Abstract
Adenosine, a purine nucleoside, is present at high concentrations in tumors where it contributes to the failure of immune cells to eliminate cancer cells. The mechanisms responsible for the immunosuppressive properties of adenosine are not fully understood. We tested the hypothesis that adenosine’s immunosuppressive functions in human T lymphocytes are in part mediated via modulation of ion channels. The activity of T lymphocytes relies on ion channels. KCa3.1 and Kv1.3 channels control cytokine release and, together with TRPM7, regulate T cell motility. Adenosine selectively inhibited KCa3.1, but not Kv1.3 and TRPM7, in activated human T cells. This effect of adenosine was mainly mediated by A2A receptors as KCa3.1 inhibition was reversed by SCH58261 (selective A2A receptor antagonist), but not by MRS1754 (A2B receptor antagonist) and it was mimicked by the A2A receptor agonist CGS21680. Furthermore, it was mediated by the cAMP/PKAI signaling pathway as adenylyl-cyclase and PKAI inhibition prevented adenosine effect on KCa3.1. The functional implication of the effect of adenosine on KCa3.1 was determined by measuring T cell motility on ICAM-1 surfaces. Adenosine and CGS21680 inhibited T cell migration. Comparable effects were obtained by KCa3.1 blockade with TRAM-34. Furthermore, the effect of adenosine on cell migration was abolished by pre-exposure to TRAM-34. Additionally, adenosine suppresses IL-2 secretion via KCa3.1 inhibition. Our data indicate that adenosine inhibits KCa3.1 in human T cells via A2A receptor and PKAI thereby resulting in decreased T cell motility and cytokine release. This mechanism is likely to contribute to decreased immune surveillance in solid tumors.
Keywords: KCa3.1 channels, Adenosine, Adenosine receptors, motility
INTRODUCTION
Adenosine is an anti-inflammatory purine nucleoside that is released by cells in response to stress and hypoxia (1). Although adenosine provides a protective mechanism that controls inflammation, it becomes an “immunological problem” in tumors (2, 3). Indeed, accumulation of adenosine is characteristic of the hypoxic microenvironment of solid tumors where it can reach levels 100-fold those of normal subcutaneous tissues (4). Importantly, because of its known immune suppressive properties, adenosine has thus been associated with tumor progression, enhanced metastatic potential and poor prognosis.
Adenosine is synthesized extracellularly by the phosphohydrolysis of ATP and ADP to AMP through the enzyme ectonucleoside triphosphate diphosphohydrolase 1 (CD39) followed by breakdown of AMP to adenosine by the ecto-5′ nucleotidase (CD73) (5). Accumulation of adenosine in the tumor microenvironment is facilitated by hypoxia and by regulatory T cells (Treg) (5, 6). Hypoxia increases CD73 expression and reduces the expression adenosine degrading enzymes (5, 7). Treg cells, which are distinguished from other T cell subsets by the dual expression of CD39 and CD73, produce adenosine and utilize it to suppress effector T cells (8, 9). Adenosine signaling is mediated by four subtypes of G protein coupled receptors: A1, A2A, A2B and A3. Of these the A2A receptor is the predominant subtype in T lymphocytes and plays an important role in mediating adenosine’s immune suppressive effects: its stimulation impairs T cell activation and effector functions such as proliferation and secretion of cytokines like IFN-γ and IL-2 (10–15). In the tumor setting in particular it has been shown that activation of A2A receptors results in decreased immune surveillance and tumor survival (16). Consequently, A2A receptor knock-down leads to T cell dependent tumor rejection (16). Another important activity of adenosine, which further contributes to its immune suppressive properties, is its ability to inhibit T cell trafficking (17). Recent findings in human lung mast cells suggested that the effect of adenosine and A2A receptor stimulation on cell migration may involve KCa3.1, a Ca2+-activated K+ channel also expressed in lymphocytes (18). While many aspects of adenosine-regulation of T cell function are now understood, the effects of adenosine on ion channels expressed in the T lymphocytes and their functional consequences are not known.
Ion channels are important regulators of T cell effector functions (i.e. cytokine release and proliferation) and motility (19). The main ion channels expressed in T cells are two K+ channels (the voltage-dependent Kv1.3 and the calcium activated KCa3.1), the Ca2+-release activated Ca2+ channel (CRAC) and the Ca2+- and Mg2+-permeant transient receptor potential melastatin 7 (TRPM7) channel. These channels act in concert to finely tune membrane potential and Ca2+ signaling (20, 21). Unequivocal evidence exists of the importance of ion channels in T cell activation as blockade of these channels inhibit cytokine production/release (20, 21). The importance of ion channels in T cell motility is less understood, while, in other cell types, ion channels have been shown to regulate various aspects of cell migration including cell volume and F-actin polymerization/depolymerization necessary for the propulsion of the cell (19, 22, 23). We have recently shown that KCa3.1 and TRPM7 regulate the motility of activated human T cells (24).
The current study was undertaken to study the effects of adenosine on ion channels in T lymphocytes and their downstream functional outcomes. We herein present evidence that adenosine inhibits KCa3.1 channels in activated human T cells thereby decreasing T cell motility and cytokine release. This mechanism is likely to contribute to decreased immune surveillance in hypoxic and adenosine-rich tumors.
MATERIALS AND METHODS
Cells
CD3+ T cells were isolated from venous blood by E-rosetting (StemCell Tech., Vancouver, Canada) followed by Ficoll-Paque density gradient (ICN Biomedicals, Aurora, OH, USA) centrifugation as previously described (25). T cells were maintained in RPMI medium supplemented with 10% pooled male human AB serum (Intergen, Milford, MA, USA), 200 U/ml penicillin, 200 μg/ml streptomycin and 10 mM Hepes (24). T cell were activated with either 4–10 μg/ml phytohemaglutinin (PHA, Sigma-Aldrich, St. Louis, MO, USA) in the presence of peripheral blood mononuclear cells (PBMC) for 72 hr or plate bound mouse anti-human CD3 (10 μg/ml) and mouse anti-human CD28 (10 μg/ml) antibodies (BD Biosciences, San Jose CA) for 72–96 hr. Blood was obtained from either healthy volunteers or blood bank donors (unutilized blood units from the Hoxworth Blood Bank Center, Cincinnati, OH, USA). Informed written consent was obtained from the healthy volunteers. The study was approved by the University of Cincinnati Institutional Review Board.
