Abstract
Hepatitis A virus (HAV) and hepatitis E virus (HEV) infection in developing countries are associated with contaminated food or water. Although Thailand is non-endemic for HEV, sporadic infections may occur from zoonotic transmission. Individuals between 7 months to 69 years (mean age = 32.8) from predominantly Islamic Narathiwat (n = 305) and swine farm-dense Lop Buri (n = 416) provinces were screened for anti-HEV and anti-HAV antibodies by commercial enzyme-linked immunosorbent assay and automated chemiluminescent microparticle immunoassay, respectively. Seroprevalence and relative antibody titers were analyzed according to age groups. HAV IgG antibody positive rates in Lop Buri and Narathiwat residents were 39.9% and 58%, respectively (p < 0.001). Greater than 90% of individuals >50 years old in both provinces possessed anti-HAV IgG. In contrast, seroprevalence for anti-HEV IgG was much higher in Lop Buri (37.3%) than in Narathiwat (8.9%) (p < 0.001). Highest anti-HEV IgG prevalence was found among 21-30 year-olds (50%) in Lop Buri and 41-50 year-olds (14.1%) in Narathiwat. In summary, fewer individuals possessed anti-HEV IgG in Narathiwat where most residents abstained from pork and fewer swine farms are present. Therefore, an increased anti-HEV IgG seroprevalence was associated with the density of swine farm and possibly pork consumption. Adults were more likely than children to have antibodies to both HEV and HAV.
Introduction
Hepatitis E virus (HEV) is a non-enveloped positive-sense RNA virus and the sole member of the genus Hepevirus in the family Hepeviridae. HEV infection had been associated with poor sanitation and unsafe drinking water. Each year approximately 20 million individuals are infected, resulting in approximately 56,000 deaths [1]. HEV infection is generally self-limiting but may cause acute liver failure with low mortality among healthy individuals but significantly higher mortality in pregnant women [2]. Symptoms can include fever, nausea, vomiting, abdominal pain and jaundice [3]. Although there are four HEV genotypes, only one serotype exist [4]. Genotypes 1 and 2 are often found in developing countries and infect only humans, while genotypes 3 and 4 are found in both humans and animals and have been identified in developed countries [5].
Like HEV, hepatitis A virus (HAV) is transmitted by contaminated food or water and is often associated with poor sanitation. In addition, HAV can be transmitted through close-contact with an infectious person. It is also a non-enveloped positive-sense RNA virus belonging to the family Picornaviridae in the genus Hepatovirus. There are 6 genotypes of HAV (designated I to VI) in which genotype I, II, and III have been identified in human [6]. Like HEV, there is only one HAV serotype [4]. Although several effective vaccines are available and HAV infection is self-limiting, HAV still affects 1.4 million individuals annually [7]. HAV infection of children in developing countries is generally asymptomatic and provides immunity from reinfection during adulthood [8]. However, HAV outbreaks do occur in industrialized countries as a result of contaminated produce including green onions, tomatoes and berries [9,10].
Although HEV is commonly transmitted via fecal-oral route in endemic regions, autochthonous HEV infections in developed countries are increasingly recognized [11]. HEV infection can be problematic in the immunocompromised as a result of organ transplantation [12] or blood transfusion [13]. Furthermore, HEV can be acquired from the consumption of pork liver food products [14]. Reverse-transcription polymerase chain reaction (RT-PCR) for viral RNA is useful in detecting active infection and the presence of HEV-specific immunoglobulins can indicate past exposure to HEV. Anti-HEV IgM can persist for several months and decline after resolution from infection, but anti-HEV IgG remains detectable for years [15].
Previous studies have shown that urban residents and occupations requiring higher educations are associated with lower seroprevalence of HEV as these factors may be surrogates for better sanitation and hygiene [16, 17]. In addition, evidence linking the consumption of pork products and increased risk for HEV infection is of particular concern [11]. In Southeast Asia, few studies have examined the association between HEV seroprevalence and either consumption of pork or occupational exposure to pigs. We therefore examined anti-HEV antibodies in two similar-sized yet demographically different provinces of Thailand where swine farm densities are different due to local norms. We also assessed the prevalence of antibodies against HAV in these communities for comparison.
Materials and Methods
This study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB No. 377/57) followed the Helsinki Declaration on medical research. Written informed consent was obtained from study participants or their parents and data such as age, gender and address was collected. Serum samples were analyzed at the Center of Excellence in Clinical Virology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University. All samples were treated as anonymous.
