Abstract
Insufficient default mode network (DMN) suppression was linked to increased rumination in symptomatic Major Depressive Disorder (MDD). Since rumination is known to predict relapse and a more severe course of MDD, we hypothesized that similar DMN alterations might also exist during full remission of MDD (rMDD), a condition known to be associated with increased relapse rates specifically in patients with adolescent onset. Within a cross-sectional functional magnetic resonance imaging study activation and functional connectivity (FC) were investigated in 120 adults comprising 78 drug-free rMDD patients with adolescent- (n = 42) and adult-onset (n = 36) as well as 42 healthy controls (HC), while performing the n-back task. Compared to HC, rMDD patients showed diminished DMN deactivation with strongest differences in the anterior-medial prefrontal cortex (amPFC), which was further linked to increased rumination response style. On a brain systems level, rMDD patients showed an increased FC between the amPFC and the dorsolateral prefrontal cortex, which constitutes a key region of the antagonistic working-memory network. Both whole-brain analyses revealed significant differences between adolescent-onset rMDD patients and HC, while adult-onset rMDD patients showed no significant effects. Results of this study demonstrate that reduced DMN suppression exists even after full recovery of depressive symptoms, which appears to be specifically pronounced in adolescent-onset MDD patients. Our results encourage the investigation of DMN suppression as a putative predictor of relapse in clinical trials, which might eventually lead to important implications for antidepressant maintenance treatment.
Keywords: Default mode network, Functional magnetic resonance imaging, Major depressive disorder, Remission, Rumination, Working memory
Highlights
-
•
Reduced default mode network suppression persists even after remission of MDD symptoms.
-
•
Adolescent-onset rMDD patients show stronger alterations likely reflecting the more severe disease course.
-
•
The anterior-medial prefrontal cortex appears to be the mediator of these brain network changes in rMDD patients.
-
•
Reduced default mode network suppression is related to increased rumination.
1. Introduction
Major Depressive Disorder (MDD) constitutes the second leading cause of disability worldwide and is associated with a substantial socio-economic burden (Licinio and Wong, 2011; Vos et al., 2012; Becker and Kleinman, 2013; Ferrari et al., 2013). The sequelae of MDD comprise several emotional as well as cognitive symptoms, which are considered to merely reflect a bias towards negatively-valenced information processing leading to adverse emotional responsiveness, attention, and dysfunctional executive function (Nolen-Hoeksema, 1991; Beck, 2008; Disner et al., 2011; Elliott et al., 2011; Millan et al., 2012). Imaging studies have provided compelling evidence of altered emotion networks in MDD encompassing the amygdala and the anterior cingulate cortex (Drevets et al., 1997; Pezawas et al., 2005; Price and Drevets, 2012), highlighting the superior role of the cortical midline structures in the pathogenesis of MDD and antidepressant treatment response (Liotti et al., 2002; Lozano et al., 2008; Kupfer et al., 2012). Recently, more attention has been paid to cognitive control mechanisms in patients with concurrent major depressive episodes (MDEs) (Harvey et al., 2005; Rose et al., 2006; Matsuo et al., 2007; Walsh et al., 2007; Fitzgerald et al., 2008; Schlosser et al., 2008; Sheline et al., 2009; Vasic et al., 2009; Davey et al., 2012b; Rodriguez-Cano et al., 2014) demonstrating increasingly converging evidence of less default mode network (DMN) suppression during performance of attention-demanding tasks (Sheline et al., 2009; Disner et al., 2011; Anticevic et al., 2012; Rodriguez-Cano et al., 2014). While the DMN (Gusnard et al., 2001; Raichle et al., 2001) is physiologically activated at rest and deactivated during goal-directed cognition, insufficient DMN suppression has been repeatedly associated with goal-irrelevant functions such as self-referential thought, introspective processing or rumination (Mason et al., 2007; Hamilton et al., 2011; Anticevic et al., 2012; Marchetti et al., 2012; Nejad et al., 2013). Complementary evidence of dysfunctional DMN activation in MDD has also been detected at rest (Greicius et al., 2007; Sheline et al., 2010; Zhang et al., 2011; Davey et al., 2012a; Zhu et al., 2012; Connolly et al., 2013; Guo et al., 2013; Li et al., 2013; Sambataro et al., 2013; Dutta et al., 2014; Ho et al., 2014).
While an abundance of imaging research on cognitive control or functioning was conducted in patients with concurrent major depressive episodes (MDEs), studies dedicated to remitted MDD (rMDD) are relatively sparse and inconclusive (Walsh et al., 2007; Okada et al., 2009; Schoning et al., 2009; Kerestes et al., 2012a, 2012b; Nixon et al., 2012; Li et al., 2013; Norbury et al., 2013; Smoski et al., 2013; Jacobs et al., 2014; Young et al., 2014). Nonetheless, rMDD is of significant clinical interest since it often represents a euthymic state with increased relapse risk (Bhagwagar and Cowen, 2008; Kendler and Gardner, 2010) and, together with information on the number of previous MDEs, guides clinical recommendations for antidepressant maintenance treatment (APA, 2013). Apart from providing complementary evidence to disease concepts of symptomatic MDD, the study of rMDD raises the possibility to elucidate the neurobiological and psychological mechanisms underlying maintenance of remission as well as determinants of relapse (Marchetti et al., 2012). While the majority of above-mentioned rMDD studies focused on possible alterations in task-positive networks, the involvement of task-negative DMN regions gains increasing attention (Jacobs et al., 2014; Li et al., 2013). Specifically, the extent of DMN suppression, which has been shown to be crucial for goal-directed cognition and to be dysfunctional in symptomatic MDD (Disner et al., 2011; Hamilton et al., 2011; Anticevic et al., 2012; Nejad et al., 2013; Leech and Sharp, 2014), has yet to be thoroughly studied in this specific patient group (Marchetti et al., 2012). Additionally, several limitations present in previously published rMDD studies such as concomitant antidepressant treatment (Walsh et al., 2007; Schoning et al., 2009; Nixon et al., 2012; Li et al., 2013), moderate sample size (Okada et al., 2009; Norbury et al., 2013), or incomplete remission (Walsh et al., 2007; Schoning et al., 2009) make it difficult to draw final conclusions.
Hence, we conducted a cross-sectional functional magnetic resonance imaging (fMRI) study in a large sample of adult fully remitted and drug-free MDD patients as well as adult healthy controls (HC) without any previous psychiatric life-time diagnosis. Additionally, we investigated possible behavioral and neural differences with respect to age of MDD onset, because adolescent-onset MDD contrasts clinically with its adult-onset counterpart with regard to chronicity, severity, vulnerability, and stress-sensitivity (Harrington et al., 1990; Klein et al., 1999; Weissman et al., 1999; Aalto-Setala et al., 2002; Gilman et al., 2003; Zisook et al., 2007; Kendler et al., 2009; Pajer et al., 2012; Schosser et al., 2012; Ramirez et al., 2015). The main goal of this study was to assess both task-positive (Cole et al., 2014) and task-negative (Anticevic et al., 2012) differences of neural networks that are engaged or suppressed during working-memory (WM) performance. Based on recent findings demonstrating a relationship between reduced DMN suppression and increased rumination in symptomatic MDD (Hamilton et al., 2011), we hypothesized to observe similar deficits in full remission, a condition, where maladaptive self-referential processing has been associated with onset of depressive symptoms (Nolen-Hoeksema, 1991; Michl et al., 2013). Moreover, we expected that adolescent-onset rMDD patients would be more severely affected than those with adult-onset given the more deleterious course of adolescent-onset MDD.
