Abstract
Background and objectives
Development of cirrhosis and hepatocellular carcinoma (HCC) are critical milestones in the natural history of chronic hepatitis B virus (HBV) infection. There are no prospective data on the risk of these critical milestones in HBV patients in Singapore. The efficacy and justification of HCC surveillance is determined by the rate of HCC development. Our study aims to determine the rates of cirrhosis and HCC in HBV patients in Singapore and hence the appropriateness of HCC surveillance.
Materials and methods
A total of 673 HBV patients were enrolled between March 2003 and March 2004 and followed up for 10 years with regular surveillance for HCC using α-fetoprotein and abdominal ultrasound.
Results
Overall, 62.6% of the patients were men, mean age 56.4 years. In all, 31% were hepatitis B e antigen-positive and 14.9% had cirrhosis at baseline. Seventy-four patients developed cirrhosis and 42 patients developed HCC after 10 years. The overall 10-year incidence of cirrhosis and HCC was 16.2% (1.6%/year) and 7.8% (0.8%/year), respectively. The overall incidence of HCC in cirrhotics was 29.7% (3.0%/year), highest within a year of diagnosis of cirrhosis (7.9%). The rate of cirrhosis was significantly higher in those aged more than 55 years (P=0.001). Sex and hepatitis B e antigen status did not affect the rate of cirrhosis. Factors with significantly higher overall rates of HCC were age 55 years or more (P=0.001), male sex (P=0.001), and baseline α-fetoprotein of 4.1 µg/l or more (P<0.0001). However, age more than 55 years was not significant in the development of HCC in cirrhotics.
Conclusion
The rate of cirrhosis in HBV patients in Singapore is about 1.6% per year. The rate of HCC is about 0.8% per year overall and 3.0% per year in cirrhotics, which justifies HCC surveillance.
Keywords: chronic hepatitis B, cirrhosis, hepatocellular carcinoma, surveillance
Introduction
Hepatitis B virus (HBV) infection is a significant healthcare problem, with more than two million individuals exposed to the virus and an estimated 350 million chronic hepatitis B (CHB) carriers globally 1. Approximately 75% of these individuals reside in Asia. In Asia alone, the prevalence of chronic HBV infection ranges from 2 to 5% in countries such as Japan and up to 20% in Taiwan 2.
CHB is the most common cause of chronic liver disease and cirrhosis in Singapore 3. The developments of cirrhosis and/or hepatocellular carcinoma (HCC) are critical milestones in the natural history of CHB. Once a CHB patient with cirrhosis decompensates, quality of life and prognosis become significantly poorer 4. In Singapore, HCC is the third most common cause of cancer death in men and fourth in women. In addition, the financial burden of HBV infection in Singapore is estimated to be US$279 million per annum, with 58% attributable to direct cost or equivalent to 12% of the national healthcare expenditure in 2003 5. From both clinical and economic perspectives, it is important to determine the rates of cirrhosis and HCC development in our local context and whether surveillance programs are indicated to detect these critical developments earlier, more reliably, and more cost-effectively. The reported incidences of HCC in a general HBV population and in a cirrhotic population were ∼1% per annum and 3.2% per annum, respectively 6–8. However, there are currently no prospective data in Singapore on the rate of development of cirrhosis and HCC in our CHB patients.
The aim of this 10-year prospective study was to determine the rates of development of cirrhosis and HCC in HBV-infected patients in Singapore and hence the appropriateness of surveillance for HCC.
Materials and methods
Study cohort
A total of 673 HBV-infected patients seen at the Department of Gastroenterology and Hepatology outpatient clinics in the Singapore General Hospital from 1 March 2003 to 1 March 2004 were enrolled in a prospective cohort. Patients with CHB monoinfection (i.e. no HCV coinfection) aged 21 years and older were included. Those who had HBV flares at enrollment were excluded because the high alanine transaminase values would confound the cohort’s aggregate baseline alanine transaminase value. These patients were followed up for 10 years. Analysis of the study data was carried out on 1 March 2013. A total of 128 patients were lost to follow-up, and another seven were excluded because of HBV flare-up at enrollment. After these exclusions, a total of 538 HBV-infected patients were included. There were 35 deaths during the 10-year follow-up period. All patients provided consent and the study was approved by the hospital’s institutional review board.
