Abstract
Introduction
Asthma affects 30 million people in Western Europe, leading to substantial burden on healthcare systems and economies. REcognise Asthma and LInk to Symptoms and Experience (REALISE™) was a large European survey across 11 countries assessing patient attitudes and behaviors towards their asthma. The present study utilizes REALISE™ data to understand resource use and absenteeism in asthma.
Methods
Data were collected on absenteeism and healthcare resource use from 8000 asthma patients (aged 18–50 years) across the 11 countries. All data were patient reported. Odds ratios (ORs) were calculated against the country with the lowest proportion of respondents for hospitalization (as a proxy for lowest resource use).
Results
Patient characteristics were broadly similar across countries. However, self-reported asthma control status varied. More than 50% of respondents in most countries considered primary healthcare professionals (HCPs), i.e., general practitioners and nurses, the main HCP they see about their asthma. However, in some countries, specialists or nurses were considered the main HCP. Hospitalization was lowest amongst patients in the Netherlands. Resource use and productivity loss varied widely across the countries; ORs for hospitalization ranged from 1 in Sweden to 4 in Norway and for productivity loss from 0.6 in Sweden to 2.6 in Italy, compared with the Netherlands.
Conclusion
This study quantified utilization of healthcare resources in asthma (number of visits of HCPs, hospitalization, and accident and emergency visits) as well as absenteeism and showed that differences exist across countries. The differences in primary care and specialist use suggest a possible difference in healthcare delivery across countries.
Funding
Mundipharma International Limited, Cambridge, UK.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-015-0204-6) contains supplementary material, which is available to authorized users.
Keywords: Asthma, Patient-reported, Productivity, Resource use
Introduction
Asthma is a common chronic disease of the respiratory system affecting approximately 30 million people in Western Europe [1] and is a serious public health issue. Both direct and indirect per patient costs associated with asthma are high [2, 3] and total asthma care costs are estimated at £18 billion per year in Europe [4]. Resource use due to asthma varies across several European countries but is generally high [2, 4, 5]. For example, in France and Spain, estimated per patient costs for a 3-month period were up to €537.9 and €556.8, respectively [2]. Furthermore, absence from work or education due to asthma is also common [2, 5, 6]. In France and Spain, estimated indirect costs accounted for up to 63% and 59% of total costs, respectively [2].
Patients with asthma may achieve various levels of control of their asthma, depending on several factors, which may include severity, exposure to triggers, treatments and patient adherence to treatment. The Global Initiative for Asthma (GINA) has developed recommendations to help establish local guidelines that inform and support health professionals’ assessment and management of asthma patients. The GINA report defines and categorizes asthma control levels as ‘well controlled’, ‘partly controlled’, and ‘uncontrolled’ based on daytime symptoms, normal activities affected by symptoms, nighttime awakenings, and reliever inhaler use [7]. The recommendations are that management and treatment should be driven by these control levels [7]. However, despite GINA recommendations and existing local guidelines, there are no standard management algorithms or practices for asthma patients across Europe.
The REcognise Asthma and LInk to Symptoms and Experience (REALISE™) survey investigated asthma control and evaluated patient perception of control and attitudes to asthma in a large European Union-based population. The data were collected from adult patients with asthma requiring treatment across 11 European countries and included 8000 individuals [8]. In addition, the survey also collected data on patient utilization of healthcare resources and time off work, providing a unique opportunity to assess the resource burden and lost productivity associated with asthma.
Using results from the REALISE™ study, the objective of this research was to understand the burden of asthma by reviewing resource use [number of visits of healthcare professionals (HCPs), hospitalization, and accident and emergency (A&E) visits] and lost productivity (absence from work or education) in 11 European countries.
