Abstract
In this study, we analyzed the impact that spontaneous seizures might have on the plasma membrane expression, composition and function of GABAA receptors (GABAARs). For this, tissue of chronically epileptic rats was collected within 3 hours of seizure occurrence (≤3 hours group) or at least 24 hours after seizure occurrence (≥24 hours group). A retrospective analysis of seizure frequency revealed that selecting animals on the bases of seizure proximity also grouped animals in terms of overall seizure burden with a higher seizure burden observed in the ≤3 hours group. A biochemical analysis showed that although animals with more frequent/recent seizures (≤3 hours group) had similar levels of GABAAR at the plasma membrane they showed deficits in inhibitory neurotransmission. In contrast, tissue obtained from animals experiencing infrequent seizures (≥24 hours group) had increased plasma membrane levels of GABAAR and showed no deficit in inhibitory function. Together, our findings offer an initial insight into the molecular changes that might help to explain how alterations in GABAAR function can be associated with differential seizure burden. Our findings also suggest that increased plasma membrane levels of GABAAR might act as a compensatory mechanism to more effectively maintain inhibitory function, repress hyperexcitability and reduce seizure burden. This study is an initial step towards a fuller characterization of the molecular events that trigger alterations in GABAergic neurotransmission during chronic epilepsy.
Keywords: GABAAR regulation, epilepsy, spontaneous seizures, epileptogenesis
INTRODUCTION
GABAA receptors (GABAAR) are heteropentameric ion channels with heterogeneous physiological, pharmacological and targeting properties that result from the combinatorial assembly of a family of homologous subunits including: α(1–6), β(1–3), γ(1–3), ε(1–3), δ, θ, and π (Macdonald and Olsen, 1994; Sieghart et al., 1999; Jacob et al., 2008). Under physiological conditions, receptors located at synaptic sites mostly contain γ subunits, have low affinity for GABA and mediate fast inhibitory neurotransmission; whereas receptors located at extrasynaptic sites primarily contain δ subunits (instead of γ subunits), exhibit high affinity for GABA and mediate tonic inhibitory neurotransmission (Glykys and Mody, 2007b; Luscher et al., 2011). Impairment of GABAAR-mediated neurotransmission and increased neuronal excitability play a critical role during epileptogenesis and during generation of spontaneous seizures (Sperk et al., 2004; Fritschy, 2008). During the latent period that precedes the onset of spontaneous seizures, animal models of TLE demonstrate increased excitability that is associated with a reduction in the number of GABAARs present at the plasma membrane (El-Hassar et al., 2007; Goodkin et al., 2008; Terunuma et al., 2008; González et al., 2013). During the chronic stage, epileptic animals experiencing spontaneous seizures also show altered inhibitory neurotransmission (Brooks-Kayal et al., 1998) but the molecular mechanisms linked to alterations in inhibitory neurotransmission (i.e., the manifestation of spontaneous seizures) are poorly understood.
Altered inhibitory neurotransmission in animals experiencing recurrent spontaneous seizures appears to stem, at least in part, from changes in gene expression and cellular distribution of GABAARs (Brooks-Kayal et al., 1998; Cossart et al., 2001). Receptors present in granule cells from dentate gyrus (DG) of chronically epileptic animals show increased GABAAR-mediated current density, zinc blockade and clonazepam augmentation (Gibbs et al., 1997; Leroy et al., 2004). GABAARs isolated from epileptic tissue and ectopically expressed in Xenopus oocytes show a characteristic ‘rundown’ of GABAAR-dependent currents that is independent of changes in membrane potential or receptor affinity (Palma et al., 2007; Mazzuferi et al., 2010). This ‘rundown’ effect can be detected immediately after the first spontaneous seizure and appears to be linked to alterations in GABAAR assembly (Palma et al., 2007; Mazzuferi et al., 2010). Altered subunit expression directly impacts channel assembly and changes the cellular distribution of GABAAR promoting the impairment of inhibitory neurotransmission (Brooks-Kayal et al., 1998; Peng et al., 2004; Lund et al., 2008). In this study, we analyzed the impact that unpredictable spontaneous seizures might have on the plasma membrane expression, composition and function of GABAARs expressed in the DG of chronically epileptic rats. Our studies provide an initial characterization of molecular changes that can occur during the chronic phase of epilepsy and its possible association with seizure burden.
MATERIALS AND METHODS
Animal Subjects
Male Sprague Dawley rats (Charles River, Wilmington, MA) were housed in a temperature-controlled vivarium with food and water ad libitum. Animal procedures were performed in accordance with Institutional Animal Care and Use Committee regulations and approved protocols by the University of Colorado Anschutz Medical Campus.
Pilocarpine-Induced Status Epilepticus
To minimize the peripheral effects of pilocarpine, rats were injected intraperitoneally with scopolamine (1 mg/kg) 30 minutes before administration of pilocarpine (385 mg/kg). According to a standard protocol, if rats did not exhibit convulsive seizures within 1 h of pilocarpine injection a maximum of two subsequent doses of pilocarpine (192.5 mg/kg) were given in order to produce equivalence in seizures between animals (Brooks-Kayal et al., 1998; Shumate et al., 1998). Diazepam (6 mg/kg; Hospira, Lake Forest, IL) was administered 1 h after the onset of SE in order to stop seizure progression and additional doses (3 mg/kg) were administered every 2 h until rats stopped seizing. Control rats were handled similarly but treated with a subconvulsive dose of pilocarpine (38.5 mg/kg) and 1/10 of the dose of diazepam (0.6 mg/kg).
Electrode Implantation
Animals were implanted with intracranial EEG electrodes approximately 4 weeks after pilocarpine treatment in order to characterize seizure burden. Rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (7 mg/kg) and then placed in a stereotaxic apparatus. Depth of anesthesia was monitored and maintained using isoflurane (1–4%) throughout the surgery. The area of incision was anesthetized with 1% lidocaine and cleaned before a midsagittal incision was made. The skin was retracted to drill small burr holes to accommodate the stainless steel screws that were used as electrodes (recording, ground and reference). For analysis of seizure burden, two screws were placed bilaterally 4 mm caudal to Bregma and 2.5 mm lateral from midline over the temporolimbic cortices. Reference and ground electrodes were placed bilaterally behind lambda (i.e., over the cerebellum). For the acquisition of recordings to evaluate spike frequency and theta power, depth-recording electrodes fashioned from PFA-coated stainless-steel wire (0.008″ diameter A-M Systems, Carlsborg, WA) were positioned in the pyramidal cell layer of the right CA1 (2.5 mm lateral, 4.0 mm caudal, 2.5 mm depth). The EEG recording electrode (i.e., a watch screw) was positioned below the dura of the left hemisphere over the temporo-limbic cortex (2.5 mm lateral, 4.0 mm caudal). Reference and ground electrodes (stainless steel screws) were placed bilaterally behind lambda (i.e., over the cerebellum). Dental acrylic was used to secure the electrodes in a plastic connector (Plastics-One, Roanoke, VA) according to standard methods (Zhang et al., 2004; Grabenstatter et al., 2014). Animals were allowed to recover from surgery for 1 week before proceeding with any further experimentation.
EEG Acquisition and Analysis
Epileptic and control rats were placed in a recording chamber equipped with flexible cables attached to a commutator (i.e., electric swivel) to allow free animal movement. Animals were recorded 24 hours/day for about 7 days prior to sacrifice using an automatic Pinnacle digital video-EEG system. EEG signals were sampled at 2 kHz, amplified by 100X, and band-pass filtered between 0.3 Hz and 650 Hz. Data analyses were performed off-line by a trained technician. Electrographic recordings were examined to identify electrographic seizures and potential artifacts as follows: seizures differed from background noise by the presence of EEG signals with progression of spike frequency; large-amplitude (at least three times baseline); and high-frequency activity (minimum of 5 Hz) that lasts at least 10 sec. Behavioral characterization of seizures was based on the Racine scale (Racine, 1972), seizures scored as class 2 or below were considered non-convulsive and seizures class 3 or above were categorized as convulsive. Seizure classification was conducted based on seizure number and type (convulsive or non-convulsive). Animals were considered epileptic when one or more spontaneous seizures detected in the EEG recordings showed a clear behavioral correlate.
To analyze the effects of spontaneous seizures on biochemical and electrophysiological analysis, the last 24 hours of EEG recordings were quickly reviewed before animals were euthanized. Tissue of epileptic animals was collected either ≤3 hours from the last seizure or ≥24 hours after the last seizure. Thus, tissue for one group of animals was collected only if seizure activity was observed during the previous 3 hours (≤3 hours group) and tissue for the second group was collected only if no seizure activity was observed in the last 24 hours (≥24 hours group). A post hoc analysis of seizure frequency showed that these two groups of chronically epileptic animals have differential seizure burden and revealed that the ≤3 hours group had more frequent seizures than the ≥24 hours group. Both groups of epileptic animals were composed of animals sacrificed after convulsive and non-convulsive seizures but while 92.3% of the final seizures detected in the ≥24 hours group were convulsive only 46.67% of the last seizures detected in the ≤3 hours group were convulsive.