Clinical data collection and follow-up
These patients were followed up at 3–6-monthly intervals with their respective primary gastroenterologists. Liver biochemistry and serum α-fetoprotein (AFP) were monitored every 3–6 months (including around the time of enrollment), and liver imaging consisting of abdominal ultrasonography was performed every 6–12 months in a physician-driven HCC surveillance program. Coagulation profiles and HBV DNA quantifications were not collected as our national guidelines for the management of CHB patients require only liver biochemistry and serum AFP measurements. Endoscopy data were not collected as not all patients undergo endoscopy, but only when a patient has cirrhosis and a low platelet count. Transient elastography was not yet available in our hospital at the time of the study enrollment. Where indicated, dynamic computed tomography (CT) or MRI of the liver was performed to clarify ultrasonographic findings.
Study definitions
The diagnosis of cirrhosis in this study was made on the basis of histology and/or imaging with supportive clinical evidence such as ascites, varices, splenomegaly, or evidence of hypersplenism. A nontargeted biopsy of the liver parenchyma is performed when the imaging is suggestive of cirrhosis, but without supporting radiological or clinical features of portal hypertension. The diagnosis of HCC was made on the basis of dynamic CT or MRI scan of the liver and/or histology. Targeted biopsy of a suspicious or indeterminate nodule in the liver is performed when the diagnosis of HCC cannot be confirmed on dynamic CT or MRI scan.
Statistical analysis
All statistical analyses were carried out using SPSS, version 19.0 (IBM Corp., Armonk, New York, USA). Mean and SD or median when appropriate were calculated for continuous variables and percentages were used for categorical variables. Qualitative and quantitative differences between subgroups were analyzed using the χ2-test for categorical parameters and the Mann–Whitney test for continuous parameters as appropriate. The Kaplan–Meier method was used to estimate the incidences of cirrhosis and HCC and differences between factors were evaluated using the log-rank test.
Results
Demographics
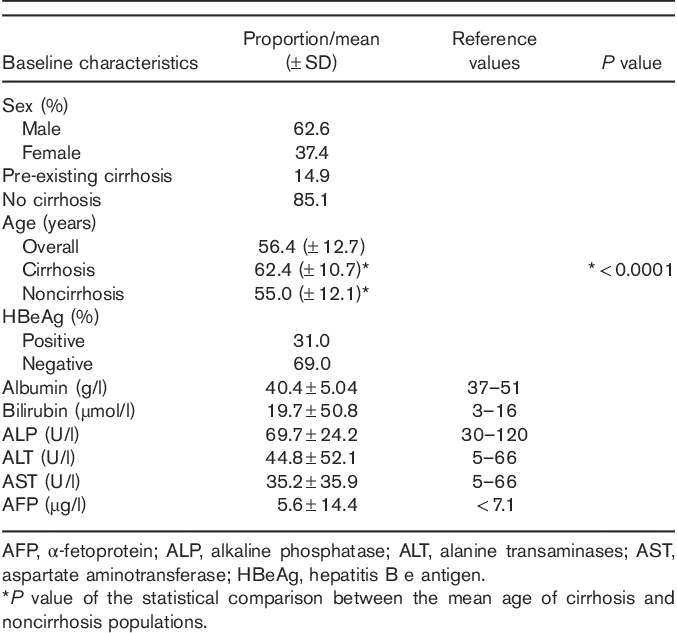
A total of 538 patients were analyzed. Demographic presentation is shown in Table 1. The majority (62.6%) were men, mean age 56.4 years (±12.7). Approximately one-third (31.0%) were hepatitis B e antigen (HBeAg) positive and 14.9% had pre-existing cirrhosis at enrollment. Patients with cirrhosis were significantly older (62.4±10.7 vs. 55.0±12.1; P<0.0001).
Table 1.
Baseline characteristics
Development of cirrhosis and HCC
There were a total of 154 patients with cirrhosis after 10 years. Eighty patients with pre-existing cirrhosis or cirrhosis diagnosed within 6 months of enrollment were excluded from analysis. The overall incidence of cirrhosis over 10 years was 16.2% or at a rate of about 1.6% per annum (Fig. 1). Forty-two patients developed HCC after 10 years. The overall incidence of HCC over 10 years was 7.8% or at a rate of about 0.8% per annum. In patients with cirrhosis, the incidence of HCC development was 29.7% over 10 years (about 3.0%/annum). Furthermore, the risk of HCC development is highest within the first year of diagnosis of cirrhosis (7.9%) and decreased to a constant 3% per annum thereafter (Fig. 1).