Methods
Survey
The REALISE™ survey is a quantitative online survey conducted in 11 European countries (Austria, Belgium, Finland, France, Germany, Italy, the Netherlands, Norway, Spain, Sweden, and the UK) between July and October 2012. Full details of the survey design are published elsewhere [8]. The survey population was drawn from validated online consumer panels that met International Organization for Standardization 20252 quality standards; multiple panels were used to reduce potential bias. Respondents who had participated in market research surveys within the previous 3 months were excluded. Eligible respondents were aged 18–50 years, had clinically diagnosed asthma, at least two prescriptions for asthma in the previous 2 years, and previously used social media. The target survey population was 8000; additional respondents were not recruited once this number was achieved. Each respondent received an incentive for participating in the research.
The survey collected data on asthma control, asthma management, resource use (e.g., number of hospitalizations) and work productivity (absence from work/education). All data, including confirmation of asthma diagnosis, were patient-reported. Respondents were asked whether they had an asthma diagnosis from a doctor and whether they had two or more prescriptions for their asthma in the past 2 years. Asthma control was assessed using the four GINA criteria: daytime symptoms, nighttime symptoms, limitations of daily activities, and need of reliever inhaler.
This article does not contain any new studies with human or animal subjects performed by any of the authors. Data were managed in accordance with the Data Protection Act (UK, 1998).
Data Analysis
Responses relating to demographics and smoking status, asthma severity, resource use, and absenteeism are reported and summary tables produced. Continuous variables are presented as means. Categorical variables are presented as counts and proportions. When calculating means in situations where the response options were categorical, counts of ≥5 or ≥10 (as an answer option) were assumed to be 5 or 10, respectively (i.e., the lowest value in the range). Respondents were asked which HCP they visited most frequently about their asthma; respondents who answered “I do not see a healthcare professional about my asthma” were excluded from answering the next question of how many times in the past year the respondent had visited the HCP. Answers of “less than once a year” were assumed to be zero during the previous 12 months.
Resource use was measured by quantifying how many times a patient reported seeing their main HCP for their asthma or being treated in an A&E department in the previous year. In addition, the number of hospitalizations with an overnight stay relating to asthma during the previous year was reported by each respondent. Number of days of hospitalization was not reported. Productivity loss was measured in days off work or education. To calculate relative resource use and productivity, odds ratios (ORs) were calculated against the country with the lowest proportion of respondents for hospitalization (as a proxy for lowest resource use). The OR was calculated by dividing the country ratio (respondents using resource versus respondents not using resource) by the reference country ratio (respondents using resource versus respondents not using resource) across all countries.
Productivity and resource use by type of HCP were further analyzed in cross-tabulations. Analyses were performed using SPSS version 9.0 (SPSS, Inc., Chicago, IL, USA). As there were no a priori hypotheses, and to control for type I family-wise error rate (the probability of at least 1 incorrect rejection of null), effect sizing was determined using the false discovery rate method [9]. The proportion of errors among those tests whose null hypotheses were rejected was set at 5% and P values were calculated to determine statistical significance.
Results
Survey Population and Respondent Characteristics
The analysis included 8000 respondents aged 18–50 years. France, Spain, Italy, Germany, and the UK reported the largest samples (N ≥ 1000). Table 1 shows the respondents’ mean age, sex, and smoking status as well as self-reported clinical factors (e.g., controlled GINA status, asthma exacerbation), and ownership and use of preventer inhaler by country.
Table 1.