Study area and population
The subjects were recruited between March 1 and October 31, 2014 during a separate hospital-based, cross-sectional national study on the impact of hepatitis A, B, and C. Lop Buri and Narathiwat were chosen as target sites for central and southern part of Thailand, respectively (Fig 1). Individuals between 6 months to 60 years were healthy volunteers or patients who attended pediatrics or general medicine clinics with no known immunological or chronic conditions. A total of 721 individual samples (311 males, 410 females; mean age 32.8 ± 17.0) were obtained from residents residing in Lob Buri and Narathiwat. Samples from 416 individuals (214 males, 202 females) between the ages of 7 months to 69 years (mean age 32.8 ± 17.2) were obtained from King Narai Hospital and Tha Wung district hospital in Lop Buri. Samples from 305 individuals (97 males, 208 females) between the ages of 3–59 years (mean age 32.8 ± 16.8) were obtained from Narathiwat Ratchanakarin Hospital and represented all but two districts (Sukhirin and Waeng) of Narathiwat.
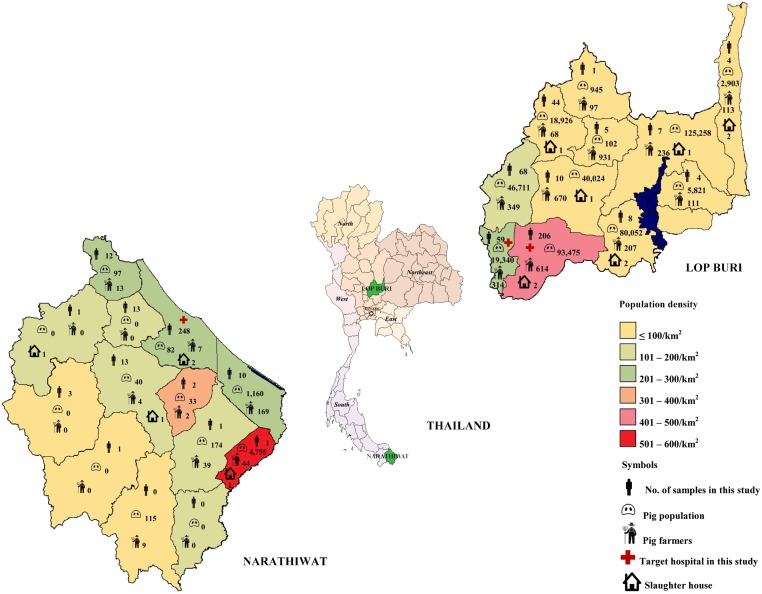
Fig 1. Geographical and population characteristics in the provinces of Lop Buri and Narathiwat.
Map indicates the locations of Lop Buri and Narathiwat with information on their respective population density. The number of samples from individuals residing in each district is indicated on the map. Approximate pig population, pig farmers, and slaughter houses in each district are noted.
Seroprevalence assay
Serum samples were analyzed for HEV IgG antibody using 96-well plate ELISA (Anti-Hepatitis E Virus ELISA IgG, Euroimmun, Lübeck, Germany) according to manufacturer’s instructions. The limit of detection for the anti-HEV IgG test was 0.1 IU/mL. HAV IgG antibodies were measured by automated chemiluminescent microparticle immunoassay (ARCHITECT HAVab-IgG, Abbott Laboratories, Abbott Park, IL) which utilized HAV-coated paramagnetic microparticles to detect anti-HAV IgG. The resulting chemiluminescent reaction was measured as relative light units (RLUs) compared to cutoff signal (S/CO). Samples with S/CO values > 1.00 were considered reactive for IgG anti-HAV, while specimens with S/CO values < 1.00 were considered nonreactive.
Data analysis
Gender and anti-HEV or anti-HAV antibody results were recorded as frequency and percentage with mean age and standard deviations (SD). Chi-square test provided comparison between groups of categorical variables. Student’s t-test provided comparison between groups of continuous variables. The analyses were performed on SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Study population of Lop Buri and Narathiwat
Thailand is located in Southeast Asia adjacent to neighboring Myanmar, Laos, Cambodia, and Malaysia. With a population of ~63 million, the majority of Thais practices Buddhism (94.6%) and Islam (4.6%), of which the latter predominates in southern Thailand. Thailand specializes in agriculture and livestock-based farming including pork production. Large-scale swine farms are primarily located in central Thailand (~57%), while very few small and mostly family farms are located in the south [18] (Fig 1).
Although Lop Buri and Narathiwat have comparable population size, there are differences in demographics and socio-economic differences (Table 1). Lop Buri is a central province ~154 km from the capital Bangkok. The total population is ~756,127 and predominantly Buddhist (99.8%) [19]. There are estimated 434,386 animals and 2,881 farmers, some on commercial scale. This density is relatively high given that there are swine farms in nearly all provincial districts [20,21].
