Supplemental Digital Content is available in the text.
Keywords: chlorogenic acid, extracellular signal-regulated kinases, 5-fluorouracil, reactive oxygen species, synergism
Abstract
There is an urgent need to search for novel chemosensitizers in the field of cancer therapy. Chlorogenic acid (CGA), a type of polyphenol present in the diet, has many biological activities. The present study is designed to explore the influence of CGA on the effects of 5-fluorouracil (5-FU) on human hepatocellular carcinoma cells (HepG2 and Hep3B). Treatment with 5-FU induced the inhibition of hepatocellular carcinoma cells’ proliferation, and the combined treatment with CGA enhanced this inhibition. 5-FU also increased the production of reactive oxygen species (ROS). The combination of 5-FU and CGA led to a more prominent production of ROS and significantly inactivated ERK1/2, although CGA and 5-FU exerted no significant changes when used alone. A previous report has shown that ROS are upstream mediators that inactivate ERK in hepatocellular carcinoma cells. Combined with our results, this indicates that the combination of 5-FU and CGA leads to the inactivation of ERK through the overproduction of ROS. This mediates the enhancement of 5-FU-induced inhibition of hepatocellular carcinoma cells’ proliferation, that is, CGA sensitizes hepatocellular carcinoma cells to 5-FU treatment by the suppression of ERK activation through the overproduction of ROS. CGA has shown potential as a chemosensitizer of 5-FU chemotherapy in hepatocellular carcinoma.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers, is associated with the third highest cancer-related mortality rate worldwide, and accounts for 695 900 deaths per year. Half of these deaths are estimated to occur in China 1. Transcatheter arterial chemoembolization and regional hepatic arterial infusion chemotherapy are the two most important therapeutic strategies for HCC 2–4. 5-Fluorouracil (5-FU) is the most commonly used chemotherapeutic drug that is used in transcatheter arterial chemoembolization and hepatic arterial infusion chemotherapy to inhibit the growth of HCC cells. 5-FU is an analogue of uracil with a fluorine atom instead of a hydrogen atom at the C-5 position. The mechanism of cytotoxicity of 5-FU has been considered to involve the misincorporation of its metabolites into RNA and DNA and the inhibition of the thymidylate synthase. Unfortunately, cancer cells often develop chemoresistance to this drug 5. Combination chemotherapy has been considered a superior treatment strategy that offers the potential for enhanced efficacy 6. Considerable effort is needed toward the development of new adjuvant therapies that can enhance chemotherapeutic efficacy.
Chlorogenic acid (CGA), one of the most abundant polyphenol compounds in the human diet, is an ester in which the acid part of caffeic acid is bound to the hydroxyl group at position 5′ of quinic acid (5′-caffeoylquinic acid). Coffee is the major dietary source of CGA, the amount of which ranges from 70 to 350 mg in a 200 ml cup of coffee 7. Epidemiological studies have shown that CGA has many biological properties, including antioxidant, anti-inflammatory, antiviral, and anticancer activities, and may be responsible for the reduced risk of some chronic diseases 8–13. Our previous study also showed that CGA could protect against carbon tetrachloride-induced liver fibrosis through its anti-inflammatory action by the toll-like receptor4 (TLR4)/myeloid differentiation factor88 (MyD88)/nuclear factor-κB (NF-κB) pathway 14. In addition, CGA may prevent diabetes and cardiovascular diseases, and it has been reported that patients with viral hepatitis who drank coffee every day experienced a reduction in the incidence of HCC 15–17. This may be because of the antioxidant properties of CGA. However, other studies have shown that CGA induces genotoxic effects on human cancer cells. This may be mediated by a prooxidant mechanism by which CGA induces topo–DNA complexes because the oxidant hydrogen peroxide can induce topoisoperase-mediated DNA damage 7. However, the exact underlying mechanism of CGA remains elusive.
In the present study, we evaluated the effects of the combination of CGA and 5-FU on the human HCC cell lines HepG2 and Hep3B. The results indicated that CGA could enhance the 5-FU-induced inhibition of HCC cells’ proliferation. In addition, this may be because of the inactivation of the extracellular signal-regulated kinases (ERK) by the CGA-induced overproduction of reactive oxygen species (ROS). CGA is a potential chemosensitizer of 5-FU chemotherapy in HCC.
