Abstract
Cortical motor simulation supports the understanding of others' actions and intentions. This mechanism is thought to rely on the mirror neuron system (MNS), a brain network that is active both during action execution and observation. Indirect evidence suggests that (alpha/beta) mu suppression, an electroencephalographic (EEG) index of MNS activity, is modulated by reward. In this study we aimed to test the plasticity of the MNS by directly investigating the link between (alpha/beta) mu suppression and reward. 40 individuals from a general population sample took part in an evaluative conditioning experiment, where different neutral faces were associated with high or low reward values. In the test phase, EEG was recorded while participants viewed videoclips of happy expressions made by the conditioned faces. Alpha/beta mu suppression (identified using event-related desynchronisation of specific independent components) in response to rewarding faces was found to be greater than for non-rewarding faces. This result provides a mechanistic insight into the plasticity of the MNS and, more generally, into the role of reward in modulating physiological responses linked to empathy.
Keywords: EEG, Empathy, Mirror neuron system, Mu, Beta, Reward
Highlights
-
•
Reward value has previously been shown to modulate metrics of empathy.
-
•
An evaluative conditioning task was used to alter the reward value of faces.
-
•
EEG was recorded in response to happy expressions of the conditioned faces.
-
•
Greater reward value of faces was associated with greater (alpha/beta) mu suppression.
-
•
Our results provide an EEG correlate of a direct link between reward and empathy.
1. Introduction
According to simulation theories, cortical motor simulation is fundamental to interpret the actions and intentions of others (Gallese and Goldman, 1998). The mirror neuron system (MNS), which maps the correspondence between the perceived and executed actions, has been proposed as the neural substrate of cortical motor simulation (Rizzolatti and Sinigaglia, 2010). Alpha-mu (8–12 Hz) and beta-mu (12–25 Hz) rhythms generated over the sensorimotor cortex desynchronize (are suppressed) both during execution and observation of actions, and therefore have been proposed as an electroencephalographic (EEG) index of mirror-like activity (Arnstein et al., 2011; Braadbaart et al., 2013; Pineda, 2005). Importantly, mu suppression has been shown to occur during observation of facial gestures in infant monkeys (Ferrari et al., 2012), and when viewing emotional faces in humans (Moore et al., 2012). This neural mirroring of emotions has been hypothesized to facilitate the understanding of emotions experienced by others, which in turn might support the ability to empathize (Iacoboni, 2009; Niedenthal, 2007; Pfeifer et al., 2008). Consistent with this speculation, Woodruff et al. (2011) and Hoenen et al. (2013) found an association between mu suppression and empathic abilities.
An electrophysiological study by Caggiano et al. (2012) reported that the activity recorded from single mirror neurons was modulated by the reward value ascribed to the observed object on which the motor act was performed. This result was the first direct evidence for the role of reward in determining the plasticity of the mirror neuron response. There is substantial indirect evidence for the plasticity of the MNS response in humans using different task manipulations (e.g., group membership (Gutsell and Inzlicht, 2010), familiarity (Liew et al., 2011; Oberman et al., 2008)), which can be potentially argued to alter the reward value of the stimuli. E.g., in-group members are considered more rewarding than out-group members and are associated with greater reward response (Brewer, 1979; Chen et al., 2014), and greater familiarity is associated with greater liking and ‘reward’ (Hansen and Wänke, 2009). More specifically, MNS activation is enhanced in response to in-group members and familiar stimuli since these stimuli might be more rewarding to us than out-group, or unfamiliar stimuli. Based on these findings, it is reasonable to hypothesize that the extent of (alpha/beta) mu suppression will be modulated by the reward value of the observed stimuli.
A link between empathy-related responses and reward has previously been demonstrated by Sims et al. (2012), who showed that spontaneous facial mimicry is modulated by the reward value associated with the imitated social stimuli. In this study, the reward value of neutral faces was manipulated using an evaluative conditioning paradigm before emotional facial expressions made by the same faces were presented to the participants in a passive viewing task. Participants showed greater spontaneous mimicry (measured using facial electromyography; EMG) in response to happy expressions performed by the faces associated with high reward as compared to those associated with low reward. Further evidence of the modulatory influence of reward value on mimicry comes from a recent neuroimaging study (Sims et al., 2014) that used the same paradigm as Sims et al. (2012). Results showed that functional connectivity between the ventral striatum, a region associated with reward processing, and the inferior frontal gyrus, a region involved in mimicry, was stronger when participants observed happy facial expressions of faces conditioned with high reward compared to those conditioned with low reward. Since spontaneous mimicry has been proposed to be a marker of empathy (Dimberg et al., 2011; Sonnby-borgström et al., 2003), this indicates the existence of a link between the reward and empathy systems.
The current study aimed at providing direct evidence of the relationship between motor cortical stimulation and reward by examining how reward modulates (alpha/beta) mu suppression. To this end, the reward value ascribed to neutral faces was experimentally manipulated using a paradigm similar to Sims et al. (2012, 2014). In light of previous results, we hypothesized that (alpha/beta) mu suppression would be more pronounced in response to happy expressions displayed by faces conditioned with high reward compared to those produced by faces conditioned with low reward.