Spectral analysis was performed on 30-min inter-ictal segments of data using routines written in Visual Basic that computed the average of multiple Fourier Transforms using a rectangular window with segments of 32768 points. As the sampling rate was 2 kHz, this provided excellent frequency resolution. The frequency was divided into bands as follows: theta (4–8 Hz), alpha (8–13 Hz), and beta-gamma (13–30Hz). Spike analysis was performed on the same segments using routines written in Visual Basic that first filtered the data using a window sync filter with a high frequency limit of 70 Hz and a low frequency limit of 1 Hz. Determination that a peak electrical response was a true spike included the following criteria: (1) amplitude greater than 3 standard deviations from the mean; (2) full width at half maximum of the peak being between 5 and 200 milliseconds; and, (3) the maximum slope greater than 4 times the mean slope. For each animal, data was randomly obtained from resting animals during both sleep and wake cycles. The selected segments of EEG recordings were located at least one hour before or after any detected seizure activity and thus correspond to samples of inter-ictal EEG.
Cell Surface Biotinylation
This protocol was adapted in our laboratory from previously reported methods (Grosshans et al., 2002; Gonzalez et al., 2007; Holman and Henley, 2007; González et al., 2013). Hippocampal slices (400 μm) were prepared using a McIlwan tissue chopper. To label plasma membrane proteins, freshly prepared slices were gently agitated for 30 min at 4°C in bubbled aCSF containing 1 mg/ml Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockford, IL). After quenching unreacted biotin, slices were microdissected to isolate the cornus ammonis 1 (CA1) as previously described (Silva et al., 2001; González et al., 2013). Tissue was lysed in RIPA buffer containing protease and phosphatase inhibitors by brief sonication and agitation at 4°C for 30 min and cleared of cell debris by centrifugation at 15,000 × g for 20 min. One aliquot of lysate (200 μl) was mixed with 4X Laemmli buffer (200 μl) and saved as “lysate fraction”. A second aliquot was mixed with an equal volume of Ultralink avidin-conjugated beads (Thermo Scientific, Rockford, IL) and incubated overnight at 4°C with constant agitation. After incubation beads were washed, once with RIPA buffer, twice with a high-salt buffer (50 mM Tris, 5 mM EDTA, 500 mM NaCl, 0.1% Triton X-100, pH 7.5), and once with a no-salt buffer (50 mM Tris, pH 7.5). Biotinylated proteins were recovered in 2X Laemmli buffer (400 μl) after incubating the beads at 37°C for 30 min. Proteins in the biotinylated fraction were diluted to the same extent than proteins in the total lysate, so that immunoreactivity in the lysate and biotinylated fractions is proportional when equal volumes are analyzed.
Immunoisolation of GABAA Receptors
Microdissected DG was obtained as described above. Lysates were obtained by passing the tissue through a 21G needle (25X) followed by agitation at room temperature (15 min) and then at 4°C (90 min). Lysates were centrifuged at 15,000 × g for 20 min to remove cell debris and pre-cleared by shaking with 40 μl of sepharose beads (1 h at 4°C). 300–350 μg of protein were incubated with 5 μg of mouse monoclonal antibodies for α1 (NeuroMab, Davis, CA) or β2/3 (Millipore, Billerica, MA) subunits or with 5 μg of non-immune mouse IgG (Santa Cruz Biotech, Santa Cruz, CA). After overnight incubation at 4°C, immune complexes were mixed with 25 μl of protein G-sepharose beads (GE Health Care, Piscataway, NJ) and incubated for 2 h at 4°C. Beads were washed with RIPA buffer and proteins were released in 25 μl of 2X Laemmli buffer by boiling for 3 min.
Western Blot
Samples were separated in SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Blots were blocked for 1 h at room temperature with 5% non-fat dry milk in Tris-buffered saline (pH 7.4) plus 0.05% Tween 20 and then incubated overnight at 4°C with primary antibodies diluted in 1% non-fat dry milk. After washing primary antibodies, blots were incubated with a secondary antibody for 1 h at room temperature. Immunoreactive bands were visualized using Super Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA) and film. After scanning the films, immunoreactive bands of the appropriate size were quantified using Image J software (National Institutes of Health, Bethesda, MD, USA). In some cases, to estimate potential variability in protein content and loading, blots were stripped and probed with an anti-actin antibody from Sigma (St. Louis, MO). Immunoreactivity for the protein of interest was normalized to actin immunoreactivity and compared to control values. Monoclonal mouse anti-β2/3 (clone 62-3G1) and polyclonal rabbit antibodies anti-α1 and anti-α4 were from Millipore (Billerica, MA). Polyclonal rabbit anti-γ2 was from Phosphosolutions (Aurora, CO). Anti-mouse or anti-rabbit secondary antibodies conjugated to horseradish peroxidase were from Jackson Immunoresearch laboratories (West Grove, PA) or GE Health Care (Piscataway, NJ), respectively.
Electrophysiology
Transversal hippocampal acute slices (300 μm) from age-matched control and pilocarpine-treated rats of 6 to 12 months of age were obtained using a slicing vibratome (Leica VT1200s). Brains were removed quickly and placed in cold (4°C) oxygenated (95% O2, 5% CO2) sucrose-modified artificial cerebral spinal fluid (ACSF) containing (in mM): Sucrose (45), NaCl (87), Glucose (25), NaHCO3 (25), KCl (2.5), NaH2PO4 (1.25), MgCl2 (7), and CaCl2 (0.5), pH=7.4 and 300–310 mOsm. For all experiments, slices were placed in a submerged slice chamber at room temperature and continuously perfused at 1–2 ml/min with ACSF containing (in mM): NaCl (130), Glucose (10), NaHCO3 (25), KCl (3.5), NaH2PO4 (1.25), MgCl2 (2), CaCl2 (2), pH=7.4 and 300–305 mOsm. Slices were visualized on a fixed stage upright microscope (Eclipse FNI, Nikon) equipped with 10X and 60X objectives using differential interference contrast (DIC) optics, infrared illumination and an infrared-sensitive camera (Cool SnapEZ, Photometrics, Tucson, AZ, USA). Whole-cell patch clamp recordings were performed with glass pipettes with an access resistance of 3–5 MΩ when filled with intracellular solution. Data were acquired with a Multiclamp 700B amplifier and digitized with a Digidata 1440A, using pClamp 10.2 acquisition software (Molecular Devices, Union City, CA, USA). All recordings were made from dentate granule cell (DGCs) identified physiologically using prolonged depolarizing and hyperpolarizing current injections (1s) to determine basic biophysics and electrical properties.
GABAergic tonic currents and phasic spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in voltage clamp and isolated by blocking ionotropic glutamatergic transmission with 6,7-dinitroquinoxaline-2,3-dione (DNQX) (25μM final concentration, AMPA/kainate antagonist, Tocris Bioscience) and DL-2-amino-5-phosphonopentanoic acid (APV) (50μM final concentration, NMDA receptor antagonist, Tocris Bioscience) in the ACSF. To determine GABAergic tonic currents and the effect of α1-GABAA receptor blockade on sIPSCs, a K-Gluconate based intracellular solution was used (in mM): K-Gluconate (70), KCl (70), HEPES (10), EGTA (4), MgCl2 (2), Mg-ATP (4), Na-GTP (0.3) and agonist drugs were bath applied. This intracellular solution allows detection of GABAergic currents as downward when the holding potential is near rest (−60 mV).
Tonic currents were acquired and analyzed by taking ten-second samples from voltage clamp recordings (Vh = −60 mV, K-Gluconate based solution) at each experimental condition (baseline (IBSLN) and δ-dependent (ITHIP, δ-subunit specific GABAR agonist, THIP 5 μM)). To minimize bias from phasic events, a Gaussian distribution was fit to the right side of an all-points histogram from each sample from a point 1–3 pA left of the peak (Glykys and Mody, 2007a). The Gaussian peak determined the mean current for the sample. Tonic specific δ-subunit current capacity was calculated from the difference in mean baseline and THIP currents (ITHIP − IBSLN). To evaluate α1-GABAA receptor-mediated phasic conductance, action potential-dependent spontaneous IPSCs were recorded with a K-Gluconate based internal solution alone (sIPSCs). IPSCs were analyzed for changes in frequency, amplitude, and kinetics before and after the application of zolpidem, an agonist that favors α1-GABAAR (200 nM).