Fig. 1.
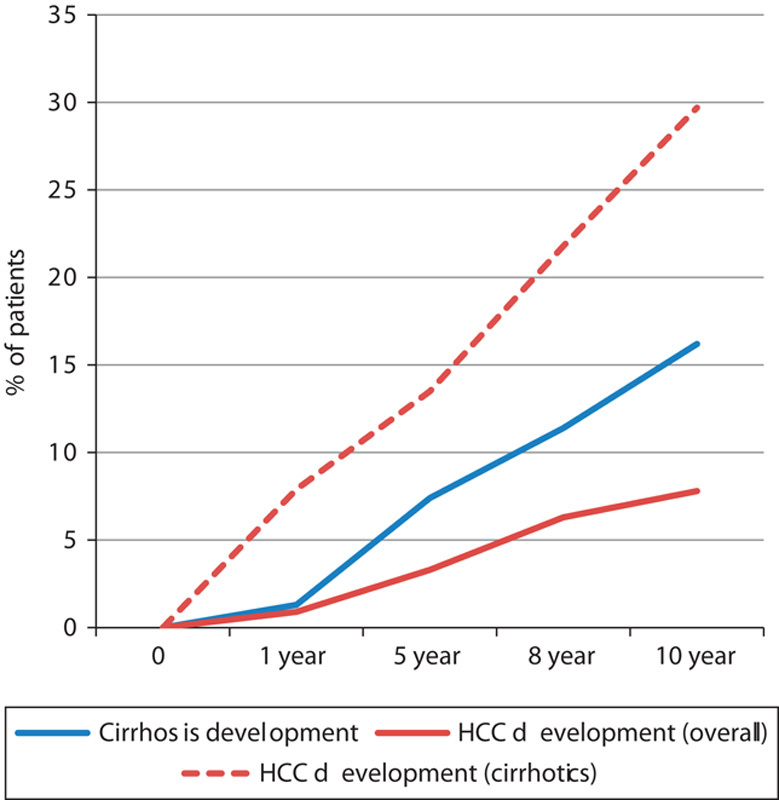
Rates of development of cirrhosis and HCC. HCC, hepatocellular carcinoma.
Effect of age
The annual rate of development of cirrhosis in patients aged 55 years and older was significantly higher. After 10 years, 21.8% developed cirrhosis compared with 10.3% (P=0.001) among those younger than 55 years of age. The annual rates of development of cirrhosis were 2.2 and 1.0%, respectively (Fig. 2).
Fig. 2.
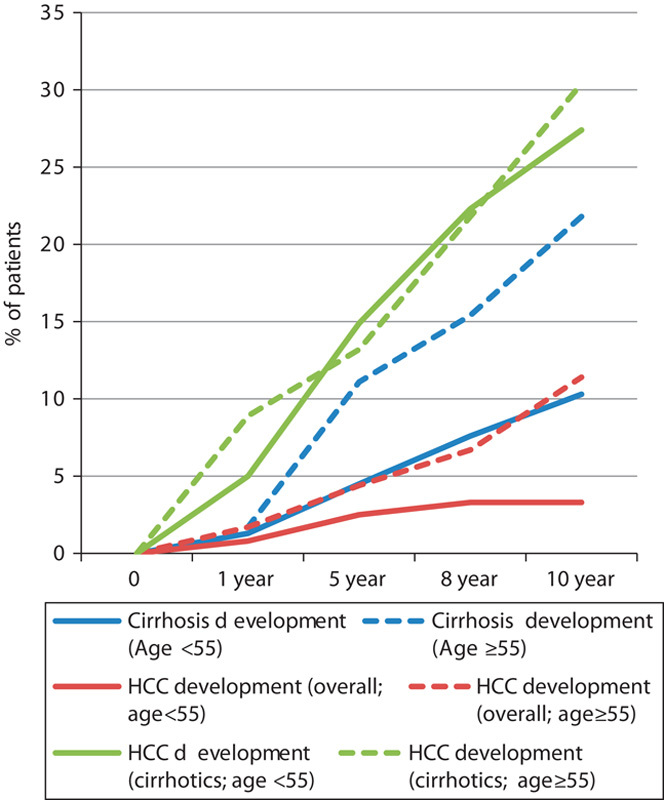
Effect of age on the rate of development of cirrhosis and HCC. Cirrhosis development age<55 versus age≥55 (log-rank P=0.001); HCC development (overall) age<55 versus age≥55 (log-rank P=0.001); HCC development (cirrhotics) age<55 versus age≥55 (log-rank P=0.507). HCC, hepatocellular carcinoma.
