Abstract
Dietary fiber that is intrinsic and intact in fiber-rich foods (eg, fruits, vegetables, legumes, whole grains) is widely recognized to have beneficial effects on health when consumed at recommended levels (25 g/d for adult women, 38 g/d for adult men). Most (90%) of the US population does not consume this level of dietary fiber, averaging only 15 g/d. In an attempt to bridge this “fiber gap,” many consumers are turning to fiber supplements, which are typically isolated from a single source. Fiber supplements cannot be presumed to provide the health benefits that are associated with dietary fiber from whole foods. Of the fiber supplements on the market today, only a minority possess the physical characteristics that underlie the mechanisms driving clinically meaningful health benefits. In this 2-part series, the first part (previous issue) described the 4 main characteristics of fiber supplements that drive clinical efficacy (solubility, degree/rate of fermentation, viscosity, and gel formation), the 4 clinically meaningful designations that identify which health benefits are associated with specific fibers, and the gel-dependent mechanisms in the small bowel that drive specific health benefits (eg, cholesterol lowering, improved glycemic control). The second part (current issue) of this 2-part series will focus on the effects of fiber supplements in the large bowel, including the 2 mechanisms by which fiber prevents/relieves constipation (insoluble mechanical irritant and soluble gel-dependent water-holding capacity), the gel-dependent mechanism for attenuating diarrhea and normalizing stool form in irritable bowel syndrome, and the combined large bowel/small bowel fiber effects for weight loss/maintenance. The second part will also discuss how processing for marketed products can attenuate efficacy, why fiber supplements can cause gastrointestinal symptoms, and how to avoid symptoms for better long-term compliance.
LARGE INTESTINE EFFECTS OF FIBER SUPPLEMENTS
The large intestine is composed of the cecum (most proximal portion, receives liquid residue from distal ileum), the colon (ascending, transverse, descending, and sigmoid), the rectum, and the anus. Approximately 1500 mL of liquid residue arrives in the large intestine daily, and normally, more than 90% of the water and electrolytes are gradually reabsorbed, resulting in formed stool.1 The motor events of the large intestine are ≈ 95% segmental (“mixing” waves) that facilitate the absorption of water and electrolytes, whereas the remaining ≈ 5% are propagating contractions (peristalsis).2 Propagating contractions occur over a wide range of amplitudes and transit rates, from frequent low-amplitude (10 mm Hg), rapidly propagating (up to 17 cm/s) waves that act like a “squeegee” to propel gas past other luminal contents to infrequent high-amplitude (>100 mm Hg), slowly propagating (≤1 cm/s) contractions that are lumen-occluding events, propelling all contents.2 This difference in wave form drives the observed differences in transit rates: Gas can transit the entire gastrointestinal tract in less than 1 hour (flatulence ≈ 14 episodes per day) versus hard stools, which can require days to transit the large bowel (infrequent bowel movements).2,3
LARGE INTESTINE EFFECTS: FIBER SUPPLEMENTS IN CONSTIPATION, DIARRHEA, AND IRRITABLE BOWEL SYNDROME
There are 2 mechanisms by which fiber supplements can improve constipation: (1) mechanical stimulation/irritation of the colonic mucosa and (2) gel-dependent/viscous water-holding capacity that resists dehydration. Both mechanisms require a fiber supplement that is relatively nonfermented, so that most of the fiber remains intact and present in stool throughout the large intestine.
The first mechanism is mechanical stimulation/irritation of the gut mucosa by the particles of insoluble fiber. The mechanical stimulation/irritation results in secretion of mucous and water, resulting in larger/softer stools and faster transit through the large bowel. This mechanism is proportional to particle size and shape—large coarse particles have a significant laxative effect, whereas small smooth particles do not.3–8 This effect was discerned by assessing wheat bran milled to different size/shaped particles versus plastic particles cut to match.6,8 The plastic particles had the same laxative effect as the wheat bran, and the magnitude of the effect was dependent upon the particle size/shape. Insoluble fiber has no water-holding/gel-forming capacity, so insoluble fiber supplements cannot be of benefit for attenuating loose/liquid stools in diarrhea. The mucosal stimulating/irritating effect of insoluble particles can actually make symptoms of diarrhea and irritable bowel syndrome (IBS) worse.9,10
The second mechanism is high water-holding capacity that resists the water-absorbing/dehydrating effects of the large bowel. Nonviscous soluble fibers, like wheat dextrin and inulin, are fermented (not present in stool throughout the large bowel) and have no water-holding capacity and thus do not provide a laxative benefit at physiologic doses.11–24 Wheat dextrin actually has is a constipating effect at physiologic doses (eg, 10-15 g/d).23 Most gel-forming fibers (eg, guar gum, Acacia gum, β-glucan from oats, and barley) are also fermented in the large bowel, resulting in loss of their viscous/gelled nature3,4,25 and no laxative effect. Once fermented, the fiber is no longer intact and present in stool, lacking the water-holding capacity required for soluble fibers to improve stool form and symptoms in constipation, diarrhea, or IBS.