Respondents’ characteristics by country
| Characteristic | Respondent % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUT (N = 468) | BEL (N = 303) | FIN (N = 473) | FRA (N = 1024) | DEU (N = 1000) | ITA (N = 1014) | NLD (N = 855) | NOR (N = 240) | ESP (N = 1020) | SWE (N = 603) | UK (N = 1000) | |
| Mean age (years) | 33.7 | 33.9 | 32.9 | 34.8 | 34.3 | 35.4 | 36.1 | 32.9 | 34.1 | 36.5 | 34.2 |
| Female (%) | 56 | 57 | 59 | 65 | 57 | 59 | 72 | 57 | 55 | 61 | 70.5 |
| Current smokers (%) | 26 | 23 | 27 | 26 | 24 | 17 | 27 | 23 | 21 | 17 | 21 |
| Controlled GINA status | 28 | 16 | 24 | 17 | 15 | 17 | 18 | 21 | 21 | 26 | 25 |
| Respondents who required at least one course of steroid tablets in last year due to asthma exacerbationa | 28 | 39 | 32 | 60 | 41 | 59 | 37 | 37 | 53 | 31 | 34 |
| Respondents who required two or more courses of steroid tablets in last year due to asthma exacerbationa | 19 | 25 | 19 | 44 | 32 | 45 | 25 | 31 | 41 | 19 | 20 |
| Patients who have a preventer inhaler | 39 | 34 | 55 | 46 | 40 | 28 | 45 | 44 | 38 | 59 | 55 |
| Respondents who use their preventer inhaler every dayb | 50 | 46 | 43 | 44 | 61 | 40 | 61 | 42 | 25 | 51 | 51 |
All characteristics were self-reported by the respondents. Base N = 8000
AUT Austria, BEL Belgium, DEU Germany, ESP Spain, FIN Finland, FRA France, GINA Global Initiative for Asthma, ITA Italy, NOR Norway, NLD The Netherlands, SWE Sweden, UK United Kingdom
aProxy for asthma exacerbation
bOnly respondents who have a preventer inhaler answered this question
The mean age range was 32–37 years and between 17% and 26% of respondents were current smokers: patient demographics were broadly similar across countries and statistically significant differences were reported in only a few instances, e.g., respondents in the Netherlands were older than in other countries. The proportion of female respondents was higher in the Netherlands and the UK compared to other countries in the study. The most commonly reported comorbidities were depression and high blood pressure/hypertension, reported by up to 30% of respondents in some countries. Chronic obstructive pulmonary disease, cancer, heart disease, diabetes, and rheumatoid arthritis were reported by less than 10% of respondents in most countries.
On average, 20% of respondents in this study had a controlled GINA status (self-reported), this ranged from 15% in Germany to 28% in Austria. Self-reported asthma exacerbation, indicated by the requirement of at least one course of steroid tablets in the previous 12 months, was highest among respondents in Italy compared with respondents in other countries. In each country in the study, at least one in four respondents possessed a preventer inhaler. Of these respondents, daily use of preventer inhaler was highest in the Netherlands (61%) and Germany (61%) and lowest in Spain (25%). Additionally, between 19% (UK) and 32% (Spain) of respondents owned a combination inhaler (i.e., combination inhaled corticosteroid/long-acting beta agonist) and between 7% (UK) and 30% (Belgium) of respondents took oral treatment to help manage their asthma.
HCPs That Respondents Would Consult as the Main HCP for Their Asthma
In all countries, except for Austria, most respondents considered general practitioners (GPs) to be the main HCP they would consult about their asthma (Fig. 1). The UK was unique among the respondent sample, in that over 25% of respondents considered a nurse to be their main asthma HCP and fewer respondents (4%) would consider seeing a specialist. In contrast, at least 10% of respondents in all other countries considered a specialist as the main HCP they would consult about their asthma; this was highest in Austria (50%) followed by Germany (42%), Spain (37%), and Italy (35%).
Fig. 1.
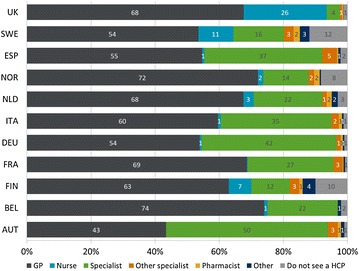
Main healthcare professional respondents consulted about their asthma, by country. Patients were asked: “Which healthcare professional would you consider to be the main person who you see about your asthma?” Base: N = 8000, numbers shown are percentages. AUT Austria, BEL Belgium, FIN Finland, FRA France, DEU Germany, GP general practitioner, HCP healthcare professional, ITA Italy, NLD The Netherlands, NOR Norway, ESP Spain, SWE Sweden, UK United Kingdom
Self-Reported Resource Use and Absence From Work or Education Amongst the Respondents in Different Countries
Table 2 reports resource use and absenteeism by country, relative to the Netherlands (that had the lowest resource use as measured by proxy indicator of hospitalization).
