Abstract
Introduction
Adherence to diabetes medication has been linked to improved glycemic levels and lower costs, but previous research on adherence has typically involved oral antidiabetic medication or insulin. This study examines how adherence and persistence to once-daily liraglutide impact glycemic control and economic outcomes in a real-world population of adult type 2 diabetes (T2D) patients.
Methods
A retrospective cohort study using administrative claims data from July 2009 through September 2013. Patients aged ≥18 years with T2D treated with liraglutide were identified (index date = first liraglutide prescription). Adherence was based on the proportion of days covered (PDC); with PDC ≥0.80 classified as adherent. Non-persistent patients were those with a gap in therapy of >90 days. Lab results for glycated hemoglobin (A1C) were used to identify whether patients achieved target levels of <7.0% and ≤ 6.5%, or experienced a reduction of ≥1.0% in A1C from pre-index (baseline) to post-index (follow-up). Logistic regression was used to estimate the likelihood of achieving the A1C goals, adjusted for baseline characteristics. Diabetes-related medical, pharmacy, and total costs were modeled and estimated for the adherence and persistence cohorts.
Results
A total of 1321 patients were identified. The mean PDC was 0.59 and 34% of patients were classified as adherent, while 60% were persistent over 12 months of follow-up. Adherent and persistent patients were more likely to achieve each of the A1C goals than their non-adherent and non-persistent counterparts after adjusting for patient characteristics. Adherence and persistence were associated with higher adjusted diabetes-related pharmacy and total healthcare costs during follow-up; whereas persistent patients had significantly lower diabetes-related medical costs than non-persistent patients.
Conclusions
Adherence and persistence to liraglutide are associated with improved A1C outcomes. Persistent patients showed significantly lower medical costs versus those discontinuing liraglutide. Total healthcare costs were higher for adherent and persistent cohorts driven by higher pharmacy costs.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-015-0199-z) contains supplementary material, which is available to authorized users.
Keywords: Adherence, Diabetes, Glycemic control, Economic outcomes, Hemoglobin A1C, Liraglutide, Persistence
Introduction
In 2012, 29.1 million people in the US had diabetes (roughly 9% of the US population), including over 8 million who were undiagnosed [1]. Type 2 diabetes (T2D) represents the large majority of diabetes cases (90–95%) [1]. The economic burden of diabetes is substantial, with roughly $245 billion in total direct and indirect costs in the US in 2012, including $176 billion in direct medical costs [1, 2]. Diabetes is the leading cause of blindness, end-stage renal disease, and non-traumatic lower-extremity amputations, and is a major risk factor for coronary artery disease and stroke [3]. In 2010, diabetes was the seventh leading cause of death in the US [1].
Treating diabetes may involve adjustments to diet and lifestyle, as well as pharmacotherapy. The main goal of treatment is to maintain blood glucose to reduce the risk of known complications [4]. The American Diabetes Association recommends that the target level for glycated hemoglobin (A1C) is less than 7% [5], although only 57% achieved that goal in 2003–2006 [6]. The American Association of Clinical Endocrinologists recommends a target for A1C of ≤6.5% [7].
Metformin (a biguanide) is recommended as first-line treatment [8], with a variety of therapies available as second- and third-line agents if treatment with metformin is insufficient to achieve desired A1C levels or if patients exhibit intolerance to metformin. Common classes of these second- and third-line antidiabetic agents include sulfonylureas, meglitinides, thiazolidinediones, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium–glucose co-transporter 2 inhibitors and insulins. Liraglutide is a long-acting, GLP-1 receptor agonist administered through a once-daily injection.