2. Methods and materials
2.1. Participants
Study volunteers recruited by online advertisements, announcements on bulletin boards or word of mouth were invited to the outpatient clinic of the Department of Psychiatry and Psychotherapy, Medical University of Vienna (MUV), Vienna, Austria, to participate in this cross-sectional fMRI study. Study procedures were approved by the Ethics Committee (EC) of the MUV (EC Number: 11/2008) according to the Declaration of Helsinki (WMA, 2013). All participants, who were adult and fully capable to give written informed consent, were considered and financially compensated for their expenditure of time.
After a comprehensive clinical assessment comprising previous history, neurological and medical examinations involving electrocardiography, blood pressure measurement and routine laboratory testing, all subjects underwent a thorough psychiatric examination. Diagnoses were evaluated according to the German version of the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I) (Wittchen et al., 1997). Depressive symptoms were assessed by the 21-item version of the Hamilton Rating Scale for Depression (HAM-D) (Hamilton, 1960). Only healthy participants without any previous or concurrent Axis I disorder were enrolled in this study, whereas previous single or multiple MDEs without any other present or previous axis I disorders were mandatory for inclusion of rMDD patients. In order to exclude cases with questionable clinical significance, only patients reporting previous antidepressant treatment (antidepressant medication, psychotherapy, or both) were included. Only rMDD patients who remitted and discontinued any antidepressant treatment at least three months prior to study enrollment were considered. Based on recent recommendations for considering remission and normal levels of functionality (Romera et al., 2011) a total HAM-D score ≤5 was required for all subjects. A complete list of further inclusion- and exclusion criteria is available in the Supplemental Information. Consecutively, 78 adult rMDD patients were enrolled in this study. With respect to age of onset, adolescent-onset was defined as ≤19 years and adult-onset as >19 years (Pajer et al., 2012). 42 adult HC chosen from a larger sample were automatically age- and gender-matched for both adult rMDD subgroups comprising 42 adolescent- and 36 adult-onset patients using an optimal full matching procedure (Hansen and Olsen Klopfer, 2006).
2.2. Paradigm
Prior to scanning, subjects received a standardized instruction and computerized training for the classical digit variant of the n-back task (Callicott et al., 1999) with two complexity conditions. The n-back task was designed to engage subjects to constantly update their mental set while minimizing interference from incoming stimuli (digits 1–4). Briefly, subjects had to follow a simple visual instruction ahead of each task block that indicated if they had to recall any number seen two presentations before (two-back; 2B) or if they had to identify the digit currently seen (zero-back; 0B). This WM paradigm consisted of four 2B blocks and four 0B blocks in total, each lasting for 30 s. Fourteen digits were pseudo-randomly presented for 1000 ms and followed by a 1000 ms inter-stimulus-interval at the corners of a trapeze-shaped array within each block. Visual stimuli were displayed using standard software (Presentation 10.3, http://www.neurobs.com/) and presented via a back-projection screen by a beamer placed outside the scanner room. Performance data represented as percentage (accuracy) as well as latencies (given in milliseconds) of correct responses were recorded via response buttons of an MRI-compatible response box fixed on the right thigh. Subjects replied with their right hand, using the middle finger for digit 1, the ring finger for digit 2, the index finger for digit 3 and the little finger for digit 4.
2.3. Demographic, clinical and behavioral data analyses
Demographic and psychometric differences between subgroups (adolescent- and adult-onset rMDD patients, HC) were investigated utilizing a one-way analysis of variance (ANOVA) or a chi-squared contingency table test. The German vocabulary scale “Wortschatztest” (WST) (Schmidt and Metzler, 1992), an operationalization of verbal intelligence, was assessed to confirm comparable education levels between the investigated subgroups. Additionally, response style questionnaire (RSQ) (Nolen-Hoeksema, 1991) data were available for a subsample of 39 participants (adolescent-onset rMDD patients: n = 14, adult-onset rMDD patients: n = 9, HC: n = 16), and were utilized to study the relationship between the personality trait rumination and imaging results within a post-hoc regression analysis. Clinical differences between the rMDD subgroups were tested by a two-sample t-test or a two-sample Wilcoxon test, where appropriate.
In accordance with previous studies employing the n-back task (Rose et al., 2006; Schoning et al., 2009), WM performance represented by accuracy and reaction time (RT) was recorded after stimulus onset for each complexity condition and examined by a repeated-measures ANOVA using one within-subject factor (WM load: two levels) and one between-subject factor (group: three levels). Behavioral analyses including scanner motion parameters were also examined by an ANOVA. Including age and gender in an additional analysis of co-variance (ANCOVA) did not alternate these results. All analyses were performed in R (version 3.1.1, http://cran-r-project.org/). The significance threshold was set at p < 0.05, two tailed.
2.4. Imaging
A detailed description of image acquisition and preprocessing is available in the supplemental information. Briefly, fMRI data processing was carried out using AFNI (http://afni.nimh.nih.gov/afni/) implemented into an R framework (http://cran.r-project.org/) (Boubela et al., 2012). Second-level activation analysis was conducted utilizing a mixed-effects meta-analysis approach (3dMEMA), which takes advantage of both beta-coefficients and t-values thereby accounting for between-subject variability and also the precision estimates of individual subject analyses (Chen et al., 2012). Age and gender were modeled as covariates of no interest in all group comparisons. Functional connectivity (FC) analysis was performed for two 4 mm spherical seeds representing maximal task-negative and -positive group differences (anterior-medial prefrontal cortex, amPFC: 5, 46, 12; dorsolateral PFC, dlPFC: −25, 26, 38). Fisher z-transformed correlation maps were used as dependent variable within a general linear model for between group comparisons using age and gender as covariates of no interest. Second-level results have been corrected for multiple comparisons in a whole-brain analysis of regions activated by the task for activation, and within a whole-brain mask for FC analysis using family-wise error (FWE) rates. Proportional variance estimation (s2) for regression analysis of behavioral imaging correlates was based on the package ‘relaimpo’ (relative importance metrics).
3. Results
3.1. Demographics and behavior
Demographic, clinical as well as behavioral data of all subjects are displayed in Table 1. No significant group differences were detected with respect to age (F(2, 117) = 1.35, p = 0.11), gender (χ2(5) = 3.9, p = 0.56), years of education (F(2, 117) = 0.88, p = 0.42), and the WST (F(2, 116) = 0.85, p = 0.43). HAM-D scores differed significantly between the subgroups (F(2, 117) = 6.45, p = 0.002) given the large sample size. It is noteworthy, that all subjects were below a conservative HAM-D cut-off ≤5, considered to indicate clinical relevance (Romera et al., 2011).
Table 1.