Table 2.
Odds ratio and proportions (%) of respondents that reported utilization of healthcare resource or taking at least 1 day off work or education during the previous 12 months by country
| Country | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUT (N = 468) | BEL (N = 303) | FIN (N = 473) | FRA (N = 1024) | DEU (N = 1000) | ITA (N = 1014) | NLD (N = 855) | NOR (N = 240) | ESP (N = 1020) | SWE (N = 603) | UK (N = 1000) | |
| HCP visitsa OR [95% CI] |
1.7 [0.99, 2.87] |
1.5 [0.75, 2.76] |
0.9 [0.28, 3.1] |
2 [1.23, 3.1] |
3.7 [2.23, 5.96] |
2.5 [1.59, 3.8] |
1 [0.57, 1.76] |
0.9 [0.39, 1.98] |
1.7 [1.08, 2.82] |
0.4 [0.19, 0.73] |
1.4 [0.84, 2.39] |
| HCP visitsa (%) | 80 | 78 | 69 | 83 | 90 | 86 | 71 | 68 | 81 | 48 | 77.5 |
| Hospitalization OR [95% CI] |
1.6 [1.03, 2.34] |
1.3 [0.79, 2.1] |
1.8 [1.21, 2.67] |
2 [1.41, 2.73] |
2.7 [1.96, 3.71] |
2.8 [2.06, 3.89] |
1 [0.68, 1.47] |
4 [2.62, 5.94] |
2.1 [1.52, 2.92] |
1 [0.67, 1.54] |
1.2 [0.87, 1.77] |
| Hospitalization (%) | 10 | 8 | 11 | 12 | 16 | 17 | 6.5 | 22 | 13 | 7 | 8 |
| A&E visits OR [95% CI] |
1.5 [1.08, 2.05] |
1.2 [0.78, 1.7] |
3.9 [2.91, 5.12] |
1.5 [1.14, 1.93] |
3.9 [3.04, 4.98] |
3.3 [2.56, 4.2] |
1 [0.74, 1.34] |
3.6 [2.58, 5.11] |
4.3 [3.35, 5.47] |
2.1 [1.61, 2.84] |
1.2 [0.92, 1.59] |
| A&E visits (%) | 16.5 | 29.5 | 34 | 16 | 34 | 30 | 12 | 32.5 | 36 | 22 | 14 |
| Absence from work/education OR [95% CI] |
0.7 [0.51, 0.89] |
0.8 [0.55, 1.06] |
0.9 [0.65, 1.11] |
0.9 [0.74, 1.14] |
1.2 [0.98, 1.51] |
2.6 [2.07, 3.18] |
1 [0.79, 1.27] |
1.8 [1.26, 2.47] |
1.3 [1.02, 1.58] |
0.6 [0.44, 0.76] |
0.7 [0.56, 0.88] |
| Absence from work/education (%) | 27 | 30 | 32 | 34 | 40 | 59 | 36 | 49 | 41 | 24 | 28 |
The proportion of respondents that reported utilization of healthcare resource or taking at least one day off work or education during the previous 12 months was calculated for each country. ORs were calculated from the proportions in relation to the Netherlands (the country with the lowest proportion of respondents for hospitalization—a proxy for lowest resource use) by dividing the country ratio (respondents using resource versus respondents not using resource) by the reference country ratio (respondents using resource versus respondents not using resource across all countries). OR and 95% CI are reported
A&E accident and emergency, AUT Austria, BEL Belgium, CI confidence interval, DEU Germany, ESP Spain, FIN Finland, FRA France, HCP healthcare professional, ITA Italy, NLD The Netherlands, NOR Norway, OR odds ratio, SWE Sweden, UK United Kingdom
aOnly respondents who reported a main HCP answered this question
Proportionally fewer respondents in Sweden and Finland reported visiting their main HCP in the previous 12 months compared with those in the Netherlands, while visits to the main HCP were up to 3.7 times (Germany) more likely in other countries. Hospitalization was up to four times more likely in Norway and A&E visits were up four times more likely in Norway, Finland, Germany, and Spain compared with the Netherlands. Several countries including Sweden and the UK reported less absenteeism compared with the Netherlands; only Germany, Spain, Italy, and Norway reported more.