Electrophysiology
Patch-clamp experiments were performed in activated T cells using Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) in whole-cell configuration as previously described (26). KCa3.1 currents were recorded with an external solution of the following composition (in mM): 160 NaCl, 4.5 KCl, 2.0 CaCl2, 1.0 MgCl2 and 10 HEPES, pH 7.4, and the pipette solution was composed of (mM): 145 K-aspartate, 10 K2EGTA, 8.5 CaCl2, 2 MgCl2, and 10 HEPES, pH 7.2, 290–310 mOsm. The KCa3.1 currents were induced by 200 ms ramp depolarization from −110 mV to +50 mV from a holding potential (HP) of −70 mV every 10 s. The macroscopic conductance of KCa3.1 channels (GKCa3.1) was calculated as a ratio of the linear fraction of macroscopic current slope to the slope of ramp voltage stimulus:
The slope conductance was measured between −100 mV and −60 mV to avoid contamination by the Kv1.3 current (27). Kv1.3 currents were recorded with an external solution of the following composition (in mM): 150 NaCl, 5 KCl, 2.5 CaCl2, 1.0 MgCl2, 10 glucose and 10 HEPES, pH 7.4, 310 mOsm. The pipette solution was composed of (mM): 134 KCl, 1 CaCl2, 2 MgCl2, 10 EGTA, and 10 HEPES, pH 7.2 (24). The Kv1.3 currents were measured by 200 ms depolarizing voltage steps to +50 mV from a HP of −120 mV every 30 s. The external solution for TRPM7 currents had the following composition (in mM): 135 CH3SO3Na, 5 CsCl, 1 CaCl2, 10 Hepes, pH 7.4, 280 mOsm. The pipette solution contained (in mM): 120 CH3O3SCs, 5 CsCl, 3.1 CaCl2, 10 BAPTA, 10 HEPES, pH 7.2, with an estimated free Ca2+ concentration of 100 nM. The TRPM7 currents were elicited by ramp depolarization from −100 mV to +100 mV from a HP of 0 mV every 15 s (24).
Cell Migration on ICAM-1 surfaces
Cell migration was measured on ICAM-1 (intercellular adhesion molecule-1) coated glass coverslips. Coverslips were prepared either by direct deposition of ICAM-1Fc on glass or by deposition of ICAM-1Fc on PNMP (poly(o-nitrobenzyl methacrylate-r-methyl methacrylate-r-poly(ethylene glycol) methacrylate), a biocompatible random terpolymer (24, 28). The former surfaces were fabricated as follows. Briefly, glass coverslips were coated with ICAM-1Fc (5 μg/ml, R&D Systems Inc., Minneapolis MN) in PBS overnight at 4°C. The next day, the coverslips were blocked for 2 h at room temperature in a solution of 2.5% BSA in PBS (28). For fabrication of ICAM-1 surfaces on PNMP, the biotinylated PNMP was spun-coated on glass coverslips to achieve a thin layer of 200 nm. Subsequently, streptavidin was attached, followed by the deposit of biotinylated anti-IgG/Fc fragment and, last, ICAM-1 (24).
T cells were seeded on the ICAM-1 coverslips at a density of 2–3 × 105 cells/coverslip. Experiments were performed in either RPMI (without phenol red) or a physiological Ca2+ solution containing the following (in mM): 155 NaCl, 4.5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.4. Cells were maintained in a cell culture incubator at 37°C for 2 h before starting the migration experiments. After this time, the coverslips were transferred to a microscopy chamber heated at 35°C. Time-lapse microscopy was performed using the InCytIm3XMTD imaging system (Intracellular Imaging, Cincinnati, OH, USA) and bright field images were acquired at the rate of 20 images/min (24). The analysis and cell tracking was performed using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Polarized motile cells were considered for analysis, using the criteria described earlier (24). Briefly, polarized T cells were defined as those displaying a leading edge and trailing uropod. Of these, the cells that were able to move away from their initial position with a minimum mean velocity of 1.5 μm/min were defined as “migrating cells” and included in our analysis. Cells that moved around a fixed contact point (i.e. did not travel any significant distance) or cells that remained at the same coordinate were excluded.
The same type of random amoeboid migration and comparable range of migration velocities were observed regardless the type of ICAM-1 surfaces and medium used (24) (Supplemental Fig. S1).
IL-2 ELISA
anti-CD3/anti-CD28 pre-activated CD3+ T cells (1 × 10 cells) were incubated with 1 μM adenosine, 250 nM TRAM-34 or 1 μM adenosine together with 250 nM TRAM-34. For these experiments, adenosine was added in the presence of 30 μM adenosine deaminase inhibitor erythro-9-(2-hydroxy-3-nonyl)-adenine hydrochloride (EHNA) (29). Cells were then restimulated with CD3/CD28 antibodies for 24 h, following which the supernatants were collected and IL-2 levels were measured using the Human IL-2 ELISA Kit II (BD Biosciences, San Jose CA) according to the manufacturer’s instructions.
Chemicals
ShK was obtained from Bachem Americas, Inc. (Torrance, CA, USA), TRAM-34 was a kind gift from Dr. Heike Wulff (Department of Pharmacology, UC Davis, CA, USA). All other chemicals were obtained from Sigma Aldrich (St. Louis MO).
Data Analysis
All data are presented as means ± SEM unless otherwise specified. Statistical analyses were performed using either Student’s t-test (paired or unpaired) or One-way ANOVA for differences across groups. Post hoc testing for the One-Way ANOVA was determined by Holm-Sidak method. Statistical analysis was performed using SPSS Sigma Stat 3.0 software; p < 0.05 was defined as significant.
RESULTS
Adenosine inhibits KCa3.1 channels in human CD3+ T cells
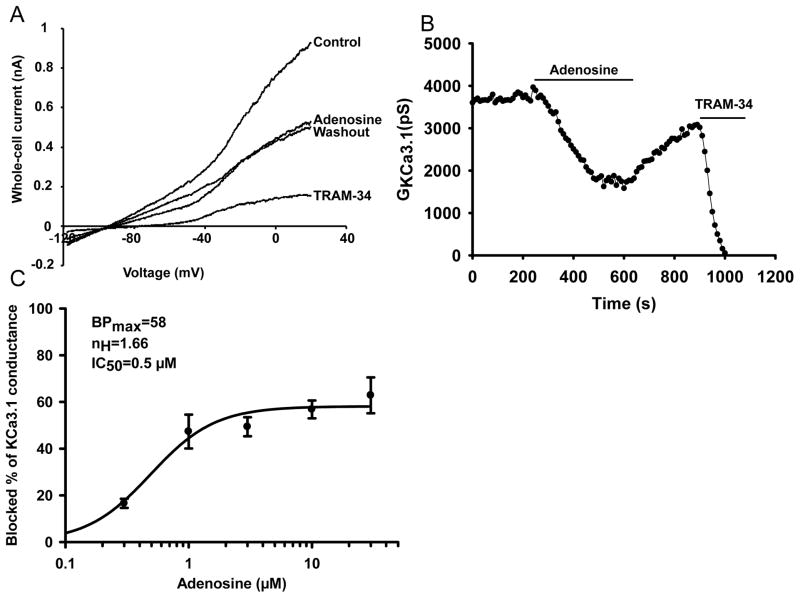
Electrophysiological experiments were conducted to study the effect of adenosine on ion channels in human T lymphocytes. We initially focused on KCa3.1, as it has been reported that adenosine inhibits this channel in lung mast cells (18). Experiments were performed to determine whether 1 μM adenosine inhibits KCa3.1 channels in human T lymphocytes (Fig. 1). Similar concentrations of adenosine have been measured in solid tumors (4). KCa3.1 currents were measured in T cells while the cells were progressively exposed to adenosine and, after adenosine wash-out, to the specific KCa3.1 blocker TRAM-34 (24, 30) (Fig. 1A–B). This blocker is highly selective for KCa3.1 with a IC50 of 25 nM for KCa3.1 vs. 5000 nM for Kv1.3 (30). TRAM-34 was used as positive control in all the studies presented in this manuscript. Actual KCa3.1 current recordings are shown in Fig. 1A. The KCa3.1 channel activity was determined from the current recordings by measuring the KCa3.1 conductance (GKCa3.1, the ratio between the current and the voltage). GKCa3.1 was calculated by fitting the linear portion of the current traces between −100 and −60 mV. In this voltage range the current recorded is purely ascribable to KCa3.1 as it is devoid of contamination by currents through the Kv1.3 channel that are also expressed in T cells (20). Application of adenosine produced a rapid inhibition of KCa3.1 channels in activated CD3+ primary T cells (Fig. 1A–B). The time-course of the effect of adenosine on KCa3.1 activity is shown in Fig. 1B. The response to adenosine developed immediately upon exposure, reached steady-state after ca. 3 min and was partially reversible (Fig. 1A–B). Fig. 1C shows that adenosine inhibited KCa3.1 current in a dose dependent manner with a maximum blockade of 58 %, Hill coefficient (nH) of 1.66 and IC50 of 0.5 μM.