Materials and methods
Materials
CGA was purchased from Sigma-Aldrich (St Louis, Missouri, USA) and was dissolved in sterile H2O, and 5-FU was purchased from Sigma-Aldrich and was dissolved in DMSO; all were stored at−20°C. The antibodies against phospho-ERK1/2 (Thr202/Tyr204), ERK1/2 and β-actin, were purchased from Cell Signaling Technology Inc. (Danvers, Massachusetts, USA).
Cell culture
The human HCC cell lines, HepG2 and Hep3B, were purchased from the American Type Culture Collection, were kindly provided by Prof. Kuwano, and were cultured in DMEM medium supplemented with 10% fetal bovine serum (PAA Laboratories, Pasching, Austria) at 37°C in a humidified atmosphere of 95% air and 5% CO2; the medium was changed every 2 days. Cells in the mid log phase were used in this study.
Assessment of cell viability
HepG2 cells were seeded at a concentration of 2×105 cells per well into a six-well plate. After treatment, HepG2 cells were collected and incubated with 0.4% Trypan blue dye. The stained and unstained cells were counted on a hemocytometer separately under a light microscope 18. Cell viability was calculated according to the following formula:
 |
A cell counting kit-8 (CCK-8) assay was also used to determine cell viability 19. HepG2 and Hep3B Cells were seeded in 96-well flat-bottom microtiter plates at a density of 2×103 cells per well. After 48 h of treatment, the cells were incubated with CCK-8 (10 μl of the CCK-8 solution in 90 μl fresh medium) at 37°C for 1 h. The absorbance of the solution was measured using spectrophotometry at 450 nm with a microtiter plate reader (Bio-TEK, Hercules, California, USA). Cell viability was determined according to the following equation:
Measurement of intracellular ROS
Intracellular ROS generation was assessed using 5-(and-6-)chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) (Invitrogen, Carlsbad, California, USA) 20. The cells were washed in PBS and then incubated with 5 μmol/l CM-H2DCFDA at 37°C for 5 min in the dark. The fluorescence of dichlorofluorescein was detected using an epifluorescence microscope (Nikon, Tokyo, Japan) at an excitation wavelength of 488 nm. To avoid photooxidation of the indicator dye, the fluorescence images were collected using a single rapid scan with identical parameters, such as contrast and brightness, for all samples. Flow cytometry was also used to quantitatively determine the intracellular ROS levels. Cells were harvested and incubated in 10 μmol/l CM-H2DCFDA for 30 min at 37°C. Then, the cells were washed and resuspended in PBS. Fluorescence was detected using FACScan (Becton Dickinson, Franklin Lakes, New Jersey, USA).
Analysis of apoptosis
Hoechst33342 and propidium iodide (PI) staining were used to detect apoptosis as described previously 21. Briefly, staining was performed to identify the nuclei. Nuclear morphology was investigated by fluorescence microscopy (Nikon) and representative images were captured. A condensed or a fragmented nucleus is a morphological characteristic of apoptosis. Early apoptotic nuclei had a bright blue fluorescent appearance, and the late apoptotic cell membrane was damaged, which appeared red because of the PI dye. Cell apoptosis was also assayed using an apoptosis detection kit. Cells were harvested and stained with Annexin V–FITC (Annexin V) and PI. Apoptosis was defined according to Annexin V+/PI− (early apoptosis) and Annexin V+/PI+ (late apoptosis) as determined using FACScan (Becton Dickinson).
Western blot analysis
Cells were harvested and lysed in a lysis buffer composed of 20 mmol/l Tris (pH 7.4), 1 mmol/l EGTA, 150 mmol/l NaCl, 5 mmol/l MgCl2, and 1% NP-40, and supplemented with protease inhibitor cocktail (Roche, Basel, Switzerland) and phosphatase inhibitor cocktail (Roche). The protein concentrations were assayed using the BCA Protein Assay Kit (Pierce, Rockford, Illinois, USA). Cell lysates (20 μg protein/lane) were separated using SDS-PAGE and transferred onto a PVDF membrane 22. The blotted membranes were blocked with 5% skim milk for 30 min and were incubated with the appropriate primary antibodies (1 : 1000 dilution for ERK1/2 and phospho-ERK1/2; 1 : 2000 dilution for β-actin). The immunoreactive bands were visualized by enhanced chemiluminescence with horseradish peroxidase-conjugated IgG secondary antibodies. The band density was measured using densitometry (G:BOX) and quantified using Quantity One 1-D analysis software (Bio-Rad, Hercules, California, USA).