Table 1. Comparison of socio-economic data between Lop Buri and Narathiwat.
| Lop Buri a , b , c , d , e | Narathiwat a , b , f , g , h | ||
|---|---|---|---|
| Area | 6,200 km2 | 4,475 km2 | |
| Population | 756,127 | 766,145 | |
| Population density | 120/km2 | 170/km2 | |
| Religion | Buddhism | 99.76% | 17.0% |
| Christianity | 0.11% | 1.0% | |
| Islam | 0.13% | 83.0% | |
| Education | No education | 5.84% | 4.87% |
| Less than elementary level | 3.78% | 4.49% | |
| Elementary level | 48.59% | 56.94% | |
| Lower secondary education | 16.58% | 14.44% | |
| Upper secondary level (general) | 9.81% | 9.91% | |
| Upper secondary level (vocational) | 4.06% | 0.81% | |
| Occupation | Agricultural and fishery workers | 29.04% | 33.75% |
| Service provider and seller | 35.78% | 22.85% | |
| Income Per Capita (Baht) | 95,412 | 71,786 | |
| Health system | Hospital (total bed) | 16 (1,769) | 13 (1,040) |
| Health care worker | 2,705 | 1,853 | |
| Pig population | 434,386 | 6,456 | |
| Pig farmers | 2,881 | 287 |
aNational Statistical Office;
bInformation Technology and Vocational Manpower Center;
cLop Buri Governor’s Office;
dLop Buri Provincial Public Health Office;
eLopburi Provincial Livestock Office;
fNarathiwat National Statistical Office;
gNarathiwat Provincial Public Health Office;
hNarathiwat Provincial Livestock Office.
In contrast, the southern-most province of Narathiwat is located immediately north of Malaysia. The total population is ~762,622 and most residents are Muslim Thais (83%). There are an estimated 287 farmers who keep 6,456 animals, mostly for local consumption by non-Muslims [22].
Anti-HEV antibody prevalence and titers
We found that the overall anti-HEV IgG positive rate was 37.3% in Lop Buri and 8.9% in Narathiwat (p <0.001) (Table 2). Mean age of individuals with positive anti-HEV antibody was 36.4 ± 14.6 in Lop Buri and 38.1 ± 15.9 in Narathiwat (p = 0.58). Although the anti-HEV IgG positive rates were generally below 50.0%, seroprevalence in Lop Buri was much higher than Narathiwat (37.3% VS 8.9%; p < 0.001) especially among adults (Fig 2A).
Table 2. Anti-HEV and anti-HAV IgG seropositive rates in Lop Buri and Narathiwat population associated with gender and age group.
| Total subjects | Mean age | No. of test | anti-HEV IgG positive (%) | anti-HAV IgG positive (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lop Buri | Narathiwat | Lop Buri | Narathiwat | p-value a | Lopburi | Narathiwat | p-value a | |||
| Sex | ||||||||||
| Male | 311 | 214 | 97 | 37.9 | 8.3 | <0.001 | 37.4 | 56.7 | 0.002 | |
| Female | 410 | 202 | 208 | 36.6 | 9.1 | <0.001 | 41.1 | 58.7 | 0.001 | |
| Age | ||||||||||
| <5 | 38 | 2.5±1.2 | 24 | 14 | 20.8 | 7.1 | 4.2 | 28.6 | 0.031 | |
| 5–10 | 63 | 7.3±1.8 | 36 | 27 | 8.3 | 3.7 | 5.6 | 22.2 | 0.049 | |
| 11–20 | 83 | 15.8±3.3 | 44 | 39 | 22.7 | 5.1 | 0.023 | 4.6 | 18.0 | |
| 21–30 | 149 | 25.7±2.6 | 76 | 73 | 50.0 | 9.6 | <0.001 | 7.9 | 42.5 | <0.001 |
| 31–40 | 106 | 35.2±2.8 | 78 | 28 | 42.3 | 3.6 | <0.001 | 28.2 | 78.6 | <0.001 |
| 41–50 | 141 | 45.4±2.9 | 77 | 64 | 46.8 | 14.1 | <0.001 | 76.6 | 82.8 | |
| >50 | 141 | 55.5±3.2 | 81 | 60 | 37.0 | 10.0 | <0.001 | 91.4 | 90.0 | |
| Total | 721 | 32.8±17.0 | 416 | 305 | 37.3 | 8.9 | <0.001 | 39.9 | 58.0 | <0.001 |
a p-value <0.05 denotes statistical significance.