Figure 1. Adenosine inhibits KCa3.1 channels in activated human T cells.
(A) Whole-cell currents were recorded in an activated T cell before (control) and after addition of 1 μM adenosine, then after washout of adenosine and in the presence of TRAM-34 (10 μM). Currents were elicited by ramp depolarization from −110 mV to +50 mV from a HP −70 mV every 10 s. (B) Representative experiment showing the time course of the change in GKCa3.1 after treatment with adenosine, washout with external solution and subsequent addition of TRAM-34 (same experiment as in A). (C) Dose-response curve of adenosine for KCa3.1 channels in activated T cells. At each concentration blocked percentage of KCa3.1 conductance (BP) was determined and plotted against the corresponding adenosine concentration (each data point is given as mean ± SEM for 5–8 cells from 3 donors). Hill equation was fitted to the points (solid line, , where BPmax: maximum blocked percentage, [ADO]: concentration of adenosine, IC50: half-maximal inhibitory concentration, nH: Hill coefficient), and the results of the fitting are indicated in the insert.
Since potassium channels share a very similar pore structure, a great deal of inhibitory molecules can exert the same effect on different K+ channels. Consequently, the specificity of adenosine for KCa3.1 was tested by measuring its effect on the other K+ channel expressed in T cells, Kv1.3. As shown in Fig. 2A, Kv1.3 currents were not inhibited by adenosine, but perfusion with tetraethylammonium (TEA; 10 mM, a general blocker of K+ channels) resulted in ca. 40% reduction of the Kv1.3 peak current in accordance with previous findings (n=17, 5 donors, Fig. 2B) (31). TEA, although not a selective blocker of Kv1.3, was chosen because it is a reversible blocker and can thus be applied as positive control before and after addition of adenosine to demonstrate that the lack of adenosine effect was not an artifact produced by the perfusion system. A more selective blocker for Kv1.3 was not necessary in the patch-clamp experiments because the low free Ca2+ concentration of the pipette solution used for Kv1.3 recording prevented KCa3.1 activation. Overall, these data indicate that adenosine selectively affects KCa3.1 channels.
Figure 2. Adenosine has no effect on Kv1.3 channel current in T lymphocytes.
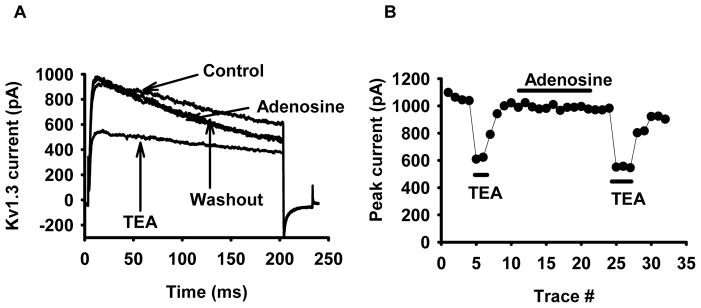
(A) Whole-cell Kv1.3 currents were recorded in a PHA-activated lymphocyte before and after application of 1 μM adenosine and in the presence of 10 mM TEA in the bath (after adenosine washout). Kv1.3 currents were evoked by 200-ms-long depolarizing pulses from a HP of −120 mV to +50 mV every 30 s. (B) Time-course of Kv1.3 peak current upon adenosine (1μM) and TEA (10 mM) application obtained for the cell in panel A. Similar results were obtained in 5 donors, and n=17 cells.
Adenosine inhibits KCa3.1 channels via A2A receptors
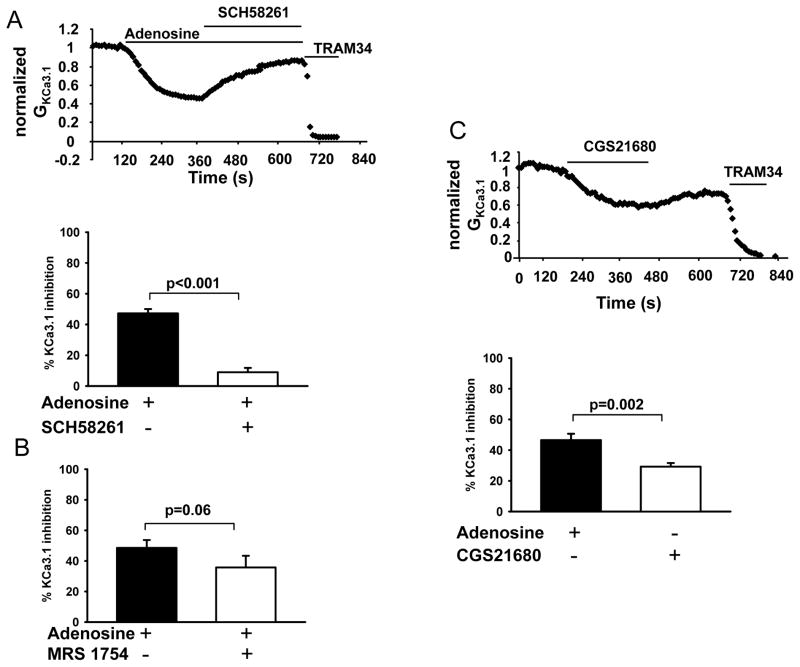
Adenosine signaling is mediated by G-protein coupled adenosine receptors. The A2A receptor is the predominant isoform expressed in human and mouse T cells, with A2B and A3 receptors expressed in lower abundance (10). Hence, we next determined whether A2A receptors mediate the inhibition of KCa3.1 channels induced by adenosine in activated human T cells. KCa3.1 channel activity was measured before and after exposure to adenosine, followed by SCH58261, a selective A2A receptor competitive antagonist, in the presence of adenosine (32). The KCa3.1 channel activity is reported as GKCa3.1 normalized to maximum control conductance (before addition of the drug). As shown in Fig. 3A, treatment with adenosine reduced KCa3.1 activity by 50%, commensurate with our findings in Fig. 1. Addition of SCH58261 to the bath, after KCa3.1 steady-state inhibition by adenosine, returned the KCa3.1 activity to 90% of its baseline value. At this point, addition of TRAM-34 produced a complete block of KCa3.1. The bottom panel of Fig. 3A summarizes these results of n=12 experiments in 4 donors. Contrary to the effect of A2A antagonist, the specific A2B antagonist MRS1754 did not reverse the effect of adenosine indicating that the effect of adenosine on KCa3.1 channels is not mediated by the A2B receptor (Fig. 3B) (33). The role of A2A receptors in adenosine-induced inhibition of KCa3.1 was further confirmed by treating the cells with the A2A receptor agonist CGS21680: it induced a rapid and significant decrease in KCa3.1 conductance (Fig. 3C, top) (34). The degree of inhibition by CGS21860 was less as compared to that reported earlier, which can be attributed to the difference in expression system (native T cells vs. over-expressed receptors in CHO) and the readout method (binding assay vs. patch-clamp technique) (Fig. 3C) (35).