Demographic, clinical, and behavioral characteristics (n = 120).
| rMDD Patients |
HC (n = 42) | F/χ2 | p | ||
|---|---|---|---|---|---|
| Adolescent-onset (n = 42) | Adult-onset (n = 36) | ||||
| Age, y | 26.0 (4.8) | 27.6 (5.7) | 25.3 (4.2) | 1.345 | 0.110 |
| Females | 60% | 53% | 60% | 3.9 | 0.560 |
| Education, y | 12.5 (1.1) | 12.7 (0.7) | 12.7 (0.8) | 0.878 | 0.418 |
| WST | 33.7 (2.7) | 33.1 (2.3) | 33.9 (3.2) | 0.853 | 0.429 |
| HAM-D | 1.6 (1.5) | 1.9 (1.6) | 0.7 (1.3) | 6.449 | 0.002** |
| Accuracy 0B | 0.98 (0.04) | 0.98 (0.04) | 0.98 (0.03) | 0.239 | 0.788 |
| Accuracy 2B | 0.73 (0.18) | 0.76 (0.16) | 0.81 (0.15) | 2.213 | 0.114 |
| Reaction time 0B | 604.64 (106.05) | 638.70 (135.43) | 561.93 (129.22) | 3.367 | 0.038* |
| Reaction time 2B | 495.84 (204.02) | 476.34 (213.66) | 476.03 (259.82) | 0.150 | 0.861 |
Continuous variables are presented as mean (standard deviation). Abbreviations: **, highly significant (p < 0.01); *, significant (p < 0.05); rMDD, remitted Major Depressive Disorder; HC, healthy controls; WST, Wortschatztest; HAM-D, Hamilton Depression Rating Scale; MDE, Major Depressive Episode; y, years; 0B, zero-back; 2B, two-back.
As expected, there was a significant effect of WM load (2B minus 0B) for both accuracy (F(1, 117) = 216.87, p < 0.001) and RT (F(1, 117) = 34.14, p < 0.001). No significant main effects were observed for both accuracy (F(2, 117) = 2.00, p = 0.14) and RT (F(2, 117) = 0.52, p = 0.59) between all three investigated groups. No significant interaction effects for group and WM load were detected for either accuracy (F(2, 117) = 2.41, p = 0.094) or RT (F(2, 117) = 1.22, p = 0.298). Also here, although all subjects showed adequate cognitive performance (0B accuracy > 80%), a separate condition analysis revealed subtle but significant subgroup differences for 0B RT (F(2, 117) = 3.37, p = 0.038) given the large sample size.
With respect to scanner motion, we observed rather low maximum translation parameters (mean 0.46 mm, SD = 0.28 mm) with strongest movement of 1.32 mm. Furthermore, we did not observe any significant difference of maximal displacement between all three groups (F(2, 117) = 1.86, p = 0.16).
3.2. Clinical data of rMDD patients
Clinical data of rMDD patients are displayed in Table S1. Adolescent-onset rMDD patients exhibited more previous MDEs (p = 0.001), a higher cumulative number of months of MDEs (p = 0.009), a longer duration of illness (t(76) = 4.83, p < 0.0001), a longer duration of remission (p = 0.036), and a longer time-span since termination of psychotherapy (p = 0.006) than adult-onset rMDD patients. No significant differences were found for HAM-D scores (t(76) = −0.96, p = 0.34), cumulative number of months of antidepressant medication (p = 0.37), cumulative number of psychotherapy units (p = 0.62), and duration of the last MDE in months (p = 0.99) between the rMDD subgroups.
3.3. Task activation patterns within the groups
To test if the employed paradigm recruited both, the task-positive WM network (WMN) as well as the task-negative DMN, in our study, we performed a within-group comparison for WM load (2B minus 0B). In all three groups, significantly increased activation was detected in the dlPFC, the ventrolateral PFC (vlPFC) as well as the inferior parietal lobe (Table 2, Fig. S1). Concurrently, significant load-dependent deactivation was found in the amPFC, posterior cingulate cortex (PCC), as well as in the adjacent frontal-, temporal-, and parahippocampal gyrus (Table 2, Fig. S1). While the overall peak of activation was clearly found in the dlPFC, maximal deactivation was located in the amPFC in all three groups (Table 2, Fig. S1).
Table 2.
Activation main effects of the n-back task in rMDD patients and HC (n = 120).
| Region | BA | Cluster (mm3) | z | p | x | y | z |
|---|---|---|---|---|---|---|---|
| dlPFC | 9, 45, 46 | 71,650 | 13.00 | <0.001** | 1 | 9 | 52 |
| IPL | 40 | 36,846 | 12.70 | <0.001** | 38 | −46 | 41 |
| amPFC | 10 | 18,318 | −11.96 | <0.001** | −6 | 48 | 12 |
| PCC | 29, 30 | 6417 | −12.26 | <0.001** | −6 | −52 | 21 |
| MidSupITempG | 21 | 1957 | −10.53 | <0.001** | −58 | −8 | −8 |
| Insula | 13 | 1591 | −10.60 | <0.001** | 36 | −19 | 21 |
| vlPFC | 24, 32, 33 | 1434 | 10.76 | <0.001** | −16 | −2 | 23 |
| Insula | 13 | 837 | −10.36 | <0.001** | −34 | −19 | 21 |
| PHG | 28, 35 | 523 | −9.55 | <0.001** | −25 | −22 | −12 |
| IMidFG | 11, 47 | 241 | −9.32 | <0.001** | −38 | 31 | −8 |
Positive z-scores indicate an increase and negative z-scores a decrease of activation; x, y, z are coordinates in Talairach space (LPI). Abbreviations: **, highly significant (FWE corrected p < 0.01); *, significant (FWE corrected p < 0.05); rMDD, remitted Major Depressive Disorder; HC, healthy controls; BA, Brodmann Area; FWE, family-wise error rate; dlPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobe; amPFC, anterior-medial prefrontal cortex; PCC, posterior cingulate cortex; MidSupITempG, middle, superior and inferior temporal gyrus; vlPFC, ventrolateral prefrontal cortex; PHG, parahippocampal gyrus; IMidFG, inferior and middle frontal gyrus.
3.4. Regional activation differences between rMDD patients and HC
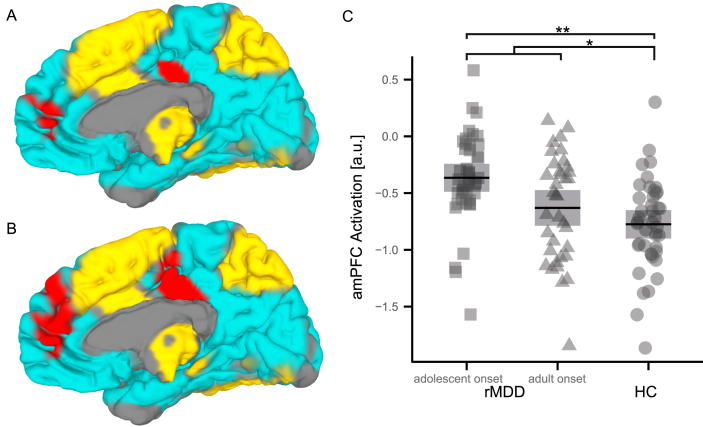
In comparison to HC, rMDD patients showed a significantly diminished deactivation of DMN regions with punctum maximum in the amPFC, while no significant group differences were found in typical WM areas (Fig. 1A and C, Fig. S2A; Table 3). Significant effects were even more pronounced, when comparing HC to adolescent-onset rMDD patients, where the latter exhibited significantly reduced DMN deactivation, which was most prominent in the amPFC, the PCC, and the temporal lobe (Fig. 1B and C, Fig. S2B; Table 3). Interestingly, adolescent-onset rMDD patients differed similarly from adult-onset rMDD patients, though not reaching significance (Fig. 1C, Fig. S2D; Table 3). In contrast, activation patterns of adult-onset rMDD patients did not significantly differ from HC (Fig. 1C, Fig. S2C; Table 3).
Fig. 1.
