Abstract
Objectives
This study aimed to [1] confirm that nonobese adolescents with polycystic ovary syndrome (PCOS) have higher anti-Mullerian hormone (AMH) than controls; [2] examine the relationship of AMH with PCOS features and hormonal profile; and [3] approximate an AMH value that discriminates between adolescents with PCOS and controls.
Design
Case-control study.
Setting
Subspecialty ambulatory clinic.
Patients
Thirty-one nonobese adolescent girls (age 13–21 years), 15 with PCOS diagnosed using the National Institutes of Health (NIH) criteria and 16 healthy control subjects. Subjects and controls were comparable for body mass index z-score, age and ethnicity.
Main outcome measure(s)
AMH in PCOS subjects and control groups, correlation of AMH with hormonal parameters.
Results
AMH was higher in PCOS subjects (4.4 ±3.4 ng/mL) than in controls (2.4 ±1.3 ng/mL), when adjusted for menstrual age. In the entire group (PCOS and controls), AMH correlated with androgens, ovarian size and the presence of polycystic ovary (PCO) appearance. There was no difference in average ovarian size between PCOS (7.1 ±2.6 cm3) and controls (6.7 ±1.8 cm3). PCOS subjects were 1.49 times more likely to have AMH >3.4 ng/mL (confidence interval 0.98–2.26 ng/mL).
Conclusions
Our data suggest that AMH may be a useful adjunct in the diagnosis of PCOS in adolescents.
Keywords: adolescent health, anti-Mullerian hormone, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in adolescents and women of reproductive age and affects between 6% and 10% of these populations when using the strictest diagnostic criteria (1, 2). Diagnostic features of PCOS include hyper-androgenism and ovulatory dysfunction (3). Additionally, individuals with PCOS often develop features of the metabolic syndrome (4, 5). Although PCOS commonly occurs in adolescence, its diagnosis is not straightforward because menstrual irregularity, hirsutism and acne are frequently transient and nonprogressive in this age group (4). Whereas premature diagnosis of PCOS may lead to unnecessary treatment and psychological distress, correct diagnosis in adolescence will allow for early intervention, leading to improvement of symptoms and prevention of comorbidities.
Stringent diagnostic criteria have recently been proposed for the diagnosis of PCOS in adolescents and require hyperandrogenism, chronic anovulation and polycystic ovaries (PCO) by ultrasound (4). The accepted definition of PCO is based on transvaginal ultrasound in adults and may not be applicable to adolescents (6) since transabdominal ultrasound is generally used in adolescents and ovarian size may be smaller in affected adolescents with PCOS than in affected adults (7).
Anti-Mullerian hormone (AMH) is a growth and differentiation factor produced by ovarian granulosa cells, predominantly from growing preantral and early antral follicles, and may be elevated in adolescents with PCOS (8–10). Production of AMH begins in the fetus. Levels are low until age 8 years, rise until puberty and, at age 25, begin to decline steadily until menopause (11). The close relationship with follicle number suggests that AMH may serve as a marker for the presence of PCOS (10, 12). Indeed, adolescents with PCOS have a higher antral follicle count and a larger ovarian size than adolescents without PCOS (7), which is supportive of this premise.
Although there have been several studies that examine AMH in adults with PCOS, there are few that examine an exclusively nonobese adolescent population. The objectives of this study were to [1] confirm that non-obese adolescents with PCOS have higher AMH than controls, [2] examine the relationship of AMH with associated PCOS features and hormonal profile, and [3] approximate an AMH value that discriminates between adolescents with PCOS and controls.
Materials and methods
Subjects
The study group comprised 31 nonobese [body mass index (BMI) < 95th percentile] adolescent girls (15 with PCOS and 16 controls) recruited from the pediatric endocrinology and adolescent clinics and via posted flyers at Columbia University Medical Center (CUMC) in New York City. The subjects in this analysis were recruited as part of a larger case-control study and had AMH levels, hormonal values and trans-abdominal pelvic ultrasounds available. Informed consent was obtained from subjects 18 years and older and from a parent or guardian for subjects under age 18 years. Assent was also obtained from subjects under age 18. The protocol was approved by the Institutional Review Boards of CUMC. All authors complied with the World Medical Association Declaration of Helsinki regarding the ethical conduct of research involving human subjects.