Adherence to diabetes medications is generally poor [9, 10]. Several studies have demonstrated a link between adherence and diabetes-related outcomes, including A1C levels [4, 10–15]. In one study, the lower A1C achieved from greater adherence was comparable to that achieved with additional medication [16]. Persistence to prescription fills has also shown to be associated with a reduction in costs and rates of hospitalizations within Medicare patients [17]. However, previous research on adherence and persistence to diabetes medication is primarily limited to oral antidiabetic (OAD) medications or insulin. In short, there is a paucity of data examining the association of adherence or persistence and subsequent clinical outcomes and costs within the GLP-1 receptor agonist therapeutic space. To expand the current knowledge and understanding of the clinical and economic outcomes associated with liraglutide, we sought to examine the impact of both adherence and persistence to once-daily liraglutide on glycemic control and healthcare costs in a real-world adult T2D population.
Methods
Study Design and Data Sources
A retrospective cohort study was conducted using administrative claims from a large, US health plan affiliated with Optum during July 2009 through September 2013. The administrative claims database includes demographic information as well as medical data from physician and facilities or hospitals and pharmacy data in the form of prescription medication claims. There were approximately 18.5 million commercially insured adult enrollees covered during the study period. Individuals included in the database are geographically diverse across the US, with the greatest representation in the South and Midwest regions. Claims include International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, Current Procedural Terminology procedure codes, Healthcare Common Procedure Coding System codes, site of service codes, and health plan and patient costs. Outpatient pharmacy data includes National Drug Codes for dispensed medications, quantity dispensed, drug strength, days’ supply, and costs. Outpatient lab results (including A1C) are available in linked laboratory data for a subset of the population.
Patient Selection
Adult commercial health plan members with T2D who were treated with liraglutide were included in the study. Specifically, those with at least one pharmacy claim for liraglutide between January 01, 2010 and September 30, 2012 were identified, and the index date was defined as the date of the first liraglutide claim. An indication of T2D was based on ICD-9-CM diagnosis codes or claims for OADs during the 180 days prior to the index date (see “Appendix 1” for the algorithm). Additionally, subjects were required to be at least 18 years old as of the index year and have continuous enrollment in the health plan with medical and pharmacy benefits for 180 days prior to the index date (baseline period) and for 365 days following the index date (follow-up period). Subjects with claims for GLP-1 agents during the baseline period or evidence of pregnancy or gestational diabetes during either the baseline or follow-up periods were excluded. Finally, subjects were required to have at least one A1C lab result during the period 45 days prior to the index date through 7 days after the index date and at least one A1C lab result between 275 and 455 days after the index date (365 days post-index ±90 days).
Study Measures
Adherence to liraglutide was based on the proportion of days covered (PDC), which has gained favor as the preferred adherence measure. The PDC is used by the Pharmacy Quality Alliance (PQA) in its most recent quality measures [18], and the Centers for Medicare and Medicaid Services (CMS) use PDC as a quality measure of Part D plans in their Quality Evaluation System [19]—a measure endorsed by the National Quality Forum. The PDC is calculated as the number of days the medication is available to the patient divided by the number of days in the follow-up period [12]. For this study, the PDC was dichotomized into ≥0.80 (adherent) and <0.80 (non-adherent). The threshold of 0.80 is commonly used, including by both the PQA and CMS [18, 19]. For completeness, as well as for use in sensitivity analyses, the Medication Possession Ratio (MPR) was also calculated [20] and dichotomized into adherent (MPR ≥0.80) and non-adherent (<0.80) binary measures. Persistence to liraglutide was defined by the continuation or discontinuation of the medication as measured via the days’ supply reported on pharmacy claims. Discontinuation was defined as a gap in therapy of at least 90 days and represented non-persistence. The time to discontinuation was calculated as the number of days from the index date to the run-out date of the last fill before the gap in therapy. Adherence and persistence were defined separately and represent different aspects of medication usage; each subject in the study cohort was defined as either adherent or non-adherent and also as either persistent or non-persistent.