Age was also significant in the overall rate of development of HCC as the overall annual rate of HCC development in patients aged 55 years and older was significantly higher (1.1 vs. 0.3%; P=0.001). In the cirrhosis subgroup, the rates of HCC development were similar in both age groups (3.0 vs. 2.7%; P=0.507) (Fig. 2).
Effect of sex
There was no effect of sex on the rate of development of cirrhosis. However, men were at a significantly higher risk of developing HCC both overall (1.1 vs. 0.3%; P=0.001) and in the cirrhotic population (3.6 vs. 1.7%; P=0.005). The overall rate of HCC development in men over 10 years was 10.9% compared with 2.9% in women. In the cirrhotic population, 35.7% of men developed HCC compared with 16.7% of women (Fig. 3).
Fig. 3.
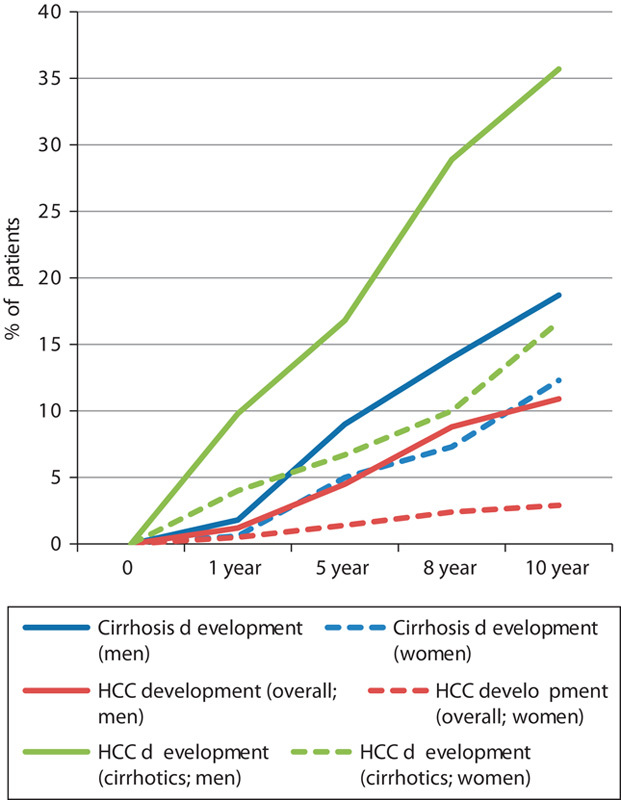
Effect of sex on the rate of development of cirrhosis and HCC. Cirrhosis development men versus women (log-rank P=0.061); HCC development (overall) men versus women (log-rank P=0.001); HCC development (cirrhotics) men versus women (log-rank P=0.005). HCC, hepatocellular carcinoma.
Effect of HBeAg status
There was no effect of HBeAg status on both the overall rate of development of cirrhosis (19.6 vs. 15.5%; P=0.135) and HCC (8.6 vs. 7.8%; P=0.506). The rate of development of HCC in the subgroup analysis of cirrhotics was also not significantly affected by HBeAg status (32.9 vs. 28.3%; P=0.996).
Effect of AFP
The annual rate of HCC development was significantly higher in patients with AFP more than or equal to 4.1 µg/l both in the cirrhotic and in the overall populations Overall, among patients with AFP more than or equal to 4.1 µg/l at baseline, 18.5% developed HCC over 10 years compared with only 2.3% among those with AFP less than 4.1 µg/l (P<0.0001). Similarly, in cirrhotic patients, those with AFP 4.1 µg/l or more at baseline developed HCC more frequently over 10 years (43.9 vs. 7.7%; P<0.0001). These translate to annual rates of development of HCC in patients with AFP 4.1 µg/l or more at baseline to be 1.9% (overall) and 4.4% (cirrhotics) (Fig. 4).
