would not be possible.
Supported in part by the Thrasher Research Fund, NICHD R01 HD47511, NIH RR01070, and the South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, and NIH/NCRR Grant Number UL1 RR029882.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 17th Vitamin D Workshop as a poster presentation on June 20, 2014 in Chicago, IL.
References
- 1.Baker AM, Haeri S, Camargo CA, Jr., Stuebe AM, Boggess KA. First-trimester maternal vitamin D status and risk for gestational diabetes (GDM) a nested case-control study. Diabetes/metabolism research and reviews. 2012;28(2):164–8. doi: 10.1002/dmrr.1282. Epub 2011/08/06. doi: 10.1002/dmrr.1282. PubMed PMID: 21818838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooke OG, Brown IRF, Bone CDM, Carter ND, Cleeve HJW, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. British medical journal. 1980;1:751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Brit Med J. 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maxwell J, Ang L, Brooke O, Brown I. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. Br J Obstet Gynaecol. 1981;88:987–91. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 5.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau J, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstetrics and gynecology. 1986;68:300–4. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Cockburn F, Belton N, Purvis R, Giles M, Brown J, Turner T, Wilkinson E, Forfar J, Barrie W, McKay G, Pocock S. Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. British medical journal. 1980;231:1–10. doi: 10.1136/bmj.281.6232.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marya R, Rathee S, Dua V, Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res. 1988;88:488–92. [PubMed] [Google Scholar]
- 8.Marya R, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecologic and obstetric investigation. 1981;12:155–61. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- 9.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. Epub 2011/06/28. doi: 10.1002/jbmr.463. PubMed PMID: 21706518; PubMed Central PMCID: PMC3183324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, Bivens B, Davis DJ, Smith PG, Murphy M, Shary JR, Hollis BW. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208(2):137, e1–e13. doi: 10.1016/j.ajog.2012.10.888. Epub 2012/11/08. doi: 10.1016/j.ajog.2012.10.888. PubMed PMID: 23131462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, Hamilton SA, Hollis BW. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. The Journal of steroid biochemistry and molecular biology. 2013;136:313–20. doi: 10.1016/j.jsbmb.2013.01.002. Epub 2013/01/15. doi: 10.1016/j.jsbmb.2013.01.002. PubMed PMID: 23314242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12(1):19–28. doi: 10.2174/138945011793591608. Epub 2010/08/28. doi: BSP/CDT/E-Pub/00160 [pii]. PubMed PMID: 20795940. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, Simhan HN. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. American journal of epidemiology. 2014;179(2):168–76. doi: 10.1093/aje/kwt237. doi: 10.1093/aje/kwt237. PubMed PMID: 24124195; PubMed Central PMCID: PMC3873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodnar LM, Simhan HN, Catov JM, Roberts JM, Platt RW, Diesel JC, Klebanoff MA. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology. 2014;25(2):207–14. doi: 10.1097/EDE.0000000000000039. doi: 10.1097/EDE.0000000000000039. PubMed PMID: 24457526; PubMed Central PMCID: PMC4053531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal Vitamin D Deficiency Increases the Risk of Preeclampsia. 2007:3517–22. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei SQ, Audibert F, Hidiroglou N, Sarafin K, Julien P, Wu Y, Luo ZC, Fraser WD. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG: An International Journal of Obstetrics & Gynaecology. 2012 doi: 10.1111/j.1471-0528.2012.03307.x. no-no. doi: 10.1111/j.1471-0528.2012.03307.x. [DOI] [PubMed] [Google Scholar]
- 17.Powe CE, Seely EW, Rana S, Bhan I, Ecker J, Karumanchi SA, Thadhani R. First Trimester Vitamin D, Vitamin D Binding Protein, and Subsequent Preeclampsia. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.110.158238. HYPERTENSIONAHA.110.158238. doi: 10.1161/hypertensionaha.110.158238. [DOI] [PMC free article] [PubMed] [Google Scholar]