Clinical assessment
The diagnosis of PCOS was made using the National Institutes of Health (NIH) criteria [3]. Inclusion criteria for all subjects were [1] between the ages of 13 and 21 years, [2] BMI less than the 95th percentile for age and [3] menarche at least 2 ears before enrollment. Inclusion criteria for PCOS subjects were [1] oligomenorrhea (cycle interval > 45 days) (13) or amenorrhea (absent menses > 3 months), [2] clinical or laboratory evidence of hyperandrogenemia and [3] no evidence of 21-hydroxylase deficiency (14) or of other hormonal, adrenal or gonadal disorders. Inclusion criteria for controls were [1] eumenorrhea, [2] absence of significant hirsutism or acne, and [3] no evidence of hormonal, adrenal or gonadal disorder. Exclusion criteria for all subjects included [1] current or past pregnancy; [2] use of an oral contraceptive pill or metformin within 3 months of the protocol; [3] gestational history complicated by maternal diabetes, multiple gestation, preterm delivery or small for gestational age; [4] chronic medical conditions; or [5] glucocorticoid or other hormonal therapy.
Assessment of subjects was performed at the Pediatric Outpatient Unit of the Clinical Research Resource at CUMC. Assessments were performed on any cycle day. Height, weight, heart rate and blood pressure were measured; BMI was calculated and BMI z-scores were determined using reference data (15). Degree of hirsutism was assessed by the use of the Ferriman-Gallwey hirsutism scale (16), and severity of acne was determined by a subjective scale of 0–3 (0 = no acne, 1 = mild, 2 = moderate, 3 = severe).
Blood samples were drawn between 8 and 9 a.m. after an overnight fast for AMH, glucose, insulin, dehydroepiandrosterone sulfate (DHEAS), androstenedione, total T, free T and E2. Trans-abdominal pelvic ultrasound was used to measure ovarian size and to assess the presence of PCO appearance. All ultrasounds were read by the same reader (JPL). Ovarian size was determined as the average of the left and right ovaries. In the event of a discrepancy of >10 cm3 between the left and the right ovary (e.g., dominant follicle in ovulating subjects), the smaller ovary was used to determine ovarian size. PCO appearance was a subjective appearance of multiple follicles on the periphery of the ovary. Dual-energy X-ray absorptiometry (Lunar Prodigy, GE, Madison, WI, USA) was used to determine percentage body fat.
Assays
AMH was measured in duplicate by Gen II ELISA following the manufacturer’s instructions (Beckman Coulter, Brea, CA, USA). All other hormonal laboratory tests were measured by Esoterix Laboratories (Calabasas Hills, CA, USA): DHEAS by radioimmunoassay after hydrolysis; E2, androstenedione and total T by high-performance liquid chromatography with tandem mass spectrometry; and free T by equilibrium dialysis.
Statistical analysis
Comparison of groups for continuous variables including age, height, weight, BMI z-score, age at menarche and menstrual age (MA) was performed using the Student’s t-test. MA-adjusted analysis of covariance was used to analyze differences in AMH and hormone levels. Results for continuous variables are presented as mean ± standard deviations. Relationships between AMH and PCOS markers in the entire group were evaluated by Pearson correlations.
A discriminant analysis was performed to approximate a cutoff value for AMH that would distinguish PCOS and controls (17). The analysis used a cross-validation algorithm. The discriminant function was derived by the non-parametric method from the iterative process of classifying each subject based on the discriminant function calculated from the remaining subjects. The sensitivity, specificity, positive and negative predictive value, and odds ratio were calculated from the discriminant analysis-derived cross-clarification table.