In contrast to the fiber supplements discussed above, psyllium is not fermented in the gut3,26 and retains its water-holding gelled structure throughout the large bowel. Although psyllium has often been reported as fermentable, there exists a significant discrepancy between in vitro data and human (clinical) experience. Under in vitro test conditions, psyllium is mixed with stool and homogenized in high-speed mechanical blender.27–29 The hydrated/gelled psyllium is exposed to rapid mechanical shearing forces that destroy the physical structure of the gel matrix, artificially rendering psyllium nonviscous/fermentable. In contrast, 5 well-controlled clinical studies show that psyllium is not fermented in the human gut.30–34 The 5 clinical studies assessed the fermentation of psyllium versus a negative control (placebo), a positive control (lactulose), and/or comparative fiber supplements (eg, methylcellulose, guar gum, pectin, cellulose), assessing breath gas, flatulence, and/or short-chain fatty acid production. All 5 studies showed that the psyllium gel was not fermented.26 This retained gel allows psyllium to provide a dichotomous effect as a stool normalizer: softens hard stool in constipation (softer bulkier stools that are easier to pass, increased transit rate, improved bowel movement frequency)3,7,35 and improves the consistency of loose/liquid stools in diarrhea (formed stools, slower transit rate, decreased urgency, less frequent bowel movements)36–39
In a randomized, double-blind, clinical study of 170 patients with chronic idiopathic constipation, psyllium was shown to be superior to docusate for increasing stool water content (softer stools; P = .007) and the frequency of bowel movements (P = .02).7 The American College of Gastroenterology Chronic Constipation Task Force systematically reviewed the available clinical evidence regarding the use of fiber supplements in chronic constipation and concluded that there was insufficient clinical evidence to support a recommendation for calcium polycarbophil, methylcellulose, or bran but concluded that psyllium was the only fiber supplement with sufficient clinical evidence to support a recommendation for treatment of chronic constipation.40 Furthermore, a recent (2013) comprehensive review on the effects of fiber in functional bowel disorders concluded that a recommendation for psyllium was best supported by the available clinical evidence.9 This conclusion was in agreement with an earlier systematic review conducted by the American College of Gastroenterology Task Force on IBS, which also concluded that psyllium was effective for IBS.41
SMALL INTESTINE EFFECTS COUPLED WITH LARGE INTESTINE EFFECTS: SATIETY IS INFLUENCED BY GEL FORMATION (SMALL INTESTINE), WHEREAS WEIGHT LOSS/MAINTENANCE IS FURTHER INFLUENCED BY RESISTANCE TO FERMENTATION (GELREMAINS INTACT IN THE LARGE INTESTINE)
A high level of dietary fiber consumption (eg, replacement) has been associated with a 30% reduction in the risk of gaining weight or developing obesity.42–44 As discussed in previous sections, however, care must be taken when attributing the health benefits of dietary replacement to fiber supplements. A recent comprehensive review of available clinical data concluded that resistant starch (soluble, nonviscous, fermentable; eg, wheat dextrin) had no significant effect on satiety or weight loss at physiologic doses.45 A yearlong study in 97 adolescents has been quoted as demonstrating weight loss for a “prebiotic” fiber supplement (soluble, nonviscous, fermentable), but a closer look at the data shows that the prebiotic fiber group (8 g/d) was not different from baseline for body mass index.46 In contrast, gel-forming fibers (eg, guar gum, pectin, and psyllium) have been shown to increase satiety and reduce subsequent energy intake.47–49 A well-cited clinical study demonstrated that apples were significantly more satiating than fiber-free apple juice, even though the juice provided the same level of carbohydrate as the apples.50 Pectin is the gel-forming fiber in apples and has been shown to increase satiety.51
Gel-forming fibers may influence satiety by several mechanisms, including delayed degradation and absorption of nutrients in the small bowel, leading to a “sustained” delivery of nutrients, and delivery of nutrients to the distal ileum with subsequent stimulation of feedback mechanism like the “ileal brake” phenomenon (slows gastric emptying and small bowel transit) and decreased appetite.3,4,52–54 Studies have used an insoluble fiber or a soluble nonviscous fiber as a negative control, reinforcing the assertion that the effect on satiety is a gel-dependent phenomenon.49,55–59 Satiety is often assessed in short-term clinical studies as a tool or mechanism for predicting the potential for decreased energy intake and weight loss, but the end therapeutic goal is weight loss (or prevention of weight regain). A review of the effects of fiber supplements on weight loss60 identified 17 placebo-controlled clinical studies, most of which maintained subjects on energy-restricted diets and fiber supplements (mostly insoluble fiber), provided 3 times daily before meals. Fiber supplement intake ranged from 4.5 to 20 g/d, and the results showed that only 1 of 17 studies provided evidence of weight loss greater than placebo,60 supporting the previous conclusion that insoluble fiber has no significant clinical effects in the small bowel.