Resource Use and Absence From Work or Education by HCP
Overall, 17% of respondents who considered a specialist as their main asthma HCP reported being hospitalized at least once in the previous 12 months, compared with 10% of respondents who considered a GP and 10% who considered a nurse their main asthma HCP (P ≤ 0.05; Fig. 2). Respondents who considered a specialist their main asthma HCP (rather than a GP or a nurse) were more likely to be treated at A&E at least once in the previous 12 months (32% specialist, 21% GP, 17% nurse, P ≤ 0.05). Additionally, these respondents also take ≥1 day off work or education (38% specialist, 30% GP, 22% nurse, P ≤ 0.05; Fig. 2).
Fig. 2.
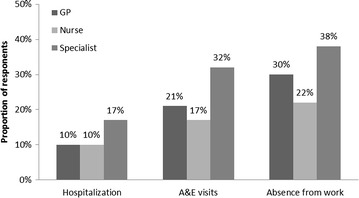
Proportions of respondents that reported being treated in A&E, hospitalized or taking time off work or education during the previous 12 months, categorized by patient feedback on their main healthcare professional. A&E accident and emergency, GP general practitioner
Discussion
This study used the results of the REALISE™ study to quantify some key healthcare resource use components (number of visits of HCPs, hospitalization, and A&E visits) across 11 European countries and showed that utilization varied widely. Primary care plays a pivotal part in the management of asthma in all countries with at least three in five respondents considering a GP or a nurse their main asthma HCP. However, the use of acute services as indicated by hospitalization or A&E events was up to four times higher across other countries in Europe compared to the Netherlands. These results indicate that there might be a higher burden on secondary healthcare in these countries, or that primary care needs further investment and development to avoid the need for patients to go to A&E.
The heterogeneity of HCPs consulted by patients reported in the REALISE™ study could be due to country-specific asthma policies or overall differences in healthcare system structures. In the UK, Sweden, and Finland for example, asthma nurses are an integral part of the disease management [10–12], whereas for example in Germany, asthma nurses do not form part of the management plan [13]. If there is no negative impact on patients’ asthma control, consulting GPs or nurses more frequently than specialists could indicate overall lower costs to healthcare systems. However, whether such a ‘gatekeeper’ approach is more appropriate for health systems compared to having the direct access to specialists is unclear and is the subject of much debate in the literature [14].
In addition, in Germany many patients are treated by specialists as German treatment guidelines stipulate that patients should be treated by specialists in cases where: asthma is uncontrolled, long-term treatment with oral corticosteroids is necessary, patient underwent previous emergency treatment, comorbidities exist, suspicion of work-related asthma exists, or treatment with a specific regimen is indicated [13]. In Germany, 42% of respondents considered a specialist their main HCP they would consult about their asthma. This further underpins our findings supporting the validity of our sample frame.
The REALISE™ survey was not specifically designed to assess healthcare burden in the different countries. However, numbers of hospitalizations per year were comparable to the European National Health and Wellness Survey which had a similar study design (countries included were France, Germany, Italy, Spain, and the UK; study design included self-administered, web-based questionnaires for asthma patients aged ≥18 years who were identified through an internet-based consumer panel with a sampling frame that reflected the quotas based on sex and age demographic distribution of each country) [5]. In the REALISE™ study, number of hospitalizations per patient annually ranged from 0.12 in the Netherlands to 0.85 in Norway compared with a mean of 0.14–0.31 for “at least well controlled” and “not well controlled” patients across the five countries in the European National Health and Wellness Survey [5]. However, proportions of respondents who reported incidences of A&E visits and hospitalizations due to asthma in the REALISE™ study were larger than in cost-of-illness studies conducted in Italy, France, and Spain [2, 15]. However, the latter study only included asthma patients treated by GPs [2], while the Italian study was conducted prior to 2000, therefore comparability between studies is limited [15].