Fig. 4.
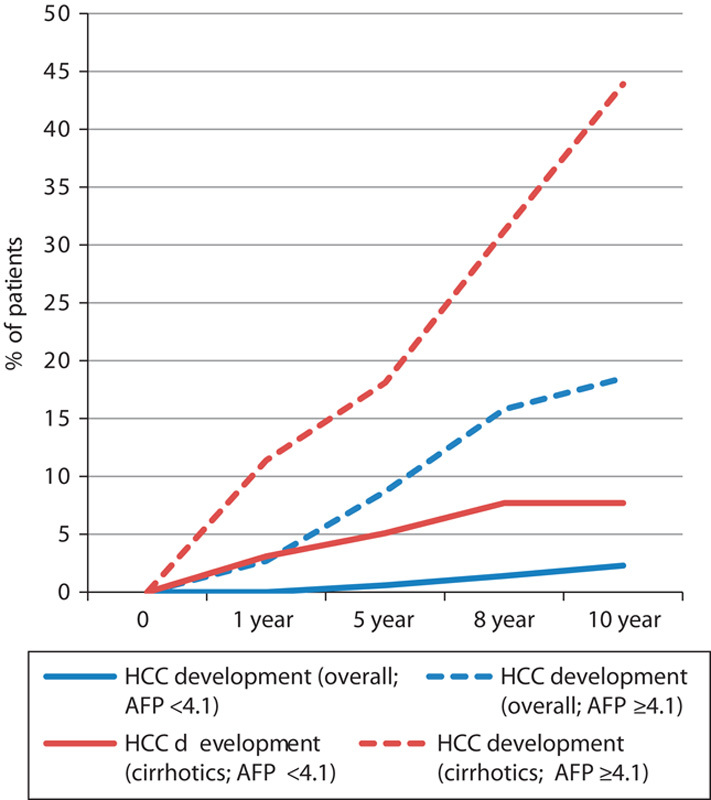
Effect of baseline serum AFP on the rate of development of cirrhosis and HCC. HCC development (overall) AFP<4.1 versus AFP≥4.1 (log-rank P<0.0001); HCC development (cirrhotics) AFP<4.1 versus AFP≥4.1 (log-rank P<0.0001). AFP, α-fetoprotein; HCC, hepatocellular carcinoma.
Antiviral treatment
A total of 190 (35.3%) patients were prescribed some form of antiviral treatment. Of these, seven received interferon therapy in combination with nucleos(t)ide analog or as monotherapy. The remaining 183 patients received nucleos(t)ide analog alone. The majority of the patients (88.7%) received antiviral treatment only after they developed cirrhosis. Entecavir was most commonly prescribed (45.4%), followed by lamivudine (40.4%). The 10-year cumulative rate of HCC development in patients on antiviral treatment was 5.4% (9/167).
Discussion
The rates of development of cirrhosis and HCC in CHB patients in this first ever prospective study in Singapore are 1.6% per annum and 0.8% per annum, respectively. In cirrhotic patients, the incidence of HCC development is 3% per annum. These results are comparable with rates reported worldwide, in Asia, America, and Europe 9–14.
HCC surveillance comprising 6-monthly surveillance serum AFP and abdominal ultrasound has been shown in a large randomized-controlled trial of 18 816 patients to reduce HCC-related mortality by 37% 15. The decision to subject a patient to HCC surveillance is determined by the individual’s risk for HCC, which is related to the incidence of HCC in that particular population. Cost-effectiveness models suggest that an intervention is considered cost-effective if it provides gains of life expectancy of more than 3 months at a cost of less than US$50 000 per year of life saved 16. A cost-effectiveness study in Child–Pugh’s A CHB cirrhosis patients suggests that HCC incidence of 1.5% per annum or greater would justify HCC surveillance 17. Similar rates are also proposed for HCV-related cirrhosis and stage-4 primary biliary cirrhosis 18. As CHB patients are at risk of HCC development even in the absence of cirrhosis, major associations such as the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) recommend that the threshold incidence for efficacy of HCC surveillance should be 0.2% per year in select CHB populations such as Asian men older than 40 years of age, Asian women older than 50 years of age, African/North American blacks, and CHB patients with a family history of HCC 18,19. Compared with the incidence of HCC in western populations of about 0.1–0.4% per annum 6,20, our HCC development rate of 0.8% per annum is higher. The results of our study justify HCC surveillance in our CHB patients.