Activation Differences between rMDD Patients (n = 78) and HC (n = 42). Significantly decreased DMN deactivation (red) in rMDD patients compared to HC (A, C). Maximal effects are being found in the amPFC. Task activation (yellow) and deactivation (cyan) is presented as underlay of group comparison results in order to outline the DMN. Adolescent-onset rMDD patients exhibit significant and even more pronounced DMN deactivation decreases with punctum maximum in the amPFC and the PCC compared to HC (B, C). Plot (C) summarizes the significance of FWE corrected group comparisons for the amPFC and further visualizes the intermediate position of adult-onset rMDD patients compared to adolescent-onset MDD patients and HC. Abbreviations: **, highly significant (p < 0.01); *, significant (p < 0.05); 95% CI, 95% confidence interval; FWE, family-wise error rate; rMDD, remitted Major Depressive Disorder; HC, healthy controls; DMN, default-mode network; amPFC, anterior-medial prefrontal cortex; PCC, posterior cingulate cortex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Activation differences between rMDD patients (n = 78) and HC (n = 42).
| Region | BA | Cluster (mm3) | z | p | x | y | z |
|---|---|---|---|---|---|---|---|
| rMDD patients (n = 78) vs. HC (n = 42) | |||||||
| PCG | 3, 40 | 1760 | −3.87 | <0.001** | −38 | −32 | 58 |
| amPFC | 10 | 1571 | −3.61 | <0.001* | 8 | 48 | 12 |
| STG | 42 | 1184 | −3.52 | <0.001* | 58 | −11 | 10 |
| PCC | 24 | 1027 | −3.44 | <0.001+ | 3 | 22 | 32 |
| Adolescent-onset rMDD patients (n = 42) vs. HC (n = 42) | |||||||
| amPFC | 10 | 6976 | −4.33 | <0.001** | 5 | 46 | 12 |
| PCC | 24 | 5730 | −4.20 | <0.001** | 5 | −17 | 36 |
| STG | 42 | 1561 | −3.70 | <0.001* | 58 | −13 | 10 |
| STG | 43 | 1100 | −3.45 | <0.001+ | −51 | −6 | 14 |
| STG | 3, 40 | 1016 | −3.43 | <0.001+ | −38 | −32 | 58 |
Positive/negative z-scores represent increased/decreased deactivation of rMDD patients versus HC (two-back vs. zero-back); x, y, z are coordinates in Talairach space (LPI). Abbreviations: **, highly significant (FWE corrected p < 0.01); *, significant (FWE corrected p < 0.05); +, trend-wise significant (FWE corrected + p < 0.10); BA, Brodmann area; rMDD, remitted Major Depressive Disorder; HC, healthy controls; FWE, family-wise error rate; PCG, postcentral gyrus; amPFC, anterior-medial prefrontal cortex; PCC, posterior cingulate cortex; STG, superior temporal gyrus.
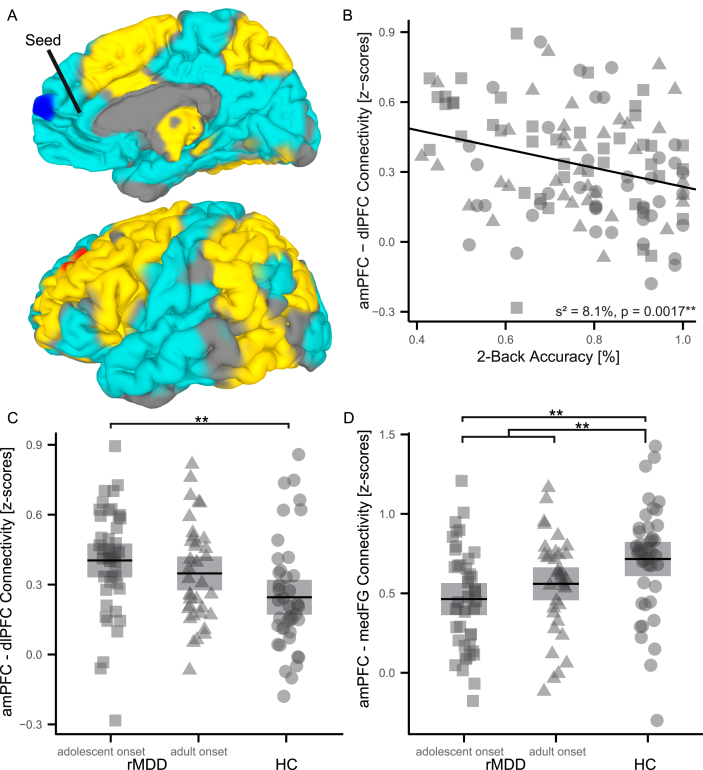
3.5. Brain systems differences between rMDD patients and HC
To investigate the degree of coupling between the DMN and the WMN, we calculated FC for regions with maximal task-negative (amPFC) and -positive (dlPFC) group differences. Analogous to activation results, FC analyses revealed significant differences between adolescent-onset rMDD patients and HC. Compared to HC, adolescent-onset rMDD patients exhibited significantly reduced coupling of the amPFC with most brain regions (Fig. 2A and D, Fig. S3; Table 4) except the dlPFC (Fig. 2A and C, Fig. S3; Table 4). Inversely, the dlPFC as seed region mirrored this result demonstrating increased FC with the amPFC and decreased FC between the dlPFC and the remaining brain regions (Fig. S4; Table 4). In contrast, adult-onset rMDD patients exhibited a qualitatively similar, but less-pronounced coupling pattern for both seed regions, which did not significantly differ from HC or adolescent-onset rMDD patients (Fig. 2C and D, Fig. S3E and F, Fig. S4E and F).
Fig. 2.
Differences in functional connectivity between adolescent-onset rMDD patients (n = 42) and HC (n = 42). The amPFC has been used as seed region (5, 46, 1) for FC analyses based on working memory paradigm data after removing task-based co-activation. The figure displays significantly increased (red) coupling of the amPFC with the dlPFC (A, C) and significantly decreased coupling (blue) with the mFG (A, D) in adolescent-onset rMDD patients compared to HC. Task activation (yellow) and deactivation (cyan) is presented as underlay of group comparison results in order to outline the DMN (A). A significant negative correlation was detected between 2B accuracy and amPFC-dlPFC coupling (B). Plots (C, D) summarize the significance of FWE corrected group comparisons for amPFC-dlPFC as well as amPFC-mFG FC and further visualize the intermediate position of adult-onset rMDD patients compared to adolescent-onset rMDD patients and HC. Abbreviations: **, highly significant (p < 0.01); *, significant (p < 0.05); 95% CI, 95% confidence interval; FWE, family-wise error rate; rMDD, remitted Major Depressive Disorder; HC, healthy controls; FC, functional connectivity; amPFC, anterior-medial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; mFG, medial frontal gyrus; DMN, default-mode network; 2B, two-back. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Differences in functional connectivity between adolescent-onset rMDD patients (n = 42) and HC (n = 42).
| Region | Seed | BA | Cluster (mm3) | z | p | x | y | z |
|---|---|---|---|---|---|---|---|---|
| MedFG | amPFC | 10 | 1687 | −3.52 | <0.001** | 3 | 59 | 17 |
| dlPFC | amPFC | 9, 8 | 1446 | 3.52 | <0.001* | −27 | 33 | 43 |
| Precuneus | dlPFC | 31 | 4745 | −4.22 | <0.001** | 25 | −41 | 36 |
| SupMidFG | dlPFC | 8, 9 | 2409 | −4.44 | <0.001** | 38 | 35 | 36 |
| amPFC | dlPFC | 10 | 2388 | 3.94 | <0.001** | −10 | 46 | 1 |
| MidFG | dlPFC | 6 | 2273 | −4.23 | <0.001** | 27 | 3 | 41 |
| SupMidFG | dlPFC | 10 | 1257 | −3.44 | <0.001* | −27 | 48 | 17 |
| SupMidFG | dlPFC | 6 | 1246 | −3.95 | <0.001* | −18 | −7 | 65 |
Positive/negative z-scores represent increased/decreased functional connectivity. Seeds are in the amPFC (5, 46, 12) and the dlPFC (−25, 26, 38). Significance is indicated by * (FWE corrected **p < 0.01, *p < 0.05); x, y, z are coordinates in Talairach space (LPI). Abbreviations: BA, Brodmann Area; rMDD, remitted Major Depressive Disorder; HC, healthy controls; dlPFC, dorsolateral prefrontal cortex; amPFC, anterior-medial prefrontal cortex; SupMidFG, superior and middle frontal gyrus; MidFG, middle frontal gyrus; MedFG, medial frontal gyrus.