Proportions of respondents reporting hospitalization, A&E visits and absence from work varied across the countries. Similar to previous reports [16–18] patient-reported asthma control was low, but resource use and work absence appeared to be higher in those countries with low control. This could be due to differences in asthma control levels: in Germany, resource use was high but the proportion of respondents reporting controlled asthma status was low relative to other countries.
In addition, overall resource use was higher for respondents who considered a specialist their main asthma HCP. It is likely that these respondents have more severe asthma than respondents who consult a GP or nurse. However, due to the study design and analysis plan, we were unable to adjust for any possible contributing factors such as the level of asthma control or severity in this analysis and recommend that future studies incorporate stratified analyses or regression techniques in order to address possible confounding. This would allow the research to identify the drivers for higher resource use which could be the level of asthma control as has been previously suggested [2, 15]. Patient demographics, such as age and gender distribution were similar across most countries and therefore adjustments were not incorporated in the between country analyses.
The REALISE™ study represents one of the largest surveys of asthma patients thus far. However, there are a number of limitations to be considered. Respondents were not randomized and only patients aged 18–50 years who used social media participated in the study; therefore, this sample might not fully represent the wider patient population. In addition, the survey was conducted online in a sample population that uses social media and as such may be biased towards this sample of patients. However, there is no evidence to suggest that these groups of people are more or less likely to respond and have any different asthma status or control. Respondents’ data were based on self-reported answers without clinical verification, therefore inaccurate response or recall bias cannot be excluded. In addition, respondents only reported how often they visited their main HCP; however, visits to HCPs not considered their main HCP were not recorded. The large sample should account for country heterogeneity limiting over-representation of specific patient-types. The respondent characteristics, proportion of smokers, and proportion of females, broadly matched that in previous European studies; only the mean age was lower which might be due to the age range set by the inclusion criteria and the restriction to patients that use social media [2, 3, 6, 18]. The REALISE™ study did not capture the purpose of visits to a GP, nurse, or specialist therefore routine and review visits as well as urgent visits might be included.
Conclusions
The present analysis of the REALISE™ data suggests differences in asthma control status, HCP, and resource use across 11 European countries. More research will be required to validate these results in order to enable comparison between the health care systems in the different countries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship for this study and article processing charges were funded by Mundipharma International Limited (Cambridge, UK). All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. We thank Emily Taylor, Incite Marketing Planning Limited (London, UK) for assistance in data analysis and Caoimhe McKerr (Adelphi Values, Bollington, UK) for editorial assistance. Support for this assistance was funded by Mundipharma International Limited.