Statistical analysis
Statistical analysis was carried out using one-way analysis of variance with a least significant difference post-hoc test; a P value less than 0.05 was considered statistically significant. Experiments were conducted in triplicate and data were expressed as the mean±SEM.
Results
CGA enhanced 5-FU-induced inhibition of hepatocellular carcinoma cell proliferation
The effect of CGA on HepG2 cells was first observed using a Trypan blue dye-exclusion assay. Below 250 μmol/l, no significant changes were noted with respect to the cell viability of the HepG2. However, as the dose increased, CGA induced a significant inhibition of HepG2 cell viability; 500 μmol/l CGA decreased viability to 61.56% and 1 mmol/l decreased viability to 38% (Fig. S1, Supplemental digital content 1, http://links.lww.com/ACD/A98). On the basis of these results and in combination with our previous experiments 14, we chose 250 μmol/l CGA for use in the following experiments.
A total of 20 μmol/l 5-FU was added to observe the effect of 5-FU treatment on HepG2 cells. After 48 h, the viability of HepG2 cells decreased to 62.75% according to Trypan blue staining (Fig. 1a) and to 57.2% according to the CCK-8 assay (Fig. 1b). The combination treatment of CGA and 5-FU induced a more marked decrease to 39.39% (Fig. 1a) and 33.43% (Fig. 1b) according to Trypan blue staining and the CCK-8 assay, respectively; combination treatment led to an ∼1.6 times increase than the use of 5-FU alone, although few changes were observed with CGA alone. These results suggested that CGA could enhance the 5-FU-induced inhibition of HepG2 proliferation.
Fig. 1.
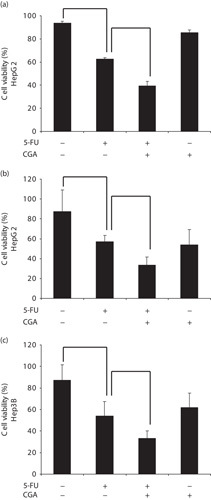
CGA enhanced 5-FU-induced inhibition of hepatocellular carcinoma cell proliferation. HepG2 and Hep3B cells were treated with control, 20 μmol/l 5-FU, 250 μmol/l CGA, and with the combination of 20 μmol/l 5-FU and 250 μmol/l CGA for 48 h at 37°C. (a) Trypan blue dye-exclusion assay was used to observe the changes in the viability of HepG2 cells. (b) HepG2 cell viability was measured using the CCK-8 assay. (c) Hep3B cell viability was also measured using the CCK-8 assay. Experiments were conducted in triplicate. Data are shown as the mean±SEM (n=3). *P<0.05. **P<0.01 compared with the control. CCK-8, cell counting kit-8; CGA, chlorogenic acid; 5-FU, 5-fluorouracil.
The effect of combination treatment with CGA (250 μmol/l) and 5-FU (20 μmol/l) was also observed in Hep3B cells. After 48 h, the viability of Hep3B cells decreased to 33.33% (Fig. 1c). These results supported the idea that CGA could enhance the 5-FU-induced inhibition of HCC cell proliferation.