3.6. Post hoc behavioral analysis
To investigate the relationship between behavioral and imaging data, we performed a post hoc linear regression analysis of accuracy and neural activation on a regional as well as brain systems level while controlling for age and gender. We correlated the strongest group difference for deactivation (amPFC) and FC (amPFC was used as seed region), and observed a significant negative relationship between 2B accuracy and amPFC-dlPFC coupling (s2 = 8.1%; t(116) = −3.22, p = 0.0016, Fig. 2B). In addition, we found a trend-wise significant positive correlation of 0B accuracy with amPFC-medial frontal gyrus (mFG) coupling (s2 = 2.9%; t(116) = 1.93, p = 0.0557) as well as a negative correlation with the amPFC activation (s2 = 2.3%; t(116) = −1.7, p = 0.0896).
3.7. Post hoc response style analysis
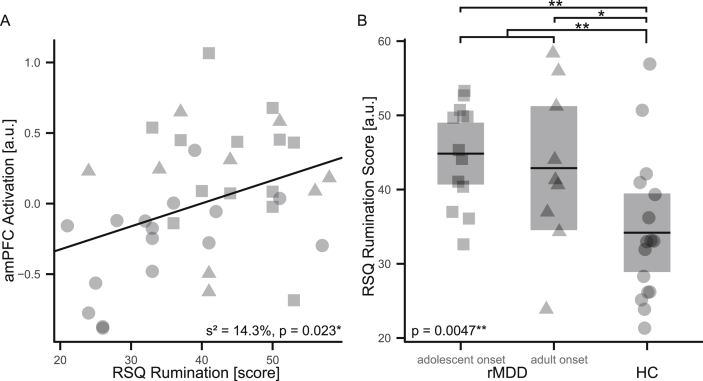
A post-hoc regression analysis of RSQ data and imaging results indicated that reduced DMN deactivation is significantly associated with an increased ruminative response style (s2 = 14.3%, t(35) = 2.39, p = 0.023; Fig. 3A). Moreover, rumination differed significantly between subgroups (F(2, 35) = 6.26, p = 0.0047) with maximal rumination values in adolescent-onset rMDD patients and lowest in HC, while adult-onset rMDD patients showed intermediate scores (Fig. 3B). Similar to previous analyses, age and gender were modeled as covariates of no interest.
Fig. 3.
Post hoc response style analysis. A reduction of amPFC deactivation is significantly associated with RSQ score increases (A) in all study participants with available RSQ data (n = 39). Both rMDD subgroups show significantly higher RSQ scores than HC with more pronounced effects in adolescent-onset rMDD patients (B). Studied subgroups are depicted by symbols: adolescent-onset (square) and adult-onset rMDD patients (triangle), HC (circle). Abbreviations: **, highly significant (p < 0.01); *, significant (p < 0.05); s2, estimated proportion of explained variance; rMDD, remitted Major Depressive Disorder; HC, healthy controls; amPFC, anterior-medial prefrontal cortex; RSQ, Response Style Questionnaire.
4. Discussion
The main goal of the present study was to investigate putative functional alterations of neural networks that are engaged or suppressed during WM performance in a large sample of adult drug-free rMDD patients and HC. Briefly, in comparison to HC, rMDD patients showed a significantly diminished deactivation of DMN nodes, which are known to be suppressed during externally directed WM performance (Anticevic et al., 2012). Moreover, the present study revealed that such reduced suppression of the DMN was significant only in adolescent-onset rMDD patients, putatively reflecting the more chronic course of early-onset MDD from a neurobiological perspective. On a brain systems level, adolescent-onset rMDD patients showed increased amPFC-dlPFC coupling, while all other connections within the WMN and DMN regions showed an inverse directionality. This tight coupling might underlie decreased reciprocal inhibition between the anterior parts of these antagonistic networks. Moreover, the fact that the strongest DMN disinhibition was detected in the amPFC underlines the superior role of this cortical midline structure in the dynamic interplay between several large-scale neural circuitries, encompassing the fronto-parietal central executive network, cingulo-opercular salience network (SN), and the medial prefrontal-medial parietal DMN, which has been shown to be dysfunctional in depression (Lemogne et al., 2012; Li et al., 2013; Liston et al., 2014) and other major neuropsychiatric disorders (Meyer-Lindenberg and Tost, 2012; Chen et al., 2013; Vilgis et al., 2014). In detail, the present findings are in line with the idea that a dysfunctional amPFC interferes with bottom-up processes during WM-related computations in MDD patients. As a consequence, top-down control mechanisms are likely initiated to down-regulate the DMN system, but fail to completely suppress its activity (Disner et al., 2011; Anticevic et al., 2012).
The key finding of this study is the reduced DMN suppression in MDD patients existing even after full recovery, which mirrors findings in symptomatic- (Rose et al., 2006; Greicius et al., 2007; Sheline et al., 2009; Sheline et al., 2010; Disner et al., 2011; Zhang et al., 2011; Anticevic et al., 2012; Davey et al., 2012a; Zhu et al., 2012; Connolly et al., 2013; Guo et al., 2013; Li et al., 2013; Sambataro et al., 2013; Dutta et al., 2014; Rodriguez-Cano et al., 2014) and euthymic- (Walsh et al., 2007; Schoning et al., 2009; Smoski et al., 2013) MDD patients. This reduction of DMN suppression, which is accompanied by increased ruminative response style in our study, might therefore be interpreted as increased self-referential processing as well as insufficient inhibition of conflicting computations in line with previous literature (Mason et al., 2007; Hamilton et al., 2011; Anticevic et al., 2012; Marchetti et al., 2012; Nejad et al., 2013). It is noteworthy that rumination has been found to be mediated by midline cortical structures of the DMN (Northoff et al., 2006; Hamilton et al., 2011; Northoff et al., 2011; Nejad et al., 2013) and to predict MDD onset, severity (Liotti et al., 2002; Northoff et al., 2011), and duration (Nolen-Hoeksema, 1991; Michl et al., 2013). Hence, we are tempted to speculate that the dysfunctional DMN suppression present in non-symptomatic MDD patients could also render the biological signature of increased relapse likelihood. This assumption is further supported by significantly pronounced DMN suppression deficits in adolescent-onset rMDD patients, who are prone to a more severe and chronic course compared to MDD patients with later onset, as repeatedly suggested in previous studies (Harrington et al., 1990; Klein et al., 1999; Weissman et al., 1999; Aalto-Setala et al., 2002; Zisook et al., 2007; Kendler et al., 2009; Pajer et al., 2012; Schosser et al., 2012; Ramirez et al., 2015).