2. Materials and methods
2.1. Participants
Forty adults (20 females) between 18 and 44 years of age (M=24.53; SD=5.73) were recruited from the University of Reading campus area. All participants had normal or corrected-to-normal vision and all but two were right-handed. None of the participants reported current neurological or psychiatric disorders, or history of regular drug/substance use. Four participants reported a history of depression and one participant had a history of eating disorder. From the original sample, 10 participants were excluded in the analysis due to regular cigarette consumption (n=2), low performance in the oddball task (i.e. score lower than 75%, see Section 3; n=3), and insufficient data after artifact removal (n=5). Regular smokers were excluded as smoking has been associated with alterations in reward processing (Martin-Sölch et al., 2001; Ohmura et al., 2005). All participants gave written informed consent and were financially remunerated for their participation. The study was approved by the School of Psychology and Clinical Language Sciences Research Ethics Committee of the University of Reading.
2.2. Stimulus material
Stimuli in the conditioning phase consisted of static images of four faces (2 male, 2 female) with neutral expressions (Fig. 1). During the test phase, 4000 ms video clips of the same identities showing happy dynamic facial expressions were presented. In addition, 4000 ms clips of 6 different identities displaying happy expressions were used as oddball stimuli. Dynamic expressions were preferred over static pictures as they enhance spontaneous mimicry and are more ecologically valid (Krumhuber et al. 2013; Sato et al., 2008). All stimuli were selected from the standardized Mindreading set (Baron-Cohen et al. 2004; available at www.jkp.com/mindreading). These stimuli show sufficient inter-rater reliability and external validity (Golan et al., 2006; Golan and Baron-Cohen, 2006), and have been used in previous research (Sims et al., 2014, 2012). Images of French playing cards were used for the card guessing game in the conditioning phase. Specifically, only cards with values 2–7 of each of the four suits (spades, hearts, diamonds and clubs) were included in the paradigm. Cards with values 8, 9 and 10, as well as face cards, were not used in order to increase the difficulty of the task.
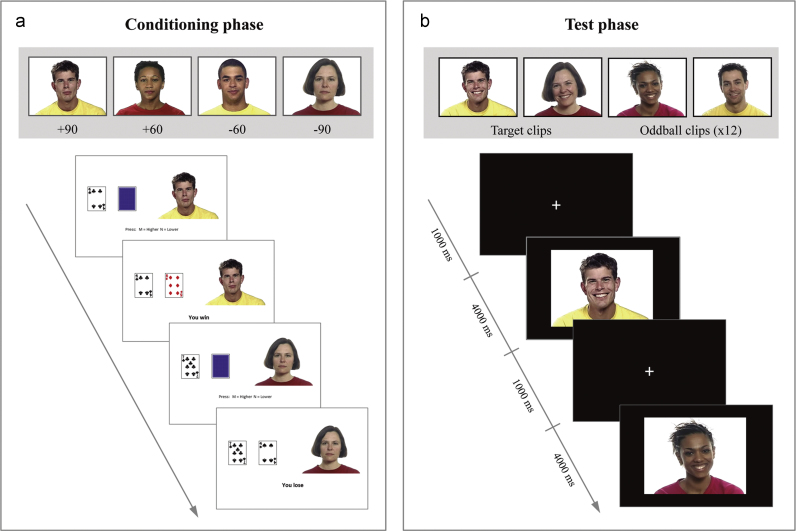
Fig. 1.
(a) Top panel: example of the four neutral faces that were associated with different reward values (90% win, 60% win, 60% loss, 90% loss) during the conditioning phase. The first face corresponds to the High Reward condition, and the fourth face to the Low Reward condition. Bottom panel: example of two trials of the conditioning phase in which the participants had to predict whether the face down card would be of lower or higher value than the face up card. Following their key response, feedback was displayed. (b) Top panel: examples of the target clips (High Reward and Low Reward conditioned faces) and two of the oddball clips presented during the test phase. Bottom panel: example of two trials of the test phase. A fixation cross appeared during 1000 ms before the presentation of the 4000 ms clips of happy facial expressions made by the target or oddball faces. Participants had to make a keypress response every time an oddball clip was presented.
2.3. Procedure
The study protocol was based on Sims et al. (2012). After EEG electrodes placement, participants were seated in front of a computer screen and introduced to the experimental procedure. Task instructions were displayed on the screen, and also read aloud by the experimenter to make sure that participants understood the procedure. During all experimental phases, participants were in the room alone to avoid distraction due to experimenter effects. Participants performed a supervised 8-trials practice session before beginning an evaluative conditioning task (see following section), followed by the test phase. The test phase consisted of an oddball task (see following section) during which emotional expressions of previously conditioned faces were viewed.
2.3.1. Conditioning phase
An implicit reward conditioning paradigm in the form of a card guessing game was used to associate faces with high and low reward value (Fig. 1). At the beginning of each trial, participants were shown two cards, one face up and one face down. The task was to predict whether the face-down card was of greater or smaller value than the first card by pressing one of two keys on a keyboard. Correct predictions won 6 pence, while incorrect predictions cost 2 pence. No money was won or lost if the cards were of equal value. After each response, feedback indicating whether participants had won, lost or drawn the round was displayed for 4000 ms. Participants were told at the beginning of the session that they would receive the monetary winnings after completion of the experiment. The total amount of money won was shown after completion of the card game. In each trial, one of four target faces was displayed on the right side of the cards. The reward value associated with each face was manipulated by adjusting the number of trials in which participants won or lost money in the presence of this particular face. In the High Reward condition, participants won 90% of the trials paired with the associated face; in the Low Reward condition, participants lost 90% of the trials in which the low rewarding face was presented. Two additional conditions in which participants won or lost 60% of the trials, respectively, were used to prevent participants from detecting the underlying structure of the game. The remaining trials in all conditions were “draw” trials (i.e. the two cards were of the same value). The card game consisted of 160 trials (40 trials per condition) and presentation of trials was randomized. The faces in the four conditions were counterbalanced across participants. To ensure that participants paid attention to the faces while playing the card game, they were told that during the test phase a simple memory task involving the same faces would be performed.