CGA increased 5-FU-induced ROS production in hepatocellular carcinoma cells
Because the exact mechanism of action of CGA remains elusive, we next examined the change in the generation of ROS in HepG2 cells using a fluorescein-labeled dye, CM-H2DCFDA, as shown in Fig. 2. With the lower concentration (below 250 μmol/l), no significant increase was observed in the production of intracellular ROS by CGA after 24 h (Fig. S2, Supplemental digital content 2, http://links.lww.com/ACD/A99). However, 250 μmol/l CGA induced little ROS production (Fig. 2a–d). A total of 20 μmol/l 5-FU treatment induced significant ROS production (Fig. 2a–e), and the combination of CGA and 5-FU led to an even more prominent production of ROS (Fig. 2a–f). These results suggested that CGA could enhance the 5-FU-induced ROS production in HepG2 cells. CGA also enhanced the 5-FU-induced ROS production in Hep3B cells (Fig. 2c). In addition to their role as critical molecules in intracellular signal transduction 23, ROS can also inhibit proliferation by ERK inactivation 6,24,25. Next, we detected the phosphorylation of ERK in the cell lines.
Fig. 2.
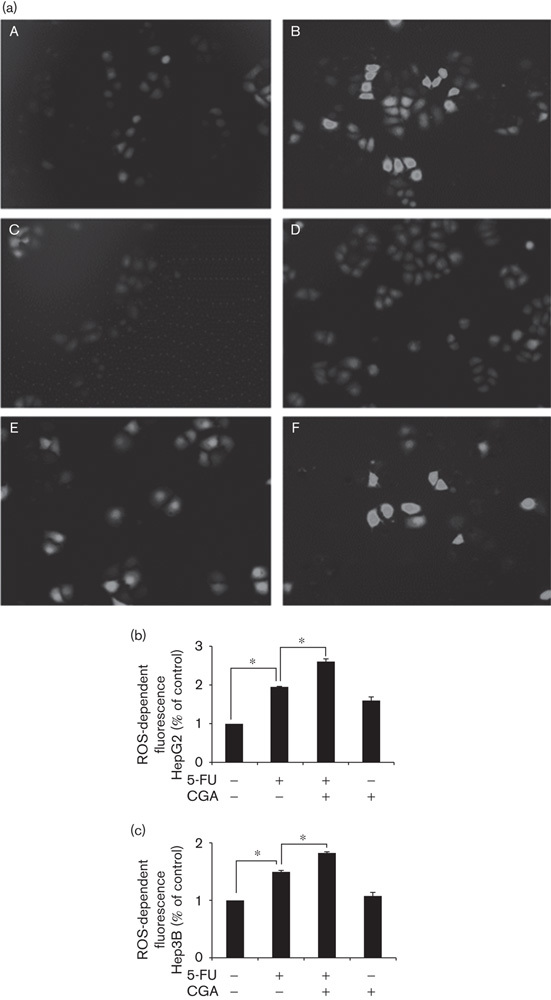
CGA increased 5-FU-induced ROS production in hepatocellular carcinoma cell. HepG2 cells were treated with control (A), 3% H2O2 as the positive control (B), 125 μmol/l CGA (C), 250 μmol/l CGA (D), 20 μmol/l 5-FU (E), and a combination of 20 μmol/l 5-FU and 250 μmol/l CGA (F) for 24 h. (a) Intracellular ROS production was detected using the ROS detection probe CM-H2DCFDA. The fluorescence intensity of ROS was directly measured using fluorescence microscopy (magnification, ×200). (b) Intracellular ROS production in HepG2 cells treated with 20 μmol/l 5-FU, 250 μmol/l CGA, and the combination of 20 μmol/l 5-FU and 250 μmol/l CGA was quantified using flow cytometric analysis. (c) Intracellular ROS production in Hep3B cells treated with 20 μmol/l 5-FU, 250 μmol/l CGA, and the combination of 20 μmol/l 5-FU and 250 μmol/l CGA was also quantified using flow cytometric analysis. Experiments were conducted in triplicate. Data are shown as the mean±SEM (n=3). *P<0.05 compared with the control. CCK-8, cell counting kit-8; CGA, chlorogenic acid; CM-H2DCFDA, 5-(and-6-)chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; 5-FU, 5-fluorouracil; ROS, reactive oxygen species.