In our study, HCC rates were highest within the first year of diagnosis of cirrhosis because of seven cases of HCC being detected within a year of diagnosis of cirrhosis. Of these seven cases, four were diagnosed concomitantly with both HCC and cirrhosis on CT/MRI. The remaining three patients who were diagnosed to have cirrhosis 2, 5, and 8 months after HCC were most likely missed because of inaccuracy before the HCC diagnosis.
The high rate of HCC development in the first year may be because of prevalent cancers in patients who were not under routine HCC surveillance as cirrhosis may not have been diagnosed previously. This can also be explained by the limited sensitivity of early detection of cirrhosis or poor detection of early cirrhosis on routine US surveillance. Cirrhotic changes could have also been misinterpreted as fatty infiltration, leading to a false diagnosis of fatty liver disease coexisting with CHB. With the increasing availability and sensitivity of noninvasive liver stiffness measurement techniques, cirrhosis may be detected earlier and with greater sensitivity and accuracy.
It is traditionally believed that HBeAg-positive status is associated with an increased risk of HCC 21. Our observation of the lack of effect of HBeAg status on HCC development is similar to a Hong Kong study that reported similar findings 22. In that study by Yuen and colleagues of 820 patients followed up for a mean of 76.8 months, there was no difference in the overall risk of HCC between patients who were positive for HBeAg (n=356) compared with those positive for anti-HBe (n=464) at presentation (P=0.54). Out of 356 patients, 144 underwent HBeAg seroconversion, but there was again no difference in the overall risk of HCC development in this subgroup compared with the initial two groups.
In our study, patients aged 55 years or older were at a significantly higher risk of developing cirrhosis over 10 years (P=0.001). Thus, as expected, the overall HCC rate was significantly higher in patients aged 55 years or older. In patients who were cirrhotic, age did not influence the risk of developing HCC.
Our data also showed that patients with a baseline AFP of 4.1 µg/l or more have a significantly higher risk of developing HCC. This is an important finding because although an AFP level of 4.1 µg/l is within the normal range, a baseline AFP level of 4.1 µg/l or more has been shown in our study to carry a significant risk of developing HCC. We hope to explore whether we can combine the threshold values of the various baseline factors, including an AFP level of 4.1 µg/l or more, to predict the risk of developing HCC.
As most of the patients were started on antiviral treatment only after they developed cirrhosis, there was a huge imbalance in the number of patients with cirrhosis (and a higher risk of developing HCC) in the treatment compared with the nontreatment groups. Thus, it was not possible for us to conclude whether antiviral treatment had any impact on the rates of cirrhosis or HCC development. In addition, those who received antiviral treatment were started on treatment at various time points of their follow-up as lamivudine, entecavir, and tenofovir were available in our pharmacy only after 2004, 2006, and 2011, respectively. We could not implement a standardized protocol for hepatitis B treatment as the medications are costly and totally self-funded.
Similar to several other longitudinal studies, male sex was also found to be associated with an increased risk of HCC development both in cirrhotics and in the overall cohort 23,24. It, however, had no effect on the development of cirrhosis.
The strengths of our study are the relatively large sample size and the long duration of prospective follow-up. However, there are several limitations in this study. The diagnosis of liver cirrhosis should ideally be made by liver biopsy. However, in this clinical study, a clinical diagnosis of cirrhosis was made on the basis of imaging evidence with concomitant clinical evidence. Furthermore, many patients refused diagnostic liver biopsy in view of the risks associated with the invasive procedure. Being an academic tertiary institution, there could also have been a referral bias in this study cohort. Risk factors for HCC development such as family history, obesity, and smoking have not been adjusted for as these data were unfortunately not collected. Nonetheless, these limitations notwithstanding, the results of our study do provide additional data on cirrhosis and HCC in CHB patients in Singapore, a country that is endemic for hepatitis B with a relatively high prevalence of CHB infection. With potent antiviral agents such as entecavir and tenofovir showing ability to lower the incidence of HCC and reverse fibrosis and cirrhosis, these rates are expected to decrease in the coming years as the effects of antiviral therapy start to bear fruit 25–27.
