Abstract
Dietary fiber that is intrinsic and intact in fiber-rich foods (eg, fruits, vegetables, legumes, whole grains) is widely recognized to have beneficial effects on health when consumed at recommended levels (25 g/d for adult women, 38 g/d for adult men). Most (90%) of the US population does not consume this level of dietary fiber, averaging only 15 g/d. In an attempt to bridge this “fiber gap,” many consumers are turning to fiber supplements, which are typically isolated from a single source. Fiber supplements cannot be presumed to provide the health benefits that are associated with dietary fiber from whole foods. Of the fiber supplements on the market today, only a minority possess the physical characteristics that underlie the mechanisms driving clinically meaningful health benefits. The first part (current issue) of this 2-part series will focus on the 4 main characteristics of fiber supplements that drive clinical efficacy (solubility, degree/rate of fermentation, viscosity, and gel formation), the 4 clinically meaningful designations that identify which health benefits are associated with specific fibers, and the gel-dependent mechanisms in the small bowel that drive specific health benefits (eg, cholesterol lowering, improved glycemic control). The second part (next issue) of this 2-part series will focus on the effects of fiber supplements in the large bowel, including the 2 mechanisms by which fiber prevents/relieves constipation (insoluble mechanical irritant and soluble gel-dependent water-holding capacity), the gel-dependent mechanism for attenuating diarrhea and normalizing stool form in irritable bowel syndrome, and the combined large bowel/small bowel fiber effects for weight loss/maintenance. The second part will also discuss how processing for marketed products can attenuate efficacy, why fiber supplements can cause gastrointestinal symptoms, and how to avoid symptoms for better long-term compliance.
There is general agreement with the statement that “fiber is good for you.”1–5 There is also general agreement that most people do not consume enough fiber in their diet and therefore would benefit from eating more fiber. Both statements are true when applied to fiber-rich whole foods (eg, fruits, vegetables, legumes, whole grains). The statements become less accurate, and less broadly applicable, when applied to fiber supplements. An important distinction is the difference between replacement and supplement. If patients are compliant with their clinician’s advice to change their eating habits, and a substantial portion of a given diet is replaced by high-fiber foods, then both the total calories consumed and the glycemic index of the diet6 should be significantly reduced. The resulting health benefits would support an association between consuming a wide variety of fiber types from whole food sources (eg, fruits, vegetables, legumes, whole grains) and observed improvements in health. This is in large part how epidemiologic studies show that a diet high in fiber consumption from whole foods is strongly associated with a reduced risk of heart attack, stroke, and cardiovascular disease.7,8
It remains unclear, however, how much of the observed health benefit is actually attributable to the increase in dietary fiber, versus how much is attributable to a reduction in calories, elimination of less healthy dietary components, and an increased consumption of health-promoting constituents of the fruits, vegetables, and whole grains that are independent of the fiber component. Although it can still be argued that the optimal goal is to dramatically change the typical American diet to one that is replaced with high levels of fruits, vegetables, and whole grains, decades of experience show that only a minority of people consume the recommended levels of dietary fiber. The Institute of Medicine’s Adequate Intake guideline recommends 14 g of dietary fiber per 1000 kcal consumed, which is about 25 g/d for women and 38 g/d for men.9 In contrast to this recommendation, most (90%) of the US population does not consume enough dietary fiber. The average American consumes only 15 g of dietary fiber per day, and for those on a low-carbohydrate diet, total fiber intake may be less than 10 g/d.10
FIBER SUPPLEMENTS ARE AN ISOLATED FIBER SOURCE AND DO NOT PROVIDE MANY OF THE HEALTH BENEFITS ASSOCIATED WITH MEAL REPLACEMENT
In an attempt to overcome the gap between recommended levels of fiber consumption versus what is actually consumed in a typical American diet, fiber supplements have become a popular option as a convenient, concentrated source of fiber. Given that a fiber supplement is an isolated fiber source consumed in addition to an existing diet, and many of the additional benefits described above for meal replacement are not provided by adding an isolated fiber source, it becomes essential to have a more in-depth understanding of the unique physiochemical characteristics of each fiber supplement and how these characteristics are, or are not, associated with 1 or more clinically meaningful health benefits. The term fiber supplement implies that regular (daily) consumption will provide health benefits that may be missing from a low-fiber diet, but for most fiber supplements, this implication is not supported by clinical data. Health benefits derived from fiber supplements are primarily a function of the fiber’s physical effects in the small bowel (eg, cholesterol lowering, improved glycemic control, satiety/weight loss) and large bowel (improved stool form and reduced symptoms in constipation, diarrhea, and irritable bowel syndrome [IBS]).
FIBER SUPPLEMENTS CAN BE DIVIDED INTO 4 CLINICALLY MEANINGFUL DESIGNATIONS
There are 4 main characteristics of fiber supplements that drive clinical efficacy: solubility, degree/rate of fermentation, viscosity, and gel formation. Solubility defines whether a fiber supplement will dissolve in water (soluble) or remain as discreet insoluble particles.3,11 For soluble fibers, viscosity refers to the ability of some fibers to “thicken” when hydrated, in a concentration-dependent manner (Figure 1).3,11–15 Gel formation refers to the ability of a subset of soluble viscous fibers to form cross-links, resulting in a viscoelastic gel when hydrated (Figure 2).3,11–13
FIGURE 1.