Our data are further underlined by a recently published imaging study of remitted depressed young adults demonstrating that hyperconnectivities of the DMN and the SN with cognitive control networks are related to rumination and sustained attention (Jacobs et al., 2014). Similar neural alterations of the medial prefrontal cortex (mPFC) have been observed in another recent study in rMDD patients reporting an association between dysfunctional deactivation of the dorsomedial PFC and impaired autobiographical memory (Young et al., 2014). Our results resembling the critical role of the mPFC in neural alterations being found in symptomatic-as well as euthymic MDD patients (Young et al., 2014; Jacobs et al., 2014) hint at the possibility that neural dysfunction may persist even after full recovery of MDD. The mPFC is known to represent a critical neural hub that is involved in self-referential processing such as autobiographical memory, rumination, and cognitive control. It is noteworthy that one important function of the mPFC is to integrate contextual information of autobiographical memory in order to imagine the future (Schacter and Addis, 2007; Euston et al., 2012). The context-dependent autobiographical memory has been shown to be negatively-biased and less specific in symptomatic- and to some degree also in remitted MDD patients (Euston et al., 2012; Laxton et al., 2013; Young et al., 2014), and is therefore thought to reflect a specific cognitive style, which might predict antidepressant treatment response and depression relapse (Nolen-Hoeksema, 1991; Liotti et al., 2002; Northoff et al., 2011; Michl et al., 2013; Andrews-Hanna et al., 2014). Together with the known stress-sensitivity of the mPFC, conditions of uncontrollable stress paralleled by an exacerbation of helplessness and rumination could therefore be responsible for depression relapse (Nolen-Hoeksema, 1991; Amat et al., 2005).
While there is little doubt today that functioning of DMN-related brain regions is affected in MDD and other psychiatric disorders, the role of the DMN and the regional specialization of anterior and posterior parts of the human medial cortex has been increasingly investigated in the context of various mental processes (Amodio and Frith, 2006; Beckmann et al., 2009; Andrews-Hanna et al., 2014; Bzdok et al., 2014; Leech and Sharp, 2014). Accordingly, neural alterations linked to symptoms like rumination as demonstrated for the mPFC and the DMN clearly show that underlying neural signatures are not limited to DSM-defined boundaries. Hence, such neural changes observed in symptomatic as well as euthymic MDD are thought to reflect disease-related changes of neural systems that might be of diagnostic value in future neurobiology-based diagnostic systems (Nestler and Hyman, 2010; Insel, 2012; Kapur et al., 2012).
In contrast to the marked neural activation differences between rMDD patients and HC, the main effects on WM performance were statistically indistinguishable between the groups, which is in line with the majority of previous reports (Walsh et al., 2007; Schoning et al., 2009; Kerestes et al., 2012a, 2012b; Norbury et al., 2013). However, the non-isomorphism between brain function and behavior present in this study could further be related to the chosen maximal WM-load of the behavioral paradigm (2B), which might change, when WM-load increases (e.g. 3B, 4B) or stress-related environmental distractors, requiring a more rigorous control of the DMN, are involved. Our post hoc analysis of the relation between behavioral and imaging data revealed a significant negative correlation between amPFC-dlPFC coupling and 2B accuracy. This finding indicates that less accuracy is accompanied by DMN-WMN hyperconnectivity, which might reflect the increased relapse risk of rMDD patients (Jacobs et al., 2014). While our results as well as limited evidence available from recent studies (Jacobs et al., 2014; Young et al., 2014) hint at the importance of DMN-WMN interplay with respect to depression relapse prediction, future longitudinal studies are clearly needed in order to validate the assumption that a dysfunctional DMN is causally related to increased relapse likelihood in MDD patients.
Despite the intriguing neural alterations observed in this large study of fully-recovered drug-free rMDD patients, some limitations may apply. To address the biological heterogeneity of MDD, we decided to sub-group our patient sample according to disease onset, which was driven by encouraging reports on distinct biological signatures of adolescent MDD patients (Pajer et al., 2012), well-known clinical differences between adolescent- and adult-onset MDD patients (Zisook et al., 2007), and the ease and precision of assessment. While competing sub-classifications such as typical/atypical depression, single/multiple episode MDD as well as others (Harald and Gordon, 2012; Lamers et al., 2012; Sung et al., 2013; Thase, 2013; Rodgers et al., 2014; Wakefield and Schmitz, 2014) exist, their biological significance is still under debate and has not fully been addressed yet (Insel, 2012; Casey et al., 2013). Thus, we are confident that our choice to subgroup patients according to disease onset is appropriate for this study type. Notably, the present cross-sectional study targets exclusively the remitted phase of MDD, and therefore does not allow a direct comparison to symptomatic MDD. Hence, all inferences being made with respect to symptomatic MDD are purely literature-based. However, we are confident that our conclusions are not too far-fetched given the multitude of imaging literature on symptomatic MDD.
Summarizing, this study demonstrates that DMN alterations persist even after full recovery of MDEs. Observed neural activation patterns were related to rumination, which is a well-established indicator of MDD severity (Hamilton et al., 2011). Brain systems level analyses mirrored the aberrant DMN suppression in rMDD patients and underlined the specific role of the amPFC as mediator of these effects (Lemogne et al., 2012; Chen et al., 2013; Li et al., 2013). In contrast to adult-onset rMDD patients, adolescent-onset rMDD patients exhibited significant local as well as systems level findings, which might reflect the more detrimental clinical course of this diagnostic subgroup. In conclusion, this study demonstrating reduced DMN suppression in rMDD patients encourages the investigation of DMN suppression measures as putative relapse predictor in future clinical trials, which might eventually lead to important implications for antidepressant maintenance treatment.
Role of the funding source
This work was partly funded by the Special Research Project SFB-35 (project number F3514-B11) and the Program Clinical Research (project number KLI148-B00) of the Austrian Science Fund (FWF), and the Oesterreichische Nationalbank, Anniversary Fund (project number 13903).
Contributors
Lucie Bartova, Bernhard M. Meyer, Kersten Diers, Ulrich Rabl, and Lukas Pezawas designed research. Lucie Bartova, Bernhard M. Meyer, Kersten Diers, Ulrich Rabl, Christian Scharinger, Ana Popovic, Klaudius Kalcher, Roland N. Boubela, Gerald Pail, Dominik Mandorfer, Christian Windischberger, and Ewald Moser performed research. Bernhard M. Meyer, Kersten Diers, Ulrich Rabl, Klaudius Kalcher, Roland N. Boubela, and Lukas Pezawas analyzed data. Lucie Bartova, Bernhard M. Meyer, Kersten Diers, Ulrich Rabl, Ana Popovic, Julia Huemer, Siegfried Kasper, Nicole Praschak-Rieder, Harald H. Sitte, Ewald Moser, Burkhard Brocke, and Lukas Pezawas wrote the paper.
Conflict of interest
Lucie Bartova, Bernhard M. Meyer, Kersten Diers, Ulrich Rabl, Christian Scharinger, Ana Popovic, Klaudius Kalcher, Roland N. Boubela, Julia Huemer, Dominik Mandorfer, Christian Windischberger, Nicole Praschak-Rieder, Burkhard Brocke and Lukas Pezawas report no conflicts of interest. Harald H. Sitte has received honoraria for lectures and consulting from Astra Zeneca, Lundbeck, Nycomed, Ratiopharm, Roche, Sanofi-Aventis, Serumwerk Bernburg, Torrex-Chiesi Pharma. Gerald Pail has worked as a consultant for AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Lundbeck, MSD and Servier and he has served on speakers' bureaus for AstraZeneca, Bristol-Myers Squibb, Lundbeck, Nycomed and Servier. He has been employed by Lundbeck from 2005 to 2007 and has received educational grants by Lundbeck and Servier. Siegfried Kasper has received grant/research support from Bristol Myers-Squibb, Eli Lilly, GlaxoSmithKline, Lundbeck, Organon, Sepracor and Servier; he has served as a consultant or on advisory boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Lundbeck, Merck Sharp and Dome (MSD), Novartis, Organon, Pfizer, Schwabe, Sepracor, and Servier; and he has served on speakers' bureaus for Angelini, AstraZeneca, Bristol Myers-Squibb, Eli Lilly, Janssen, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, Sepracor, and Servier. Ewald Moser has received an “Unrestricted research Grant” by Siemens Healthcare, which is unrelated to the present work.