Figure 3. Adenosine inhibits KCa3.1 channels via A2A receptors.
(A) Top: Normalized GKCa3.1 (relative to the maximum conductance prior to the addition of adenosine) for a representative T cell upon perfusion with 1 μM adenosine alone and together with 1 μM SCH58261. TRAM-34 (10 μM) was added at the end of the experiments as positive control. SCH58261 was applied to the bath with adenosine still present in the bath, after the steady-state inhibition of KCa3.1 by adenosine was achieved. Bottom: Blocked percentage of GKCa3.1 after treatment with 1 μM adenosine alone and adenosine plus 1 μM SCH58261 (n=12, 4 donors). (B) Blocked percentage of GKCa3.1 in cells treated with 1 μM adenosine alone and after addition of 50 nM A2B receptor antagonist MRS1754 in the presence of adenosine (n=8, 3 donors). (C) Top: receptor Representative normalized GKCa3.1 are plotted before and after addition of A2A agonist CGS21680 (1 μM) (10 μM TRAM-34 was added at the end of experiment). Bottom: Comparison of the percent inhibition of GKCa3.1 in cells treated with 1 μM adenosine (n=21, 6 donors) or 1 μM CGS21680 (n=21, 6 donors).
In T cells, A2A signaling is mediated by cAMP and PKA. The downstream effect of the A2A receptor is the activation of adenylate cyclase and, consequently, cAMP production and PKA activation (13). cAMP, along with the PKA isoform PKAI, negatively regulates cytokine production (36). Experiments were thus designed to determine whether an identical signaling pathway is involved in adenosine-induced inhibition of KCa3.1. The effect of adenosine on KCa3.1 was prevented by the adenylate cyclase inhibitor 2′,5′-dideoxyadenosine (37). We observed a 52% inhibition of KCa3.1 channel activity with adenosine, and this inhibition was reduced to 30% when 100 μM 2′5′-dideoxyadenosine was added to the pipette solution to deliver to the cells. Furthermore, a similar effect was observed with the PKAI inhibitor Rp-8Br-cAMP (100 μM, Fig. 4B) (38).
Figure 4. Inhibition of KCa3.1 by adenosine is mediated by the cAMP/PKAI signaling pathway.
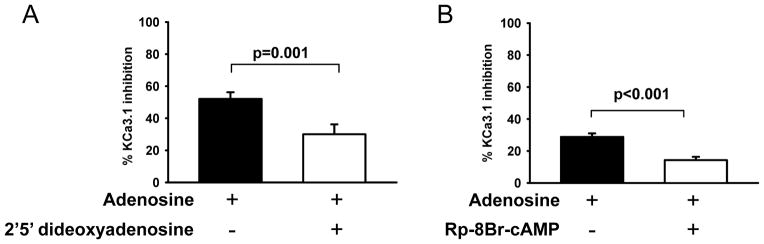
Percentage inhibition of GKCa3.1 values are shown in T cells perfused with 1 μM adenosine alone (n=14, 3 donors) or in presence of either the (A) adenyl cyclase inhibitor 2′5′-dideoxydenosine (100 μM, n=13, 3 donors) or the (B) PKAI inhibitor Rp-8Br-cAMP (100 μM, n=12, 3 donors) in the patch-clamp pipette solution. Both, 2′5′ dideoxydenosine and Rp-8Br-cAMP were added to the pipette solution and delivered into the cells, before adenosine addition, by diffusion once in the whole-cell configuration.
Overall, our results demonstrate that A2A receptors and cAMP/PKAI mediate the inhibitory effect of adenosine on KCa3.1 channels in activated CD3+ T cells. Still, the functional implications of KCa3.1 modulation by adenosine are not understood. Recent reports have indicated that adenosine inhibits cell motility, but the mechanisms are poorly understood although KCa3.1 has been implicated in the effect of adenosine on the motility of human lung mast cells (18, 39). KCa3.1 has been shown to play a vital role in the migration of activated T cells (24). Therefore, we investigated the effect of adenosine on T cell migration and the possible role of KCa3.1.
Adenosine inhibits T cell migration
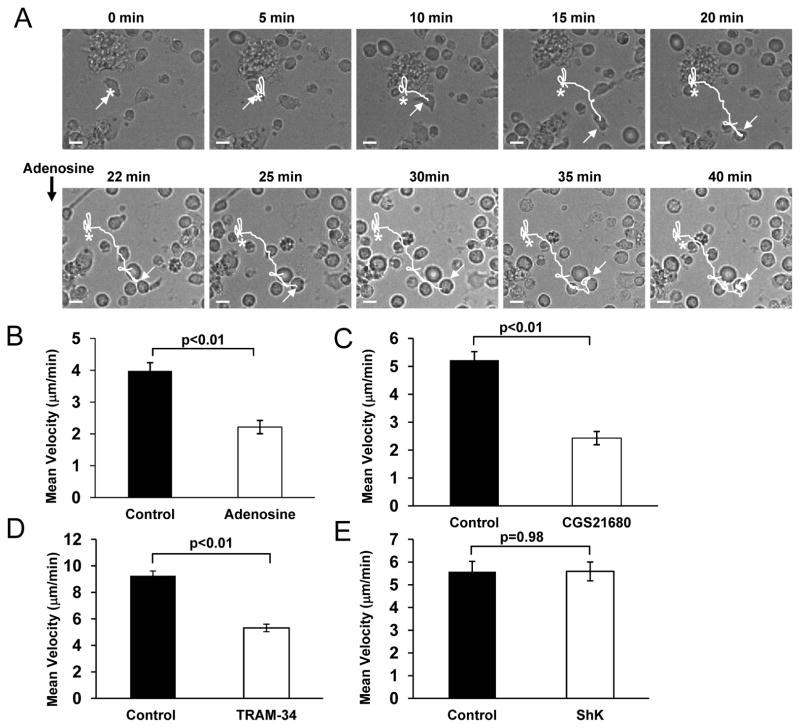
The motility of T cells was studied using ICAM-1 surfaces without chemokine gradient. ICAM-1 interacts with the integrin lymphocyte function-associated antigen 1 (LFA-1) on the T cell membrane and LFA1 cross-linking can induce by itself T cell polarization and migration (23). Binding of ICAM-1 to LFA-1 results in LFA-1 activation and the downstream signaling cascade that leads to F-actin polymerization and forward motion (23). ICAM-1 surfaces are routinely used to study integrin-dependent migration. Integrins regulate the migration and retention of T cells during inflammation as they are involved in the extravasation of T cells into tissues and interactions with the extracellular matrix (23). To study effect of adenosine on T cell migration, the motility of single T cells on ICAM-1 surfaces was recorded for 20 min before and after application of adenosine (Fig. 5A). T cells migrate on these surfaces by random amoeboid walk, as previously described (supplemental Fig. S1A) (24). We observed that adenosine reduced the velocity of migrating T cells by 44% (Fig. 5A–B). The mean baseline velocity of 3.98 ± 0.26 μm/min decreased significantly to 2.22 ± 0.21 μm/min after addition of adenosine (n=32, 3 donors; p<0.01 Fig. 5B). The effect of adenosine was also mimicked by CGS21680. The mean baseline velocity of migrating T cells decreased by 54% in presence of CGS21680: from 5.23 ± 0.30 μm/min to 2.43 ± 0.24 μm/min (n=26, 3 donors; p<0.01 Fig. 5C).