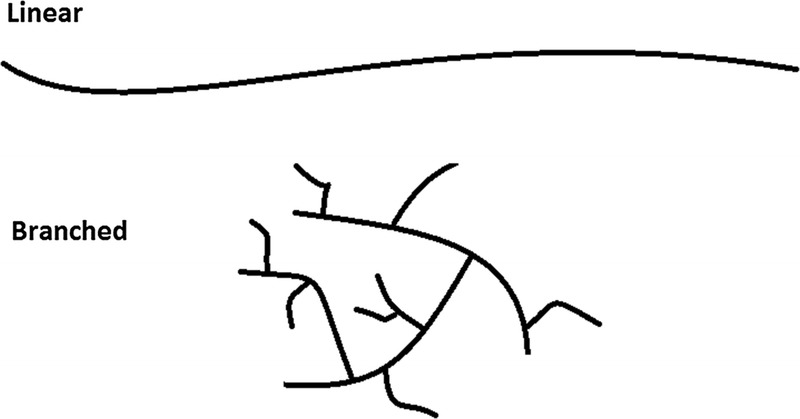
Linear versus branched polymers. Drawings representing linear and branched polysaccharides. The volume “swept out” by a fully extended linear fiber is much greater than a fiber with an equal number of sugar units (same molecular weight) but with a “bush-like,” highly branched configuration. Because the volume occupied by a polymer molecule is a function of the radius cubed, even small increases in effective hydrodynamic size can translate into a large increase in viscosity for linear fibers.
FIGURE 2.
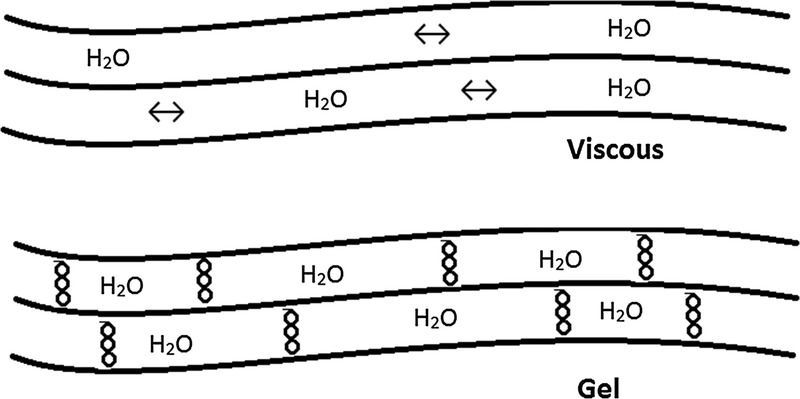
Viscous and gel-forming linear polymers. Drawings representing viscous linear polymers (top) and gel-forming linear polymers (bottom). Long-chain linear polymers orient parallel to adjacent fibers and increase viscosity in a concentration-dependent manner. Some long-chain linear polymers also can form cross-links that create a gel in a concentration-dependent manner (behave as a viscoelastic solid). Gel formation is an important driver of several metabolic health benefits for dietary fiber supplements, including cholesterol lowering, improved glycemic control, satiety, weight control, and stool normalization (soften hard stool in constipation and firm loose/liquid stool in diarrhea). (Drawings recreated with permission from John D. Keller, Jr, Keller Konsulting LLC, Freehold, NJ.)
Fermentation refers to the rate and degree to which a dietary fiber, after resisting enzymatic digestion in the small bowel, can be degraded by gut bacteria, producing fermentation byproducts such as short chain fatty acids and gas.3 Most fibers are not exclusively soluble or insoluble, so for the purposes of this review, the predominant characteristic will be discussed (eg, a fiber that is 70% soluble will be considered a soluble fiber). Using the 4 fiber characteristics described above, fiber supplements can be divided into 4 clinically meaningful categories:
Insoluble, poorly fermented (eg, wheat bran): when you think of “insoluble fiber,” think of plastic (clinical studies described later actually used plastic particles to mimic effects of wheat bran): does not dissolve in water (no water-holding capacity); poorly fermented; can exert a laxative effect by mechanical irritation/stimulation of gut mucosa if particles are sufficiently large and coarse (“plastic effect”); small smooth particles (eg, wheat bran flour/bread) have no significant laxative effect; insoluble fiber does not gel or alter viscosity and thus does not provide other (gel-dependent) fiber health benefits.
Soluble, nonviscous, readily fermented (eg, inulin, wheat dextrin, oligosaccharides, resistant starches): dissolves in water; no increase in viscosity; rapidly and completely fermented (once fermented, the fiber is no longer present in stool, rapid gas formation, increased flatulence, energy harvest [calorie uptake] from fermentation by-products); may alter the numbers of specific bacteria in the gut (eg, “prebiotic” effect); no laxative effect at physiologic doses; does not gel or alter viscosity and thus does not provide any of the gel-dependent fiber health benefits. Readily fermented fibers are part of an emerging area of science relating to their effects on the gut microbiome, but to date, the marketed fiber supplements have no established clinically meaningful health benefits.
Soluble viscous/gel forming, readily fermented (eg, β-glucan [oats, barley], raw guar gum): dissolves in water, forms a viscous gel (eg, oatmeal), increases chyme viscosity to slow nutrient absorption and improve glycemic control, lowers elevated serum cholesterol, readily fermented (gas formation, energy harvest [calorie uptake] from fermentation by-products), fermentation results in loss of gel and water-holding capacity, and thus, no significant laxative effect and no retained gel to attenuate diarrhea.