Acknowledgments
We are grateful to diploma students of the Clinical Neuroimaging Group (Supervisor: Assoc.Prof. Priv.Doz. Dr. Lukas Pezawas), Division of Biological Psychiatry, Department of Psychiatry and Psychotherapy, Medical University of Vienna for their assistance in patient recruitment and evaluation (Pia Auersperg, Roman Fronz, Joachim Manuel Gruber, Anastasia Gudakovskaja, Cordula Bettina Höfle, Johanna Kafka, Viktoria Köller, Elisabeth Kühtreiber, Franziska Mayr, Nora Bibiana Ortner, Helge Oswald, Lisa Ott, Sophia Petschnak, Matthias Pilgerstorfer, Sebastian Ribar, Marie Janina Schwidde, Andrea Spitzer, and Sandra Tockner).
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Aalto-Setala T., Marttunen M., Tuulio-Henriksson A., Poikolainen K., Lonnqvist J. Depressive symptoms in adolescence as predictors of early adulthood depressive disorders and maladjustment. Am J Psychiatry. 2002;159(7):1235–1237. doi: 10.1176/appi.ajp.159.7.1235. [DOI] [PubMed] [Google Scholar]
- Amat J., Baratta M.V., Paul E., Bland S.T., Watkins L.R., Maier S.F. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8(3):365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . American Psychiatric Association; 2013. Diagnostic and statistical manual of mental disorders: DSM-5. [Google Scholar]
- Beck A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165(8):969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Becker A.E., Kleinman A. Mental health and the global agenda. N Engl J Med. 2013;369(1):66–73. doi: 10.1056/NEJMra1110827. [DOI] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29(4):1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z., Cowen P.J. ‘It's not over when it's over’: persistent neurobiological abnormalities in recovered depressed patients. Psychol Med. 2008;38(3):307–313. doi: 10.1017/s0033291707001250. [DOI] [PubMed] [Google Scholar]
- Boubela R.N., Huf W., Kalcher K., Sladky R., Filzmoser P., Pezawas L. A highly parallelized framework for computationally intensive MR data analysis. MAGMA. 2012;25(4):313–320. doi: 10.1007/s10334-011-0290-7. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Heeger A., Langner R., Laird A.R., Fox P.T., Palomero-Gallagher N. Subspecialization in the human posterior medial cortex. Neuroimage. 2014;106C:55–71. doi: 10.1016/j.neuroimage.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott J.H., Mattay V.S., Bertolino A., Finn K., Coppola R., Frank J.A. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Craddock N., Cuthbert B.N., Hyman S.E., Lee F.S., Ressler K.J. DSM-5 and RDoC: progress in psychiatry research? Nat Rev Neurosci. 2013;14(11):810–814. doi: 10.1038/nrn3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.C., Oathes D.J., Chang C., Bradley T., Zhou Z.W., Williams L.M. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Saad Z.S., Nath A.R., Beauchamp M.S., Cox R.W. FMRI group analysis combining effect estimates and their variances. Neuroimage. 2012;60(1):747–765. doi: 10.1016/j.neuroimage.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Repovs G., Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20(6):652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.G., Wu J., Ho T.C., Hoeft F., Wolkowitz O., Eisendrath S. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Harrison B.J., Yucel M., Allen N.B. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42(10):2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Yucel M., Allen N.B., Harrison B.J. Task-related deactivation and functional connectivity of the subgenual cingulate cortex in major depressive disorder. Front Psychiatry. 2012;3:14. doi: 10.3389/fpsyt.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Simpson J.R., Jr., Todd R.D., Reich T., Vannier M. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dutta A., McKie S., Deakin J.F. Resting state networks in major depressive disorder. Psychiatry Res. 2014;224(3):139–151. doi: 10.1016/j.pscychresns.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Elliott R., Zahn R., Deakin J.F., Anderson I.M. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36(1):153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston D.R., Gruber A.J., McNaughton B.L. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P.B., Srithiran A., Benitez J., Daskalakis Z.Z., Oxley T.J., Kulkarni J. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp. 2008;29(4):490–501. doi: 10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S.E., Kawachi I., Fitzmaurice G.M., Buka L. Socio-economic status, family disruption and residential stability in childhood: relation to onset, recurrence and remission of major depression. Psychol Med. 2003;33(8):1341–1355. doi: 10.1017/s0033291703008377. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Liu F., Zhang J., Zhang Z., Yu L., Liu J. Dissociation of regional activity in the default mode network in first-episode, drug-naive major depressive disorder at rest. J Affect Disord. 2013;151(3):1097–1101. doi: 10.1016/j.jad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B.B., Olsen Klopfer S. Optimal full matching and related designs via network flows. J Comput Graph Stat. 2006;15(3):1–19. [Google Scholar]
- Harald B., Gordon P. Meta-review of depressive subtyping models. J Affect Disord. 2012;139(2):126–140. doi: 10.1016/j.jad.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Harrington R., Fudge H., Rutter M., Pickles A., Hill J. Adult outcomes of childhood and adolescent depression. I. Psychiatric status. Arch Gen Psychiatry. 1990;47(5):465–473. doi: 10.1001/archpsyc.1990.01810170065010. [DOI] [PubMed] [Google Scholar]
- Harvey P.O., Fossati P., Pochon J.B., Levy R., Lebastard G., Lehericy S. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26(3):860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Ho T.C., Connolly C.G., Henje Blom E., LeWinn K.Z., Strigo I.A., Paulus M.P. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R. Next-generation treatments for mental disorders. Sci Transl Med. 2012;4(155):155ps19. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- Jacobs R.H., Jenkins L.M., Gabriel L.B., Barba A., Ryan K.A., Weisenbach S.L. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One. 2014;9(8):e104366. doi: 10.1371/journal.pone.0104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S., Phillips A.G., Insel T.R. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Fiske A., Gardner C.O., Gatz M. Delineation of two genetic pathways to major depression. Biol Psychiatry. 2009;65(9):808–811. doi: 10.1016/j.biopsych.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Gardner C.O. Dependent stressful life events and prior depressive episodes in the prediction of major depression: the problem of causal inference in psychiatric epidemiology. Arch Gen Psychiatry. 2010;67(11):1120–1127. doi: 10.1001/archgenpsychiatry.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R., Bhagwagar Z., Nathan P.J., Meda S.A., Ladouceur C.D., Maloney K. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Res. 2012;202(1):30–37. doi: 10.1016/j.pscychresns.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R., Ladouceur C.D., Meda S., Nathan P.J., Blumberg H.P., Maloney K. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2012;42(1):29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- Klein D.N., Schatzberg A.F., McCullough J.P., Dowling F., Goodman D., Howland R.H. Age of onset in chronic major depression: relation to demographic and clinical variables, family history, and treatment response. J Affect Disord. 1999;55(2–3):149–157. doi: 10.1016/s0165-0327(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Kupfer D.J., Frank E., Phillips M.L. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379(9820):1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F., Burstein M., He J.P., Avenevoli S., Angst J., Merikangas K.R. Structure of major depressive disorder in adolescents and adults in the US general population. Br J Psychiatry. 2012;201:143–150. doi: 10.1192/bjp.bp.111.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxton A.W., Neimat J.S., Davis K.D., Womelsdorf T., Hutchison W.D., Dostrovsky J.O. Neuronal coding of implicit emotion categories in the subcallosal cortex in patients with depression. Biol Psychiatry. 2013;74(10):714–719. doi: 10.1016/j.biopsych.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C., Delaveau P., Freton M., Guionnet S., Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136(1–2):e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Li B., Liu L., Friston K.J., Shen H., Wang L., Zeng L.L. A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry. 2013;74(1):48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Licinio J., Wong M.L. Advances in depression research: 2011. Mol Psychiatry. 2011;16(7):686–687. doi: 10.1038/mp.2011.74. [DOI] [PubMed] [Google Scholar]