Figure 5. Adenosine inhibits T cell migration via A2A receptors.
(A) Representative time-lapse bright-field microscopy of an activated T cell migrating on an ICAM-1 surface before and after addition of adenosine (1 μM). The asterisk indicates the starting point; the arrow points at the migrating cell, the dotted line shows the projected path and the continuous line the covered distance. Scale bar is 5 μm. (B–C) Effect of adenosine and A2A receptor agonist CGS21680 on T cell migration (both 1 μM). T cell migration was measured in the same T cell before and after treatment with either 1 μM adenosine (B, n=32, 3 donors) or 1 μM CGS21680 (Panel C, n=26, 3 donors). The results were obtained tracking individual cells for 20 min before and after drug application. (D–E) Responses to KCa3.1 and Kv1.3 specific blockers. The experiments were conducted as indicated in B–C, using 25 nM TRAM-34 (Panel D, n=9, 2 donors) or 10 nM ShK (Panel E, n=6, 1 donor).
Similar to adenosine, and in accordance with our earlier reported findings, the specific KCa3.1 blocker TRAM-34 inhibits T cell migration in activated CD3+ cells (Fig. 5D) (24). We observed a 43% decrease in the mean velocity, which decreased from 9.26 ± 0.35 μm/min to 5.32 ± 0.28 μm/min after addition of 25 nM TRAM-34 (n=9, 2 donors, p<0.01, Figure 5D). This effect of TRAM-34 was produced by a concentration that, similarly to adenosine 1 μM, inhibits 50% of the KCa3.1 current. No further inhibition was produced by higher concentrations of TRAM-34 500 nM TRAM-34 induced a 48% decrease in the mean velocity which decreased from 4.01 ± 0.36 μm/min to 2.08 ± 0.18 μm/min after TRAM-34, n=8, 1 donor, p<0.01) In agreement with previous findings, the motility of migrating activated human T cells did not depend on Kv1.3 as treatment with the Kv1.3 specific blocker ShK did not change the mean velocity (p=0.98, Fig. 5E) (24). These findings raise the possibility that adenosine-effect on motility may be due to KCa3.1 inhibition. Still, we cannot exclude that the effect of adenosine on T cell motility may involve the other channel that regulates the motility of activated human T cells, TRPM7 (24). Since the effect of adenosine on TRPM7 is not known, we studied whether TRPM7 channel activity is influenced by adenosine.
Adenosine does not modulate TRPM7 currents
TRPM7 currents were measured in activated T cells before and after addition of adenosine. 2-APB, a non-specific TRPM7 blocker, was used as positive control. The actual TRPM7 current recordings are illustrated in Fig. 6A. The effect of adenosine and 4-APB is reported as percentage of TRPM7 inhibition, measured at +100 mV. As shown in Fig. 6, there was no reduction in TRPM7 current when cells were perfused with 1 μM adenosine (n=6), while the current was largely inhibited by the TRPM7 inhibitor 2-aminoethoxydiphenyl borate (2-APB) at 250 μM concentration (24). Thus, out of the two channels modulating the migration of activated T cells, only KCa3.1 is inhibited by adenosine. This further supports the possibility that the effect of adenosine on T cell motility is mediated by KCa3.1. Conclusive evidence could only be drawn if the effect of adenosine on T cell migration is abolished by KCa3.1 blockade.
Figure 6. Adenosine does not affect TRPM7 current.
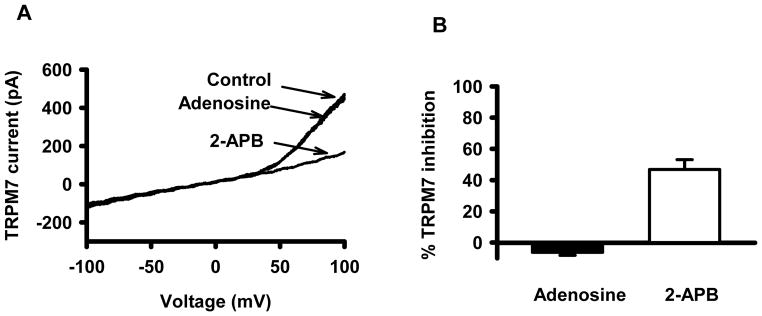
(A) Current traces of TRPM7 channels in a T cell in the absence or presence of 1 μM adenosine and 200 μM 2-APB were registered upon 200-ms-long voltage-ramp protocol ranging from −100 to +100 mV (HP was set 0 mV). (B) The inhibited percentage of TRPM7 current upon application of adenosine and 2-APB (see Result also).
The effect of adenosine on T cell migration is conveyed by KCa3.1
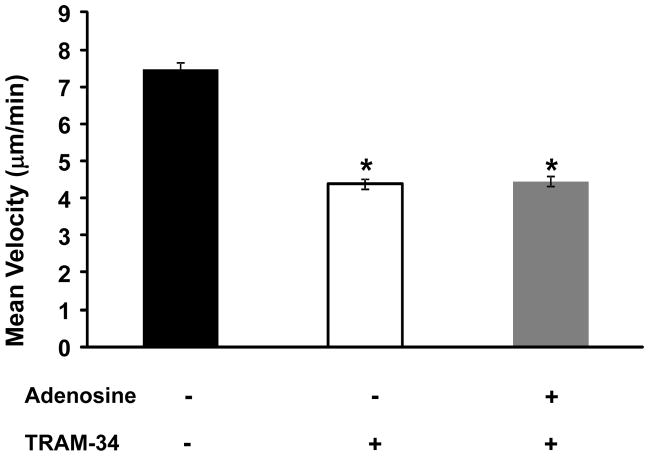
Experiments were performed to determine the effect of adenosine on T cell migration in presence of TRAM-34. The following experimental protocol was implemented: The migration of a single T cell was recorded for 6 minutes in regular medium (without adenosine and/or TRAM-34). After this time TRAM-34 (500 nM, 20-fold higher than the IC50 value) was added to the bath solution and motility was measured for 6 more minutes (30). This interval is sufficient to obtain a steady-state inhibition of KCa3.1 currents and T cell motility (24). Cells were then exposed to adenosine in the presence of TRAM-34 and the motility was followed for 6 more minutes. The corresponding velocities in normal bath solution (no drugs), in the presence of TRAM-34 and TRAM-34 with 1 μM adenosine are shown in Fig. 7. Overall, we observed a 40% decrease in the mean velocity of T cells from a baseline value of 7.47 ± 0.20 μm/min to 4.39 ± 0.14 μm/min after addition of TRAM-34 (n=12, 3 donors, p<0.01). Addition of adenosine to the bath solution in the presence of TRAM-34 did not further decrease the mean velocity of the cells (4.46 ± 0.14 μm/min, n=12, 3 donors, p=0.76 as compared to TRAM-34 treated group). In these experiments, adenosine alone produced a 40% inhibition of the velocity (from 6.10 ± 0.33 μm/min to 3.69 ± 0.22 μm/min (n=6 cells from 1 donor, p<0.01). Similar effects were observed in T cells that were activated by T cell receptor (TCR) stimulation with CD3/CD28 antibodies (Supplemental Fig. S2). Overall, these data show no additive or synergistic effect of adenosine on T cell motility in presence of TRAM-34 indicating that KCa3.1 is the effector protein that mediates adenosine-induced inhibition of T cell migration.