Soluble viscous/gel forming, nonfermented (ie, psyllium): dissolves in water; forms a viscous gel; increases chyme viscosity to slow nutrient absorption and improve glycemic control, lowers elevated serum cholesterol; not fermented (no gas production, no calorie harvest from fermentation by-products); because it is not fermented, it remains gelled throughout the large bowel, providing a dichotomous “stool-normalizing” effect: softens hard stool in constipation (relieves/prevents constipation) and firms/forms loose/liquid stool in diarrhea (relieves/prevents diarrhea), and normalizes stool form in IBS.
SMALL INTESTINAL EFFECTS: IMPROVED GLYCEMIC CONTROL AND CHOLESTEROL LOWERING ARE GEL-DEPENDENT HEALTH BENEFITS
The small intestine is ≈7 m long and divided anatomically into 3 regions: duodenum, jejunum, and ileum. The mucosa of the small intestine is studded with millions of small villi, each covered with ≈1000 microvilli per 0.1 μm2, making the small intestine the largest body surface exposed to the outside world (approximately 250 m2, roughly the size of a tennis court).3,16 Delivery of acidic nutrients into the duodenum (proximal small bowel) stimulates pancreatic secretions (inorganic = water, bicarbonate, and electrolytes; organic = digestive enzymes) as well as the release of bile from the gall bladder. The total quantity of fluid absorbed by the small bowel each day is a combination of fluids consumed (≈1.5 L/d) and the digestive juices secreted (≈6-7 L/d). In the fed state, the motor activity of the small bowel predominantly consists of segmental (mixing) contractions.3,16 These segmental contractions mix chyme back and forth, exposing food particles to digestive enzymes and bile and facilitating exposure of digested nutrients to the absorptive brush border of the mucosa for absorption. Chyme, the liquid content of the small intestine, is normally very low in viscosity and is easily mixed with digestive enzymes for degradation and easily exposed to the villi for absorption of nutrients. The very large surface area of the mucosa normally results in efficient absorption of nutrients, which occurs early in the proximal small bowel.3,16
Introduction of insoluble fiber (eg, wheat bran) or soluble nonviscous fiber (eg, inulin, wheat dextrin) has no significant effect on the rate of nutrient absorption in the small bowel because neither type forms a gel to alter the viscosity of chyme. In contrast, introduction of a soluble viscous, gel-forming fiber (eg, guar gum, psyllium, high-molecular-weight β-glucan) will significantly increase the viscosity of chyme in a dose-dependent manner, which will slow the mixing of chyme with digestive enzymes. This will lead to a slowing of the degradation of complex nutrients into simple, absorbable components and also slow turnover of chyme at the villi, all of which will slow the absorption of glucose and other nutrients.3 This slowing of nutrient degradation and absorption lowers peak serum glucose concentration after a meal and delivers nutrients further into the small bowel for absorption.
An effective gelling fiber can delay the absorption of nutrients long enough to deliver nutrients to the distal ileum, where they are not normally present. Nutrients delivered to the distal ileum stimulate mucosal receptors to initiate a cascade of metabolic responses, 1 of which is the release of glucagon-like peptide-1 into the blood stream. Glucagon-like peptide-1 is a short-lived (≈2-minute half-life) peptide that significantly decreases appetite, increases insulin secretion, decreases glucagon secretion (a peptide that stimulates glucose production in the liver), increases pancreatic β-cell growth (cells that produce insulin), improves insulin production and sensitivity, and slows gastric emptying and small bowel transit via a feedback loop called the “ileal brake” phenomenon.3 All of the above metabolic responses are therapeutic targets for treating type 2 diabetes mellitus. Taken together, the sum of these phenomenon leads to a gel-dependent improvement in glycemic control for patients with type 2 diabetes and those at risk for developing the disease (eg, metabolic syndrome).3,14,17–22
SMALL INTESTINAL EFFECTS: SHORT-TERM AND LONG-TERM IMPROVEMENTS IN GLYCEMIC CONTROL ARE GEL-DEPENDENT HEALTH BENEFITS
There are 2 primary methods for assessing the effects of fiber supplements on glycemic control: an acute postprandial study and a long-term assessment of glycemic control. The acute postprandial test (glucose tolerance test) provides a glucose load (eg, 50 g glucose solution) with and without fiber supplementation. Blood glucose concentrations are drawn at frequent, predetermined intervals over a few hours to assess the rate of glucose absorption. Glucose is normally very rapidly absorbed in the most proximal region of the small intestine, resulting in a relatively fast rise in blood glucose and a high peak concentration. Because of a short lag in insulin response, this is typically followed by rapid decline in glucose with a transient excursion below the baseline level (Figure 3A,C). This transient hypoglycemia is caused by the lag in insulin response, which tends to stay elevated past the point where the blood glucose concentration has returned to baseline.
FIGURE 3.