Combination of 5-FU and CGA inhibited MAPK/ERK activation in hepatocellular carcinoma cells
The mitogen-activated protein kinase signaling pathway plays a key role in cell proliferation 26. Aberrant regulation of this pathway is related to the pathogenesis of several human cancers and contributes toward resistance to cancer drugs 27,28. The activation of ERK1/2 was examined according to the level of phosphorylated ERK1/2 using immunoblot. After 24 h of treatment, no significant changes were observed in the activation of ERK1/2 in HepG2 cells by 5-FU or CGA alone. However, the combined treatment of both 5-FU and CGA markedly inhibited the phosphorylation of ERK1/2 in HepG2 cells (Fig. 3). A similar phenomenon was observed in Hep3B cells (Fig. 4). ROS have been reported to be upstream mediators of ERK inactivation and contribute toward subsequent cell growth arrest 24. Combined with our data, it was suggested that the combination of 5-FU and CGA led to the inactivation of ERK through the overproduction of ROS, which mediated the enhancement of 5-FU-induced inhibition of HCC cells’ proliferation; that is, CGA sensitized HCC cells to 5-FU treatment by the suppression of ERK activation through ROS overproduction.
Fig. 3.
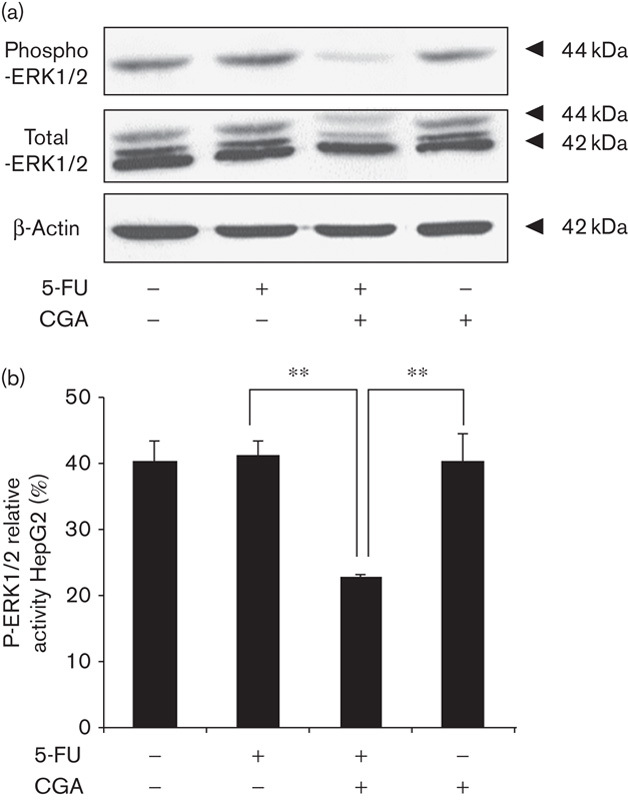
Activation of ERK1/2 was inhibited in HepG2 cells by the combination of 5-FU and CGA. HepG2 cells were treated as shown in Fig. 1 for 24 h. (a) Cell lysates were prepared and subjected to immunoblot analysis to determine the level of total ERK1/2 and phospho-ERK1/2. (b) The intensity of the band was quantified using densitometric imaging. Experiments were conducted in triplicate. Data are shown as the mean±SEM (n=3). **P<0.01 compared with the control. CGA, chlorogenic acid; ERK, extracellular signal-regulated kinase; 5-FU, 5-fluorouracil.
Fig. 4.