A 6-month study compared the effects of viscosity on weight loss by assessing a viscous, gel-forming, nonfermented fiber (psyllium) versus a less viscous, readily fermented fiber (partially hydrolyzed guar gum).61 This randomized controlled clinical study included 141 patients with metabolic syndrome. Patients were maintained on a restricted diet alone (American Heart Association Step 2 diet, negative control) or the restricted diet supplemented with psyllium or partially hydrolyzed guar gum (both dosed 3.5 g twice a day with breakfast and dinner). The control group showed gradual loss in weight over the first 4 months, followed by weight regain (Figure). After 2 months, the guar gum treatment group showed a marked weight reduction (−2.4 kg vs baseline), but this reversed to weight regain over the following 4 months (Figure). In contrast, the psyllium treatment group showed gradual and sustained weight loss across the entire 6-month test period (Figure). At 6 months, weight loss for the psyllium treatment group was −3.3 kg versus baseline, −2.1 kg versus control, and −1.76 kg versus guar gum (P < .01 for all 3 comparisons).61 The data suggest that 2 fiber characteristics, high viscosity/gel forming and nonfermented (no calorie harvest from fiber), may play key roles in the long-term weight loss.61
Figure.
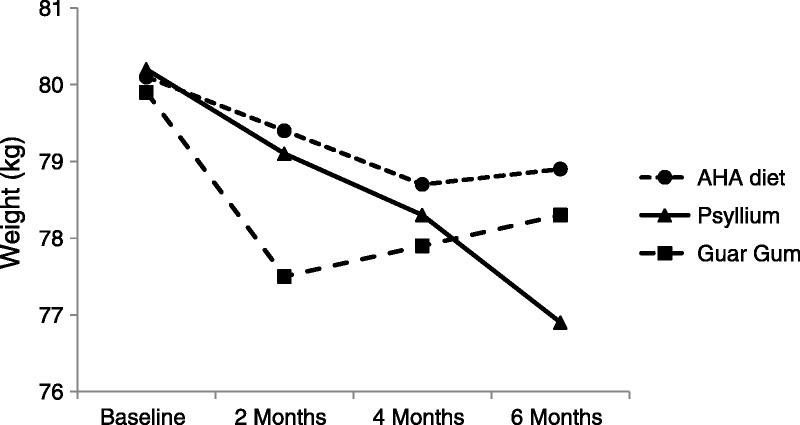
Shows the results of a 6-month study in patients with Metabolic Syndrome. A restricted diet alone showed a modest weight loss over 4 months, followed byweight re-gain. In combinationwith the restricted diet, partially hydrolyzed guar gum (3.5g bid), a less viscous readily fermented fiber, showed amarkedweight loss at 2 months followed by weight re-gain over the following 4 months. In contrast, psyllium (3.5 g twice a day), a viscous, gel-forming, non-fermented fiber supplement, inaddition to therestricteddiet, showed sustained weight loss over the 6-month study. Source: McRorie J, Fahey G. A review of gastrointestinal physiology and the mechanisms underlying the health benefits of dietary fiber: matching an effective fiber with specific patient needs. Clin Nurs Stud. 2013;1(4):82-92.
It is worth noting that the gel-forming fibers in the study above also improved other objective clinical measures of metabolic syndrome. After 6 months of treatment, both the psyllium and guar gum treatment groups showed significant improvement in fasting plasma glucose (−28% vs −11%, respectively), fasting plasma insulin (−20% vs −11%, respectively), hemoglobin A1c (−10% vs −10%, respectively), and low-density lipoprotein (LDL) cholesterol (−8% vs −8%, respectively).61 Only the psyllium group exhibited a significant improvement in plasma triglyceride concentration (−13.3%) and systolic (−3.9%) and diastolic (−2.6%) blood pressure. At the conclusion of the study, 12.5% of patients in the psyllium group no longer qualified for a diagnosis of metabolic syndrome, versus 2.1% of patients in the guar gum group and 0% of patients in the diet-alone group.61 Taken together, these data support that a soluble viscous, gel-forming fiber supplement can be an effective cotherapy for treating metabolic syndrome.
DEGREE OF PROCESSING CAN AFFECT FIBER GELLING BEHAVIOR AND EFFICACY IN MARKETED PRODUCTS
Although it has been clearly shown that raw gel-forming fibers (eg, guar gum, high-molecular-weight β-glucan) can exhibit significant health benefits, it is important to consider how the degree of processing to make a final marketed product may alter the viscosity/gelling capacity of a fiber supplement.3,57,59,62–64 For example, 2 clinical studies investigated the effects of β-glucan from oat bran, either baked into bread and cookies (study 1) or provided as a raw fiber in orange juice (study 2), on serum cholesterol concentrations in 48 subjects with hypercholesterolemia.64 In the first study, subjects completed a 3-week baseline with control bread and cookies rich in wheat fiber (insoluble, negative control), followed by a randomized 4-week treatment period: remained on the control fiber products (negative control) or switched to bread and cookies enriched with β-glucan (5.9 g/d). The β-glucan baked into bread and cookies had no effect on serum LDL-cholesterol (not different from negative control). In contrast to these results, study 2 provided a lower dose of β-glucan (5 g/d) in orange juice, which significantly decreased LDL-cholesterol concentration versus the wheat fiber control (P < .001). The authors concluded that food matrix, food processing, or both could adversely affect the cholesterol-lowering efficacy of β-glucan.64 This emphasizes the importance of recognizing that not all marketed fiber supplements will provide the clinical efficacy of the original raw fiber.