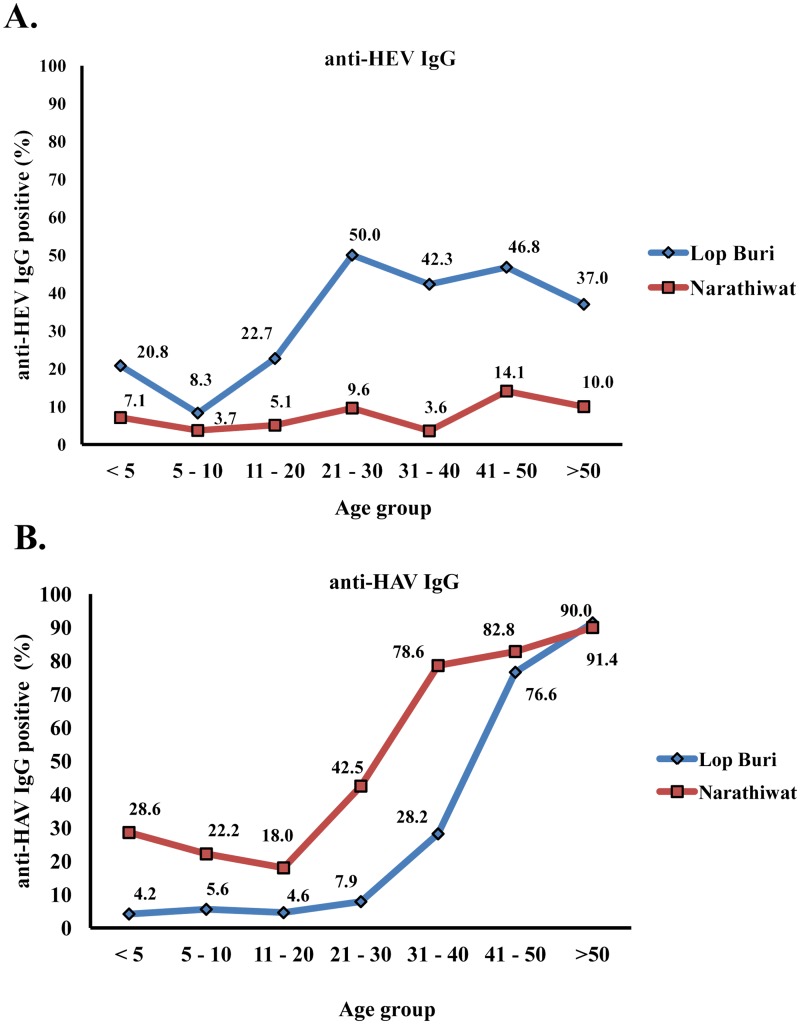
Fig 2. Distribution of seroprevalence for Lop Buri and Narathiwat by age group.
(A) anti-HEV IgG antibody and (B) anti-HAV IgG antibody prevalence in different age groups.
When individuals were categorized into 7 age groups, highest seroprevalence was observed among those between 21–30 in Lop Buri (50.0%) and 41–50 (14.1%) in Narathiwat. Age group with the lowest prevalence of anti-HEV IgG was 5–10 year-olds in Lop Buri (8.3%) and 31–40 year-olds in Narathiwat (3.6%). The overall anti-HEV titers among the different age groups were similar in both provinces (4.4±5.7 IU/mL in Lop Buri and 4.4±4.7 IU/mL in Narathiwat; p = 0.992).
Anti-HAV antibody prevalence and titers
For comparison, we also aimed to assess the seroprevalence of HAV, another food borne virus that is endemic in Thailand. We found that the overall anti-HAV antibody was 39.9% in Lop Buri and 58.0% in Narathiwat (p < 0.001). Seropositive rates were similar between men and women in both Lop Buri (41.1% versus 38.8%; p = 0.69) and in Narathiwat (58.7% versus 56.7%; p = 0.80). The mean age of individuals with positive anti-HAV antibody was significantly different between Lop Buri (47.1 ± 10.9) and Narathiwat (40.6 ± 14.5) (p < 0.001). Moreover, seropositive rate of anti-HAV IgG was consistently higher among Narathiwat residents in all age groups compared to residents of Lop Buri (Fig 2B). The presence of anti-HAV for both provinces was highest among individuals >50 years in which ≥ 90% tested positive.
The overall S/CO titers of anti-HAV IgG antibody in residents of both provinces were slightly different but not statistically significant (12.4±2.8 in Lop Buri and 13.0±3.4 in Narathiwat; p = 0.051). Furthermore, there were no significant differences in the anti-HAV titers among different age groups.
Discussion
HEV infection from porcine zoonosis has long been suspected of contributing to the entero-transmissible form of hepatitis similar to that caused by HAV [23]. In addition, occupational exposure to pigs has been linked to HEV infection [24]. In this study, the exposure risk to HEV was examined from a different perspective using religion as a surrogate for evaluating disease risk. We hypothesized that the likelihood of detecting HEV antibodies in individuals from two similarly sized regions may differ based on diet, religious and social norms. We found that although both HEV and HAV are foodborne, the seroprevalence of anti-HAV IgG was significantly higher in Narathiwat than in Lop Buri. In contrast, relatively low seroprevalence (3–14%) for anti-HEV IgG among individuals of different age groups in Narathiwat contrasted with higher seropositive rates (8–50%) found among Lop Buri residents.