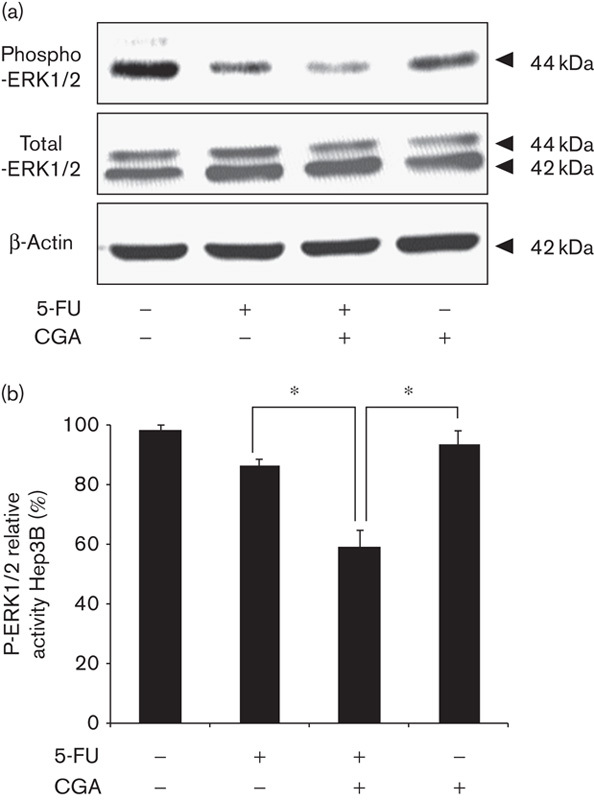
Activation of ERK1/2 was inhibited in Hep3B cells by the combination of 5-FU and CGA. Hep3B cells were treated as shown in Fig. 1 for 24 h. (a) Cell lysates were prepared and subjected to immunoblot analysis to determine the level of total ERK1/2 and phospho-ERK1/2. (b) The intensity of the band was quantified using densitometric imaging. Experiments were conducted in triplicate. Data are shown as the mean±SEM (n=3). *P<0.05 compared with the control. CGA, chlorogenic acid; ERK, extracellular signal-regulated kinase; 5-FU, 5-fluorouracil.
Discussion
There is an urgent need to search for novel chemosensitizers in the field of cancer therapy. CGA is one of the most abundant polyphenols in the human diet, and according to some epidemiological studies, has been shown to reduce the risk of some chronic diseases. In our experiment, we used the human HCC cell lines, HepG2 and Hep3B, and observed the 5-FU-induced inhibition of HCC cell proliferation. The combined treatment with CGA enhanced this inhibition (Fig. 1). 5-FU stimulated the overproduction of ROS, and the combination of 5-FU and CGA led to an even more prominent overproduction of ROS (Fig. 2). Moreover, the combination of 5-FU and CGA led to inactivated ERK1/2, although no significant changes were observed after the use of 5-FU alone or CGA alone (Figs 3 and 4). In combination with previous reports 24, our results suggest that CGA could enhance the 5-FU-induced inhibition of HCC cell proliferation by the inactivation of ERK1/2 through the overproduction of ROS.
CGA is a common dietary polyphenol that can be found in many plant species and is a major component of coffee. Epidemiological studies have shown that CGA exerts many biological effects and may play a role in the development of several chronic diseases associated with coffee consumption. It has been reported that CGA may protect against oxidant stress in the liver by the promotion of the Nrf2/ARE antioxidant system, which synthesizes the antioxidant and phase II detoxifying enzymes 29. In addition, the anticancer activities of CGA have also been shown to be increasingly more conspicuous. CGA has been shown to protect the mouse epidermal cell line JB6 against environmental carcinogen-induced carcinogenesis through the upregulation of cellular antioxidant enzymes and the suppression of NF-κB, activator protein-1, and mitogen-activated protein kinase activation 30. CGA inhibits the migration of U-87 glioma cells 31. CGA also inhibits the proliferation of human colon cancer cells and liver cancer cells 32. In addition, CGA was cytotoxic to the human HCC cell line (HCCLM3) at doses between 400 and 800 μg/ml 33. Burgos-Moron et al. 7 reported that CGA induced DNA damage in normal and cancer cells, which indicates the possible carcinogenic and therapeutic potential of CGA. Therefore, the exact underlying mechanism of CGA remains elusive. In our study, at a lower concentration, no significant changes were observed in the viability of HepG2 cells. However, a high dosage of CGA decreased cell viability and led to cell death (data not shown), which is also what was observed in the report by Burgos-Moron and colleagues. Therefore, it was better to explore the optimal dosage of CGA so that it can be used in a clinical setting in the future.