2.3.2. Test phase
After conditioning, participants were presented with 4000 ms clips of happy expressions of the same faces previously associated with high and low reward value. In addition, clips of 6 novel faces (oddballs) displaying happy expressions were displayed (Fig. 1). Participants were asked to press a key every time they spotted a novel face. A total of 72 trials were presented during the test phase: target clips (High Reward and Low Reward conditions) were presented 30 times each, and oddball clips appeared twice. The order of presentation of the target and oddball clips was pseudorandomized such that the same condition was never presented more than three times in a row. A fixation cross appeared for 1000 ms before each clip. The use of an oddball task instead of passive observation was chosen to ensure that attention was directed to the target facial stimuli. Behavioral performance in the oddball task was assessed for each participant individually (see Section 3).
2.4. EEG measurement
Continuous EEG data was recorded using Brain Vision Recorder 1.10 (Brain Products, Munich, Germany) from 26 active AG/AgCL electrodes (Fp1, Fp2, F1, F2, F3, F4, Fz, FC1, FC2, FC5, FC6, Pz, P3, P4, P7, P8, CP1, CP2, CP5, CP6, C3, C4, Cz, O1, O2, Oz) embedded in EEG caps (BrainCaps, Brain Products GmbH, Munich, Germany). Electrode placement was done according to the International 10/20 System (Jasper, 1958) and all electrodes were referenced to an electrode located at the left earlobe. An additional electrode was placed at the right earlobe for offline re-referencing to the average of the right and left earlobe electrodes. Electrooculogram (EOG) was recorded using two electrodes placed below (vertical EOG) and on the outer canthi of the left eye (horizontal EOG). High chloride gel was used to facilitate conductance between the cap electrodes and the scalp. Impedances were kept below 5 kΩ. EEG data was amplified with an EEG amplifier (BrainAmp MR plus, Brain Products GmbH, Munich, Germany) and digitized at a sampling rate of 1000 Hz. High-pass filter was set at 0.01 Hz and low-pass filter at 100 Hz, with a notch filter of 50 Hz.
2.5. EEG data processing
BrainVision Analyzer 2.0.1 (Brain Products, Munich, Germany) and EEGLAB v.13 (Delorme and Makeig, 2004) were used for offline EEG data preprocessing. EEG data was filtered with a 30 Hz low-pass filter and a 0.5 Hz high-pass filter, re-referenced to the average of the right and left earlobes, and down-sampled to 512 Hz. Independent component analysis (ICA) was ran in order to remove components visually identified as ocular artifacts (Jung et al., 2000). EEG continuous data was then epoched into 4750 ms timebins (−750 ms to 4000 ms with reference to the onset of the clip) for each of the two conditions of interest (High Reward, Low Reward). Oddball trials were not included in the analysis since these were included solely to ensure that participants attended to the stimuli. Trials contaminated with EEG artifacts exceeding ±75 µV in any electrode were rejected. A minimum of 80% of the total number of trials in each condition for each participant was required for inclusion in the analyses. In accordance with this criterion, data from 5 participants were excluded. After exclusion, the average number of artifact-free trials per participant was 28.82 (SD=1.44) for the Low Reward condition, and 28.90 (SD=1.65) for the High Reward condition. There was no significant difference between the two conditions in the number of trials included in the analysis, t(29)=−0.372, p=0.712.
2.6. Independent component clustering
Independent component clustering has been proven to be a successful method to identify mu clusters in previous studies (Bowers et al., 2013; Moore et al., 2012; Vukovica and Shtyrov, 2014; Zhang et al., 2014). Since the clustering method requires the same number of independent components (ICs) per each participant, a second ICA was run on the artifact-clean epoched data. Brain sources of each of the ICs were localized using the DIPFIT2 toolbox in EEGLAB (Oostenveld and Oostendorp, 2002; available from sccn.ucsd.edu/eeglab/dipfit.html), which fits an equivalent current dipole model using a non-linear optimization technique (Oostendorp and van Oosterom, 1989). Electrode coordinates were registered to the standard Boundary Element Model (BEM) of the Montreal Neurological Institute (MNI) average brain. The BEM is a representative model since it is composed of three 3-D surfaces (skin, skull, cortex) extracted from the MNI.
In order to maximize the subsequent ICs clustering process, several measures were computed for each IC. Component power spectra were calculated by averaging fast Fourier transform spectra from −750 to 4000 ms (window length of 2432 points) using the spectopo function. Event related potential (ERPs) were calculated using a −200 ms baseline correction. Finally, time-frequency decomposition was calculated using a Mortlet wavelet with the number of cycles linearly rising from a minimum of 3 cycles at 7 Hz to a maximum of ~5 cycles at 25 Hz. Power was calculated for 100 log-spaced frequencies ranging from 7 to 25 Hz and along 200 temporal bins (from −750 ms to 4000 ms). Moment to moment oscillatory change between baseline and test phase (Event-related spectral perturbations; ERSPs) was calculated using a 750 ms common baseline (−750 to 0 ms with reference to the stimulus onset) for each of the two conditions. A decrement in post-stimulus power corresponds to an event-related desynchronization (ERD; suppression) of the frequency band, while an increment corresponds to an event-related synchronization (ERS).