Conclusion
The rate of cirrhosis in HBV-infected patients in Singapore is about 1.6% per annum and the overall rate of development of HCC in HBV-infected patients in Singapore is about 0.8% per annum. The rate of development of HCC in patients with HBV cirrhosis in Singapore is about 3.0% per annum. Our rates of development of HCC justify HCC surveillance in our patients with CHB infection.
Acknowledgements
The authors would like to thank all doctors from the Department of Gastroenterology and Hepatology at Singapore General Hospital.
All authors contributed equally to the update and maintenance of the database. Zhongxian Poh and Chee-Kiat Tan contributed equally to the data and statistical analyses of this study as well as to the writing of the manuscript; Boon-Bee George Goh and Pik-Eu Jason Chang contributed to the refinement of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hepatitis B: World Health Organization Fact Sheet 204. 2000: World Health Organization. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/. [Accessed 26 November 2013].
- 2.Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia–Pacific region. J Gastroenterol Hepatol 2000; 15 (Suppl):E3–E6. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Wong GW, Chang J. Epidemiology of patients with liver cirrhosis in a single tertiary centre in Singapore [abstract PP596]. Hepatol Int 2010; 4:261–262. [Google Scholar]
- 4.Hui AY, Chan HL, Leung NW, Hung LC, Chan FK, Sung JJ. Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol 2002; 34:569–572. [DOI] [PubMed] [Google Scholar]
- 5.Ong SC, Lim SG, Li SC. How big is the financial burden of hepatitis B to society? A cost-of-illness study of hepatitis B infection in Singapore. J Viral Hepat 2009; 16:53–63. [DOI] [PubMed] [Google Scholar]
- 6.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008; 48:335–352. [DOI] [PubMed] [Google Scholar]
- 7.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer 1988; 61:1942–1956. [DOI] [PubMed] [Google Scholar]
- 8.Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver 1989; 9:235–241. [DOI] [PubMed] [Google Scholar]
- 9.Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology 1988; 8:493–496. [DOI] [PubMed] [Google Scholar]
- 10.Huo T, Wu JC, Hwang SJ, Lai CR, Lee PC, Tsay SH, et al. Factors predictive of liver cirrhosis in patients with chronic hepatitis B: a multivariate analysis in a longitudinal study. Eur J Gastroenterol Hepatol 2000; 12:687–693. [DOI] [PubMed] [Google Scholar]
- 11.Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, Mak CW, et al. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology 2013; 144:933–944. [DOI] [PubMed] [Google Scholar]
- 12.McMahon BJ, Alberts SR, Wainwright RB, Bulkow L, Lanier AP. Hepatitis B-related sequelae. Prospective study in 1400 hepatitis B surface antigen-positive Alaska native carriers. Arch Intern Med 1990; 150:1051–1054. [DOI] [PubMed] [Google Scholar]
- 13.Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, et al. Natural history and prognostic factors for chronic hepatitis B. Gut 1991; 32:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fattovich G. Natural history of hepatitis B. J Hepatol 2003; 39 (Suppl 1):S50–S58. [DOI] [PubMed] [Google Scholar]
- 15.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422. [DOI] [PubMed] [Google Scholar]
- 16.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992; 146:473–481. [PMC free article] [PubMed] [Google Scholar]
- 17.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child–Pugh class A cirrhosis. Am J Med 1996; 101:422–434. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. AASLD practice guidelines: management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufou JF, et al. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–943. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-Tapias JM, Costa J, Mas A, Bruguera M, Rodés J. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology 2002; 123:1848–1856. [DOI] [PubMed] [Google Scholar]
- 21.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002; 347:168–174. [DOI] [PubMed] [Google Scholar]
- 22.Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009; 50:80–88. [DOI] [PubMed] [Google Scholar]
- 23.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127 (Suppl 1):S35–S50. [DOI] [PubMed] [Google Scholar]
- 24.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 2001; 48:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013; 58:98–107. [DOI] [PubMed] [Google Scholar]
- 26.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010; 52:886–893. [DOI] [PubMed] [Google Scholar]
- 27.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381:468–475. [DOI] [PubMed] [Google Scholar]