Conflict of interest
Monica Fletcher has received reimbursement for travel and accommodation from Almirall UK and Novartis AG. Education for Health received honoraria from Almirall, Boehringer, Ingelheim, Novartis AG and Novartis UK for advisory boards attended by Monica Fletcher. It also received educational grants from Almirall, Chiesi and TEVA, and grants for research in the last 5 years from Abbott Laboratories, AstraZeneca, Novartis AG, Pfizer and UCB. Ashok Jha is an employee of Mundipharma International. William Dunlop is an employee of Mundipharma International. Louise Heron is an employee of Adelphi Values. Verena Wolfram is an employee of Adelphi Values. Adelphi Values received funding from Mundipharma International. Louise Heron and Verena Wolfram declare that they have no conflict of interest. Thys Van der Molen has acted as a consultant and board member for Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma, Novartis and Teva, and as a consultant for MSD. His research department at the University of Groningen has received grants and support for research in respiratory disease from the following organizations in the past 5 years: AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Lung Foundation Netherlands, Merck, Mundipharma, Novartis, Nycomed and Stichting Astma Bestrijding. He has received honoraria for lectures/speaking from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Merck, Mundipharma, Novartis and Teva and payment for travel/accommodation/meeting expenses from Boehringer Ingelheim, Mundipharma, Novartis and Teva. David Price has acted as a consultant and board member for Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda Pharma, Merck, Mundipharma, Napp, Novartis, Nycomed, Pfizer, Sandoz and Teva. He or his research team has received grants and support for research in respiratory disease from the following organizations in the previous 5 years: Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Novartis, Nycomed, Orion, Pfizer, Takeda, Teva and the UK National Health Service. He has received honoraria for lectures/presentations from Activaero, Almirral, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Novartis, Merck, Mundipharma, Pfizer, Takeda and Teva and for manuscript preparation from Merck, Mundipharma and Teva. He owns stock/stock options in AKL Limited, Research in Real Life and its subsidiary social enterprise Optimum Patient Care. He has received payment for travel/accommodation/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors. Data were managed in accordance with the Data Protection Act (UK, 1998).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4s–12s. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.Doz M, Chouaid C, Com-Ruelle L, et al. The association between asthma control, health care costs, and quality of life in France and Spain. BMC Pulm Med. 2013;13:15. doi: 10.1186/1471-2466-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vervloet D, Williams A, Lloyd A, Clark T. Costs of managing asthma as defined by a derived Asthma Control TestTM score in seven European countries. Eur Respir Rev. 2006;15(98):17–23. doi: 10.1183/09059180.06.00009803. [DOI] [Google Scholar]
- 4.European Respiratory Society, European Lung White Book. http://www.erswhitebook.org/about/. Accessed July 22, 2014.
- 5.Demoly P, Annunziata K, Gubba E, Adamek L. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur Respir Rev. 2012;21(123):66–74. doi: 10.1183/09059180.00008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dal Negro RW, Micheletto C, Tosatto R, Dionisi M, Turco P, Donner CF. Costs of asthma in Italy: results of the SIRIO (Social Impact of Respiratory Integrated Outcomes) study. Respir Med. 2007;101(12):2511–2519. doi: 10.1016/j.rmed.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Asthma GIf. GINA Report, Global strategy for asthma management and prevention, 2012. 2014.
- 8.Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 10.Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61(8):663–670. doi: 10.1136/thx.2005.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindberg M, Ahlner J, Ekstrom T, Jonsson D, Moller M. Asthma nurse practice improves outcomes and reduces costs in primary health care. Scand J Caring Sci. 2002;16(1):73–78. doi: 10.1046/j.1471-6712.2002.00054.x. [DOI] [PubMed] [Google Scholar]
- 12.Network TBTSSIG. British guideline on the management of asthma. 2008. http://www.sign.ac.uk/pdf/qrg101.pdf. Accessed July 23, 2014.
- 13.Bundesärztekammer, Kassenärztliche Bundesvereinigung, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, Nationale VersorgungsLeitlinie Asthma. 2013. http://www.awmf.org/uploads/tx_szleitlinien/nvl-002k_S3_Asthma_2013-09.pdf. Accessed July 20, 2014.
- 14.Holland W. Tackling assessment of the performance of health services. Eurohealth. 2007;13(4):1–3. [Google Scholar]
- 15.Accordini S, Corsico AG, Braggion M, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160(1):93–101. doi: 10.1159/000338998. [DOI] [PubMed] [Google Scholar]
- 16.Cazzoletti L, Marcon A, Janson C, et al. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol. 2007;120(6):1360–1367. doi: 10.1016/j.jaci.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeire PA, Rabe KF, Soriano JB, Maier WC. Asthma control and differences in management practices across seven European countries. Respir Med. 2002;96(3):142–149. doi: 10.1053/rmed.2001.1241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