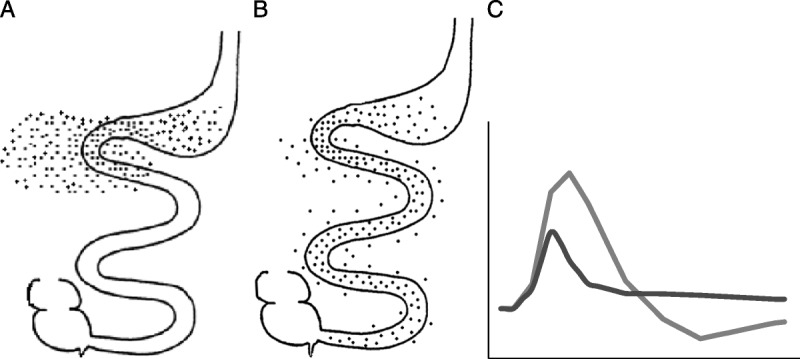
Absorption of nutrients in the small bowel is delayed by viscous fiber. Diagrams of nutrient absorption in the small bowel. Nutrients normally absorb very early in the proximal small bowel (A). Introduction of a viscous, gel-forming fiber (eg, guar gum, psyllium, high-molecular-weight β-glucan) can delay nutrient absorption to more distal regions of the small bowel (B). Rapid nutrient absorption (C: grey line, corresponds with A) is reflected by the higher peak concentration of blood glucose followed by a transient hypoglycemic trough below baseline. With the introduction of a viscous, gel-forming soluble fiber, the delay in nutrient absorption (C: black line, corresponds with B) results in an attenuation of glucose excursions: lower peak concentration of blood glucose, and attenuated hypoglycemic trough. (Drawings recreated with permission from Thomas Wolever, PhD, University of Toronto.)
It has been established for more than 3 decades that the viscosity of a gel-forming fiber supplement is highly correlated with minimizing the high and low excursions of postprandial glucose. In a study published in 1978,23 volunteers underwent glucose (50 g) tolerance tests with and without the addition of several fiber supplements, including guar gum. Native guar gum is a highly viscous, gel-forming fiber, and it exhibited a clinically meaningful decrease in both postprandial blood glucose and insulin concentrations. This beneficial response, however, was abolished when the guar gum was hydrolyzed to a nonviscous form, as is typically marketed today—partially hydrolyzed guar gum. The study showed that a reduction in postprandial blood glucose was highly correlated with the viscosity of a gel-forming fiber (r = 0.926; P < .01), as was a slowing of small bowel transit (r = 0.885; P < .02). Taken together, a high-viscosity, gel-forming fiber supplement (eg, raw guar gum, high-molecular-weight β-glucan, psyllium) can provide a clinically meaningful effect on elevated blood glucose level in a viscosity/dose-dependent manner, but nonviscous soluble fiber supplements (eg, wheat dextrin, inulin) do not provide a gel-dependent, clinically meaningful glycemic benefit.1,3
The second method, long-term assessment of glycemic control, entails multimonth, well-controlled, randomized clinical studies that assess a fiber’s effects on glycemic control in the target population. These long-term studies provide evidence of a sustained effect and should be the standard for establishing a clinically meaningful therapeutic response for a given fiber supplement. Numerous multimonth (2–6 months) clinical studies have demonstrated that consumption of a soluble, gel-forming fiber supplement with meals can improve glycemic control (lowers fasting blood glucose, insulin, and hemoglobin A1c concentrations) in subjects at risk for type 2 diabetes (eg, metabolic syndrome) and in patients being treated for type 2 diabetes.1,3,24–38
An example is an 8-week, placebo-controlled clinical study that evaluated psyllium (5.1 g BID) for improved glycemic control in 49 patients being treated for type 2 diabetes (fasting blood glucose and hemoglobin A1c at baseline: psyllium group, 208 mg/dL and 10.5; placebo group, 179 mg/dL and 9.1).38 After 8 weeks of treatment, fasting blood glucose for the psyllium group showed a significant decrease (−89.7 mg/dL; P < .05) versus placebo. Hemoglobin A1c also showed a significant decrease (−3.0) versus placebo.38 Note that the improvement in glycemic control observed with psyllium was above that already conferred by a restricted diet and stable doses of a sulfonylurea and/or metformin. The long-term effects of an effective gel-forming fiber on fasting blood glucose concentrations are proportional to baseline glycemic control: there is no significant effect on normal blood glucose concentrations in healthy subjects.39 A moderate effect in patients with prediabetes and metabolic syndrome (eg, −19.8 mg/dL for psyllium 3.5 g BID; −9 mg/dL for guar gum 3.5 g BID),26 and a larger effect in patients with type 2 diabetes (eg, psyllium, −35.0 to −89.7 mg/dL).34,38 Note that gel-forming fiber supplements will not directly cause hypoglycemia (suppression of glucagon by glucagon-like peptide-1 does not occur at hypoglycemic levels), but for patients being treated for type 2 diabetes, fasting blood glucose should be closely monitored with the initiation of an effective fiber therapy as the fiber supplement may reduce the required dose of hypoglycemic drugs.