ROS are upstream mediators of ERK inactivation 6,24. Zhang et al. 25 have reported that ROS inhibit ERK phosphorylation in Hep-2 cells after 9-hydroxypheophorbide α-mediated photodynamic therapy. Belkaid et al. 31 also reported that CGA inhibits sphingosine-1-phosphate-induced ERK phosphorylation in U-87 glioma cells. Our study simultaneously showed the overproduction of ROS and the inactivation of ERK with a combination of 5-FU and CGA, which was considered the mechanism of enhanced 5-FU-induced inhibition of HCC cell proliferation. There was no significant apoptosis in the three treatment groups (Fig. S3, Supplemental digital content 3, http://links.lww.com/ACD/A100). Therefore, we speculate that CGA may inactivate ERK through the overproduction of ROS, which mediates the enhancement of 5-FU-induced inhibition of HCC cell proliferation. The Raf/MEK/ERK cascade has also been identified to be related closely to the induction of drug resistance during cancer therapy 34. Our results strongly suggest that CGA could improve the efficiency of 5-FU treatment in cases of HCC through the inactivation of ERK and ROS overproduction. This finding suggests that the combined use of CGA and 5-FU could be a highly efficient strategy to achieve anticancer synergism against HCC. Further in-vivo studies are needed in the future.
Conclusion
The current study shows that CGA can enhance the 5-FU-induced inhibition of HCC cell proliferation. The synergistic effect of CGA and 5-FU may be because of the inactivation of ERK by the CGA-induced overproduction of ROS. Our observations suggest that CGA is a potential chemosensitizing agent of 5-FU chemotherapy in HCC.
Supplementary Material
Acknowledgements
The authors thank Prof. Kuwano and Prof. Torii (Graduate School of Medicine, Gunma University, Maebashi, Japan) for their kind assistance. They also thank the staff of the laboratory of Genetics and Molecular Biology, School of Medicine, Xi’an Jiaotong University, for their technical support. This work was financially supported by the National Natural Science Foundation of China (81172358).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.anti-cancerdrugs.com).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011; 37:212–220. [DOI] [PubMed] [Google Scholar]
- 3.Miyaki D, Aikata H, Honda Y, Naeshiro N, Nakahara T, Tanaka M, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma according to Child–Pugh classification. J Gastroenterol Hepatol 2012; 27:1850–1857. [DOI] [PubMed] [Google Scholar]
- 4.Baek YH, Kim KT, Lee SW, Jeong JS, Park BH, Nam KJ, et al. Efficacy of hepatic arterial infusion chemotherapy in advanced hepatocellular carcinoma. World J Gastroenterol 2012; 18:3426–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Tu M, Han B, Gu Y, Xue X, Sun J, et al. Vasohibin 2 decreases the cisplatin sensitivity of hepatocarcinoma cell line by downregulating p53. PLoS One 2014; 9:e90358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan C, Chen J, Wang Y, Wong YS, Zhang Y, Zheng W, et al. Selenocystine potentiates cancer cell apoptosis induced by 5-fluorouracil by triggering reactive oxygen species-mediated DNA damage and inactivation of the ERK pathway. Free Radic Biol Med 2013; 65:305–316. [DOI] [PubMed] [Google Scholar]
- 7.Burgos-Moron E, Calderon-Montano JM, Orta ML, Pastor N, Perez-Guerrero C, Austin C, et al. The coffee constituent chlorogenic acid induces cellular DNA damage and formation of topoisomerase I- and II-DNA complexes in cells. J Agric Food Chem 2012; 60:7384–7391. [DOI] [PubMed] [Google Scholar]
- 8.Yun N, Kang JW, Lee SM. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. J Nutr Biochem 2012; 23:1249–1255. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Wang J, Ballevre O, Luo H, Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens Res 2012; 35:370–374. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Dong L, Dang X, Liu Y, Jiang J, Wang Y, et al. Effect of chlorogenic acid on LPS-induced proinflammatory signaling in hepatic stellate cells. Inflamm Res 2013; 62:581–587. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Chen J, Yu X, Tao W, Jiang F, Yin Z, et al. Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm Res 2010; 59:871–877. [DOI] [PubMed] [Google Scholar]