Figure 7. T cell migration by adenosine is regulated by KCa3.1 channels.
Mean velocity of activated T cells upon application of TRAM-34 (500 nM) alone and in combination with 1 μM adenosine (n=12, 3 donors).
Along with cell migration, KCa3.1 channels regulate cytokine release in T lymphocytes (31). Hence, we wanted to study whether KCa3.1 channel inhibition mediates the effect of adenosine on cytokine release in T cells.
Adenosine suppresses IL-2 release via KCa3.1 channel inhibition
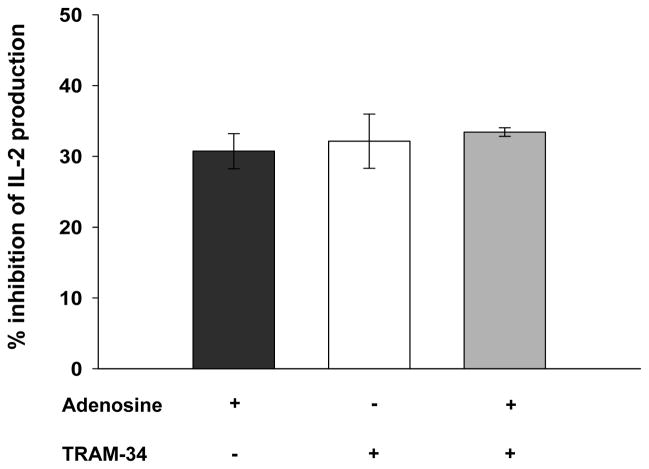
Activated T cells were re-stimulated for 24 hr with CD3/CD28 antibodies in presence of either 1 μM adenosine or 250 nM TRAM-34 or 250 nM TRAM-34 along with 1 μM adenosine. For these experiments, EHNA (30 μM) was added together with adenosine to prevent adenosine degradation and ensures its long-term efficacy (29). Untreated cells were used as controls. ELISA was then performed to measure IL-2 levels. Adenosine and TRAM-34 reduced IL-2 levels by 31 ± 3 % (n=3 donors) and 32 ± 7%, (n=3 donors), respectively (Fig. 8). Moreover, no additive effect of adenosine on IL-2 levels in the presence of TRAM-34 was detected (33 ± 1% inhibition, n=3 donors, Fig. 8). These data show that adenosine decreases IL-2 production in activated T cells and provide evidence of a role of KCa3.1 in mediating adenosine’s effect on cytokine release.
Figure 8. Inhibition of KCa3.1 channels by adenosine reduces IL-2 secretion in T cells.
CD3/CD28 antibodies activated T cells were reactivated in the presence of 1 μM adenosine, 250 nM TRAM-34 or both 1 μM adenosine and 250 nM TRAM-34 for additional 24 hrs, and IL-2 levels were measured in the supernatants by ELISA. The decreases in IL-2 levels are reported as percentage values normalized to the untreated controls. The data are the average of 3 distinct donors; each sample was measured in duplicate.
DISCUSSION
Adenosine is an anti-inflammatory signaling molecule that has been implicated in the failure of immune surveillance in solid tumors (2). Herein we show, for the first time, a role for ion channels in mediating adenosine-induced inhibition of T cell motility and IL-2 release. This finding adds ion channels to the signaling pathway that mediates the immune suppressive properties of adenosine downstream to the A2A receptor.
Adenosine has long been known to suppress the activation of T lymphocytes and secretion of pro-inflammatory cytokines (10). Moreover, adenosine has been implicated in suppressing lymphocytes trafficking in response to tissue injury or infections (10). The biochemistry of adenosine signaling that leads to inhibition of cytokine production and proliferation has been thoroughly investigated in T lymphocytes and it involves the stimulation of A2A receptor and activation of cAMP/PKAI (10). The effect of adenosine on T cell trafficking is instead less understood. Furthermore, to our knowledge, no information is available on the effect of adenosine on ion channels in T lymphocytes. This manuscript is the first report of such effect. On the contrary, adenosine has been known to regulate the activity of ion channels in other cell types. Adenosine inhibits voltage-dependent Ca2+ channels in PC12 cells and the TWIK-related-acid-sensitive K channel 1 (TASK-1) in carotid body cells (40, 41). Furthermore, ATP-sensitive K+ channels and Cl− channels in epithelial cells are also modulated by adenosine (42). Our finding indicates that human T lymphocytes respond to acute exposure to adenosine with a suppression of KCa3.1 activity. The sensitivity to adenosine is specific to KCa3.1, as other channels expressed in T cells, Kv1.3 and TRPM7, are not modulated by adenosine. Furthermore, the classic adenosinergic pathway that mediates the transcriptional effects of adenosine (A2A receptor and cAMP/PKAI) in T cells also regulates KCa3.1. Modulation of KCa3.1 by cAMP/PKA has been reported in other cell types with different outcomes. Increased cAMP levels have been shown to enhance KCa3.1 currents in human erythrocytes, Xenopus oocytes and rat submandibular acinar cells by a mechanism that appears to be dependent on PKA (43–45). Others have reported a different outcome in oocytes where they showed a potent inhibition of KCa3.1 by the catalytic subunit of PKA due to direct phosphorylation of the channel itself (46). In HEK293 cells KCa3.1 was reported to be modulated via a cAMP dependent protein kinase-independent phosphorylation (43). Similar to our finding, an inhibitory effect of adenosine and A2A receptor stimulation on KCa3.1 has been reported in human mast cells (18).