By studying the prevalence of HEV and HAV, which share the same mode of transmission, one might expect to detect similar frequency of antibodies to these viruses among Thais. Both HAV and HEV are foodborne, why then was the presence of HAV antibodies more prevalent in individuals living in Narathiwat, yet HEV seroprevalence was significantly lower? One possible answer might be the zoonotic transmission of HEV through occupational contact with swine and/or pork consumption. Buddhists comprising the majority of residents in Lop Buri have no dietary restrictions of pork products and therefore do raise swine in the farm or work in pork-processing facilities. These individuals may be exposed to HEV as swine farmers, animal transporters, abattoir workers, pork handlers, or consumers. Moreover, this province is the largest pork producer in central Thailand. Of the 226 swine farms in Lop Buri, most are large facilities that process and distribute meat to neighboring provinces including pork sold in metropolitan Bangkok. These factors may therefore increase the exposure of HEV in the Lop Buri population. In contrast, most Narathiwat residents adhere to Islam, which proscribes the consumption of pork. Persons of Islamic faith also do not engage in activities involving swine raising, butchering, and selling of pork products. As a result, individuals in Narathiwat are at a decreased risk of HEV infection. It is interesting to note that contact with swine was a risk factor for Nipah virus infection in the predominantly Islamic Malaysia when researchers observed that infected individuals were all non-Muslims [25]. Finally, evidence from comparative studies between HEV sequences isolated in swine and human strongly support zoonotic transmission in Europe such as Belgium [26], France [14], and the UK [27].
Findings from this study reaffirmed evidence that the prevalence of HEV IgG antibodies were higher among older men than other groups [11, 17]. It is not known whether being older is a risk factor for HEV seroprevalence or that having lived through certain years during times of outbreak is a risk factor as was identified in England [28]. No particular patterns can be discerned from the levels of antibody titers against HAV or HEV to suggest recent viral infection in our cohorts. It is noteworthy that higher HAV and HEV seroprevalence observed among those <5 years old found in this study was not unexpected. Transplacental transfer of anti-HAV antibodies from mothers to infants may account for some of the higher seroprevalence in young children [29]. Furthermore, relatively high seroprevalence of anti-HAV antibody in Thailand among those ≤ 2 years old (between 3%–23% depending on the province surveyed) had previously been documented [30].
Immunity to HEV is not life-long as serum anti-HEV IgG does decline several years after initial infection [31]. The duration of IgG immunity can last as long as 12 years after acute infection, but reinfection has been documented and therefore immunity to HEV may be limited [11]. The waning of HEV IgG over an individual’s lifetime is consistent with our observed decreased in seroprevalence among those >50 years old, especially in the Lop Buri cohort.
Previous efforts to determine the burden of HEV infection in Thailand using HEV seroprevalence survey found 23.3% in occupational high-risk group and 6.5% in ethnic Hmong population in the northern Thailand [16, 32]. The overall seroprevalence of 37% found in this study was higher than the 9–22% [33] from a previous Thai study, which may be due to better detection sensitivity. It was also higher than the 14% from another study, which only surveyed Thai men between the ages 18–30 [34]. Globally, prevalence of anti-HEV IgG varies and previous studies conducted in the general population included mostly adults. For example, the overall HEV seroprevalence was 5.9% in Korea [24], 4.6%–6.7% in Japan [35], and 17% in Germany [36]. Among blood donors, the rate of HEV antibodies found were 4.9% in Switzerland [37], and 3.7% in Japan [38].
Detection of HAV antibodies generally indicate past infection and its presence is generally recognized as lifelong [8]. Prior to 1980, >97% of Thais ≥16 years old tested positive for anti-HAV [39]. Improved sanitation and hygiene over the past three decades, however, have helped to reduce exposure to HAV infection and thus HAV immunity in the general population. As a result, sporadic outbreaks in recent years have rendered young Thais vulnerable to HAV infection [40]. Our previous assessment of the overall anti-HAV seroprevalence in the Thai population was 27%, but it was as high as 71% among those residing in regions bordering Myanmar [41]. Similarly, higher HAV seroprevalence in Narathiwat may result from reduced access to better sanitation or exposure to unsafe drinking water or contaminated produce. Consistent with this observation is the many documented outbreaks of HAV in southern Thailand including Narathiwat [42–44].
Hepatitis E vaccine has been tested and is licensed for use in China [45]. Despite the availability of an effective HAV vaccine [46], universal HAV vaccination in the general Thai population is currently not recommended based on cost-effective analysis [47]. In addition, HAV vaccine is not included in the vaccination schedule of most EU countries [10].
There are several limitations in this study. There were fewer men than women and fewer residents from Narathiwat than from Lop Buri in this seroprevalence survey. The seroprevalence of individuals living in these two provinces may or may not reflect the immunity to either HAV or HEV in central and southern Thailand. Furthermore, information on the occupation or religion of individuals would have been valuable. We were unable to ascertain whether pre-existing antibodies surveyed in this study resulted from recent or distant infection or whether they were specific for particular genotypes, although substantial evidence suggests that HEV genotype 3 may be the predominant strain circulating in Thailand [48–53]. Since ELISA results may differ depending on the commercial source, definitive determination of past HEV exposure may benefit from confirmation via HEV-specific cell-mediated immunity with interferon-gamma ELISPOT assay [54].