Statistical analysis
Data are presented as the mean ± SEM. The differences between groups were assessed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test or Tukey’s test for multiple comparisons. A paired student’s t-test was used when only two groups of data were compared. The Mann-Whitney non-parametric test was used to compare inhibitory event populations. In all cases, p values < 0.05 were considered significant. GraphPad InStat (GraphPad Software, Inc., San Diego, CA, USA) was used to perform statistical analysis.
RESULTS
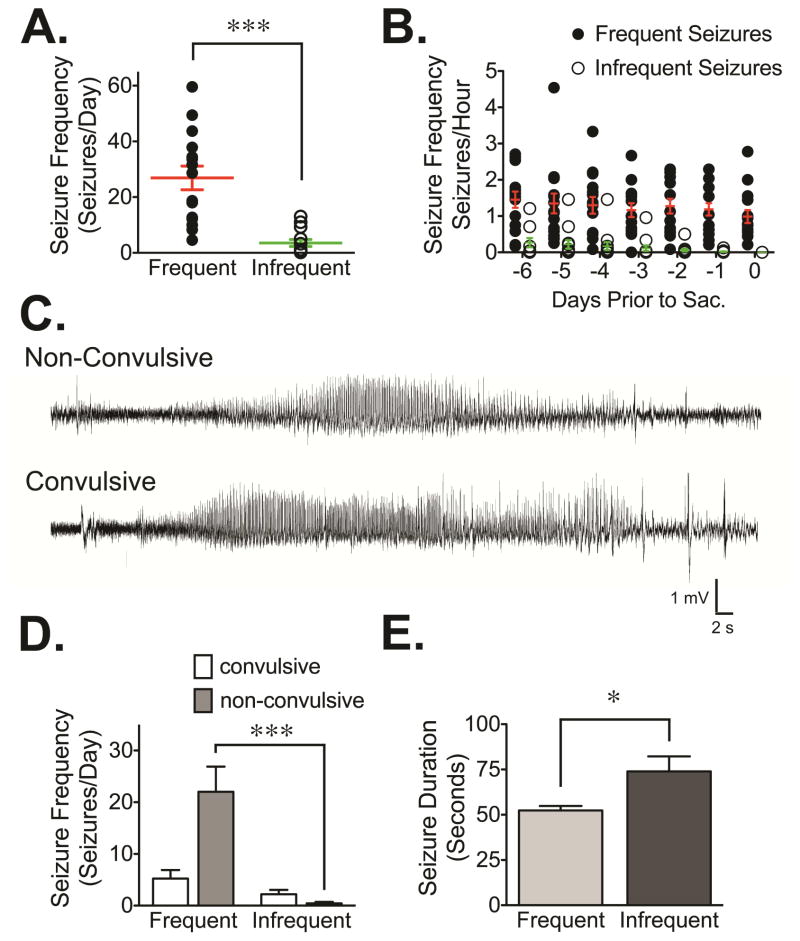
In the current study, we used brain tissue obtained from chronically epileptic rats generated using the pilocarpine model of TLE to investigate if occurrence of spontaneous seizures and/or epilepsy severity influenced the expression/function of GABAAR. More specifically, we examined the cell surface expression, composition and function of GABAAR in tissue obtained from two distinct groups of spontaneously epileptic rats. For the first group, tissue was obtained within 3 hours of seizure occurrence (≤3 hours group); and, for the second group, tissue was obtained at least 24 hours after seizure occurrence (≥24 hours group). A retrospective analysis of EEG recordings from these chronically epileptic animals revealed that, as might be expected, the overall frequency of spontaneous seizures varied considerably between the two groups. Higher spontaneous seizure frequency (‘frequent seizures’) was detected in animals included as part of the ≤3 hours group while lower spontaneous seizure frequency (‘infrequent seizures’) was observed in the ≥24 hours group (Figure 1). In the seven days prior to sacrifice, animals with frequent seizures (≤3 hours group) had on average 26.9 ± 16.5 seizures/day while animals with infrequent seizures (≥24 hours group) had on average 3.5 ± 4.6 seizures/day (Figure 1A). A similar trend in seizure burden can be seen by examining the individual days prior to tissue collection, with the seizure number being much higher in the animals collected as part of the ≤3 hours group (Figure 1B). Interestingly, a small proportion of animals from each group (13% in the ≤3 hours group and 15% in the ≥24 hours group) experienced seizure frequencies that were more similar to the epileptic group that was sacrificed based on the opposite timing. In other words, two animals from the ≤3 hours group had seizure frequencies of less than 10 seizures/day and experienced epochs of seizure freedom lasting at least 24 hours; and two animals in the ≥24 hours group had days with ~20 seizures.
Figure 1. Characterization of seizure frequency and duration in chronically epileptic rats.
(A) Summary of seizure data obtained during a 7-day monitoring period that occurred prior to sacrifice. The ≤3 hours group (n=15) demonstrated a significantly higher (p<0.0001, unpaired t-test) seizure frequency (i.e., seizures/day) relative to the ≥24 hours group (n=13) that showed infrequent seizures. (B) Seizure frequency in both groups of epileptic animals shows that the ≤3 hours group had more frequent seizures on daily bases when compared to the number of seizures showed by the ≥24 hours group. (C) Representative EEG traces detected in pilocarpine-treated rats that correspond to spontaneous seizures correlated with either non-convulsive or convulsive behaviors. (D) Electrographic seizures were classified as convulsive (Racine scale ≥ 3) or non-convulsive (Racine scale ≤ 2). The mean daily seizure frequency demonstrates a significant decrease in the rate of non-convulsive seizures in the ≥24 hours group relative to the ≤3 hours group (p<0.0001, ANOVA). (E) A significant increase in seizure duration (p=0.013) was observed in the group with infrequent seizures (≥24 hours group) relative to the group with more frequent/recent seizures (≤3 hours group).
The behavior associated with each individual electrographic seizure was analyzed and classified according to the Racine scale in order to determine the behavioral seizure severity (Racine, 1972). In this analysis, stage 3–5 behavioral seizures were reported as convulsive while stage 1–2 behavioral seizures were reported as non-convulsive (Racine, 1972). Representative EEG recordings associated with convulsive and non-convulsive seizures are presented in Figure 1C. Although the number of convulsive seizures was not statistically different between animals with frequent and infrequent seizures (5.2 ± 1.6 seizures/day in the ≤3 hours group vs 2.2 ± 0.8 seizures/day in the ≥24 hours group, Figure 1D), the number of non-convulsive seizures was dramatically increased in animals with frequent/recent seizures (22.0 ± 4.9 seizures/day in the ≤3 hours group vs 0.4 ± 0.3 seizures/day in the ≥24 hours group, p<0.0001 by ANOVA). When the average duration of the seizure events detected in both groups of epileptic animals was compared (Figure 1E), we found that the ≥24 hours group had increased seizure duration (p<0.05 by student’s t-test).