Results
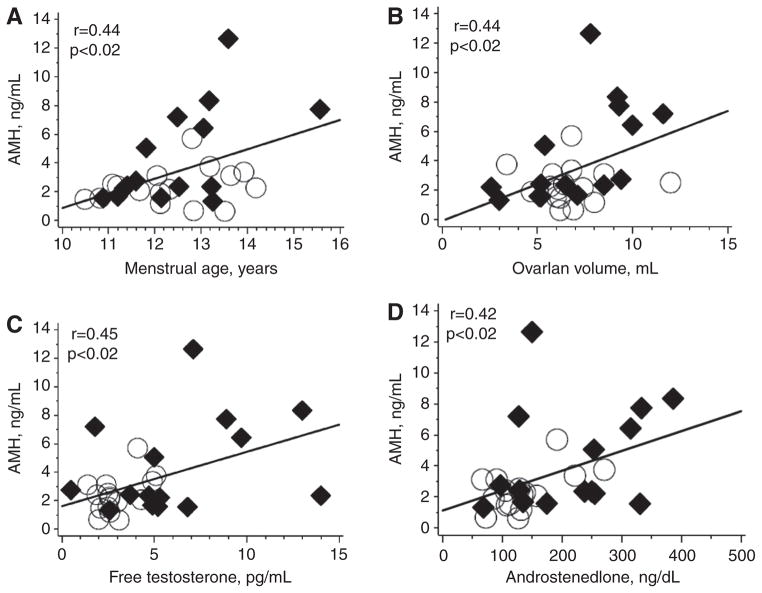
The clinical characteristics of the study population are summarized in Table 1. AMH was higher in the PCOS subjects (4.4 ±3.4 ng/mL) than in controls (2.4 ±1.3 ng/mL) (p <0.05). As expected, the PCOS subjects had higher ovarian and adrenal andogrens than the controls (p <0.05). This was also reflected in the higher Ferriman-Gallwey score in the PCOS subjects (p <0.0001) (Table 1). Ovarian size was similar in the PCOS subjects (7.1 ±2.6 cm3) and controls (6.7 ±1.8 cm3) (p =0.64). In an assessment of the entire group (n =31), AMH correlated with average ovarian size (r =0.42), PCO appearance (r =0.57), free T (r =0.46) and androstenedione (r =0.42) (p <0.03 for all) (Figure 1).
Table 1.
Characteristics of the study population.
| PCOS (n = 15) | Control (n = 16) | p-Value | |
|---|---|---|---|
| Age, years | 16.6 ± 2.1 | 18.6 ± 2.6 | < 0.03 |
| Weight, kg | 57.0 ± 8.9 | 57.0 ± 7.0 | NS |
| Height, cm | 158.4 ± 6.0 | 160.3 ± 6.3 | NS |
| BMI z-score | 0.45 ± 0.79 | 0.19 ± 0.60 | NS |
| Percentage body fat | 36.6 ± 8.4 | 34.2 ± 6.5 | NS |
| Years since menarche | 4.2 ± 2.2 | 6.3 ± 2.2 | < 0.02 |
| AMHa | 4.4 ± 3.4 | 2.4 ± 1.3 | < 0.05 |
| Mean ovarian size, cm3 | 7.1 ± 2.6 | 6.7 ± 1.9 | NS |
| Testosterone, ng/dLa | 39.9 ± 18.9 | 29.6 ± 8.9 | 0.11 |
| Free testosterone, pg/mLa | 6.2 ± 3.8 | 2.9 ± 1.1 | < 0.006 |
| DHEAS, μg/dLa | 190.5 ± 92.3 | 131.5 ± 67.2 | < 0.05 |
| Androstenedione, ng/dLa | 216.3 ± 97.8 | 134.2 ± 54.6 | < 0.003 |
| Ferriman-Gallwey scorea | 21.3 ± 6.7 | 8.6 ± 4.8 | < 0.0001 |
| Estradiol, ng/dLa | 28.1 ± 31.1 | 58.8 ± 47.6 | < 0.05 |
p-Value adjusted for menstrual age.
NS, Not significant.
Figure 1. Scatterplots of AMH with ovarian and hormonal parameters in PCOS subjects and controls.
(A) AMH vs. menstrual age; (B) AMH vs. ovarian volume; (C) AMH vs. free testosterone; (D) AMH vs. androstenedione. ◆, PCOS subjects; ○, controls subjects.