Ion channels are important regulators of T cell activation, effector functions, such as cytokine release and proliferation (20, 24), and motility (24, 47, 48). We have recently shown that KCa3.1 and TRPM7, which localize at the uropod of migrating cells, control the motility of activated human T cells (i.e. inhibition of KCa3.1 and downregulation of TRPM7 inhibit T cell motility) (24). Toyama et al. have also implicated KCa3.1 channels in T cell and macrophage migration/accumulation into atherosclerotic lesions in ApoE−/− mice in vivo (49). We have speculated that KCa3.1 and TRPM7 channels may work in concert to control the intracellular Ca2+ levels necessary for T cell forward motion (19). The role of ion channels in cell motility is well established in many cell types where it has been shown that they control the motility by regulating membrane potential, Ca2+ homeostasis and cell volume (19). Further studies are necessary to define the mechanism by which KCa3.1 and TRPM7 control T cell motility. Moreover, additional studies are necessary to establish whether KCa3.1 channels only control motility of selective T cell subsets. It has been reported that Kv1.3, the other K+ channel of T cells, plays a role in the migratory capacity of resting human T cells in vitro and rat effector memory T (TEM) cells in vivo (47, 48). It has been reported that the expression of Kv1.3 and KCa3.1 channels differs in different T cell subsets and depends on the activation state: activated CCR7− TEM cells express a high number of Kv1.3 channels and activated CCR7+ naïve and central memory T cells express high levels of KCa3.1 (50). The studies we have conducted are on a mixed population of T cells (pre-activated by PHA or CD3/CD28 stimulation) with a high prevalence of T cells expressing high levels of KCa3.1 channels (51). Thus, we cannot exclude that our finding may not be extendable to all T cell subpopulations, but only apply to those cells whose predominant K+ channel is KCa3.1. Indeed, resting human T cells, which display low KCa3.1 expression, did not polarize and migrate on ICAM-1 surfaces (data not shown) (51). Yet, quiescent T cells display also an LFA-1 with a low affinity for ICAM-1, which could explain/contribute to the lack of migration in our experimental setting (52). Furthermore, the K+ channel phenotype of different T cell subsets in disease other than autoimmunity has yet to be defined.
Although ion channels play such important role in T cell motility, very little is known about their regulation in T lymphocytes. Here we showed that adenosine and A2A receptor stimulation selectively inhibit KCa3.1 channels. Importantly, we showed that adenosine inhibits T cell migration and this effect is prevented by KCa3.1 blockade. This finding indicates that adenosine-effect on motility occurs via KCa3.1 inhibition. A role for KCa3.1 in mediating adenosine-effect on cell motility was also suggested in human lung mast cells by correlating the effects of adenosine on cell motility to those produced by KCa3.1 blockade, but no conclusive evidence were reported (18). Along with T cell motility, KCa3.1 channels are also responsible for cytokine production (31). Similarly, adenosine and A2A receptor stimulation has been shown to inhibit cytokine release (5, 53). We herein presented evidence that support a role of KCa3.1 in mediating adenosine’s blockade of cytokine.
The ability of adenosine to inhibit T cell motility and cytokine release may be part of a protective mechanism in place to reduce inflammation. Unfortunately, the same effects may contribute to the decreased immune surveillance of solid tumors. Adenosine is highly concentrated in tumor sites, where it is produced by tumor cells, stromal cells and Treg cells (3, 54). There is strong clinical evidence that immune surveillance in cancer patients correlates with the infiltration of immune cells into the tumor (3). Yet, the infiltration of tumors by effector T cells is limited and CD3+ T cells in tumors display low motility (3, 55, 56). Although trafficking of immune cells into tumors is of such importance, the factors that limit the movement of the immune cells inside the tumors are poorly defined. The data we have presented here suggest a new mechanistic paradigm by which adenosine, produced in the tumor microenvironment, reduces KCa3.1 channel activity in effector T cells thus limiting the infiltration of T lymphocytes into the tumor mass. This raises the importance of ion channels in T cells as contributing factors of the failure of the immune system caused by the tumor microenvironment. We have shown that hypoxia, which is also characteristic of the tumor microenvironment, inhibits Kv1.3 channels in T lymphocytes thus reducing Ca2+ signaling and proliferation (26, 27, 57, 58). This finding combined with the effect of adenosine on KCa3.1 raise the possibility that hypoxia and adenosine in the tumor microenvironment provide a multidirectional attack on T cells via ion channels’ inhibition, which ultimately contributes to the weakened immune defenses at the tumor site. Furthermore, our finding raise the possibility that Treg cell-mediated immune suppression is conveyed, at least in part, by KCa3.1 channels. It is well established that Treg cells generate and utilize adenosine to suppress effector T cells by acting on the A2A receptors on these cells (8). Our results indicate that KCa3.1 channels, downstream to the A2A receptor, may link Treg function to the suppression of effector T cells.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by NIH grants CA095286, AI083076 and AR060966 to LC. OS was supported in part by the European Union and the State of Hungary (TÁMOP 4.2.4. A/2-11-1-2012-0001).
Abbreviations
- GKCa3.1
Conductance of KCa3.1 channels
- ICAM-1
intercellular adhesion molecule 1
- TEA
Tetraethyl Ammonium
- 2-APB
2 aminoethoxydiphenylborate
- IC50
half-maximal inhibitory concentration
- LFA-1
Lymphocyte function-associated antigen 1
References
- 1.Kobayashi S, Zimmermann H, Millhorn DE. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. Journal of Neurochemistry. 2000;74:621–632. doi: 10.1046/j.1471-4159.2000.740621.x. [DOI] [PubMed] [Google Scholar]
- 2.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside TL. Immune responses to malignancies. The Journal of Allergy and Clinical Immunology. 2010;125:S272–283. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blay J, White TD, Hoskin DW. The Extracellular Fluid of Solid Carcinomas Contains Immunosuppressive Concentrations of Adenosine. Cancer Research. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 5.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological Control of Immune Response and Inflammatory Tissue Damage by Hypoxia-Inducible Factors and Adenosine A2A Receptors. Annual Review of Immunology. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 6.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends in Immunology. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Conrad PW, Conforti L, Kobayashi S, Beitner-Johnson D, Rust RT, Yuan Y, Kim HW, Kim RH, Seta K, Millhorn DE. The molecular basis of O2-sensing and hypoxia tolerance in pheochromocytoma cells. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:187–204. doi: 10.1016/s1096-4959(00)00326-2. [DOI] [PubMed] [Google Scholar]
- 8.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007 doi: 10.1084/jem.20062512. jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linden J, Cekic C. Regulation of Lymphocyte Function by Adenosine. Arterioscler Thromb Vasc Biol. 2012;32:2097–2103. doi: 10.1161/ATVBAHA.111.226837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic Signaling during Inflammation. New England Journal of Medicine. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacological Reviews. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 13.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 14.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 15.Ohta A, Madasu M, Kini R, Subramanian M, Goel N, Sitkovsky M. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. Journal of immunology. 2009;183:5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 16.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesdorffer CS, Malchinkhuu E, Biragyn A, Mabrouk OS, Kennedy RT, Madara K, Taub DD, Longo DL, Schwartz JB, Ferrucci L, Goetzl EJ. Distinctive immunoregulatory effects of adenosine on T cells of older humans. The FASEB Journal. 2012;26:1301–1310. doi: 10.1096/fj.11-197046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy SM, Cruse G, Brightling CE, Bradding P. Adenosine closes the K+ channel KCa3.1 in human lung mast cells and inhibits their migration via the adenosine A2A receptor. Eur J Immunol. 2007;37:1653–1662. doi: 10.1002/eji.200637024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwab A, Fabian A, Hanley PJ, Stock C. Role of Ion Channels and Transporters in Cell Migration. Physiological Reviews. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 20.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nature reviews Immunology. 2012;12:532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab A, Hanley P, Fabian A, Stock C. Potassium Channels Keep Mobile Cells on the Go. Physiology. 2008;23:212–220. doi: 10.1152/physiol.00003.2008. [DOI] [PubMed] [Google Scholar]