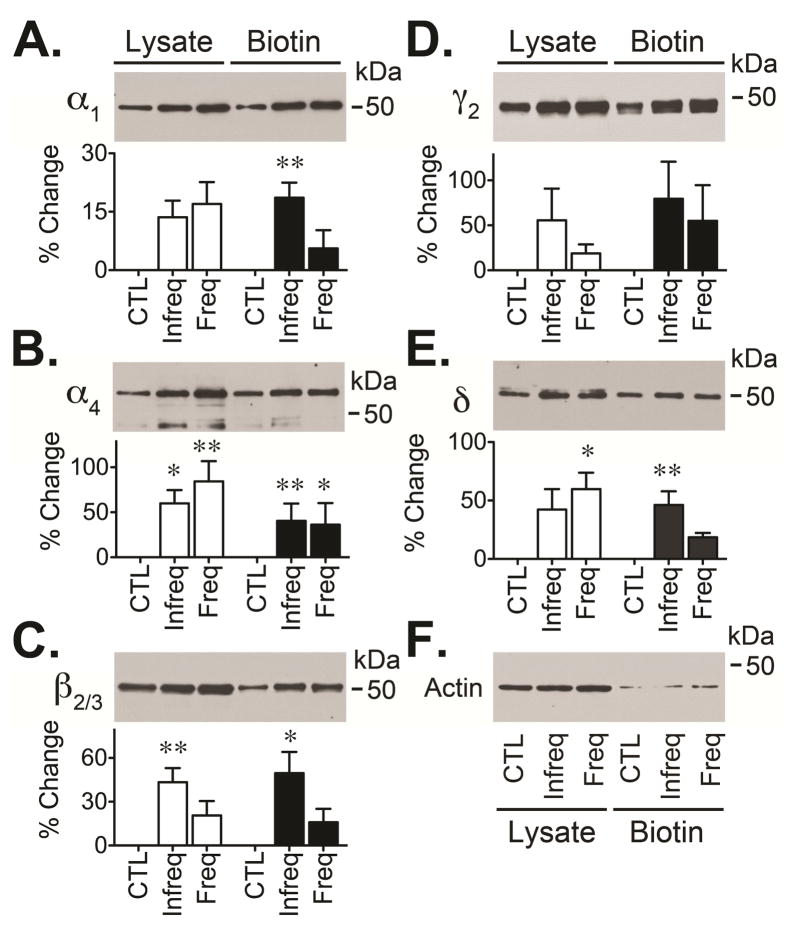
We analyzed the impact that unpredictable spontaneous seizures might have on the plasma membrane expression, receptor composition and receptor function of GABAARs expressed in the DG of these two distinctive groups of chronically epileptic rats. Alterations in GABAAR have been detected during the chronic stage of epilepsy but the specific effects that seizure occurrence might have on the plasma membrane expression of GABAARs is poorly understood. We decided to first examine the possible effect that spontaneous seizures might have on the plasma membrane expression of GABAAR. For this, we used a cell surface biotinylation assay to selectively label and isolate those receptors located at the plasma membrane (González et al., 2013). We found that in tissue from DG of hippocampus collected from animals with frequent/recent seizures (≤3 hours group) there was a significant increase in the plasma membrane expression of only the α4 subunit (136 ± 11%, p<0.05) of GABAAR (Figure 2). In contrast, when tissue was isolated from animals with less frequent/recent seizures (≥24 hours group) there was an increase in cell surface levels of several GABAAR subunits including the α1 (119 ± 4%, p<0.01), α4 (141 ± 9%, p<0.01), β2/3 (150 ± 15%, p<0.05) and δ (146 ± 12%, p<0.01) subunits (Figure 2). Since both α4 and β2/3 subunits also showed a significant increase in the protein expression detected in the whole tissue lysates, the signal detected in the biotinylated fraction was normalized to the signal detected in the total tissue lysate to estimate its relative expression at the cell surface. This analysis suggested that the change in total expression of α4 and β2/3 subunits is well correlated with an increase in the plasma membrane expression of both α4 and β2/3 subunits. Together, tthese results suggest that seizure occurrence might modify the cell surface levels of some GABAARs subunits and/or that GABAARs are more efficiently maintained at the plasma membrane when epileptic animals experience less frequent spontaneous seizures.
Figure 2. Cell surface expression of GABAARs in chronically epileptic animals.
Hippocampal slices were obtained from control (CTL) and two distinct groups of epileptic rats: one that had infrequent seizures and did not experience spontaneous seizures in the last 24 hours (≥24 hours group) and another that had frequent seizures and experienced spontaneous seizures in the last 3 hours (≤3 hours group). Slices were labeled using a cell impermeable biotinylation reagent (sulfo-NH-SS-biotin at 1 mg/ml) that allowed the isolation of cell surface proteins. Total lysate and “cell surface proteins” obtained from microdissected DG were analyzed by Western blot. Representative blots (upper panel) show the immunoreactivity for subunits α1 (A), α4 (B), β2/3 (C), γ2 (D) or δ (E). As a control, blots were also probed with an anti-actin antibody (F). GABAAR subunit immunoreactivity was normalized to the actin signal detected in the lysate before comparing the signal detected in epileptic animals to controls. Values are presented as the mean ± SEM of four to five independent experiments (lower panel) and compared by ANOVA followed by Bonferroni post hoc test (*p<0.05 or **p<0.01 represent a significant difference between epileptic animals and controls).
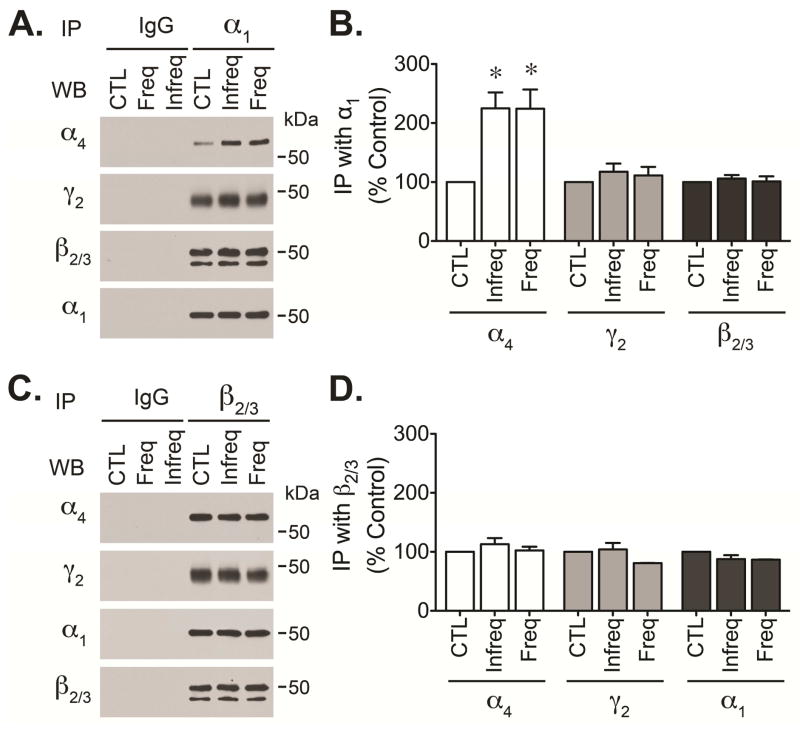
There is evidence that GABAAR composition is altered during the latent and chronic periods of epilepsy (Peng et al., 2004; Zhang et al., 2007; Lund et al., 2008). During the latent period, alteration in GABAARs assembly can be associated with changes in plasma membrane expression (Lund et al., 2008; González et al., 2013). Immunoprecipitation of GABAARs using antibodies directed against γ2 subunits revealed that GABAAR isolated from the DG show altered ratios of α1 and α4 subunits along with the substitution of γ2 subunits by δ subunits (Lund et al., 2008). To further investigate if the composition of GABAARs present in epileptic tissue is altered, we used antibodies directed against α1 and β2/3 subunits (two core subunits of pentameric GABAAR channels) to immunoisolate receptors from DG of control and chronically epileptic animals. Analysis of receptors isolated using anti-α1 antibodies revealed that α4 immunoreactivity present in α1 immunoprecipitates was increased in both groups of epileptic animals (≤3 hours group and ≥24 hours group, Figure 3A and 3B). The same samples showed no change in the immunoreactivity detected for β2/3 and γ2 subunits. Isolation of GABAARs with anti-β2/3 antibodies revealed that the levels of α1, α4 and γ2 immunoreactivity detected in β2/3 immunoprecipitates were similar in control and epileptic animals (Figure 3C and 3D). These results suggest that the amount of α4 subunits associated with α1 subunits is increased in epileptic animals but the association with other subunits like β2/3 or γ2 subunits remained unchanged. Together, our observations suggest that abundance of receptors containing a mixture of α1 and α4 subunits is increased in chronically epileptic animals but that receptor composition appears to be independent of seizure occurrence.
Figure 3. Alteration of GABAARs subunit composition in chronically epileptic animals.
GABAARs were immunoprecipitated from control (CTL) or chronically epileptic tissue from animals with infrequent (≥24 hours group) or frequent (≤3 hours group) seizures. The DG was microdissected from freshly prepared hippocampal slices and lysed in RIPA buffer. Pre-cleared lysates (300–350 μg of protein) were incubated with 5 μg of mouse monoclonal antibodies directed against α1 or β2/3 subunits, non-immune mouse IgG was used as control. Immune complexes were recovered using protein G-sepharose beads and boiled for 3 min before separation by SDS-PAGE. The immunoreactivity of α4, γ2, β2/3 or α1 subunits present in α1 or β2/3 immunoprecipitates was detected by western blot analysis. Representative blots showing α4, γ2, β2/3 and α1 immunoreactivity present in α1 (A) or β2/3 (C) immunoprecipitates. Densitometric analysis of α4, γ2 and β2/3 and α1 immunoreactivity detected in α1 (B) or β2/3 (D) immunoprecipitates presented as the mean ± SEM of three independent experiments (*p<0.05 compared to the immunoreactivity detected in control samples by students t-test).