Another example of the importance of processing (heat and pressure) on the viscosity/gel-forming capacity of a raw fiber is a double-blind, parallel-design, multicenter clinical study that randomly assigned 386 subjects to receive cereal containing wheat fiber (negative control) or 1 of 3 oat bran cereals (high, medium, and low viscosity), equaling 3 to 4 g of β-glucan daily.59 The viscosity of the β-glucan was altered by the degree of processing (heat and pressure) to which the fiber was exposed in making the cereal. The results showed that cholesterol lowering was highly correlated with the viscosity of the β-glucan: High viscosity (lower heat and pressure) was correlated with significant cholesterol lowering; low viscosity (higher heat and pressure) was correlated with diminished cholesterol lowering. Taken together, these studies demonstrate that the physicochemical properties of raw oat β-glucan were altered by processing and the degree to which a gelling fiber is processed (hydrolysis, baking, heat/pressure extrusion into cereal shapes) before marketing should be taken into consideration before recommending a particular fiber supplement or cereal.
Attempts to improve the palatability of fiber supplements can also affect the efficacy of a fiber supplement. As discussed previously, raw guar gum is normally a highly viscous, gel-forming fiber with proven gel-dependent health benefits. To improve palatability, however, a commonly marketed version is partially hydrolyzed guar gum, which is a nonviscous product that dissolves completely in water without altering viscosity. Other similar products also make claims of “dissolves completely” and/or “no viscosity” (eg, wheat dextrin, inulin), as if the nonviscous nature of the fiber represented a consumer benefit. Advertising that compares a nonviscous fiber with a gelling fiber, on the basis of relative palatability, carries an underlying implication that the 2 fiber supplements are comparable in efficacy. This implied equality is not supported by clinical data, which shows that most of fiber-related health benefits are dependent on a viscous gel. Because the term fiber supplement implies that regular (daily) consumption will provide essentially the same health benefits of a high-fiber diet, it is reasonable to require evidence of a clinically meaningful health benefit before selecting/recommending a fiber supplement.
This requirement for clinical evidence is consistent with the definition of fiber provided by the Institute of Medicine,64 in which they differentiated between “dietary fiber” and “functional fiber”: “Dietary Fiber is defined in this report as non-digestible carbohydrates and lignin that are intrinsic and intact in plants. Functional Fiber is defined as isolated, non-digestible carbohydrates that have beneficial physiological effects in humans.” Note that the Institute of Medicine definition requires that isolated carbohydrates (fiber supplements) have clinical evidence of a health benefit before being considered a functional fiber. This requirement is also consistent with the Academy of Nutrition and Dietetics position paper on the health implication of dietary fiber, in which they state “Few fiber supplements have been studied for physiological effectiveness, so the best advice is to consume fiber in foods. Look for physiological studies of effectiveness before selecting functional fibers in dietetics practice.”66 Taken together, these observations emphasize the importance of being cognizant of not only the specific fiber types that exhibit characteristics closely associated with specific health benefits (eg, viscosity/gel formation) but also the degree of processing to which the final marketed product has been exposed. For a simple and reasonable test to determine if a fiber supplement can provide gel-dependent health benefits, stir a single dose of the marketed product (usually 2–4 g of fiber) into 120 mL of water and let it stand for 15 minutes. If the fiber supplement does not dissolve in the water, then form a highly viscous gel within the allotted time, it is unlikely to have a clinically meaningful effect on cholesterol lowering, improved glycemic control, appetite control, or other viscosity/gel-related health benefits.
WHY FIBER SUPPLEMENTS CAN CAUSE GASTROINTESTINAL SYMPTOMS, AND HOW TO AVOID SYMPTOMS FOR LONG-TERM COMPLIANCE
Sensations of slight discomfort to cramping pain may be associated with an increase in consumption of dietary fiber, particularly if the patient is constipated and/or a fiber supplement is initiated at a relatively high dose.3,4 When stool is formed, and of similar consistency, there is minimal deformation with peristalsis, so there is no significant bowel wall distention. In normal individuals, this propulsion is not typically perceived unless it causes stool to fill the rectum, stimulating an urge to defecate.3,4,67 In contrast, if a propagating contraction causes a bolus of lower-viscosity fiber-rich stool to collide with more distal formed/hard stool, the lower-viscosity fiber-rich stool deforms to cause acute dilation of the bowel, stretching mechanoreceptors and causing sensations of discomfort to cramping pain. The discomfort/pain would be transient, occurring with the frequency of propagating contractions, and relieved with a bowel movement.
To facilitate long-term compliance with a fiber supplement regimen, it is important to minimize significant differences in stool viscosity. For nonconstipated subjects, this entails starting a new fiber supplement gradually, initiating dosing at no more than 3 or 4 g/d the first week, then increasing very gradually over subsequent weeks with a goal of about 10 to 15 g/d. For constipated patients, any introduction of a new fiber regimen carries a significant risk of cramping pain unless the hard stool is eliminated first. A reasonable suggestion is to first clear the hard stool from the bowel with a significant dose of an osmotic laxative (eg, polyethylene glycol). The ensuing cramping pain and potential loose stool after evacuation of the hard stool will be associated with the osmotic laxative, not a fiber supplement. Once the hard stool is cleared, gradually introduce a new fiber supplement as above. This may improve long-term compliance with a new fiber supplement.