- 12.Ji L, Jiang P, Lu B, Sheng Y, Wang X, Wang Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J Nutr Biochem 2013; 24:1911–1919. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Dong L, Bai Y, Zhao J, Zhang Y, Zhang L. Chlorogenic acid against carbon tetrachloride-induced liver fibrosis in rats. Eur J Pharmacol 2009; 623:119–124. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Dong L, Jiang J, Zhao J, Zhao G, Dang X, et al. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013; 303:107–114. [DOI] [PubMed] [Google Scholar]
- 15.Arab L. Epidemiologic evidence on coffee and cancer. Nutr Cancer 2010; 62:271–283. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Kurahashi N, Iwasaki M, Shimazu T, Tanaka Y, Mizokami M, et al. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomarkers Prev 2009; 18:1746–1753. [DOI] [PubMed] [Google Scholar]
- 17.Ohfuji S, Fukushima W, Tanaka T, Habu D, Tamori A, Sakaguchi H, et al. Coffee consumption and reduced risk of hepatocellular carcinoma among patients with chronic type C liver disease: a case–control study. Hepatol Res 2006; 36:201–208. [DOI] [PubMed] [Google Scholar]
- 18.Yedjou CG, Tchounwou PB. N-acetyl-l-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int J Environ Res Public Health 2007; 4:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu P, Wang Z, Sun X, Chen X, Zeng S, Chen L, et al. Hydrogen-rich medium protects human skin fibroblasts from high glucose or mannitol induced oxidative damage. Biochem Biophys Res Commun 2011; 409:350–355. [DOI] [PubMed] [Google Scholar]
- 20.Hou N, Torii S, Saito N, Hosaka M, Takeuchi T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology 2008; 149:1654–1665. [DOI] [PubMed] [Google Scholar]
- 21.Meng AG, Jiang LL. Pseudolaric acid B-induced apoptosis through p53-dependent pathway in human gastric carcinoma cells. J Asian Nat Prod Res 2009; 11:142–152. [DOI] [PubMed] [Google Scholar]
- 22.Tripathi DN, Jena GB. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res 2010; 696:69–80. [DOI] [PubMed] [Google Scholar]
- 23.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012; 24:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan C, Zheng W, Fu X, Li X, Wong YS, Chen T. Strategy to enhance the therapeutic effect of doxorubicin in human hepatocellular carcinoma by selenocystine, a synergistic agent that regulates the ROS-mediated signaling. Oncotarget 2014; 5:2853–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Shen B, Swinarska JT, Li W, Xiao K, He P. 9-Hydroxypheophorbide alpha-mediated photodynamic therapy induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in Hep-2 cells via ROS-mediated suppression of the ERK pathway. Photodiagnosis Photodyn Ther 2014; 11:55–62. [DOI] [PubMed] [Google Scholar]
- 26.Brunet A, Brondello JM, L’Allemain G, Lenormand P, McKenzie F, Pages G, et al. MAP kinase module: role in the control of cell proliferation. C R Seances Soc Biol Fil 1995; 189:43–57. [PubMed] [Google Scholar]
- 27.Sun L, Liu L, Liu X, Wang Y, Li M, Yao L, et al. MGr1-Ag/37LRP induces cell adhesion-mediated drug resistance through FAK/PI3K and MAPK pathway in gastric cancer. Cancer Sci 2014; 105:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westhoff MA, Fulda S. Adhesion-mediated apoptosis resistance in cancer. Drug Resist Updat 2009; 12:127–136. [DOI] [PubMed] [Google Scholar]
- 29.Xu D, Hu L, Xia X, Song J, Li L, Song E, et al. Tetrachlorobenzoquinone induces acute liver injury, up-regulates HO-1 and NQO1 expression in mice model: the protective role of chlorogenic acid. Environ Toxicol Pharmacol 2014; 37:1212–1220. [DOI] [PubMed] [Google Scholar]
- 30.Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, Ding M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem 2005; 280:27888–27895. [DOI] [PubMed] [Google Scholar]
- 31.Belkaid A, Currie JC, Desgagnes J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int 2006; 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Chen Q, He M, Mir P, Su J, Yang Q. Inhibitory effect of antioxidant extracts from various potatoes on the proliferation of human colon and liver cancer cells. Nutr Cancer 2011; 63:1044–1052. [DOI] [PubMed] [Google Scholar]
- 33.Intisar A, Zhang L, Luo H, Kiazolu JB, Zhang R, Zhang W. Anticancer constituents and cytotoxic activity of methanol-water extract of Polygonum bistorta L. Afr J Tradit Complement Altern Med 2012; 10:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget 2012; 3:1068–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