From the 800 ICs (25 ICs × 32 participants), only those with a single dipole model within the head volume accounting for 80% or greater of the variance in the independent component scalp distribution were included (563 ICs). Each of the IC measures was reduced by principal component analysis (PCA). Specifically, power spectrum was reduced to 3 PCs, ERPs to 4 PCs, scalp map gradient to 5 PCs, and averaged ERSPs to 5 PCs. The equivalent dipole location measure was up-weighted by a factor of 2 while other measures were given a weight of 1, and all features were normalized. Finally, these weighted measures were further compressed by PCA into a single 12-dimensional cluster vector. These clustering options were taken for previous reports that have utilized a similar approach to individualize right and left mu clusters (Zhang et al., 2014). ICs were then clustered by the k-means algorithm based on the 12-dimensional measure, resulting in 10 mutually exclusive IC clusters. ICs with a distance larger than 3 SDs from the mean of any cluster centroid were excluded as outliers.
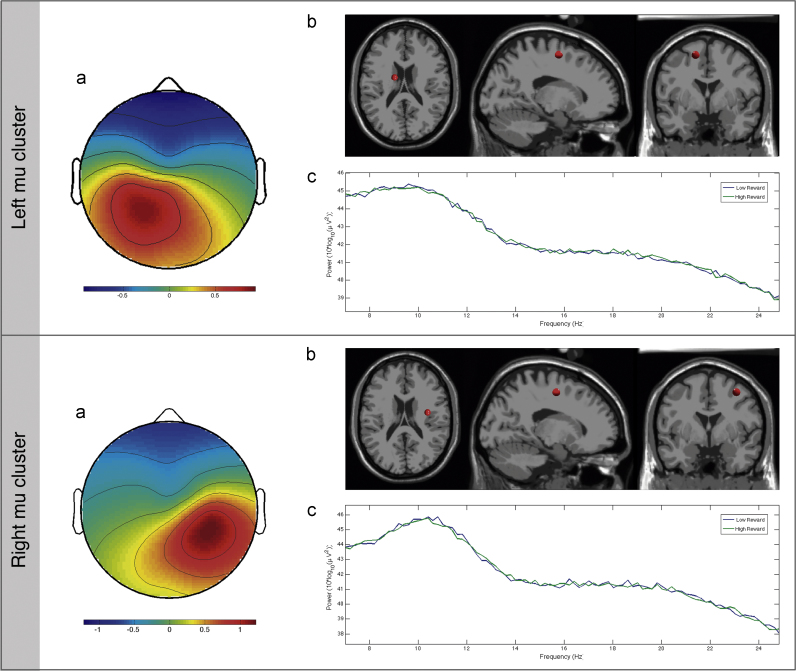
The resulting 10 clusters were visually inspected for their physiological profile. Three criteria reported in previous studies (Delorme and Makeig, 2004; Moore et al., 2012; Vukovica and Shtyrov, 2014) were used to identify the right and left mu clusters: a) topographic scalp map characterized by a lateralized left or right midline focus; b) characteristic mu spectra with peaks around 10 and 20 Hz; c) brain source localized around BA 1–4 and 6.
2.7. Data analysis
Since ERSPs data were not normally distributed, data analyses were performed using non-parametric random permutation-based (2000 permutations, chosen according to guidelines suggested by Cohen (2014) and Manly (1997)) statistics on two one-way ANOVA respectively for the left and right cluster. A correction for false discovery rate (pFDR) at p<0.05 was applied to correct for multiple comparisons.
3. Results
3.1. Behavioral performance
A performance score in the oddball task (test phase) was calculated for each participant as follows: (# correct responses – # incorrect responses) / # oddball clips)⁎100, where correct responses refers to key presses in response to oddball clips, and incorrect responses refers to key presses in response to target clips. Three participants had a score lower than 75% due to incorrect responses (i.e. key presses when target clips were presented). Since execution of motor movements could influence the magnitude of mu suppression (Frenkel-Toledo et al., 2013; Pfurtscheller et al., 2000), these participants were excluded from the analyses. The mean score in the oddball task for the remaining 37 participants was very high (M=95.72; SD=6.40), which indicates that participants were attending to the target stimuli.
3.2. ERSPs
We identified one left- and one right-hemisphere mu clusters (Fig. 2), which were composed of 76 ICs of 28 participants and 49 ICs of 26 participants, respectively. The average Talairach dipole locations were [−15, −2, 53] for the left cluster, and [35, −4, 48] for right cluster. These Talairach coordinates correspond to Brodmann area 6 (Lancaster et al., 2000), one of the regions comprising the MNS.
Fig. 2.
Cluster maps for each hemisphere in response to the individual task conditions. (a) Cluster mean topographical scalp maps; (b) cluster mean DIPFIT dipole location (c) cluster mean spectra (10×log10 (µV2/Hz)) as a function of condition.
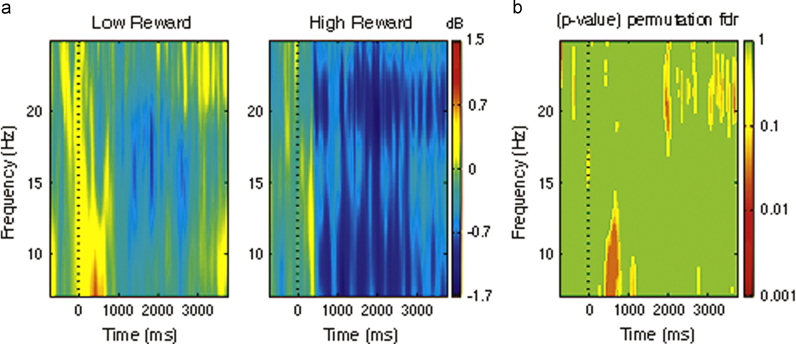
For the left mu cluster, permutation test revealed no significant difference across the two ERSPs matrices of the experimental conditions (200×100, pFDR>0.05). Importantly, significant differences were found in the right mu cluster in the alpha-mu band (7–12.5 Hz) between ~500 and 700 ms, and in the beta-mu band (12.5–25Hz) between ~2000 and 4000 ms (200×100; pFDR<0.05; Fig. 3).