Conclusions
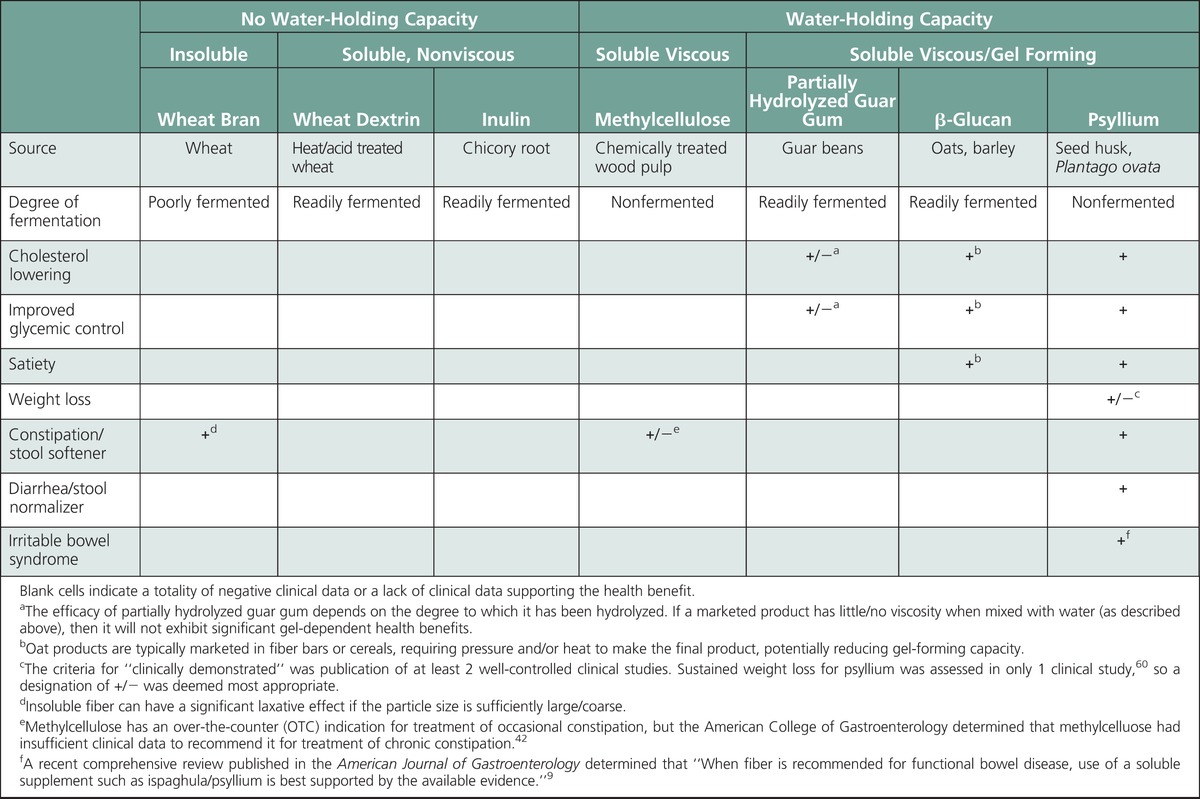
Despite a general consensus that fiber is “good for you,” it is important to recognize the difference between replacement with dietary fiber that is intrinsic and intact in whole foods and a supplement with an isolated fiber source. Fiber supplements cannot be presumed to have the same health benefits that are associated with dietary fiber that is intact and intrinsic in whole foods. The clinically proven health benefits for fiber supplements are associated with specific characteristics (eg, viscous gel), and only a minority of marketed fiber products provide health benefits (summarized in the Table). As described in part 1 of this 2-part series, the health benefits associated with fiber effects in the small bowel (eg, cholesterol lowering, improved glycemic control) are a gel-dependent phenomenon, and the degree of benefit is proportional to the viscosity of the gelling fiber. As described in part 2 of this series, the health benefits associated with fiber effects in the large bowel (eg, relief from constipation, diarrhea, IBS) are derived from 2 mechanisms: An insoluble fiber provides a mechanical stimulus proportional to particle size (eg, wheat bran—softens hard stool in constipation but can exacerbate diarrhea and IBS), whereas a soluble, nonfermented gel-forming fiber retains its high water-holding capacity throughout the large bowel to provide a stool normalizing effect (ie, psyllium—softens hard stool in constipation, firms loose/liquid stool in diarrhea, normalizes stool form in IBS). When recommending a fiber supplement, only a soluble nonfermenting, gel-forming fiber has been clinically proven to provide all of the health benefits typically associated with a fiber supplement.
TABLE.
Clinically Demonstrated Health Benefits Associated With Common Fiber Supplements
Footnotes
The author is a full-time employee of the Procter & Gamble Company, which markets a fiber product.