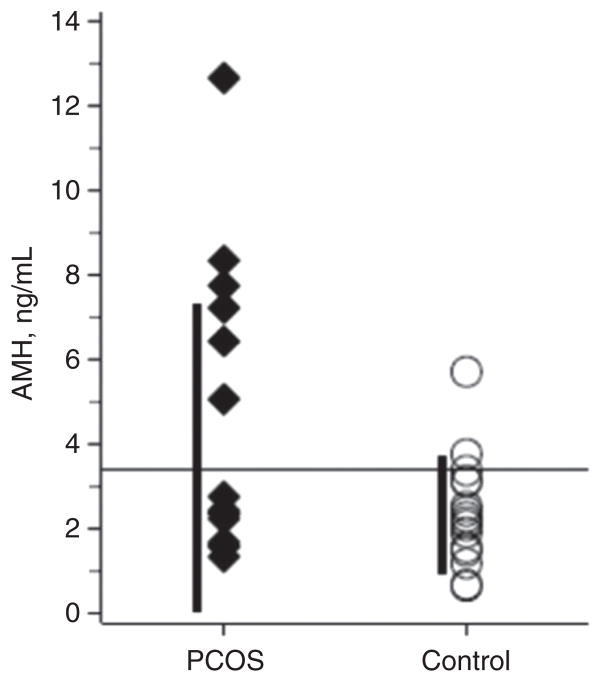
In the discriminant analysis, an AMH value of 3.4 ng/mL best distinguished between PCOS and controls (Figure 2). This value had a sensitivity of 40% and a specificity of 93.8% for predicting PCOS and had a positive predictive value of 75% and a negative predictive value of 61%. Those with PCOS were 1.49 more likely to have an AMH value > 3.4 ng/mL (confidence interval 0.98–2.26 ng/mL).
Figure 2. Discriminant analysis of AMH.
AMH in PCOS subjects and control subjects. The value that best discriminates between PCOS subjects and controls in adolescents is 3.4 ng/mL (horizontal line); however, there is an overlap between the groups. ◆, PCOS subjects; ○, control subjects.
Discussion
This is one of the first studies to address the utility of AMH in the diagnosis of PCOS in an exclusively nonobese adolescent sample. In this small group, AMH was higher in PCOS subjects than in controls and correlated with androgens and PCO appearance. A cutoff value of 3.4 ng/mL was approximated to best discriminate between PCOS subjects and controls in this small group.
This study supports several observations that have been described in adolescents and adults: [1] The close relationship between AMH and number of follicles was supported by the observed correlation between AMH and both ovarian size and PCO appearance. [2] The described relationship between AMH and androgens was supported by the correlation of AMH with both free T and androstenedione. It has been suggested that androgens may stimulate AMH production by increasing follicle number; however, it is still unclear whether the relationship is causative or simply incidental in which both androgens and AMH are byproducts of the large number of follicles in PCO (8). In fact, Villarroel et al. (18) suggest that AMH is higher in regularly menstruating adolescents with PCO than in adolescents with oligomenorrhea. In contrast to other adolescent studies, we did not find a significant difference in ovarian size between controls and PCOS subjects (7).
Several studies have been performed in adolescent and adult populations to determine an appropriate AMH cutoff for the diagnosis of PCOS. The cutoff values vary among studies because of the variables including AMH assay, PCOS diagnostic criterion and patient population; however, most authors agree that AMH has utility in the diagnosis of PCOS. The AMH cutoff value approximated in this study is within the range of values suggested by prior studies (2.8–10.7 ng/mL) (11, 19–22), and a recent meta-analysis suggested a cutoff of 4.7 ng/mL (23). One study of adults with PCOS suggested that AMH should replace PCO as a PCOS diagnostic criterion owing to the technical challenges associated with ultrasonography (11). Recently, it has been suggested that PCO should be included as a diagnostic criterion for PCOS in adolescents (4). Indeed, AMH may be an attractive alternative to ultrasonography in this age group. However, because of the significant overlap in AMH values observed between PCOS and control, it most likely cannot be used as an independent marker in the diagnosis of PCOS. A recent study of 207 adolescents showed that AMH had a low sensitivity, specificity and positive predictive value in predicting PCOS according to both the NIH and the Rotterdam Criteria (22). Explanations for these low values included selection bias and increased prevalence of menstrual irregularities in adolescents, which may confound the results.