Fig. 3.
(a) Mean right-hemisphere mu time-frequency ERSPs (event related spectral perturbations; 7–25 Hz power, dB) as a function of condition. Event-related decreases in spectral power are represented in blue and increases are represented in orange and red. (b) Significant differences between conditions in the alpha-mu (7–12.5 Hz) and beta-mu (12.5–25 Hz) ranges. Significant values at p<0.05 after FDR correction are indicated in dark orange and non-significant values in green. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Confounders analysis
To control for a possible confound due to inclusion of left-handed participants, time-frequency analysis was run a second time with a sample of right-handed participants only (n=28). All effects reported above remained unchanged.
4. Discussion
The present study investigated whether reward conditioning could modulate (alpha/beta) mu suppression, a putative EEG index of MNS, in response to emotional facial expressions. As predicted, (alpha/beta) mu suppression was stronger during observation of happy facial expressions conditioned with high reward than with low reward. These findings add to previous psychophysiological and neuroimaging data demonstrating a direct link between reward and mimicry (Sims et al., 2014, 2012). More specifically, these studies showed that spontaneous facial mimicry (measured with EMG) and functional connectivity between the ventral striatum (associated with reward processing) and the inferior frontal gyrus (involved in mimicry) were modulated by reward conditioning, that is, they were stronger in response to happy faces conditioned with high reward compared to low reward. Consistent with these earlier findings, the results of the current study show that reward conditioning effectively modulates the extent of cortical motor simulation in response to facial expressions observed in others. Given that neural mirroring of emotion and spontaneous facial mimicry have been proposed as indices of empathic abilities (Dimberg et al., 2011; Sonnby-borgström et al., 2003; Woodruff et al., 2011), these findings provide convergent validity to the link between reward and empathy across different methods (i.e. EMG, fMRI and EEG) and independent samples.
In line with our results, Brown et al. (2013) demonstrated that observation of motor actions leading to monetary reward produced stronger mu suppression than observation of punishing or neutral actions. Moreover, alpha-mu rhythm responses to observation of hand movements have been shown to vary depending on the emotional facial expressions of the actors performing the action (Cooper et al., 2013), presumably by changing the reward value ascribed to the actor. Indeed, greater mu suppression was observed in response to happy (vs. angry) expressions, which are rewarding social stimuli (Niedenthal et al., 2010; O’Doherty et al., 2003). The current results extent these findings by showing that reward-related modulation of cortical motor simulation occurs also when observing emotional facial expressions.
Interestingly, significant reward modulations of (alpha/beta) mu suppression were found only in the right hemisphere. Even though bihemispheric attenuation of the alpha-mu rhythm is a common finding (e.g., Cochin et al., 1999; Muthukumaraswamy et al., 2004; Pineda and Hecht, 2009; Cooper et al. 2013), recent data suggests that mu suppression during action-observation might be stronger in the right hemisphere (Frenkel-Toledo et al., 2013). Of note, most of the literature examining the spatial domain of mu suppression is based on observation and execution of limb movements, which might not be comparable to the mu effects in response to emotional facial stimuli. Indeed, Moore et al. (2012) found that differences in mu suppression between angry and happy faces were stronger in the right hemisphere. Moreover, neuroimaging studies examining the neural correlates of spontaneous facial mimicry have indicated a relative right lateralization of MNS regions during observation of emotional facial expressions (Lee et al., 2006; Likowski et al., 2012). In view of these findings, it could be speculated that the observed lateralization of the effects reported here is related to the right hemisphere dominance repeatedly reported in face and emotion processing (Ahern et al., 1991; Borod et al., 1998; Sato et al., 2008).
Importantly, the time-course of the cortical desynchronization appeared to be different for alpha and beta rhythms. More specifically, the reward effect in alpha-mu suppression occurred within the first 1000 ms post-stimulus onset, while the beta-mu effect occurred much later, between 2000 and 4000 ms. Several studies reported an early desynchronization of alpha-mu around the first 1000–2000 ms, which is believed to reflect an automatic motor resonance mechanism (Babiloni et al., 2002; Moore et al., 2012; Orgs et al., 2008; Streltsova et al., 2010). Moreover, Moore et al. (2012) found an earlier alpha-mu ERD for faces displaying negative compared to positive emotions. This suggests that early cortical motor simulation can be highly sensitive to the social significance of the observed stimuli.
Differently from alpha-mu, beta-mu showed a later reward-related effect. Alpha and beta ERD have shown to be similarly modulated in action observation tasks (Meyer et al., 2011; Perry et al., 2010b; Perry et al., 2010a, but cf. Cochin et al., 1999; Muthukumaraswamy et al., 2004). However, a recent study reported that beta-mu ERD during action observation showed a peculiar spatiotemporal pattern that could reflect its role in evaluating the accuracy of the simulated motor plan (Sebastiani et al., 2014). Based on this, we speculate that such assessment has to be more accurate for socially relevant stimuli (i.e. high rewarding faces) and thus requires a higher beta-mu ERD.