REFERENCES
- 1. Guyton A, Hall J. Textbook of Medical Physiology. 11th ed Philadelphia, PA: Elsevier Inc; 2006. Unit XII, Chapters 62–66. [Google Scholar]
- 2. McRorie J, Greenwood-Van Meerveld B, Rudolph C. Characterization of propagating contractions in the proximal colon of ambulatory mini pigs. Dig Dis Sci. 1998; 43( 5): 957– 963. [DOI] [PubMed] [Google Scholar]
- 3. McRorie J, Fahey G. A review of gastrointestinal physiology and the mechanisms underlying the health benefits of dietary fiber: matching an effective fiber with specific patient needs. Clin Nurs Stud. 2013; 1( 4): 82– 92. [Google Scholar]
- 4. Chutkan R, Fahey G, Wright W, McRorie J. Viscous versus non-viscous soluble fiber supplements: mechanisms and evidence for fiber-specific health benefits. J Am Acad Nurse Pract. 2012; 24: 476– 487. [DOI] [PubMed] [Google Scholar]
- 5. Greenwood-Van Meerveld B, Neeley D, Tyler K, Peters L, McRorie J. Comparison of effects on colonic motility and stool characteristics associated with feeding olestra and wheat bran to ambulatory mini-pigs. Dig Dis Sci. 1999; 44( 7): 1282– 1287. [DOI] [PubMed] [Google Scholar]
- 6. Lewis SJ, Heaton KW. Roughage revisited: the effect on intestinal function of inert plastic particles of different sizes and shapes. Dig Dis Sci. 1999; 44: 744– 748. [DOI] [PubMed] [Google Scholar]
- 7. McRorie J, Pepple S, Rudolph C. Effects of fiber laxatives and calcium docusate on regional water content and viscosity of digesta in the large intestine of the pig. Dig Dis Sci. 1998; 43( 4): 738– 745. [DOI] [PubMed] [Google Scholar]
- 8. Tomlin J, Read N. Laxative properties of indigestible plastic particles. Br Med J. 1988; 297: 1175– 1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eswaran S, Muir J, Chey W. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013; 108: 718– 727. [DOI] [PubMed] [Google Scholar]
- 10. Francis CY, Whorwell PJ. Bran and irritable bowel syndrome: time for reappraisal. Lancet. 1994; 344( 8914): 39– 40. [DOI] [PubMed] [Google Scholar]
- 11. Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004; 80( 6): 1658– 1664. [DOI] [PubMed] [Google Scholar]
- 12. Brighenti F, Casiraghi MC, Canzi E, Ferrari A. Effect of consumption of a ready-to-eat breakfast cereal containing inulin on the intestinal milieu and blood lipids in healthy male volunteers. Eur J Clin Nutr. 1999; 53( 9): 726– 733. [DOI] [PubMed] [Google Scholar]
- 13. Castiglia-Delavaud C, Verdier E, Besle JM, et al. Net energy value of non-starch polysaccharide isolates (sugarbeet fibre and commercial inulin) and their impact on nutrient digestive utilization in healthy human subjects. Br J Nutr. 1998; 80( 4): 343– 352. [DOI] [PubMed] [Google Scholar]
- 14. Costabile A, Kolida S, Klinder A, et al. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br J Nutr. 2010; 104( 7): 1007– 1017. [DOI] [PubMed] [Google Scholar]
- 15. Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995; 108( 4): 975– 982. [DOI] [PubMed] [Google Scholar]
- 16. Kleessen B, Sykura B, Zunft HJ, Blaut M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am J Clin Nutr. 1997; 65( 5): 1397– 402. [DOI] [PubMed] [Google Scholar]
- 17. Kleessen B, Schwarz S, Boehm A, et al. Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br J Nutr. 2007; 98( 3): 540– 549. [DOI] [PubMed] [Google Scholar]
- 18. Kolida S, Meyer D, Gibson GR. A double-blind placebo-controlled study to establish the bifidogenic dose of inulin in healthy humans. Eur J Clin Nutr. 2007; 61( 10): 1189– 1195. [DOI] [PubMed] [Google Scholar]
- 19. Linetzky Waitzberg D, Alves Pereira CC, Logullo L, et al. Microbiota benefits after inulin and partially hydrolized guar gum supplementation: a randomized clinical trial in constipated women. Nutr Hosp. 2012; 27( 1): 123– 129. [DOI] [PubMed] [Google Scholar]
- 20. Marteau P, Guerin-Deremaux L, Wils D, Cazaubiel M, Housez B. Short-term digestive tolerance of high-dose of NUTRIOSE®FB10 in adult. Int J Food Sci Nutr. 2011; 62( 2): 97– 101. [DOI] [PubMed] [Google Scholar]
- 21. Ramnani P, Gaudier E, Bingham M, van Bruggen P, Tuohy KM, Gibson GR. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: a human intervention study. Br J Nutr. 2010; 104( 2): 233– 240. [DOI] [PubMed] [Google Scholar]
- 22. Slavin J, Feirtag J. Chicory inulin does not increase stool weight or speed up intestinal transit time in healthy male subjects. Food Funct. 2011; 2: 72– 77. [DOI] [PubMed] [Google Scholar]