The strengths of this study are as follows: [1] Use of a nonobese cohort, which minimizes the confounding effects of excess weight. This is relevant as an inverse association between obesity and AMH has been described (8). [2] Use of the NIH criteria for the diagnosis of PCOS, thus making the overdiagnosis of PCOS less likely. The limitations of this study are as follows: [1] Assessments were not uniformly performed in the early follicular phase in menstruating subjects. Although AMH does not fluctuate with the menstrual cycle (22, 24), it has recently been shown in vitro that estradiol may repress AMH expression (25). This effect may have led to lower AMH levels in mid-cycle participants. [2] The approximated AMH cut-point has not yet been validated in a sample population. [3] This small cohort may not represent the general population and may limit our ability to detect differences between PCOS and control subjects. [4] Control subjects were older than the PCOS subjects, which we accounted for in our statistical analysis by adjusting for menstrual age. [5] The subjects comprised only nonobese adolescents; thus the results may not be applicable to obese adolescents with PCOS. [6] The transabdominal approach was used for ultrasound studies, which may have limited our assessment of the ovaries.
In conclusion, this study addresses the use of AMH as an indicator of PCOS in an exclusively nonobese adolescent population. AMH was higher in PCOS subjects than in controls and, in the entire group, correlated with androgens, PCO appearance and ovarian size. An approximated AMH cutoff value of 3.4 ng/mL best discriminated between PCOS subjects and controls; however, there was an overlap between these groups. Although AMH cannot be used on its own, it may be useful as part of an algorithm, along with clinical signs, androgen levels and ultrasound. In light of the difficulties and uncertainties associated with ovarian ultrasound in the adolescent population, AMH may have the potential to replace its use for the diagnosis of PCOS in this age group. In order to further assess the use of AMH in this population, larger studies that use magnetic resonance imaging for better resolution of ovarian size and follicle count will be needed in this population.
Acknowledgments
Grant support: This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers KL2 TR000081 and UL1 TR000040 (formerly the National Center for Research Resources, grant numbers KL2 RR024157 and UL1 RR024156), and by the John M. Driscoll, Jr., M.D. Children’s Fund Award.
The authors thank Daniel Haisenleder, PhD, Associate Professor, Medicine, Endocrinology and Metabolism, School of Medicine, University of Virginia, for running the AMH assays.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or of the John M. Driscoll, Jr., Fund.
Contributor Information
Aviva B. Sopher, Division of Pediatric Endocrinology, Diabetes and Metabolism, Columbia University Medical Center, 622 West 168 Street, PH 522 5E, New York, NY 10032, USA.
Galina Grigoriev, Division of Pediatric Endocrinology, Diabetes and Metabolism, Columbia University Medical Center, New York, NY, USA.
Diana Laura, Division of Pediatric Endocrinology, Diabetes and Metabolism, Columbia University Medical Center, New York, NY, USA.
Tamara Cameo, Division of Pediatric Endocrinology, Diabetes and Metabolism, Columbia University Medical Center, New York, NY, USA.
Jodi P. Lerner, Department of Obstetrics and Gynecology, Columbia University Medical Center, New York, NY, USA