It is worth noting that the latency of reward modulation of beta-mu ERD overlaps with the latency of facial EMG reported in Sims et al. (2012), where facial mimicry for high reward faces peaked after 2000 ms post-stimulus onset. Cortical motor simulation is believed to require an online mechanism of motor inhibition, which prevents us from executing the motor plan we are mirroring (Sommerville and Decety, 2006). In line with this idea, a recent study has demonstrated a functional dissociation between alpha and beta rhythms during cortical motor simulation (Brinkman et al., 2014). Specifically, the authors propose that alpha-mu oscillations over the sensorimotor cortex are related with the disengagement of task-irrelevant neural regions, whereas suppression of beta-mu reflects the desinhibition of cortical areas involved in movement preparation. Given the spontaneous nature of facial mimicry in response to social stimuli, the reward-related modulation of beta-mu frequency observed here could also reflect the switch from an “as-if” motor simulation to a motor facial response (Moore et al., 2012).
Finally, mu suppression has been associated with expectancy and attentional processes (Klimesch, 2012). While differences in attention were not measured in this study, eye-tracking measures used in an identical paradigm in an fMRI experiment showed no differences in gaze duration to rewarding vs. non-rewarding happy faces (Sims et al., 2014). Moreover, the (alpha/beta) mu components identified here were originated in more anterior regions as shown by both the topographical scalp maps and dipole locations, making it unlikely that the observed reward-related effects were driven by attention-based occipital alpha. Nonetheless, future experiments should involve a simultaneous measure of eye-tracking in this paradigm to rule out this potential confound. A second aspect to improve on in future studies would be to have higher spatial accuracy by using structural MRI images for the dipole fitting procedure, and by recording from a larger number of electrodes. It should be noted that this study is one of the first few to use this methodological pipeline for EEG data analysis (i.e. ICA-Dipole fitting-ICs clustering – ERSP). Hence, to ensure replicability, we closely followed the procedures used by groups that have examined mu suppression using these methods (Bowers et al., 2013; Moore et al., 2012; Vukovica and Shtyrov, 2014; Zhang et al., 2014).
Notwithstanding its limitations, the current study provides evidence of reward based modulation of cortical motor simulation. In light of the findings discussed earlier, and given the link between empathy, spontaneous facial mimicry, and cortical motor simulation (Dimberg et al., 2011; Hoenen et al., 2013; Niedenthal, 2007; Pfeifer et al., 2008; Sonnby-borgström et al., 2003; Sonnby-borgström, 2002; Woodruff et al., 2011), the current study points to a significant role of reward in modulating the extent of empathy-related responses. These results are consistent with social psychological theories that suggest a relationship between mimicry and liking (Byrne and Griffitt, 1973), as well as recent neurobiological models suggesting a top down modulation of mimicry (Wang and Hamilton, 2012). These findings thus provide a direct insight into the fundamental architecture of human social behavior and how reward helps to shape this.
Acknowledgments
We are grateful to Prof. J. Douglas Saddy and all at the CINN for their input and support at all stages of this study. ITG was supported by a scholarship from Maastricht University. MSP was supported by a fellowship from Sapienza University of Rome. This work was supported by a Medical Research Council UK New Investigator Research Grant to BC (G1100359/1).
References
- Ahern G., Schomer D., Kleefield J. Right hemisphere advantage for evaluating emotional facial expressions. Cortex. 1991;27(2):193–202. doi: 10.1016/s0010-9452(13)80123-2. [DOI] [PubMed] [Google Scholar]
- Arnstein D., Cui F., Keysers C., Maurits N.M., Gazzola V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. : Off. J. Soc. Neurosci. 2011;31(40):14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C., Babiloni F., Carducci F., Cincotti F., Cocozza G., Del Percio C., Rossini P.M. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. NeuroImage. 2002;17:559–572. [PubMed] [Google Scholar]
- Baron-Cohen S., Golan O., Wheelwright S., Hill J. Jessica Kingsley; London: 2004. Mindreading: The Interactive Guide to Emotions. [Google Scholar]
- Borod J.C., Cicero B. a, Obler L.K., Welkowitz J., Erhan H.M., Santschi C., Whalen J.R. Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology. 1998;12(3):446–458. doi: 10.1037//0894-4105.12.3.446. [DOI] [PubMed] [Google Scholar]
- Bowers A., Saltuklaroglu T., Harkrider A., Cuellar M. Suppression of the µ rhythm during speech and non-speech discrimination revealed by independent component analysis: implications for sensorimotor integration in speech processing. PLoS One. 2013;8(8):e72024. doi: 10.1371/journal.pone.0072024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braadbaart L., Williams J.H.G., Waiter G.D. Do mirror neuron areas mediate mu rhythm suppression during imitation and action observation? Int. J. Psychophysiol. : Off. J. Int. Org. Psychophysiol. 2013;89(1):99–105. doi: 10.1016/j.ijpsycho.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Brewer M.B. In-group bias in the minimal intergroup situation: a cognitive-motivational analysis. Psychol. Bull. 1979;86(2):307–324. [Google Scholar]
- Brinkman L., Stolk A., Dijkerman H.C., de Lange F.P., Toni I. Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. J. Neurosci. 2014;34(44):14783–14792. doi: 10.1523/JNEUROSCI.2039-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.C., Wiersema J.R., Pourtois G., Brüne M. Modulation of motor cortex activity when observing rewarding and punishing actions. Neuropsychologia. 2013;51:52–58. doi: 10.1016/j.neuropsychologia.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Byrne D., Griffitt W. Interpersonal attraction. Annu. Rev. Psychol. 1973;24:317–336. [Google Scholar]