- 23. van den Heuvel EG, Wils D, Pasman WJ, Saniez MH, Kardinaal AF. Dietary supplementation of different doses of NUTRIOSE-FB, a fermentable dextrin, alters the activity of faecal enzymes in healthy men. Eur J Nutr. 2005; 44: 445– 451. [DOI] [PubMed] [Google Scholar]
- 24. van Dokkum W, Wezendonk B, Srikumar TS, van den Heuvel EG. Effect of nondigestible oligosaccharides on large-bowel functions, blood lipid concentrations and glucose absorption in young healthy male subjects. Eur J Clin Nutr. 1999; 53( 1): 1– 7. [DOI] [PubMed] [Google Scholar]
- 25. Dikeman C, Fahey G. Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr. 2006; 46: 649– 663. [DOI] [PubMed] [Google Scholar]
- 26. McRorie J. Clinical data support that psyllium is not fermented in the gut [letter to the editor]. Am J Gastroenterol. 2013; 108( 9): 1541. [DOI] [PubMed] [Google Scholar]
- 27. Campbell J, Fahey G. Psyllium and methylcellulose fermentation properties in relation to insoluble and soluble fiber standards. Nutr Res. 1997; 17: 619– 629. [Google Scholar]
- 28. Kaur A, Rose D, Rumpagaporn P, et al. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “ slowly fermentable ” dietary fibers. J Food Sci. 2011; 76: H137– H142. [DOI] [PubMed] [Google Scholar]
- 29. Timm D, Stewart M, Hospattankar A, et al. Wheat dextrin, psyllium, and inulin produce distinct fermentation patterns, gas volumes, and short-chain fatty acid profiles in vitro. J Med Food. 2010; 13: 961– 966. [DOI] [PubMed] [Google Scholar]
- 30. Levitt MD, Furne J, Olsson S. The relation of passage of gas and abdominal bloating to colonic gas production. Ann Intern Med. 1996; 124: 422– 424. [DOI] [PubMed] [Google Scholar]
- 31. Marteau P, Flouri é B, Cherbut C, et al. Digestibility and bulking effect of ispaghula husks in healthy humans. Gut. 1994; 35: 1747– 1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolever T, Robb P. Effect of guar, pectin, psyllium, soy polysaccharide and cellulose on breath hydrogen and methane in healthy subjects. Am J Gastroenterol. 1992; 87: 305– 310. [PubMed] [Google Scholar]
- 33. Wolever T, ter Wal P, Spadafora P, et al. Guar, but not psyllium, increases breath methane and serum acetate concentrations in human subjects. Am J Clin Nutr. 1992; 55: 719– 722. [DOI] [PubMed] [Google Scholar]
- 34. Zumarraga L, Levitt M, Suarez F. Absence of gaseous symptoms during ingestion of commercial fibre preparations. Aliment Pharmacol Ther. 1997; 11: 1067– 1172. [DOI] [PubMed] [Google Scholar]
- 35. McRorie J, Daggy B, Morel J, Diersing P, Miner P, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998; 12: 491– 497. [DOI] [PubMed] [Google Scholar]
- 36. Eherer A, Santa Ana C, Fordtran J. Effect of psyllium, calcium polycarbophil, and wheat bran on secretory diarrhea induced by phenolphthalein. Gastroenterology. 1993; 104: 1007– 1012. [DOI] [PubMed] [Google Scholar]
- 37. Qvitzau S, Matzen P, Madsen P. Treatment of chronic diarrhoea: loperamide versus ispaghula husk and calcium. Scand J Gastroenterol. 1988; 23: 1237– 1240. [DOI] [PubMed] [Google Scholar]
- 38. Singh B. Psyllium as a therapeutic and drug delivery agent. Int J Pharm. 2007; 334: 1– 14. [DOI] [PubMed] [Google Scholar]
- 39. Washington N, Harris M, Mussellwhite A, Spiller R. Moderation of lactulose-induced diarrhea by psyllium: effects on motility and fermentation. Am J Clin Nutr. 1998; 67: 317– 321. [DOI] [PubMed] [Google Scholar]
- 40. Brandt L, Prather C, Quigley E, Schiller L, Schoenfeld P, Talley N. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol. 2005; 100: S5– S22. [DOI] [PubMed] [Google Scholar]
- 41. Brandt L, Chey W, Foxx-Orenstein A, et al. American College of Gastroenterology Task Force on IBS. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009; 104( suppl 1): S1– S35. [DOI] [PubMed] [Google Scholar]
- 42. Du H, van der ADL, Boshuizen HC, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010; 91: 329– 336. [DOI] [PubMed] [Google Scholar]
- 43. Anderson J, Baird P, Davis R, et al. Health benefits of dietary fiber. Nutr Rev. 2009; 67: 188– 205. [DOI] [PubMed] [Google Scholar]
- 44. Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003; 78: 920– 927. [DOI] [PubMed] [Google Scholar]
- 45. Higgins J. Resistant starch and energy balance: impact on weight loss and maintenance. Crit Rev Food Sci Nutr. 2014; 54: 1158– 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abrams S, Griffin I, Hawthorne K, Ellis KJ. Effect of prebiotic supplementation and calcium intake on body mass index. Pediatrics. 2007; 151( 3): 293– 298. [DOI] [PubMed] [Google Scholar]