R. Jeffrey Chang, Department of Reproductive Medicine, University of California – San Diego, La Jolla, CA, USA.
Donald J. McMahon, Division of Endocrinology, Department of Medicine, Columbia University Medical Center, New York, NY, USA
Sharon E. Oberfield, Division of Pediatric Endocrinology, Diabetes and Metabolism, Columbia University Medical Center, New York, NY, USA
References
- 1.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewilly D, Diamanti-Kandarakis E, Excobar-Morreal HF, et al. The androgen excess and PCOS Society for polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam GR, editors. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific Publications; 1992. pp. 377–84. [Google Scholar]
- 4.Carmina E, Oberfield SE, Lobo RA. The diagnosis of poly-cystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203:201.e1–5. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance independent of obesity in polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 6.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Brown M, Park AS, Shayya RF, Wolfson T, Su HI, et al. Ovarian imaging by magnetic resonance in adolescent girls with poly-cystic ovary syndrome and age-matched controls. J Magn Reson Imaging. 2013;38:689–93. doi: 10.1002/jmri.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park AS, Lawson MA, Chuan SS, Oberfield SE, Hoeger KM, et al. Serum anti-Mullerian hormone concentrations are elevated in oligomenorrheic girls without evidence of hyperandrogenism. J Clin Endocrinol Metab. 2010;95:1786–92. doi: 10.1210/jc.2009-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siow Y, Kives S, Hertweck P, Perlman S, Fallat ME. Serum mullerian-inhibiting substance levels in adolescent girls with normal menstrual cycles or with polycystic ovary syndrome. Fertil Steril. 2005;84:938–44. doi: 10.1016/j.fertnstert.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 10.Pigny P, Merlen E, Yann R, Cortet-Rudelli C, Decanter C, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–62. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 11.Eilertsen TB, Vanky E, Carlsen SM. Anti-mullerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Human Reprod. 2012;27:2494–502. doi: 10.1093/humrep/des213. [DOI] [PubMed] [Google Scholar]
- 12.Pawelczak M, Kenigsberg L, Milla S, Liu Y, Shah B. Elevated serum anti-mullerian hormone in adolescents with polycystic ovary syndrome: relationship to ultrasound features. J Pediatr Endocrinol Metab. 2012;25:983–9. doi: 10.1515/jpem-2012-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ACOG Committee Opinion. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2006;108:1323–8. doi: 10.1097/00006250-200611000-00059. [DOI] [PubMed] [Google Scholar]
- 14.Armengaud J, Charkaluk M, Trivin C, Tardy V, Breat G, et al. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94:2835–40. doi: 10.1210/jc.2009-0314. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–27. [PubMed] [Google Scholar]
- 16.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 17.Rao CR. Linear statistical inference. New York: Wiley; 1973. [Google Scholar]
- 18.Villarroel C, Merino PM, Lopez P, Eyzaguirre FC, Van Velzen A, et al. Polycystic morphology in adolescents with regular menstrual cycles is associated with elevated anti-Mullerian hormone. Hum Reprod. 2011;26:2861–8. doi: 10.1093/humrep/der223. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfield RL, Wroblewski K, Padmanabhan V, Littlejohn E, Mortensen M, et al. Antimullerian hormone levels are independently related to ovarian hyperandrogenism and polycystic ovaries. Fertil Steril. 2012;98:242–9. doi: 10.1016/j.fertnstert.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–9. doi: 10.1093/humrep/der297. [DOI] [PubMed] [Google Scholar]
- 21.Casadei L, Madrigale A, Puca F, Manicuti C, Emidi E, et al. The role of serum anti-Mullerian hormone (AMH) in the hormonal diagnosis of polycystic ovary syndrome. Gynecol Endocrinol. 2013;29:545–50. doi: 10.3109/09513590.2013.777415. [DOI] [PubMed] [Google Scholar]
- 22.Hart R, Doherty DA, Norman RJ, Franks S, Dickinson JE, et al. Serum antimullerian hormone (AMH) levels are elevated in adolescent girls with polycystic ovaries and the polycystic ovarian syndrome (PCOS) Fertil Steril. 2010;94:1118–21. doi: 10.1016/j.fertnstert.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab. 2013;98:3332–40. doi: 10.1210/jc.2013-1393. [DOI] [PubMed] [Google Scholar]
- 24.Hagen CP, Aksglaede L, Sorensen K, Main KM, Boas M, et al. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95:5003–10. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 25.Grynberg M, Pierre A, Rey R, Leclerc A, Arouche N, et al. Differential regulation of ovarian anti-Mullerian hormone (AMH) by estradiol through α- and β-estrogen receptors. J Clin Endocrinol Metab. 2012;97:E1649–57. doi: 10.1210/jc.2011-3133. [DOI] [PubMed] [Google Scholar]