SMALL INTESTINAL EFFECTS: CHOLESTEROL LOWERING IS A GEL-DEPENDENT HEALTH BENEFIT
It is well established that reducing elevated serum low-density lipoprotein (LDL)-cholesterol concentration reduces the risk of coronary artery disease.40 It has been estimated that a 1% reduction in LDL-cholesterol concentration reduces the risk of coronary artery disease by 1.2% to 2.0%.41 Similar to the gel-dependent nature of improved glycemic control with fiber supplements, lowering elevated serum cholesterol concentrations is also a gel-dependent phenomenon in the small bowel, and the effectiveness of cholesterol lowering is highly correlated with the viscosity of the gel-forming fiber: The higher the viscosity of a gel-forming fiber is, the greater the effect on lowering elevated cholesterol concentrations.37 Clinical studies have shown that the viscosity of a gel-forming fiber is actually a better predictor of cholesterol-lowering efficacy than is the quantity of fiber consumed.42 The primary mechanism by which gel-forming fibers lower serum cholesterol is by trapping and eliminating bile. Bile is secreted by the liver (normally 600–1000 mL/d) to emulsify large fat particles into many small particles for digestion by lipase enzymes and absorption across the mucosa.16 Bile is normally highly conserved, recovered in the distal ileum, and recycled up to several times within a single meal. When bile is trapped in a gel-forming fiber and eliminated via stool, the liver must produce more bile to meet digestive needs. Cholesterol is a component of bile, and the liver uses serum stores of cholesterol to generate more bile, effectively lowering serum LDL-cholesterol and total cholesterol concentration, without affecting high-density lipoprotein cholesterol.43
To assess the importance of viscosity for a gel-forming fiber in lowering elevated serum cholesterol concentration, a clinical study compared the cholesterol-lowering effects of a medium-viscosity blend of gel-forming fibers (psyllium, pectin, guar gum, and locust bean gum) with those of an equal amount (three 5-g servings per day for 4 weeks) of low-viscosity gum Arabic (Acacia gum, highly branched) in 26 patients with hypercholesterolemia.44 The medium-viscosity gel-forming blend exhibited a 10% reduction in total cholesterol concentration (P < .01) and a 14% reduction in LDL-cholesterol concentration (P < .001), with no significant change in high-density lipoprotein or triglyceride levels. In contrast, the low-viscosity gum Arabic-treated group showed no change in any plasma lipid characteristics.44 A second publication with 4 studies (duration 4–12 weeks) explored the plasma lipid-lowering effects of a variety of soluble dietary fibers.45 The studies were randomized, double-blind, placebo-controlled trials involving men and women with hyperlipidemia (plasma cholesterol >200 mg/dL). Low-viscosity gum Arabic (acacia gum) (15 g/d for 4 weeks) did not produce a significant lipid-lowering effect versus placebo. In contrast, 15 g/d of a medium-viscosity blend of soluble fibers (psyllium, pectin, guar gum, and locust bean gum) consumed for 4 weeks yielded significant reductions in total cholesterol (8.3%) and LDL-cholesterol (12.4%) concentrations (P < .001), similar to the 10 g/d high-viscosity raw guar gum. The lipid-lowering benefit of the medium-viscosity blend of soluble fibers (psyllium, pectin, guar gum, and locust bean gum) also showed a dose-response effect for reducing LDL-cholesterol concentration: placebo, +0.8%; 5 g/d, −5.6%; 10 g/d, −6.8%, and 15 g/d, −14.9% (all doses P < .01 vs placebo). The effects of the gel-forming fibers on plasma lipids were similar for both men and women. The authors concluded that the findings support the usefulness of medium- and high-viscosity gel-forming fibers as a cholesterol-lowering therapy but cautioned against ascribing cholesterol lowering benefits solely on a classification of solubility.45 As with improved glycemic control, the viscosity of the gel-forming fiber is the key driver of efficacy for lowering cholesterol in patients with hyperlipidemia.