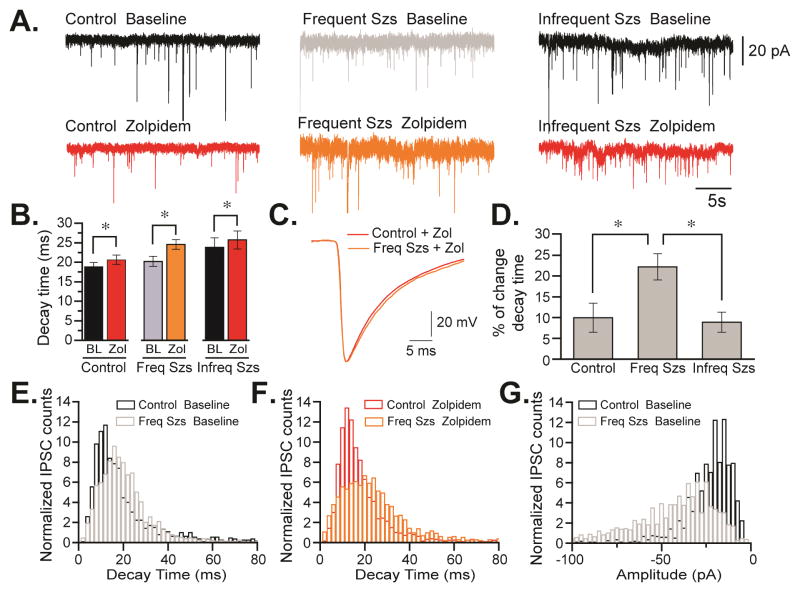
Previous studies performed in epileptic animals have demonstrated that alterations in expression, assembly or distribution of GABAAR change phasic and tonic inhibition (Brooks-Kayal et al., 1998; Zhang et al., 2007; Fritschy, 2008). For example, GABAARs expressed in dentate granule cells (DGCs) of epileptic animals are sensitive to zinc blockade, an observation compatible with the possible enrichment of receptors containing α4γ2 subunits (Gibbs et al., 1997; Brooks-Kayal et al., 1998). Here, we conducted electrophysiological studies to explore some of the functional alterations that could be associated with the changes in plasma membrane expression and assembly of GABAAR detected in epileptic animals (Figure 4). First, we examined the effects of zolpidem, an agonist that specifically interacts with the benzodiazepine-binding site of GABAARs containing α1 subunits and less efficiently interacts with α2- and α3-containing receptors (Korpi et al., 2002; Mohler et al., 2002). Measurement of sIPSCs in hippocampal DGCs from control and epileptic animals showed a significant increase in the sIPSC amplitude in the ≤3 hours group compared to the control animals (21.7 ± 4.4pA, n = 6 to 39 ± 6.7pA, n = 7, p = 0.028, Table 1). These differences were also noted in the distribution of amplitude of the sIPSC population where ≤3 hours group animals display a bigger percentage of sIPSC event populations at bigger amplitudes than control animals (p = 1.5e-5; Figure 4G). These results were not affected by the application of zolpidem in the two different animals conditions (19.5 ± 3.3pA, n = 6 to 37.6 ± 5.5pA, n = 7, p = 0.02, Table 1). These data suggest that there is a difference in a phasic inhibition that can be detected between animals that underwent frequent seizures when compared to controls.
Figure 4. Alterations in sIPSC decay kinetics in chronically epileptic animals.
(A) Representative voltage-clamp traces of DGCs from control (left) and epileptic rats that experienced frequent seizures (≤3 hours group, center) or infrequent seizures (≥24 hours group, right) before tissue collection. Recordings were performed in either ACSF (bseline) or in ACSF plus zolpidem. (B) Averaged sIPSC decay in DGCs before and after addition of zolpidem. Values are presented as the mean ± SEM of six to seven independent experiments (*p<0.05 compared to the values detected in controls by students t-test). (C) Average sIPSC trace for currents detected in granule cells from control and animals with frequent/recent seizures (≤3 hours group) in the absence and presence of zolpidem. (D) Alterations in the decay times detected in control and epileptic animals in absence and presence of zolpidem. sIPSC decay measured in animals belonging to ≤3 hours group is significantly different from control and from animals in the ≥24 hours group. Histograms of normalized sIPSC counts (decay time bins of 4 ms) detected in control and in the group with frequent seizures in the absence (E) and after addition of zolpidem (F) (*p<0.05, Mann-Whitney non-parametric test). (G) Histograms of normalized sIPSC counts (amplitude bins of −2.5 pA) detected in control and ≤3 hours group (*p<0.05, Mann-Whitney non-parametric test).
Table 1.
Summary of the frequency and amplitude of spontaneous miniature inhibitory post-synaptic currents measured in DGCs obtained from control animals, and epileptic animals with frequent/recent seizures (≤3 hours group) or infrequent seizures (≥24 hours group).
| Baseline | Zolpidem | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Rm (MΩ) | Tau (ms) | IPSC Freq (Hz) | IPSC Amp (pA) | IPSC Freq (Hz) | IPSC Amp (pA) | |
| Control | 192.0 ±15.49 | 1.03 ± 0.08 | 1.68 ± 0.85 | 21.7 ± 4.4 | 0.80 ± 0.31 | 19.5 ± 3.3 |
| Frequent Szs | 170.4 ± 17.76 | 1.16 ± 0.05 | 0.70 ± 0.40 | 39.0 ± 6.7* | 0.86 ± 0.36 | 37.6 ± 5.5* |
| Infrequent Szs | 147.7 ± 15.77 | 0.98 ± 0.09 | 1.21 ± 0.52 | 26.5 ± 4.9 | 0.77 ± 0.20 | 23.7 ± 3.4 |
p < 0.05, compared to control animals.
In addition, zolpidem application produced the broadening of sIPSCs (Figure 4). In the control group, zolpidem increased the decay time of sIPSCs from 18.82 ± 1.14 ms to 20.62 ± 1.2 ms (n = 6, p = 0.04, Figure 3B); in the ≤3 hours group went from 20.23 ± 1.29 to 24.54 ± 1.26 ms (n = 7, p = 0.0001); and in the ≥24 hours group increased from 23.8 ± 2.46 to 25.74 ± 2.31 ms (n = 6, p = 0.014). However, the increment on the decay kinetics of the ≤3 hours group (22.2 ± 3.2%, n = 7) was significantly larger than the control group (9.9 ± 3.5%, n = 6; p = 0.02, Figure 3D). In contrast, the decay values in the ≥24 hours group were similar to control (8.8 ± 2.4%, n = 6, p = 0.61). Thus, compared to control, zolpidem increased the decay time of GABAAR-mediated IPSCs by ~20% in the group with frequent/recent seizures but only by ~10% in the group with infrequent seizures (Figure 4D). To understand whether the average change in decay time seen in controls and the group with frequent seizures corresponded to a change in the distribution of sIPSC population or a subset of these events, we analyzed a histogram of the normalized sIPSC decay times measured in the absence (base line) and presence of zolpidem (Figures 4E and 4F). In rats experiencing seizures within 3 hours of tissue collection, the sIPSC count population was significantly shifted towards longer decay times when measurements were conducted in the presence of zolpidem (non-parametric Mann-Whitney test p = 0.039). Thus, currents detected in animals with more frequent/recent seizures have distinct pharmacological and kinetic properties than currents detected in control DGCs (Figure 4), suggesting that these cells could express GABAAR with altered subunit composition and perhaps a different ratio of α subunits.