- Liotti M., Mayberg H.S., McGinnis S., Brannan S.L., Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159(11):1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Liston C., Chen A.C., Zebley B.D., Drysdale A.T., Gordon R., Leuchter B. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano A.M., Mayberg H.S., Giacobbe P., Hamani C., Craddock R.C., Kennedy S.H. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H., Sonuga-Barke E.J., De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev. 2012;22(3):229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Glahn D.C., Peluso M.A., Hatch J.P., Monkul E.S., Najt P. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12(2):158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15(5):663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Michl L.C., McLaughlin K.A., Shepherd K., Nolen-Hoeksema S. Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: longitudinal evidence in early adolescents and adults. J Abnorm Psychol. 2013;122(2):339–352. doi: 10.1037/a0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J., Agid Y., Brune M., Bullmore E.T., Carter C.S., Clayton N.S. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon N.L., Liddle P.F., Worwood G., Liotti M., Nixon E. Prefrontal cortex function in remitted major depressive disorder. Psychol Med. 2012:1–12. doi: 10.1017/S0033291712002164. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Norbury R., Godlewska B., Cowen P.J. When less is more: a functional magnetic resonance imaging study of verbal working memory in remitted depressed patients. Psychol Med. 2013:1–7. doi: 10.1017/S0033291713001682. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G., Wiebking C., Feinberg T., Panksepp J. The 'resting-state hypothesis' of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35(9):1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Okada G., Okamoto Y., Yamashita H., Ueda K., Takami H., Yamawaki S. Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry Clin Neurosci. 2009;63(3):423–425. doi: 10.1111/j.1440-1819.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- Pajer K., Andrus B.M., Gardner W., Lourie A., Strange B., Campo J. Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Transl Psychiatry. 2012;2:e101. doi: 10.1038/tp.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Drabant E.M., Verchinski B.A., Munoz K.E., Kolachana B.S. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A., Ekselius L., Ramklint M. Depression in young adult psychiatric outpatients: delimiting early onset. Early Interv Psychiatry. 2015;9(2):108–117. doi: 10.1111/eip.12092. [DOI] [PubMed] [Google Scholar]
- Rodgers S., Grosse Holtforth M., Muller M., Hengartner M.P., Rossler W., Ajdacic-Gross V. Symptom-based subtypes of depression and their psychosocial correlates: a person-centered approach focusing on the influence of sex. J Affect Disord. 2014;156:92–103. doi: 10.1016/j.jad.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cano E., Sarro S., Monte G.C., Maristany T., Salvador R., McKenna P.J. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychol Med. 2014:1–11. doi: 10.1017/S0033291714000841. [DOI] [PubMed] [Google Scholar]
- Romera I., Perez V., Menchon J.M., Polavieja P., Gilaberte I. Optimal cutoff point of the Hamilton Rating Scale for depression according to normal levels of social and occupational functioning. Psychiatry Res. 2011;186(1):133–137. doi: 10.1016/j.psychres.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Rose E.J., Simonotto E., Ebmeier K.P. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 2006;29(1):203–215. doi: 10.1016/j.neuroimage.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Sambataro F., Wolf N.D., Pennuto M., Vasic N., Wolf R.C. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med. 2013:1–11. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R. Constructive memory: the ghosts of past and future. Nature. 2007;445(7123):27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schlosser R.G., Wagner G., Koch K., Dahnke R., Reichenbach J.R., Sauer H. Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage. 2008;43(3):645–655. doi: 10.1016/j.neuroimage.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Schmidt K.-H., Metzler P. 1992. Wortschatztest (WST) [Google Scholar]
- Schoning S., Zwitserlood P., Engelien A., Behnken A., Kugel H., Schiffbauer H. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp. 2009;30(9):2746–2756. doi: 10.1002/hbm.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schosser A., Serretti A., Souery D., Mendlewicz J., Zohar J., Montgomery S. European Group for the Study of Resistant Depression (GSRD)–where have we gone so far: review of clinical and genetic findings. Eur Neuropsychopharmacol. 2012;22(7):453–468. doi: 10.1016/j.euroneuro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski M.J., Keng S.L., Schiller C.E., Minkel J., Dichter G.S. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. J Affect Disord. 2013;151(1):171–177. doi: 10.1016/j.jad.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S.C., Wisniewski S.R., Balasubramani G.K., Zisook S., Kurian B., Warden D. Does early-onset chronic or recurrent major depression impact outcomes with antidepressant medications? A CO-MED trial report. Psychol Med. 2013;43(5):945–960. doi: 10.1017/S0033291712001742. [DOI] [PubMed] [Google Scholar]
- Thase M.E. The multifactorial presentation of depression in acute care. J Clin Psychiatry. 2013;74(2):3–8. doi: 10.4088/JCP.12084su1c.01. [DOI] [PubMed] [Google Scholar]
- Vasic N., Walter H., Sambataro F., Wolf R.C. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med. 2009;39(6):977–987. doi: 10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- Vilgis V., Chen J., Silk T.J., Cunnington R., Vance A. Frontoparietal function in young people with dysthymic disorder (DSM-5: persistent depressive disorder) during spatial working memory. J Affect Disord. 2014;160:34–42. doi: 10.1016/j.jad.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield J.C., Schmitz M.F. Predictive validation of single-episode uncomplicated depression as a benign subtype of unipolar major depression. Acta Psychiatr Scand. 2014;129(6):445–457. doi: 10.1111/acps.12184. [DOI] [PubMed] [Google Scholar]
- Walsh N.D., Williams S.C., Brammer M.J., Bullmore E.T., Kim J., Suckling J. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry. 2007;62(11):1236–1243. doi: 10.1016/j.biopsych.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Weissman M.M., Wolk S., Goldstein R.B., Moreau D., Adams P., Greenwald S. Depressed adolescents grown up. J Am Med Assoc. 1999;281(18):1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- Wittchen H., Wunderlich U., Gruschwitz S., Zaudig M. Hogrefe Göttingen; Germany: 1997. SKID-I, Strukturiertes Klinisches Interview für DSM-IV. [Google Scholar]
- WMA World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Young K.D., Bellgowan P.S., Bodurka J., Drevets W.C. Neurophysiological correlates of autobiographical memory deficits in currently and formerly depressed subjects. Psychol Med. 2014;44(14):2951–2963. doi: 10.1017/S0033291714000464. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., He Y. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70(4):334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang X., Xiao J., Liao J., Zhong M., Wang W. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Zisook S., Lesser I., Stewart J.W., Wisniewski S.R., Balasubramani G.K., Fava M. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. 2007;164(10):1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.