- Caggiano V., Fogassi L., Rizzolatti G., Casile A., Giese M.A. Mirror neurons encode the subjective value of an observed action. PNAS. 2012;109(29):11848–11853. doi: 10.1073/pnas.1205553109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. a., Whalen P.J., Freeman J.B., Taylor J.M., Heatherton T.F. Brain reward activity to masked in-group smiling faces predicts friendship development. Soc. Psychol. Personal. Sci. 2015:1–7. doi: 10.1177/1948550614566093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochin S., Barthelemy C., Roux S., Martineau J. Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur. J. Neurosci. 1999;11 (5):1839–1842. doi: 10.1046/j.1460-9568.1999.00598.x. 〈http://onlinelibrary.wiley.com/doi/10.1046/j.1460-9568.1999.00598.x/full〉 [DOI] [PubMed] [Google Scholar]
- Cohen M.X. 2014. Analyzing Neural Time Series Data. Theory and Practice (Issues in Clinical and Cognitive Neuropsychology) [Google Scholar]
- Cooper N.R., Simpson A., Till A., Simmons K., Puzzo I. Beta event-related desynchronization as an index of individual differences in processing human facial expression: further investigations of autistic traits in typically developing adults. Front. Hum. Neurosci. 2013;7:159. doi: 10.3389/fnhum.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dimberg U., Andréasson P., Thunberg M. Emotional empathy and facial reactions to facial expressions. J. Psychophysiol. 2011;25(1):26–31. [Google Scholar]
- Ferrari P.F., Vanderwert R.E., Paukner A., Bower S., Suomi S.J., Fox N. a. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J. Cognit. Neurosci. 2012;24(5):1165–1172. doi: 10.1162/jocn_a_00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel-Toledo S., Bentin S., Perry A., Liebermann D.G., Soroker N. Dynamics of the EEG power in the frequency and spatial domains during observation and execution of manual movements. Brain Res. 2013;1509:43–57. doi: 10.1016/j.brainres.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Gallese V., Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cognit. Sci. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Golan O., Baron-Cohen S. Systemizing empathy: teaching adults with Asperger syndrome or high-functioning autism to recognize complex emotions using interactive multimedia. Dev. Psychopathol. 2006;18(2):591–617. doi: 10.1017/S0954579406060305. [DOI] [PubMed] [Google Scholar]
- Golan O., Baron-Cohen S., Hill J. The Cambridge Mindreading (CAM) face-voice battery: testing complex emotion recognition in adults with and without Asperger syndrome. J. Autism Dev. Disord. 2006;36(2):161–182. doi: 10.1007/s10803-005-0057-y. [DOI] [PubMed] [Google Scholar]
- Gutsell J.N., Inzlicht M. Empathy constrained: prejudice predicts reduced mental simulation of actions during observation of outgroups. J. Exp. Soc. Psychol. 2010;46:841–845. [Google Scholar]
- Hansen J., Wänke M. Liking what’s familiar: the importance of unconscious familiarity in the mere-exposure effect. Soc. Cognit. 2009;27(2):161–182. [Google Scholar]
- Hoenen M., Schain C., Pause B.M. Down-modulation of mu-activity through empathic top-down processes. Soc. Neurosci. 2013;8:515–524. doi: 10.1080/17470919.2013.833550. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annu. Rev. Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Jasper H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Jung T.P., Makeig S., Westerfield M., Townsend J., Courchesne E., Sejnowski T.J. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. : Off. J. Int. Fed. Clin. Neurophysiol. 2000;111(10) doi: 10.1016/s1388-2457(00)00386-2. 〈http://www.ncbi.nlm.nih.gov/pubmed/11018488〉 1745–58. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Α-band oscillations, attention, and controlled access to stored information. Trends Cognit. Sci. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumhuber E.G., Kappas A., Manstead A.S.R. Effects of dynamic aspects of facial expressions: a review. Emot. Rev. 2013;5(1):41–46. [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Fox P.T. Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.-W., Josephs O., Dolan R.J., Critchley H.D. Imitating expressions: emotion-specific neural substrates in facial mimicry. Soc. Cognit. Affect. Neurosci. 2006;1(2):122–135. doi: 10.1093/scan/nsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew S.L., Han S., Aziz-Zadeh L. Familiarity modulates mirror neuron and mentalizing regions during intention understanding. Hum. Brain Mapp. 2011;32:1986–1997. doi: 10.1002/hbm.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likowski K.U., Mühlberger A., Gerdes A.B.M., Wieser M.J., Pauli P., Weyers P. Facial mimicry and the mirror neuron system: simultaneous acquisition of facial electromyography and functional magnetic resonance imaging. Front. Hum. Neurosci. 2012;6:214. doi: 10.3389/fnhum.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly B.F.J. 2nd ed. Chapman & Hall; London: 1997. Randomization, Bootstrap, and Monte Carlo Methods in Biology. [Google Scholar]
- Martin-Sölch C., Magyar S., Künig G., Missimer J., Schultz W., Leenders K. Changes in brain activation associated with reward processing in smokers and nonsmokers. Exp. Brain Res. 2001;139(3):278–286. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- Meyer M., Hunnius S., Van Elk M., Van Ede F., Bekkering H. Joint action modulates motor system involvement during action observation in 3-year-olds. Exp. Brain Res. 2011;211:581–592. doi: 10.1007/s00221-011-2658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A., Gorodnitsky I., Pineda J. EEG mu component responses to viewing emotional faces. Behav. Brain Res. 2012;226(1):309–316. doi: 10.1016/j.bbr.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Johnson B.W., McNair N.A. Mu rhythm modulation during observation of an object-directed grasp. Cognit. Brain Res. 2004;19(2):195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M. Embodying emotion. Science. 2007;316(5827):1002–1005. doi: 10.1126/science.1136930. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M., Mermillod M., Maringer M., Hess U. The Simulation of Smiles (SIMS) model: embodied simulation and the meaning of facial expression. Behav. Brain Sci. 2010;33(6):417–433. doi: 10.1017/S0140525X10000865. discussion 433–80. [DOI] [PubMed] [Google Scholar]