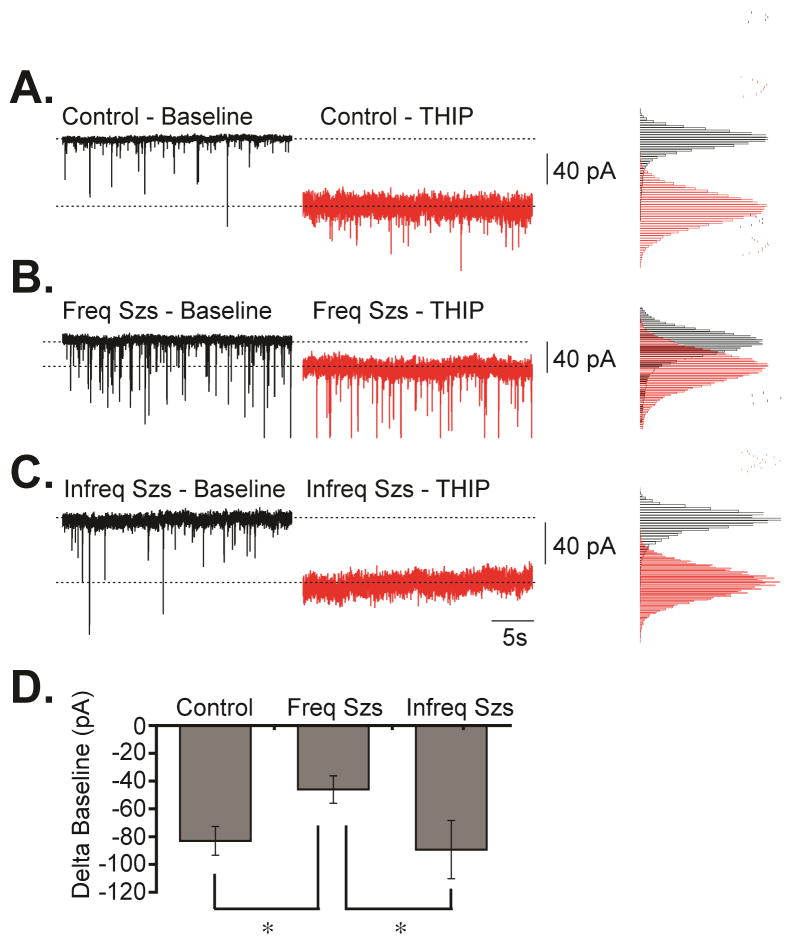
Tonic GABAergic currents in DGCs are primarily mediated by GABAAR containing δ subunits that under normal conditions are located at peri- and extra-synaptic sites (Peng et al., 2002; Glykys and Mody, 2007b). Our biochemical assays suggest that epileptic animals that did not suffer seizures in the last 24 hours before analysis have increased cell surface expression of GABAAR containing δ subunits (146% ± 12, p<0.01) when compared to controls. In contrast, if seizures occurred within 3 hours of analysis, no significant increase in plasma membrane expression was detected (119% ± 4, compared to controls) despite an increased in the total protein expression of δ subunits (160% ± 14, p<0.05). In fact, normalized values (plasma membrane/total) suggest that only a fraction (76 ± 6%, p<0.05) of δ subunits are present at the cell surface when more frequent/recent seizures occurred prior to analysis. To evaluate if these molecular changes correlate with alterations in tonic inhibition detected in DGCs, we examined the effects of 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP, Figure 5), a preferential agonist of GABAAR containing δ subunits (Brown et al., 2002; Drasbek et al., 2007). Bath application of THIP (5 μM) caused stronger inward tonic currents in controls (−83.01 ± 10.24 pA, n = 8, p = 0.013) and in animals that did not experience seizures during the last 24 hours (−80.92 ± 8.56 pA, n = 5, p = 0.023) than in animals experiencing seizures in the last 3 hours before analysis (−46.01 ± 9.89 pA, n = 7, Figure 5). Thus, it appears that a reduction in tonic currents is associated with a reduction in the levels of δ subunits present at the plasma membrane of animals that had seizures in the last 3 hours before tissue collection. This suggests that when animals did not experience seizures in the last 24 hours an increase in the plasma membrane levels of GABAAR containing δ subunits may be sufficient to achieve tonic inhibition similar to control levels. In contrast, when animals experience more frequent/recent seizures (≤3 hours group), a failure to increase the plasma membrane levels of GABAAR containing δ subunits might result in an inability to compensate and result in a reduction of tonic currents. However, we must acknowledge that a direct correlation between changes in tonic inhibition observed here and the changes in receptors containing δ subunit is complex since other receptors may contribute to tonic inhibition. In addition, other factors beyond receptor expression/assembly (i.e., extracellular GABA levels) might also impact on tonic inhibition.
Figure 5. Alterations in tonic inhibition detected in chronically epileptic animals.
Representative voltage-clamp traces obtained in granule cells from (A) control, (B) ≤3 hours group, or (C) ≥24 hours group before and after application of THIP along with the corresponding histograms for baseline currents detected before or after THIP addition. (D) Summary of the change in currents detected after THIP addition (tonic inhibition) measured in granule cells from control, ≤3 hours group or ≥24 hours group. Data is presented as the mean ± SEM of five to eight independent experiments (*p<0.05).
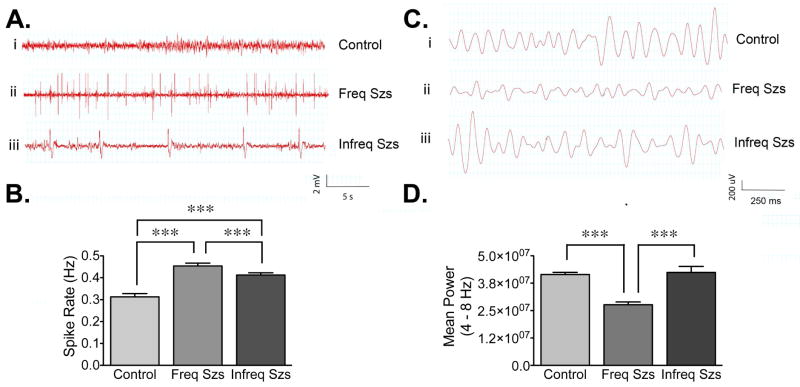
Among patients with TLE there is a very small group of individuals (2–3%) with a relatively less severe disease (Marsan and Zivin, 1970; Rosati et al., 2003). These patients, sometimes referred to as “oligospikers”, have delayed seizure onset, lower frequency of complex partial seizures, lower incidence of secondarily generalized tonic-clonic seizures and rarely develop SE. “Oligospikers” have prolonged periods of standard scalp EEG recording without spiking and few EEG tracings show inter-ictal activity (Rosati et al., 2003). Since the ≥24 hours group had infrequent seizures and appear to have a relatively less severe disease, we decided to investigate if this low seizure incidence was correlated with a decrease in spike frequency. The analysis of spike rate was conducted on 30-minute long artifact-free EEG segments obtained from chronically epileptic animals or age-matched controls that were implanted with electrodes at the pyramidal cell layer of hippocampus, within the CA1 region. To minimize the possible impact of spontaneous seizures on spike rate, EEG segments were taken during inter-ictal periods, at least 1 hour before or after the occurrence of a seizure event. This analysis showed that compared to controls, epileptic animals with frequent and infrequent seizures (≤3 hours group and ≥24 hours group) had a significant increase in spike rate (Figure 6A). However, as shown in Figure 6B, the spike rate detected in the ≤3 hours group (0.46 ± 0.01) was higher than the spike rate detected in the ≥24 hours group (0.38 ± 0.01). These observations suggest that the spike rate detected in the chronically epileptic animals is well correlated with the seizure frequency detected for each group, in a similar fashion to what has been observed in patients with TLE.
Figure 6. Characterization of electroencephalographic differences in chronically epileptic rats.
Chronically epileptic rats and age-matched controls were continuously video-EEG monitored to assess baseline spike rates and characterize electrophysiological parameters during inter-ictal intervals. (A and B) Average spike rates (i.e., spike number/second) for animals with depth-recording electrodes implanted in the pyramidal cell layer of the CA1 region of hippocampus. Representative (unfiltered) traces sampled from the CA1 region of control (Ai), ≤3 hours group (Aii), or ≥24 hours group (Aiii) detected during inter-ictal intervals (at least 1 h before or after a seizure). (B) Both epileptic groups have significantly higher spike rates (p<0.0001) relative to controls (n=47 segments from 4 animals); but the ≤3 hours group (n=106 segments from 8 animals) had significantly higher spike rate (p<0.0001) relative to the ≥24 hours group (n=60 segments from 4 animals). Significant differences were determined using a one-way ANOVA with a Tukey’s test for multiple comparisons. (C and D) Average power for the theta (4–8 Hz) bandwidth was measured for the 30-minute inter-ictal segments and the 30-minute control epochs used above. Representative traces (filtered between 4–8 Hz) of theta activity sampled from CA1 regions of animals in the control (Ci), ≤3 hours group (Cii), or ≥24 hours group (Ciii) detected during inter-ictal intervals. (D) In the pyramidal layer of the CA1 region of hippocampus, the average theta power was significantly lower in the ≤3 hours group relative to control rats and the ≥24 hours group (p<0.0001). Significant differences were evaluated using a using one-way ANOVA with Tukey’s test for multiple comparisons.
Another EEG abnormality reported in animal models and patients with TLE is the alteration in the power spectra of the theta rhythm (Chauviere et al., 2009; Ge et al., 2013). The largest theta amplitude in hippocampus is detected in stratum lacunosum moleculare of the CA1, a region where afferents from entorhinal cortex form synapses with distal dendrites of pyramidal cells (Buzsaki, 2002). Since our implanted electrodes were positioned near the pyramidal cell layer and more likely recorded the global CA1 theta field, we calculated the theta power for this region. The average power for the theta bandwidth (4–8 Hz) was measured using the same 30-minute inter-ictal intervals used to calculate the spike rate in control and epileptic rats (Figure 6C). Compared to controls and to the animals with infrequent seizures that appear to have less severe epilepsy (≥24 hours group), the EEG traces from the epileptic animals with frequent/recent seizures (≤3 hours group) displayed a decrease in absolute theta power (Figure 6D). In addition, both the alpha (8–13 Hz) and beta/gamma (13–30 Hz) frequencies were also decreased in the group with more frequent/recent seizures (data not shown), but the change in theta frequency was the most substantial. Together, these observations suggest that increased seizure frequency is associated with a decline in theta power. However, results correlating seizure frequency with changes in spike frequency and theta power must be interpreted with caution because a number of biological cycles and environmental factors can influence both theta power and spike frequency. In these experiments, we did not control other influencing factors, but instead, attempted to demonstrate differences in the baseline and inter-ictal activity that can be detected during continuous EEG recordings from two distinct groups of epileptic animals. Thus, we cannot directly attribute differences in seizure frequency to spike rate increases or changes in theta power.