- 47. Archer B, Johnson S, Devereux H, Baxter A. Effect of fat replacement by inulin or lupin-kernel fibre on sausage patty acceptability, post-meal perceptions of satiety and food intake in men. Br J Nutr. 2004; 91: 591– 599. [DOI] [PubMed] [Google Scholar]
- 48. Tiwary C, Ward J, Jackson B. Effect of pectin on satiety in healthy US army adults. J Am Coll Nutr. 1997; 16: 423– 428. [DOI] [PubMed] [Google Scholar]
- 49. Wanders A, van den Borne J, de Graaf C, et al. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev. 2011; 12: 724– 739. [DOI] [PubMed] [Google Scholar]
- 50. Haber GB, Heaton KW, Murphy D, Burroughs LF. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet. 1977; 2: 679– 682. [DOI] [PubMed] [Google Scholar]
- 51. Di Lorenzo C, Williams C, Hajnal F, Valenzuela J. Pectin delays gastric emptying and increases satiety in obese subjects. Gastroenterology. 1988; 95( 5): 1211– 1215. [DOI] [PubMed] [Google Scholar]
- 52. Howarth N, Saltzman E, Roberts S. Dietary fiber and weight regulation. Nutr Rev. 2001; 59: 129– 139. [DOI] [PubMed] [Google Scholar]
- 53. Pereira M, Ludwig D. Dietary fiber and body-weight regulation: observations and mechanisms. Pediatr Clin North Am. 2001; 48: 969– 980. [DOI] [PubMed] [Google Scholar]
- 54. Slavin J. Dietary fiber and body weight. Nutrition. 2005; 21: 411– 418. [DOI] [PubMed] [Google Scholar]
- 55. Kim J, Cha YJ, Lee KH, Park E. Effect of onion peel extract supplementation on the lipid profile and antioxidative status of healthy young women: a randomized, placebo-controlled, double-blind, crossover trial. Nutr Res Pract. 2013; 7( 5): 373– 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwartz SE, Levine RA, Singh A, Scheidecker JR, Track NS. Sustained pectin ingestion delays gastric emptying. Gastroenterology. 1982; 83( 4): 812– 817. [PubMed] [Google Scholar]
- 57. Vuksan V, Jenkins AL, Rogovik AL, Fairgrieve CD, Jovanovski E, Leiter LA. Viscosity rather than quantity of dietary fibre predicts cholesterol-lowering effect in healthy individuals. Br J Nutr. 2011; 106: 1349– 1352. [DOI] [PubMed] [Google Scholar]
- 58. Wanders AJ, Jonathan MC, van den Borne JJ, et al. The effects of bulking, viscous and gel-forming dietary fibers on satiation. Br J Nutr. 2013; 109: 1330– 1337. [DOI] [PubMed] [Google Scholar]
- 59. Wolever T, Tosh S, Gibbs A, et al. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. Am J Clin Nutr. 2010; 92: 723– 732. [DOI] [PubMed] [Google Scholar]
- 60. Anderson JW. Dietary fiber and associated phytochemicals in prevention and reversal of diabetes. In: Pasupuleti VK, Anderson JW, eds. Nutraceuticals, Glycemic Health and Type 2 Diabetes, Ames, IA: Blackwell Publishing Professional, 2008: 111– 142. [Google Scholar]
- 61. Cicero AFG, Derosa G, Bove M, Imola F, Borghi C, Gaddi AV. Psyllium improves dyslipidemaemia, hyperglycaemia and hypertension, while guar gum reduces body weight more rapidly in patients affected by metabolic syndrome following an AHA step 2 diet. Mediterr J Nutr Metab. 2010; 3: 47– 54. [Google Scholar]
- 62. Anderson J, Floore T, Geil P, Spencer D, Balm T. Hypocholesterolemic effects of different bulk-forming hydrophilic fibers as a adjuncts to dietary therapy in mild to moderate hypercholesterolemia. Arch Intern Med. 1991; 151: 1597– 1602. [PubMed] [Google Scholar]
- 63. Anderson J, Zettwoch N, Feldman T, Tietyen-Clark J, Oeltgen P, Bishop C. Cholesterol-lowering effects of psyllium hydrophilic mucilloid for hypercholetserolemic men. Arch Intern Med. 1988; 148: 292– 296. [PubMed] [Google Scholar]
- 64. Kerkhoffs D, Hornstra G, Mensick R. Cholesterol-lowering effect of β-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when β-glucan is incorporated into bread and cookies. Am J Clin Nutr. 2003; 78: 221– 227. [DOI] [PubMed] [Google Scholar]
- 65.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, Carbohydrates, Fiber, Fat, Fatty Acids Cholesterol, Protein and Amino Acids. Washington, DC: National Academies Press; 2002. [Google Scholar]
- 66. Slavin J. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008; 108: 1716– 1731. [DOI] [PubMed] [Google Scholar]
- 67. McRorie J, Zorich N, Riccardi K, et al. Effects of olestra and sorbitol consumption on objective measures of diarrhea: impact of stool viscosity on common gastrointestinal symptoms. Regul Toxicol Pharmacol. 2000; 31( 1): 59– 67. [DOI] [PubMed] [Google Scholar]