Also similar to the gel-dependent effect glycemic control, the potential for a cholesterol-lowering benefit is highly influenced by the baseline cholesterol level: Gel-forming fibers have no appreciable effect on cholesterol concentrations in healthy subjects with normal cholesterol concentrations but exhibit a progressively greater benefit as baseline cholesterol exceeds normal concentrations. The cholesterol-lowering benefit of gel-forming fiber supplements is observed in addition to the benefits conveyed by the prescription drugs in patients already being treated for hyperlipidemia. Eight clinical studies have shown that a gel-forming fiber (psyllium) enhanced the cholesterol-lowering benefit of prescription drugs when dosed as a cotherapy to statin drugs and bile sequestrants.46–53 There are only 2 fiber supplements approved by the Food and Drug Administration to claim a reduced risk of cardiovascular disease by lowering serum cholesterol: β-glucan (oats and barley) and psyllium, both gel-forming fibers.54
An effective gel-forming fiber supplement can actually lower the required dose of a prescription statin drug. For example, in a 12-week randomized, double-blind study including 68 patients with hyperlipidemia, a low dose of simvastatin (10 mg) combined with psyllium (15 g/d, divided doses before meals) was superior to the low dose of simvastatin alone (−63 vs −55 mg/dL, respectively; P = .03) and identical to a high dose of simvastatin alone (20 mg, −63 mg/dL) for lowering elevated serum LDL-cholesterol concentration.55 Gel-forming fibers can provide an effective cotherapy for hypoglycemic and cholesterol-lowering drugs to help reduce required doses and potentially reduce side effects. As described above, low-viscosity fibers (gum Arabic/acacia gum, methylcellulose) and nonviscous supplements (eg, inulin, wheat dextrin) do not exhibit a cholesterol-lowering benefit.37,44,45,56,57
CONCLUSIONS
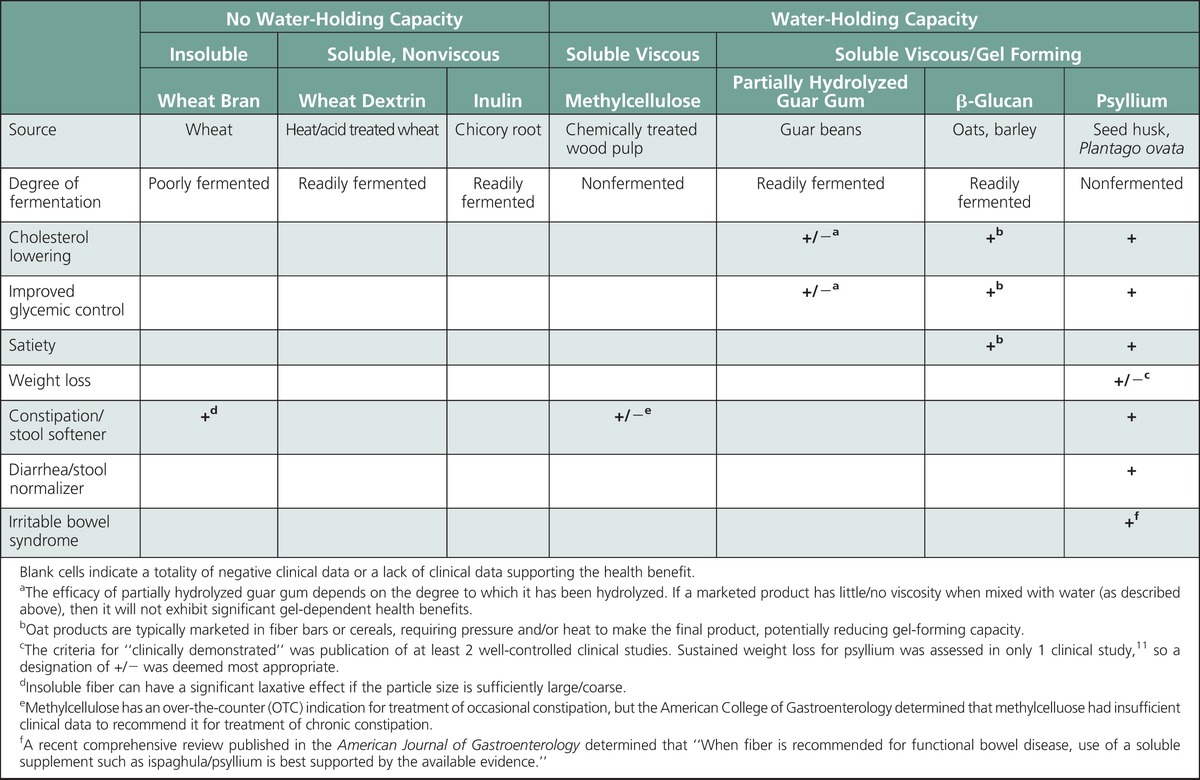
Despite a general consensus that fiber is “good for you,” it is important to recognize the difference between replacement with dietary fiber that is intrinsic and intact in whole foods and supplement with an isolated fiber source. Fiber supplements cannot be presumed to have the same health benefits that are associated with dietary fiber that is intact and intrinsic in whole foods. The clinically proven health benefits for fiber supplements are associated with specific characteristics (eg, viscous gel), and only a minority of marketed fiber products provide health benefits (summarized in the Table). Health benefits associated with fiber effects in the small bowel (eg, cholesterol lowering, improved glycemic control, satiety, weight loss) are a gel-dependent phenomenon, and the degree of benefit is proportional to the viscosity of the gelling fiber. Health benefits associated with fiber effects in the large bowel (eg, relief from constipation, diarrhea, IBS) will be discussed in detail in part 2 of this 2-part series. Briefly, large bowel effects are derived from 2 mechanisms: An insoluble fiber provides a mechanical stimulus proportional to particle size (eg, wheat bran—softens hard stool in constipation but can exacerbate diarrhea and IBS) and a nonfermented gel-forming fiber that remains intact/retains its high water-holding capacity throughout the large bowel can provide a stool-normalizing effect (ie, psyllium—softens hard stool in constipation, firms loose/liquid stool in diarrhea, normalizes stool form in IBS). When recommending a fiber supplement, only a soluble nonfermenting, gel-forming fiber has been clinically proven to provide all of the health benefits typically associated with a fiber supplement.
TABLE.
Clinically Demonstrated Health Benefits Associated With Common Fiber Supplements
Footnotes
The author is a full-time employee of the Procter & Gamble Company, which markets a fiber product.
REFERENCES
- 1. Chutkan R, Fahey G, Wright W, McRorie J. Viscous versus non-viscous soluble fiber supplements: mechanisms and evidence for fiber-specific health benefits. J Am Acad Nurse Pract. 2012; 24: 476– 487. [DOI] [PubMed] [Google Scholar]
- 2. Klosterbuer A, Roughead Z, Slavin J. Benefits of dietary fiber in clinical nutrition. J Nutr Clin Pract. 2011; 26( 5): 625– 635. [DOI] [PubMed] [Google Scholar]
- 3. McRorie J, Fahey G. A review of gastrointestinal physiology and the mechanisms underlying the health benefits of dietary fiber: matching an effective fiber with specific patient needs. Clin Nurs Stud. 2013; 1( 4): 82– 92. [Google Scholar]
- 4. Slavin J. Position of the American Dietetic Association: health implications of dietary fiber. JAMA. 2008; 108: 1716– 1731. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Agriculture. Why is it important to eat vegetables? http://www.choosemyplate.gov/food-groups/vegetables.html Accessed March 6, 2015.