DISCUSSION
In the present study, we analyzed the impact that spontaneous seizures might have on the plasma membrane expression, subunit composition and function of GABAARs expressed in the DG of hippocampus. We report that in chronically epileptic animals, the occurrence of spontaneous seizure activity is associated with biochemical and functional alterations of GABAAR. Compared to controls, epileptic animals that experienced more frequent/recent seizures (≤3 hours group) have similar levels of α1, β2/3, and δ subunits at the plasma membrane. In contrast, animals that had infrequent seizures and did not experience seizures in the last 24 hours showed increased levels of α1, β2/3, and δ subunits at the cell surface. Interestingly, animals with more frequent/recent seizures showed abnormalities in phasic and tonic inhibition while GABAAR responses in animals with infrequent seizures were similar to controls. These observations suggest that despite having “normal” levels of GABAAR subunits at the plasma membrane, epileptic animals with frequent/recent seizures might have abnormal receptors. Animals with less frequent seizures show an overall increase in the plasma membrane levels of several GABAAR subunits that perhaps translates into an enrichment of GABAARs at the plasma membrane and compensates for the functional deficit. Together, these observations suggest that epilepsy severity (i.e. frequency of spontaneous seizures) might be associated with a reduction in the efficiency of inhibitory function that in turn could be directly correlated with a reduction in GABAAR function. Alternatively, or more likely in addition, these results also suggest that the molecular characteristics of GABAARs expressed in the epileptic brain may be, at least in part, determined by seizure burden. A summary of the most salient findings of this study is included in Table 2.
Table 2.
Summary of the most salient changes observed in the different parameters examined in this study. Arrows indicate either a significant increase (↑) or a decrease (↓) and the symbol (•) represents no change in the parameter described.
| GABAAR | Frequent Seizures | Infrequent Seizures | |
|---|---|---|---|
| Cell Surface | Biotin | ||
|
| |||
| α1 | • | ↑ | |
| α4 | ↑ | ↑ | |
| β2/3 | • | • | |
| δ | • | ↑ | |
|
| |||
| Composition | Western Blot | ||
|
| |||
| IP α1 | α4 | ↑ | ↑ |
|
|
|||
| β2/3 | • | • | |
| γ2 | • | • | |
|
| |||
| Function | Phasic | ||
|
| |||
| Zolpidem | Amplitude | ↑ | • |
| Δ Decay Time | ↑ | • | |
| Population Shift | ↑ | • | |
|
| |||
| Function | Tonic | ||
|
| |||
| Δ Base line | ↓ | • | |
|
| |||
| EEG | |||
|
| |||
| Spike Rate | ↑ | ↑ | |
| Mean Power | ↓ | • | |
Animals with rare seizures (and no seizures in the last 24 hours) had an increased number of GABAAR at the plasma membrane that appears to compensate for the deficit in inhibitory neurotransmission and permits relatively normal inhibitory function. By contrast, although animals with frequent/recent seizures had similar levels of GABAARs at the plasma membrane relative to controls, the ≤3 hours group show reduced tonic inhibitory function. A possible explanation for these observations is that GABAAR stability at the plasma membrane is decreased in animals with more frequent/recent seizures. Supporting evidence for this idea comes from studies demonstrating that repetitive pharmacological activation of GABAARs isolated from epileptic tissue produces a characteristic ‘rundown’ of GABAAR-mediated currents independently of changes in receptor affinity or membrane potential (Palma et al., 2007). Coincidentally, a switch in receptor assembly altering the proportion of α4/α1 subunits incorporated into GABAARs can be detected at the same time that the ‘rundown’ effect is observed (Mazzuferi et al., 2010). The mechanism(s) contributing to a decrease in the stability of receptors at the plasma membrane of epileptic animals is unknown but alterations in the clustering of gephyrin, the main scaffolding protein required for proper anchoring of receptors to synaptic sites, might contribute to the differential presence of GABAAR at the cell surface. Tissue of chronically epileptic animals contains an increased number of gephyrin clusters and a relative excess of GABAergic synapses but those synapses are dysfunctional (Thind et al., 2010). Together, these observations suggest that gephyrin dysfunction might contribute to the differential stability and clustering of GABAAR. However, this possibility remains to be investigated.
As mention before, aberrant expression of GABAAR subunits appears to contribute to the functional alterations detected in epileptic tissue (Gibbs et al., 1997; Brooks-Kayal et al., 1998). Our analysis of receptors immunoisolated from epileptic tissue revealed that levels of α4 subunits present in α1 immunoprecipitates was increased. Since there is evidence that α4 subunits might co-assemble with α1, α2, and α3 subunits (Kern and Sieghart, 1994; Benke et al., 1997), our studies suggest that in the epileptic brain there is an increase in the proportion of GABAARs that simultaneously contain α1 and α4 subunits. The functional properties of receptors containing a mixture of α1 and α4 subunits is not known, but direct comparison of recombinant receptors formed by α1β3γ2 and α4β3γ2 subunits suggest that substitution of α1 by α4 subunits results in receptors with similar affinity for GABA that are less efficacious (Lagrange et al., 2007). An additional consequence of abnormal GABAAR assembly is the altered synaptic distribution of these “aberrant” receptors. In epileptic animals, α4 subunits increase partnership with γ subunits and result in the redistribution of α4γ2-containing receptors towards perisynaptic and/or synaptic locations (Sun et al., 2007; Zhang et al., 2007). Although the synaptic distribution of receptors containing a mixture of α subunits is unclear, our data suggest at least two possibilities: (1) α1α4βγ receptors might remain at synaptic locations and result in altered phasic inhibition that potentially include rapid desensitization and longer decay times; and/or (2) α1α4βγ receptors might be redistributed to perisynaptic and/or extrasynaptic locations and contribute to altered tonic inhibition that potentially include reduced neurosteroid sensitivity.
Altered receptor composition and differential expression of receptor subunits at the plasma membrane appears to be reflected in the electrophysiological measurements of GABAAR function carried out here. We found that zolpidem application increased the decay time of GABA-mediated currents in all three groups of animals (control, ≤3 hours group and ≥24 hours group). However, compared to animals in the control or ≥24 hours group, the decay kinetics of currents observed in animals with more frequent/recent seizures doubled and partially shifted towards slower decay times. In addition, the sIPSC amplitude was bigger in animals with more frequent/recent seizures than in controls or than in animals with infrequent seizures, suggesting than additional factors, other than the GABAAR subunit composition, might also be implicated. Thus, for example, we cannot eliminate the possibility that these animals might have increased extracellular GABA concentrations due to dysfunctional GABA transporters.
Also, our analyses showed a failure in tonic inhibition when more frequent/recent seizures occurred. All together, the results presented here suggest that although the levels of GABAAR present at the plasma membrane of animals with more frequent/recent seizures is similar to controls, GABAARs present in the ≤3 hours group appear to be functionally altered. We speculate that these functional changes may be related to differential subunit configuration. GABAARs containing α4 subunits have slower decay kinetic properties than those containing α1 subunits (Lagrange et al., 2007) and α4γ2-containing receptors with low neurosteroid sensitivity contribute to the maintenance of tonic inhibition in epileptic DG (Zhang et al., 2007; Rajasekaran et al., 2010). Increased presence of α1α4-containing GABAARs may explain, at least in part, both the longer decay times and the reduced neurosteroid augmentation of tonic currents that can be detected in animals with more frequent/recent seizures. Our results are in agreement with previous reports suggesting that the number of GABAergic synapses present in epileptic animals homeostatically increase beyond control levels but some of those synapses are dysfunctional (Payne et al., 2006; Zhang and Buckmaster, 2009; Thind et al., 2010). Together, these observations suggest that animals with infrequent seizures may display a mechanism to partially compensate the functional deficits and produce a net increase in receptor function by promoting the plasma membrane accumulation of GABAAR.