The prevalence of HEV in pigs and sequence comparison with strains isolated in Thais will provide better understanding into transmission and viral co-evolution with the porcine and human hosts. Evidence-based knowledge should facilitate policy-making decisions towards the improvement of hygienic practices, infrastructure for agribusiness, and management of enteric viral infections in the region.
Acknowledgments
We would like to thank the staff of the Center of Excellence in Clinical Virology, Faculty of Medicine, Chulalongkorn University for their excellent technical and administrative assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Research University Project, Office of Higher Education Commission (WCU001-HR-57, WCU007-HR-57, and WCU-58-006-HR), the Research Chair Grant from NSTDA, Chulalongkorn University Centenary Academic Development Project (CU56-HR01), the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (RES560530093), the Outstanding Professor of the Thailand Research Fund (DPG5480002), Center of Excellence in Clinical Virology, Chulalongkorn University, King Chulalongkorn Memorial Hospital, Siam Cement Group, and MK Restaurant Company Limited. This research is also supported by the Rachadapisek Sompote Fund of Chulalongkorn University for postdoctoral fellowship to Pattaratida Sa-nguanmoo, Rujipat Wasitthankasem and Sompong Vongpunsawad. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Hepatitis A. Fact Sheet No. 328. 2014 June [cited 27 November 2014]. In WHO website [Internet]. Switzerland: WHO 2015 -. [about 5 screens]. Available: http://www.who.int/mediacentre/factsheets/fs328/en/.
- 2. Labrique AB, Sikder SS, Krain LJ, West KP Jr, Christian P, Rashid M, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18: 1401–1404. 10.3201/eid1809.120241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aggarwal R, Jameel S. Hepatitis E. Hepatology. 2011;54: 2218–2226. 10.1002/hep.24674 [DOI] [PubMed] [Google Scholar]
- 4. Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. 10.1016/j.jhep.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 5. Smith DB, Simmonds P. International Committee on Taxonomy of Viruses Hepeviridae Study Group, Jameel S, Emerson SU, Harrison Tj, et al. Consensus proposals for classification of the family Hepeviridae. J Gen Virol. 2014;95: 2223–2232. 10.1099/vir.0.068429-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costa-Mattioli M, Cristina J, Romero H, Perez-Bercof R, Casane D, Colina R, et al. Molecular evolution of hepatitis A virus: a new classification based on the complete VP1 protein. J Virol. 2002;76: 9516–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Hepatitis E. Fact Sheet No. 280. 2014 June [cited 27 November 2014]. In WHO website [Internet]. Switzerland: WHO 2015 -. [about 5 screens]. Available: http://www.who.int/mediacentre/factsheets/fs280/en/.
- 8. Koff RS. Hepatitis A. Lancet. 1998;351: 1643–1649. [DOI] [PubMed] [Google Scholar]
- 9. Wheeler C, Vogt TM, Armstrong GL, Vaughan G, Weltman A, Nainan OV, et al. An outbreak of hepatitis A associated with green onions. N Engl J Med. 2005;353: 890–897. [DOI] [PubMed] [Google Scholar]
- 10. Gossner CM, Severi E. Three simultaneous, food-borne, multi-country outbreaks of hepatitis A virus infection reported in EPIS-FWD in 2013: what does it mean for the European Union? Euro Surveill. 2014;19. pii: 20941. [DOI] [PubMed] [Google Scholar]
- 11. Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. 10.1016/S1473-3099(08)70255-X [DOI] [PubMed] [Google Scholar]
- 12. Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssière L, et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation. 2010;89: 353–360. 10.1097/TP.0b013e3181c4096c [DOI] [PubMed] [Google Scholar]
- 13. Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384: 1766–1773. 10.1016/S0140-6736(14)61034-5 [DOI] [PubMed] [Google Scholar]
- 14. Pavio N, Merbah T, Thébault A. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg Infect Dis. 2014;20: 1925–1927. 10.3201/eid2011.140891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang F, Li X, Li Z, Harrison TJ, Chong H, Qiao S, et al. Detection of HEV antigen as a novel marker for the diagnosis of hepatitis E. J Med Virol. 2006;78: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 16. Pourpongporn P, Samransurp K, Rojanasang P, Wiwattanakul S, Srisurapanon S. The prevalence of anti-hepatitis E in occupational risk groups. J Med Assoc Thai. 2009;92: S38–42. [PubMed] [Google Scholar]