- 6. Wolever TM. Is glycaemic index (GI) a valid measure of carbohydrate quality? Eur J Clin Nutr. 2013; 67( 5): 522– 531. [DOI] [PubMed] [Google Scholar]
- 7. Mozaffarian D, Kumanyika SK, Lemaitre RN, Olson JL, Burke GL, Siscovick DS. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA. 2003; 289: 1659– 1666. [DOI] [PubMed] [Google Scholar]
- 8. Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996; 275: 447– 451. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, Carbohydrates, Fiber, Fat, Fatty Acids Cholesterol, Protein and Amino Acids. Washington, DC: National Academies Press; 2002. [Google Scholar]
- 10. Slavin J. Dietary fiber and body weight. Nutrition. 2005; 21: 411– 418. [DOI] [PubMed] [Google Scholar]
- 11. Dikeman C, Fahey G. Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr. 2006; 46: 649– 663. [DOI] [PubMed] [Google Scholar]
- 12. Anderson JW. All fibers are not created equal. J Med. 2009; 2: 87– 91. [Google Scholar]
- 13. Anderson J, Baird P, Davis R, et al. Health benefits of dietary fiber. Nutr Rev. 2009; 67: 188– 205. [DOI] [PubMed] [Google Scholar]
- 14. Guillon F, Champ MM. Carbohydrate fractions of legumes: uses in human nutrition and potential for health. Br J Nutr. 2002; 88( Suppl 3): S293– S306. [DOI] [PubMed] [Google Scholar]
- 15. Morris ER, Cutler AN, Ross-Murphy DA, Rees DA, Price J. Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydrate Polym. 1981; 1: 5– 21. [Google Scholar]
- 16. Guyton A, Hall J. Textbook of Medical Physiology. 11th ed Philadelphia, PA: Elsevier Inc; 2006. Unit XII, Chapters 62–66. [Google Scholar]
- 17. Dikeman C, Murphy M, Fahey G. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestine digesta. J Nutr. 2006; 136: 913– 919. [DOI] [PubMed] [Google Scholar]
- 18. Holst J, Deacon C, Vilsbøll T, Krarup T, Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008; 14: 161– 168. [DOI] [PubMed] [Google Scholar]
- 19. Jenkins D, Wolever T, Taylor R, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981; 34: 362– 366. [DOI] [PubMed] [Google Scholar]
- 20. Maljaars P, Peters H, Kodde A, et al. Length and site of the small intestine exposed to fat influences hunger and food intake. Br J Nutr. 2011; 106: 1609– 1615. [DOI] [PubMed] [Google Scholar]
- 21. Maljaars P, Peters H, Mela D, Masclee A. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav. 2008; 95: 271– 281. [DOI] [PubMed] [Google Scholar]
- 22. Schirra J, Goke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept. 2005; 128: 109– 115. [DOI] [PubMed] [Google Scholar]
- 23. Jenkins D, Wolever T, Leeds A, et al. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br Med J. 1978; 1: 1392– 1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson J, Allgood L, Turner C, Oelgten P, Daggy B. Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes and hypercholesterolemia. Am J Clin Nutr. 1999; 70: 466– 473. [DOI] [PubMed] [Google Scholar]
- 25. Beer M, Arrigoni E, Amado R. Effects of oat gum on blood cholesterol levels in healthy young me. Eur J Clin Nutr. 1995; 49: 517– 522. [PubMed] [Google Scholar]
- 26. Cicero AFG, Derosa G, Bove M, Imola F, Borghi C, Gaddi AV. Psyllium improves dyslipidemaemia, hyperglycaemia and hypertension, while guar gum reduces body weight more rapidly in patients affected by metabolic syndrome following an AHA Step 2 diet. Mediterr J Nutr Metab. 2010; 3: 47– 54. [Google Scholar]
- 27. Dall’Alba V, Silva FM, Antonio JP, et al. Improvement of the metabolic syndrome profile by soluble fibre—guar gum—in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr. 2013; 110( 9): 1601– 1610. [DOI] [PubMed] [Google Scholar]
- 28. Feinglos M, Gibb R, Ramsey D, Surwit R, McRorie J. Psyllium improves glycemic control in patients with type-2 diabetes mellitus. Bioactive Carbohydrates Dietary Fibre. 2013; 1: 156– 161. [Google Scholar]
- 29. Gupta RR, Agrawal CG, Singh GP, Ghatak A. Lipid-lowering efficacy of psyllium hydrophilic mucilloid in non-insulin dependent diabetes mellitus with hyperlipidemia. Indian J Med Res. 1994; 100: 237– 241. [PubMed] [Google Scholar]
- 30. Karmally W, Montez M, Palmas W, et al. Cholesterol-lowering benefits of oat-containing cereal in Hispanic Americans. J Am Diet Assoc. 2005; 105: 967– 970. [DOI] [PubMed] [Google Scholar]
- 31. Keogh G, Cooper G, Mulvey T, et al. Randomized controlled cross-over study of the effect of a highly β-glucan–enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am J Clin Nutr. 2003; 78: 711– 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kerkhoffs D, Hornstra G, Mensick R. Cholesterol-lowering effect of β-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when β-glucan is incorporated into bread and cookies. Am J Clin Nutr. 2003; 78: 221– 227. [DOI] [PubMed] [Google Scholar]
- 33. Naumann E, Van Rees A, Onning G, Oste R, Wydra M, Mensick R. β-glucan incorporated into a fruit drink effectively lowers serum LDL-cholesterol concentrations. Am J Clin Nutr. 2006; 83: 601– 605. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez-Moran M, Guerrero-Romero F, Laczano-Burciaga L. Lipid- and glucose-lowering efficacy of plantago psyllium in type II diabetes. J Diabetes Complicat. 1998; 12: 273– 278. [DOI] [PubMed] [Google Scholar]