Little is known about the molecular mechanisms behind the generation of spontaneous seizures and the possible influence that spontaneous seizures themselves might have on the process of ongoing seizure generation (Houser et al., 2012). Molecular changes resulting from seizure occurrence can be detected during seizure-free intervals, these include changes in early gene expression and phosphorylation of key mediators in cell signaling pathways (Harvey and Sloviter, 2005; Peng and Houser, 2005; Houser et al., 2008). Changes in the temporal pattern of expression of the immediate early gene c-fos have been detected following seizure occurrence (Harvey and Sloviter, 2005; Peng and Houser, 2005). Fifteen minutes after occurrence of a behavioral spontaneous seizure, Fos labeling first increases in DGCs of hippocampus and within a few hours becomes evident in interneurons to finally return to levels observed in controls (Peng and Houser, 2005). The second example is related to changes in the phosphorylation levels of the extracellular signal-regulated kinase (ERK), a highly sensitive indicator of neuronal activity (Houser et al., 2008). Mice that had not experienced spontaneous behavioral seizures in the last 24 hours show low levels of phosphorylated ERK (Houser et al., 2008) but minutes after seizure onset phospho-ERK levels increase dramatically and return to seizure-free levels within 30 min of seizure occurence (Houser et al., 2008). Here our experiments suggest that spontaneous seizures might also be involved in the regulation of the plasma membrane expression and function of GABAARs. Our observations also suggest that modulation of GABAAR by spontaneous seizures represents one of the potential mechanism by which seizure occurrence might enhance the likelihood of new “spontaneous seizures” and promote the phenomenon commonly described as “seizures beget seizures”.
A limitation of this study is that we cannot specifically differentiate whether the molecular and functional changes in GABAARs are the direct result of seizures, a cause of seizures or both. One possible interpretation is that recurrent spontaneous seizures directly modulate intracellular signaling pathways that alter the subunit expression and trafficking of GABAARs, much like what has been observed after status epilepticus (Brooks-Kayal et al., 1998; Goodkin et al., 2008; Lund et al., 2008; Terunuma et al., 2008; González et al., 2013). Alternatively, increased seizure frequency might originate from firmly established changes in receptor distribution that triggers long-term changes in the balance of excitation/inhibition, thus favoring seizure occurrence. However, a scenario where both mechanisms contribute to the perpetuation of GABAAR alterations and epilepsy progression is the more likely possibility. Additional studies are needed to better understand the complex interactions between spontaneous seizures and GABAAR alterations that occur during chronic epilepsy. This knowledge will help to better define the mechanisms underlying secondary epileptogenesis and to identify potential therapeutic targets to reduce or inhibit epilepsy progression.
CONCLUSIONS
In summary, this study found that alterations in the plasma membrane expression and assembly of GABAARs correlate with functional alterations in inhibitory neurotransmission and seizure burden. Moreover, these observations suggest that increased GABAARs expression at the plasma membrane might represent a compensatory and/or homeostatic mechanism to repress hyperexcitability and reduce seizure burden in chronically epileptic rats. These findings offer a molecular substrate to explain how alterations in GABAAR function can be associated with differential seizure frequency and susceptibility and represent an initial step towards a fuller characterization of the molecular events that trigger alterations in GABAergic neurotransmission that characterize chronic epilepsy.
HIGHLIGHTS.
Seizure activity affects the plasma membrane expression and function of GABAARs.
Seizure frequency might be associated with a reduction in the efficiency of inhibitory function.
Increased levels of GABAAR at the plasma membrane might be a compensatory mechanism to maintain inhibitory function.
Acknowledgments
Funding from The National Institutes of Health supported this work: K01-NS069583 (MIG), R01-NS053719 (MMH) and R01-NS051710 (ABK).
Footnotes
Conflict of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benke D, Michel C, Mohler H. GABAA Receptors Containing the α4-Subunit: Prevalence, Distribution, Pharmacology and Subunit Architecture In Situ. J Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Chauviere L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci. 2009;29:5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J Neurophysiol. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge M, Wang D, Dong G, Guo B, Gao R, Sun W, Zhang J, Liu H. Transient impact of spike on theta rhythm in temporal lobe epilepsy. Exp Neurol. 2013;250:136–142. doi: 10.1016/j.expneurol.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Shumate M, Coulter D. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. Journal of Neurophysiology. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007a;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007b;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Krizman-Genda E, Robinson MB. Caveolin-1 regulates the delivery and endocytosis of the glutamate transporter, excitatory amino acid carrier 1. J Biol Chem. 2007;282:29855–29865. doi: 10.1074/jbc.M704738200. [DOI] [PubMed] [Google Scholar]
- González MI, Cruz Del Angel Y, Brooks-Kayal A. Down-regulation of gephyrin and GABAA receptor subunits during epileptogenesis in the CA1 region of hippocampus. Epilepsia. 2013;54:616–624. doi: 10.1111/epi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstatter HL, Del Angel YC, Carlsen J, Wempe MF, White AM, Cogswell M, Russek SJ, Brooks-Kayal AR. The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy. Neurobiol Dis. 2014;62:73–85. doi: 10.1016/j.nbd.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE. 2002;2002:pl8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: implications for hippocampal epileptogenesis. J Comp Neurol. 2005;488:442–463. doi: 10.1002/cne.20594. [DOI] [PubMed] [Google Scholar]
- Holman D, Henley JM. A novel method for monitoring the cell surface expression of heteromeric protein complexes in dispersed neurons and acute hippocampal slices. J Neurosci Methods. 2007;160:302–308. doi: 10.1016/j.jneumeth.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Huang CS, Peng Z. Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy. Neuroscience. 2008;156:222–237. doi: 10.1016/j.neuroscience.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Zhang N, Peng Z, Huang CS, Cetina Y. Neuroanatomical clues to altered neuronal activity in epilepsy: from ultrastructure to signaling pathways of dentate granule cells. Epilepsia. 2012;53(Suppl 1):67–77. doi: 10.1111/j.1528-1167.2012.03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, Sieghart W. Polyclonal Antibodies directed against an epitope specific for the α4-subunit of GABAA receptors identify a 67-kDa protein in rat brain membranes. J Neurochem. 1994;62:764–769. doi: 10.1046/j.1471-4159.1994.62020764.x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABA(A) receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of alpha4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol. 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Marsan CA, Zivin LS. Factors related to the occurrence of typical paroxysmal abnormalities in the EEG records of epileptic patients. Epilepsia. 1970;11:361–381. doi: 10.1111/j.1528-1157.1970.tb03903.x. [DOI] [PubMed] [Google Scholar]
- Mazzuferi M, Palma E, Martinello K, Maiolino F, Roseti C, Fucile S, Fabene PF, Schio F, Pellitteri M, Sperk G, Miledi R, Eusebi F, Simonato M. Enhancement of GABA(A)-current run-down in the hippocampus occurs at the first spontaneous seizure in a model of temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2010;107:3180–3185. doi: 10.1073/pnas.0914710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Palma E, Roseti C, Maiolino F, Fucile S, Martinello K, Mazzuferi M, Aronica E, Manfredi M, Esposito V, Cantore G, Miledi R, Simonato M, Eusebi F. GABA(A)-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABA(A) “phasic” receptors. Proc Natl Acad Sci U S A. 2007;104:20944–20948. doi: 10.1073/pnas.0710522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne HL, Donoghue PS, Connelly WM, Hinterreiter S, Tiwari P, Ives JH, Hann V, Sieghart W, Lees G, Thompson CL. Aberrant GABA(A) receptor expression in the dentate gyrus of the epileptic mutant mouse stargazer. J Neurosci. 2006;26:8600–8608. doi: 10.1523/JNEUROSCI.1088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Houser CR. Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Racine R. Modification of seizure activity by electrical stimulation II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Joshi S, Sun C, Mtchedlishvilli Z, Kapur J. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis. 2010;40:490–501. doi: 10.1016/j.nbd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati A, Aghakhani Y, Bernasconi A, Olivier A, Andermann F, Gotman J, Dubeau F. Intractable temporal lobe epilepsy with rare spikes is less severe than with frequent spikes. Neurology. 2003;60:1290–1295. doi: 10.1212/01.wnl.0000058761.12715.0e. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Silva AP, Carvalho AP, Carvalho CM, Malva JO. Modulation of intracellular calcium changes and glutamate release by neuropeptide Y1 and Y2 receptors in the rat hippocampus: differential effects in CA1, CA3 and dentate gyrus. J Neurochem. 2001;79:286–296. doi: 10.1046/j.1471-4159.2001.00560.x. [DOI] [PubMed] [Google Scholar]
- Sperk G, Furtinger S, Schwarzer C, Pirker S. GABA and its receptors in epilepsy. Adv Exp Med Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the alpha4 subunit of GABA(A) receptors in an animal model of epilepsy. J Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol. 2010;518:647–667. doi: 10.1002/cne.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Raol Y, Hsu F, Coulter D, Brooks-Kayal A. Effects of Status Epilepticus on Hippocampal GABAA Receptors are Age-Dependent. Neuroscience. 2004;125:299–303. doi: 10.1016/j.neuroscience.2004.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Buckmaster PS. Dysfunction of the dentate basket cell circuit in a rat model of temporal lobe epilepsy. J Neurosci. 2009;29:7846–7856. doi: 10.1523/JNEUROSCI.6199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]