- 17. Yoon Y, Jeong HS, Yun H, Lee H, Hwang YS, Park B, et al. Hepatitis E Virus (HEV) Seroprevalence in the general population of the Republic of Korea in 2007–2009: a nationwide cross-sectional study. BMC Infect Dis. 2014;14: 517 10.1186/1471-2334-14-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charoensook R, Knorr C, Brenig B, Gatphayak K. Thai pigs and cattle production, genetic diversity of livestock and strategies for preserving animal genetic resources. Maejo Int J Sci Technol. 2013;7: 113–132. [Google Scholar]
- 19.Lop Buri Governor’s Office. Provincial development plan (2014–2017); 2013. Preprint. Available: http://www.lopburi.go.th/plan_lopburi/plan_lop57-60.pdf. Accessed 27 November 2014. [Article in Thai]
- 20.Ministry of Agriculture and Cooperatives. Information of animal breeders Year 2013; 2013. Preprint. Available: http://ict.dld.go.th/th2/images/stories/planning/2557/report_summary2013.pdf. Accessed 27 November 2014. [Article in Thai]
- 21.Lopburi Provincial Livestock Office. Livestock database Year 2013; 2013. Preprint. Available: http://pvlo-lbr.dld.go.th/. Accessed 8 February 2015. [Article in Thai]
- 22.Narathiwat Provincial Livestock Office. Livestock database Year 2013. Preprint. Available: http://pvlo-naw.dld.go.th/. Accessed 8 February 2015. [Article in Thai]
- 23. Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94: 9860–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teo CG. Hepatitis E indigenous to economically developed countries: to what extent a zoonosis? Curr Opin Infect Dis. 2006;19: 460–466. [DOI] [PubMed] [Google Scholar]
- 25. Goh KJ, Tan CT, Chew NK, Tan PS, Kamarulzaman A, Sarji SA, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med. 2000;342: 1229–1235. [DOI] [PubMed] [Google Scholar]
- 26. Thiry D, Mauroy A, Saegerman C, Thomas I, Wautier M, Miry C, et al. Estimation of hepatitis E virus (HEV) pig seroprevalence using ELISA and Western blot and comparison between human and pig HEV sequences in Belgium. Vet Microbiol. 2014;172: 407–414. 10.1016/j.vetmic.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 27. Said B, Ijaz S, Chand MA, Kafatos G, Tedder R, Morgan D. Hepatitis E virus in England and Wales: indigenous infection is associated with the consumption of processed pork products. Epidemiol Infect. 2014;142: 1467–1475. 10.1017/S0950268813002318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ijaz S, Vyse AJ, Morgan D, Pebody RG, Tedder RS, Brown D. Indigenous hepatitis E virus infection in England: more common than it seems. J Clin Virol. 2009;44: 272–276. 10.1016/j.jcv.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 29. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immun. 2012; 2012:985646 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatproedprai S, Chongsrisawat V, Chatchatee P, Theamboonlers A, Yoocharoen P, Warinsathien P, et al. Declining trend in the seroprevalence of infection with hepatitis A virus in Thailand. Ann Trop Med Parasitol. 2007;101: 61–68. [DOI] [PubMed] [Google Scholar]
- 31. Lee SD, Wang YJ, Lu RH, Chan CY, Lo KJ, Moeckli R. Seroprevalence of antibody to hepatitis E virus among Chinese subjects in Taiwan. Hepatology. 1994;19: 866–870. 37 [PubMed] [Google Scholar]
- 32. Louisirirotchanakul S, Myint KS, Srimee B, Kanoksinsombat C, Khamboonruang C, Kunstadter P, et al. The prevalence of viral hepatitis among the Hmong people of northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33: 837–844. [PubMed] [Google Scholar]
- 33. Poovorawan Y, Theamboonlers A, Chumdermpadetsuk S, Komolmit P. Prevalence of hepatitis E virus infection in Thailand. Ann Trop Med Parasitol. 1996;90: 189–196. [DOI] [PubMed] [Google Scholar]
- 34. Gonwong S, Chuenchitra T, Khantapura P, Islam D, Sirisopana N, Mason CJ. Pork consumption and seroprevalence of hepatitis E virus, Thailand, 2007–2008. Emerg Infect Dis. 2014;20: 1531–1534. 10.3201/eid2009.140418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanaka E, Takeda N, Tian-Chen L, Orii K, Ichijo T, Matsumoto A, et al. Seroepidemiological study of hepatitis E virus infection in Japan using a newly developed antibody assay. J Gastroenterol. 2001;36: 317–321. [DOI] [PubMed] [Google Scholar]