- 23.Gomez TS, Billadeau DD, Frederick WA. Advances in Immunology. Academic Press; 2008. T Cell Activation and the Cytoskeleton: You Can’t Have One Without the Other; pp. 1–64. [DOI] [PubMed] [Google Scholar]
- 24.Kuras Z, Yun YH, Chimote AA, Neumeier L, Conforti L. KCa3.1 and TRPM7 Channels at the Uropod Regulate Migration of Activated Human T Cells. PLoS ONE. 2012;7:e43859. doi: 10.1371/journal.pone.0043859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolaou SA, Neumeier L, Takimoto K, Lee SM, Duncan HJ, Kant SK, Mongey AB, Filipovich AH, Conforti L. Differential calcium signaling and Kv1.3 trafficking to the immunological synapse in systemic lupus erythematosus. Cell Calcium. 2010;47:19–28. doi: 10.1016/j.ceca.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szigligeti P, Neumeier L, Duke E, Chougnet C, Takimoto K, Lee SM, Filipovich AH, Conforti L. Signalling during hypoxia in human T lymphocytes--critical role of the src protein tyrosine kinase p56Lck in the O2 sensitivity of Kv1.3 channels. The Journal of Physiology. 2006;573:357–370. doi: 10.1113/jphysiol.2006.109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins JR, Lee SM, Filipovich AH, Szigligeti P, Neumeier L, Petrovic M, Conforti L. Hypoxia modulates early events in T cell receptor-mediated activation in human T lymphocytes via Kv1.3 channels. The Journal of Physiology. 2005;564:131–143. doi: 10.1113/jphysiol.2004.081893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. Journal of Cell Science. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- 29.Hesdorffer CS, Malchinkhuu E, Biragyn A, Mabrouk OS, Kennedy RT, Madara K, Taub DD, Longo DL, Schwartz JB, Ferrucci L, Goetzl EJ. Distinctive immunoregulatory effects of adenosine on T cells of older humans. Faseb J. 2012;26:1301–1310. doi: 10.1096/fj.11-197046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wulff H, Miller MJ, Hänsel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proceedings of the National Academy of Sciences. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahalan MD, Wulff H, Chandy KG. Molecular Properties and Physiological Roles of Ion Channels in the Immune System. Journal of Clinical Immunology. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- 32.Zocchi C, Ongini E, Conti A, Monopoli A, Negretti A, Baraldi PG, Dionisotti S. The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2a adenosine receptor antagonist. Journal of Pharmacology and Experimental Therapeutics. 1996;276:398–404. [PubMed] [Google Scholar]
- 33.Ji X-d, Kim Y-C, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A2B adenosine receptors. Biochemical Pharmacology. 2001;61:657–663. doi: 10.1016/s0006-2952(01)00531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer CA, Haxhiu MA, Martin RJ, Wilson CG. Adenosine A2A receptors mediate GABAergic inhibition of respiration in immature rats. Journal of Applied Physiology. 2006;100:91–97. doi: 10.1152/japplphysiol.00459.2005. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruppelt A, Mosenden R, Gronholm M, Aandahl EM, Tobin D, Carlson CR, Abrahamsen H, Herberg FW, Carpen O, Tasken K. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase a type I by the a-kinase anchoring protein Ezrin. J Immunol. 2007;179:5159–5168. doi: 10.4049/jimmunol.179.8.5159. [DOI] [PubMed] [Google Scholar]
- 37.Désaubry L, Shoshani I, Johnson RA. 2′,5′-Dideoxyadenosine 3′-Polyphosphates Are Potent Inhibitors of Adenylyl Cyclases. Journal of Biological Chemistry. 1996;271:2380–2382. doi: 10.1074/jbc.271.5.2380. [DOI] [PubMed] [Google Scholar]
- 38.Kuras Z, Kucher V, Gordon SM, Neumeier L, Chimote AA, Filipovich AH, Conforti L. Modulation of Kv1.3 channels by protein kinase A I in T lymphocytes is mediated by the disc large 1-tyrosine kinase Lck complex. Am J Physiol Cell Physiol. 2012;302:C1504–1512. doi: 10.1152/ajpcell.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Save S, Mohlin C, Vumma R, Persson K. Activation of adenosine A2A receptors inhibits neutrophil transuroepithelial migration. Infect Immun. 2011;79:3431–3437. doi: 10.1128/IAI.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu F, Xu J, Tse FW, Tse A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol. 2006;290:C1592–1598. doi: 10.1152/ajpcell.00546.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi S, Conforti L, Pun RY, Millhorn DE. Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. J Physiol. 1998;508(Pt 1):95–107. doi: 10.1111/j.1469-7793.1998.095br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Sun Y, Zhang W, Huang P. Apical adenosine regulates basolateral Ca2+-activated potassium channels in human airway Calu-3 epithelial cells. Am J Physiol Cell Physiol. 2008;294:C1443–1453. doi: 10.1152/ajpcell.00556.2007. [DOI] [PubMed] [Google Scholar]
- 43.Gerlach AC, Syme CA, Giltinan L, Adelman JP, Devor DC. ATP-dependent Activation of the Intermediate Conductance, Ca2+-activated K+ Channel, hIK1, Is Conferred by a C-terminal Domain. Journal of Biological Chemistry. 2001;276:10963–10970. doi: 10.1074/jbc.M007716200. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi M, Kunii C, Takahata T, Ishikawa T. ATP-dependent regulation of SK4/IK1-like currents in rat submandibular acinar cells: possible role of cAMP-dependent protein kinase. American Journal of Physiology - Cell Physiology. 2004;286:C635–C646. doi: 10.1152/ajpcell.00283.2003. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrino M, Pellegrini M. Modulation of Ca2+-activated K+ channels of human erythrocytes by endogenous cAMP-dependent protein kinase. Pflugers Arch - Eur J Physiol. 1998;436:749–756. doi: 10.1007/s004240050698. [DOI] [PubMed] [Google Scholar]
- 46.Neylon C, D’Souza T, Reinhart P. Protein kinase A inhibits intermediate conductance Ca2+-activated K+ channels expressed in Xenopus oocytes. Pflugers Arch - Eur J Physiol. 2004;448:613–620. doi: 10.1007/s00424-004-1302-5. [DOI] [PubMed] [Google Scholar]
- 47.Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, Attali B, Lider O. Extracellular K(+) and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and beta1 integrins. J Exp Med. 2000;191:1167–1176. doi: 10.1084/jem.191.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flügel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of Effector Memory T Cells during a Delayed-Type Hypersensitivity Reaction and Suppression by Kv1.3 Channel Block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 52.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 53.Csóka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Németh ZH, Haskó G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. The FASEB Journal. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mandapathil M, Szczepanski M, Harasymczuk M, Ren J, Cheng D, Jackson EK, Gorelik E, Johnson J, Lang S, Whiteside TL. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4(+) T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology. 2012;1:659–669. doi: 10.4161/onci.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL., Jr Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Research. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chimote AA, Kuras Z, Conforti L. Disruption of kv1.3 channel forward vesicular trafficking by hypoxia in human T lymphocytes. J Biol Chem. 2012;287:2055–2067. doi: 10.1074/jbc.M111.274209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conforti L, Petrovic M, Mohammad D, Lee S, Ma Q, Barone S, Filipovich AH. Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes: a possible role in T cell proliferation. Journal of immunology. 2003;170:695–702. doi: 10.4049/jimmunol.170.2.695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.