- O’Doherty J., Winston J., Critchley H., Perrett D., Burt D.M., Dolan R.J. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Ramachandran V.S., Pineda J.A. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis. Neuropsychologia. 2008;46:1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Ohmura Y., Takahashi T., Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182(4):508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Oostendorp T.F., van Oosterom A. Source parameter estimation in inhomogeneous volume conductors of arbitrary shape. IEEE Trans. Bio-Med. Eng. 1989;36:382–391. doi: 10.1109/10.19859. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Oostendorp T.F. Validating the boundary element method for forward and inverse EEG computations in the presence of a hole in the skull. Hum. Brain Mapp. 2002;17:179–192. doi: 10.1002/hbm.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgs G., Dombrowski J.H., Heil M., Jansen-Osmann P. Expertise in dance modulates alpha/beta event-related desynchronization during action observation. Eur. J. Neurosci. 2008;27:3380–3384. doi: 10.1111/j.1460-9568.2008.06271.x. [DOI] [PubMed] [Google Scholar]
- Perry A., Bentin S., Shalev I., Israel S., Uzefovsky F., Bar-On D., Ebstein R.P. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35(10):1446–1453. doi: 10.1016/j.psyneuen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Perry A., Troje N.F., Bentin S. Exploring motor system contributions to the perception of social information: Evidence from EEG activity in the mu/alpha frequency range. Soc. Neurosci. 2010;5(3):272–284. doi: 10.1080/17470910903395767. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Iacoboni M., Mazziotta J.C., Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. NeuroImage. 2008;39(4):2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G., Neuper C., Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin. Neurophysiol. : Off. J. Int. Fed. Clin. Neurophysiol. 2000;111(10):1873–1879. doi: 10.1016/s1388-2457(00)00428-4. 〈http://www.ncbi.nlm.nih.gov/pubmed/11018505〉 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Pineda J. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res. Rev. 2005;50(1):57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pineda J., Hecht E. Mirroring and mu rhythm involvement in social cognition: are there dissociable subcomponents of theory of mind? Biol. Psychol. 2009;80(3):306–314. doi: 10.1016/j.biopsycho.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Sato W., Fujimura T., Suzuki N. Enhanced facial EMG activity in response to dynamic facial expressions. Int. J. Psychophysiol. : Off. J. Int. Org. Psychophysiol. 2008;70(1):70–74. doi: 10.1016/j.ijpsycho.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Sebastiani V., de Pasquale F., Costantini M., Mantini D., Pizzella V., Romani G.L., Penna S. Della. Being an agent or an observer: different spectral dynamics revealed by MEG. NeuroImage. 2014;102:717–728. doi: 10.1016/j.neuroimage.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Sims T.B., Neufeld J., Johnstone T., Chakrabarti B. Autistic traits modulate frontostriatal connectivity during processing of rewarding faces. Soc. Cognit. Affect. Neurosci. 2014;9(12):2010–2016. doi: 10.1093/scan/nsu010. 0–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims T.B., Van Reekum C.M., Johnstone T., Chakrabarti B. How reward modulates mimicry: EMG evidence of greater facial mimicry of more rewarding happy faces. Psychophysiology. 2012;49(7):998–1004. doi: 10.1111/j.1469-8986.2012.01377.x. [DOI] [PubMed] [Google Scholar]
- Sommerville J.A., Decety J. Weaving the fabric of social interaction: articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychon. Bull. Rev. 2006;13:179–200. doi: 10.3758/bf03193831. [DOI] [PubMed] [Google Scholar]
- Sonnby-borgström M. Automatic mimicry reactions as related to differences. Scand. J. Psychol. 2002;43:433–443. doi: 10.1111/1467-9450.00312. [DOI] [PubMed] [Google Scholar]
- Sonnby-borgström M., Jonsson P., Svensoon O. Emotional empathy as related to mimicry reactions at different levels of information processing. J. Nonverbal Behav. 2003;27(1):3–23. [Google Scholar]
- Streltsova A., Berchio C., Gallese V., Umilta’ M.A. Time course and specificity of sensory-motor alpha modulation during the observation of hand motor acts and gestures: A high density EEG study. Exp. Brain Res. 2010;205:363–373. doi: 10.1007/s00221-010-2371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovica N., Shtyrov Y. Cortical motor systems are involved in second-language comprehension: Evidence from rapid mu-rhythm desynchronisation. NeuroImage. 2014;102:695–703. doi: 10.1016/j.neuroimage.2014.08.039. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hamilton A., F. de C. Social top-down response modulation (STORM): a model of the control of mimicry in social interaction. Front. Hum. Neurosci. 2012;6:153. doi: 10.3389/fnhum.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff C.C., Martin T., Bilyk N. Differences in self- and other-induced Mu suppression are correlated with empathic abilities. Brain Res. 2011;1405:69–76. doi: 10.1016/j.brainres.2011.05.046. [DOI] [PubMed] [Google Scholar]
- Zhang D., Gu R., Broster L.S., Jiang Y., Luo W., Zhang J., Luo Y.-J. Linking brain electrical signals elicited by current outcomes with future risk decision-making. Front. Behav. Neurosci. 2014;8:84. doi: 10.3389/fnbeh.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]