- 36. Faber MS, Wenzel JJ, Jilg W, Thamm M, Höhle M, Stark K. Hepatitis E virus seroprevalence among adults, Germany. Emerg Infect Dis. 2012;18: 1654–1657. 10.3201/eid1810.111756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaufmann A, Kenfak-Foguena A, André C, Canellini G, Bürgisser P, Moradpour D, et al. Hepatitis E virus seroprevalence among blood donors in southwest Switzerland. PLoS One. 2011;6: e21150 10.1371/journal.pone.0021150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fukuda S, Sunaga J, Saito N, Fujimura K, Itoh Y, Sasaki M, et al. Prevalence of antibodies to hepatitis E virus among Japanese blood donors: identification of three blood donors infected with a genotype 3 hepatitis E virus. J Med Virol. 2004;73: 554–561. [DOI] [PubMed] [Google Scholar]
- 39. Burke DS, Snitbhan R, Johnson DE, Scott RM. Age-specific prevalence of hepatitis A virus antibody in Thailand. Am J Epidemiol. 1981;113: 245–249. [DOI] [PubMed] [Google Scholar]
- 40. Poovorawan K, Chattakul P, Chattakul S, Thongmee T, Theamboonlers A, Komolmit P, et al. The important role of early diagnosis and preventive management during a large-scale outbreak of hepatitis A in Thailand. Pathog Glob Health. 2013;107: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rianthavorn P, Fakthongyoo A, Yamsut S, Theamboonlers, Poovorawan Y. Seroprevalence of hepatitis A among Thai population residing near Myanmar border. J Health Popul Nutr. 2011;29: 174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Theamboonlers A, Jantaradsamee P, Chatchatee P, Chongsrisawat V, Mokmula M, Poovorawan Y. Ann Trop Med Parasitol. 2002;96:727–734. [DOI] [PubMed] [Google Scholar]
- 43. Poovorawan Y, Theamboonlers A, Chongsrisawat V, Jantaradsamee P, Chutsirimongkol S, Tangkijvanich P. Clinical features and molecular characterization of hepatitis A virus outbreak in a child care center in Thailand. J Clin Virol. 2005;32:24–28. [DOI] [PubMed] [Google Scholar]
- 44. Wattanasri N, Ruchusatsawat K, Wattanasri S. Phylogenetic analysis of hepatitis A virus in Thailand. J Med Virol. 2005;75:1–7. [DOI] [PubMed] [Google Scholar]
- 45. Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376: 895–902. 10.1016/S0140-6736(10)61030-6 [DOI] [PubMed] [Google Scholar]
- 46. Poovorawan Y, Theamboonlers A, Safary A. Single-dose hepatitis A vaccination: comparison of different dose levels in adolescents. Vaccine. 1996;14: 1092–1094. [DOI] [PubMed] [Google Scholar]
- 47. Teppakdee A, Tangwitoon A, Khemasuwan D, Tangdhanakanond K, Suramaethakul N, Sriratanaban J, et al. Cost-benefit analysis of hepatitis a vaccination in Thailand. Southeast Asian J Trop Med Public Health. 2002;33: 118–127. [PubMed] [Google Scholar]
- 48. Keawcharoen J, Thongmee T, Panyathong R, Joiphaeng P, Tuanthap S, Oraveerakul K, et al. Hepatitis E virus genotype 3f sequences from pigs in Thailand, 2011–2012. Virus Genes. 2013;46: 369–370. 10.1007/s11262-012-0853-3 [DOI] [PubMed] [Google Scholar]
- 49. Cooper K, Huang FF, Batista L, Rayo CD, Bezanilla JC, Toth TE, et al. Identification of genotype 3 hepatitis E virus (HEV) in serum and fecal samples from pigs in Thailand and Mexico, where genotype 1 and 2 HEV strains are prevalent in the respective human populations. J Clin Mircobiol. 2005;43:1684–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siripanyaphinyo U, Laohasinnarong D, Siripanee J, Kaeoket K, Kameoka M, Ikuta K, et al. Full-length sequence of genotype 3 hepatitis E virus derived from a pig in Thailand. J Med Virol. 2009;81:657–664. 10.1002/jmv.21428 [DOI] [PubMed] [Google Scholar]
- 51. Suwannakarn K, Tongmee C, Theamboonlers A, Komolmit P, Poovorawan Y. Swine as the possible source of hepatitis E virus transmission to humans in Thailand. Arch Virol. 2010;155:1697–1699. 10.1007/s00705-010-0751-8 [DOI] [PubMed] [Google Scholar]
- 52. Wiratsudakul A, Sariya L, Prompiram P, Tantawet S, Suraruangchai D, Sedwisai P, et al. Detection and phylogenetic characterization of hepatitis E virus genotype 3 in a captive wild boar in Thailand. J Zoo Wildl Med. 2012;43:640–644. [DOI] [PubMed] [Google Scholar]
- 53. Hinjoy S, Nelson KE, Gibbons RV, Jarman RG, Chinnawirotpisan P, Fernandez S, et al. A cross-sectional study of hepatitis E virus infection in pigs in different-sized farms in northern Thailand. Foodborne Pathog Dis. 2013;10:698–704. 10.1089/fpd.2012.1369 [DOI] [PubMed] [Google Scholar]
- 54. Shata MT, Barrett A, Shire NJ, Abdelwahab SF, Sobhy M, Daef E, et al. Characterization of hepatitis E-specific cell-mediated immune response using IFN-gamma ELISPOT assay. J Immunol Methods. 2007;328: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.