- 35. Sartore G, Carlström S, Scherstén B. Dietary supplementation of fibre (Lunelax) as a means to reduce postprandial glucose in diabetics. Acta Med Scand Suppl. 1981; 656: 51– 53. [DOI] [PubMed] [Google Scholar]
- 36. Tosh SM. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr. 2013; 67( 4): 310– 317. [DOI] [PubMed] [Google Scholar]
- 37. Wolever T, Tosh S, Gibbs A, Brand-Miller J, et al. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. Am J Clin Nutr. 2010; 92: 723– 732. [DOI] [PubMed] [Google Scholar]
- 38. Ziai S, Larijani B, Akhoondzadeh S, et al. Psyllium decreased serum glucose and glycosylated hemoglobin significantly in diabetic outpatients. J Ethnopharmacol. 2005; 102: 202– 207. [DOI] [PubMed] [Google Scholar]
- 39. Bell LP, Hectorne K, Reynolds H, Balm T, Hunninghake D. Cholesterol-lowering effects of psyllium hydrophilic mucilloid. Adjunct therapy to a prudent diet for patients with mild to moderate hypercholesterolemia. JAMA, 1989; 261 (23): 3419– 3423. [DOI] [PubMed] [Google Scholar]
- 40. Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated metaanalysis. Clin Ther. 2009; 31: 236– 244. [DOI] [PubMed] [Google Scholar]
- 41. Katan MB, Grundy SM, Jones P, et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003; 78( 8): 965– 978. [DOI] [PubMed] [Google Scholar]
- 42. Vuksan V, Jenkins AL, Rogovik AL, Fairgrieve CD, Jovanovski E, Leiter LA. Viscosity rather than quantity of dietary fibre predicts cholesterol-lowering effect in healthy individuals. Br J Nutr. 2011; 106: 1349– 1352. [DOI] [PubMed] [Google Scholar]
- 43. Gunness P, Gidley M. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010; 1: 149– 155. [DOI] [PubMed] [Google Scholar]
- 44. Jensen C, Spiller G, Gates J, Miller A, Whittam J. The effect of acacia gum and a water-soluble dietary fiber mixture on blood lipids in humans. J Am Coll Nutr. 1993; 12( 2): 147– 154. [DOI] [PubMed] [Google Scholar]
- 45. Haskell WL, Spiller GA, Jensen CD, Ellis BK, Gates JE. Role of water-soluble dietary fiber in the management of elevated plasma cholesterol in healthy subjects. Am J Cardiol. 1992; 69( 5): 433– 439. [DOI] [PubMed] [Google Scholar]
- 46. Agrawal A, Tandon M, Sharma P. Effect of combining viscous fibre with lovastatin on serum lipids in normal human subjects. Int J Clin Pract. 2007; 61: 1812– 1818. [DOI] [PubMed] [Google Scholar]
- 47. Jayaram S, Prasad HB, Sovani VB, Langade DG, Mane PR. Randomised study to compare the efficacy and safety of isapgol plus atorvastatin versus atorvastatin alone in subjects with hypercholesterolaemia. J Indian Med Assoc. 2007; 105( 3): 142– 145. [PubMed] [Google Scholar]
- 48. Maciejko JJ, Brazg R, Shah A, Patil S, Rubenfire M. Psyllium for the reduction of cholestyramine-associated gastrointestinal symptoms in the treatment of primary hypercholesterolemia. Arch Fam Med. 1994; 3: 955– 960. [DOI] [PubMed] [Google Scholar]
- 49. Neal G, Balm T. Synergistic effects of psyllium in the dietary treatment of hypercholesterolemia. South Med J. 1990; 83: 1131– 1137. [DOI] [PubMed] [Google Scholar]
- 50. Roberts D, Truswell A, Bencke A, Dewar H, Farmakalidis E. The cholesterol-lowering effect of a breakfast cereal containing psyllium fibre. Med J Aust. 1994; 161: 660– 664. [PubMed] [Google Scholar]
- 51. Shrestha S, Freake H, McGrane M, Volek J, Fernandez M. A combination of psyllium and plant sterols alters lipoprotein metabolism in hypercholesterolemic subjects by modifying the intravascular processing of lipoproteins and increasing LDL uptake. J Nutr. 2007; 137( 5): 1165– 1170. [DOI] [PubMed] [Google Scholar]
- 52. Shrestha S, Volek JS, Udani J, et al. A combination therapy including psyllium and plant sterols lowers LDL cholesterol by modifying lipoprotein metabolism in hypercholesterolemic individuals. J Nutr. 2006; 136( 10): 2492– 2497. [DOI] [PubMed] [Google Scholar]
- 53. Spence J, Huff M, Heidenheim P, et al. Combination therapy with colestipol and psyllium mucilloid in patients with hyperlipidemia. Annals of Internal Medicine. 1995; 123: 493– 499. [DOI] [PubMed] [Google Scholar]
- 54.Code of Federal Regulations, Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.81. Accessed March 6, 2015.
- 55. Moreyra AE, Wilson AC, Koraym A. Effect of combining psyllium fiber with simvastatin in lowering cholesterol. Arch Intern Med. 2005; 165: 1161– 1166. [DOI] [PubMed] [Google Scholar]
- 56. Anderson J, Floore T, Geil P, Spencer D, Balm T. Hypocholesterolemic effects of different bulk-forming hydrophilic fibers as a adjuncts to dietary therapy in mild to moderate hypercholesterolemia. Arch Intern Med. 1991; 151: 1597– 1602. [PubMed] [Google Scholar]
- 57. Brownawell AM, Caers W, Gibson GR, et al. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr. 2012; 142( 5): 962– 974. [DOI] [PubMed] [Google Scholar]