Demographic characteristics of subjects included their age as of the index year, gender, and geographic region. Also collected were patient paid amounts for their index prescription fill and whether or not their index fill was obtained through a mail order. Baseline clinical characteristics included A1C at index, use of antidiabetic medications, and comorbid conditions. The baseline A1C was captured from laboratory results on claims during the 45 days prior to the index date through 7 days post-index. If multiple values were present, the A1C result closest to the index date was used. The dose of the index fill of liraglutide was obtained, as well as the specialty of the prescribing physician. Fills for insulin and OAD medications during the baseline period were identified and counted at the class level, and the overall baseline antidiabetic regimen was categorized into several groups (no therapy, OAD monotherapy, OAD combination therapy, insulin monotherapy, and insulin with OADs). Comorbid conditions during the baseline period were defined using the Clinical Classification Software managed by the Agency of Healthcare Research and Quality [21], which generates indicator variables for specific disease conditions based on ICD-9-CM diagnoses. The comorbidities of dyslipidemia, hypertension, renal disease, and non-alcohol fatty liver disease were identified during the baseline period based on diagnosis, procedure, and revenue codes appearing on medical claims. The Diabetes Complications Severity Index (DCSI) at baseline was created using ICD-9-CM codes as described in Young et al. [22] and Chang et al. [23].
During follow-up, several A1C outcomes were analyzed. The follow-up A1C result occurring within 90 days of the end of the 1-year follow-up period was captured from claims. If multiple A1C measures were available during this period, the one closest to the end of the 1-year follow-up period was retained. This A1C result was used to create indicator variables for A1C <7.0% and ≤6.5% achievement at follow-up. Additionally, the absolute change in A1C from baseline was calculated, and patients with a reduction of ≥1.0% from baseline to follow-up were identified.
Diabetes-related healthcare resource utilization during the baseline and follow-up periods was characterized by binary indicators and counts of ambulatory visits, emergency room (ER) visits, and inpatient (IP) stays related to diabetes. Visits were considered diabetes-related if they had an ICD-9-CM diagnosis code of 250.xx in any position. Consumer Price Index [24] adjusted diabetes-related healthcare costs were computed as the combined health plan and patient paid amounts. Total healthcare costs were calculated as the sum of medical costs (categorized into ambulatory visit costs, emergency services costs, IP costs, and other costs) and pharmacy costs. For medical claims, services were defined as diabetes related if they had a diagnosis of 250.xx in any position, and pharmacy costs included oral and injectable diabetes medications.
Statistical Analysis
All study variables, including baseline and outcome measures, were analyzed descriptively. Numbers and percentages were calculated for dichotomous and polychotomous variables, while means, medians, and standard deviations (SD) were calculated for continuous variables. Results were stratified by the dichotomous adherence and persistence measures. Bivariate comparisons were conducted, and appropriate tests for significance were performed based on the distribution of the variable.
Multivariate analysis of the study outcomes was conducted using appropriate regression models. Ordinary least squares regression was used to analyze the absolute reduction in A1C, while logistic regression was used to analyze dichotomous A1C outcomes (e.g., A1C goal attainment). To analyze diabetes-related costs, generalized linear models with a gamma distribution and log link were employed, utilizing Manning and Mullahy’s formulation [25]. All multivariate analyses were adjusted for key covariates, including age group, gender, health plan region, index prescriber specialty, mail-order status, patient paid amount for index fill, baseline DCSI, baseline comorbidities of interest (dyslipidemia, hypertension, renal disease, non-alcohol fatty liver disease), baseline count of OAD classes, baseline insulin use, baseline diabetes-related utilization, baseline diabetes-related costs (not included in cost models), and baseline A1C. Adjusted outcomes and average costs were predicted and bootstrapped 95% confidence intervals were estimated. Data extraction and statistical analysis were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Statement of Ethics
No identifiable protected health information was extracted or accessed during the course of this study; hence, no Institutional Review Board approval was required.
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Sample Selection and Baseline Characteristics
After applying all inclusion and exclusion criteria, the final study population included a total of 1321 liraglutide patients with T2D (Fig. 1). The mean (SD) age of the sample was 53.0 (9) years, and just over half (51%) were male (Table 1).
Fig. 1.
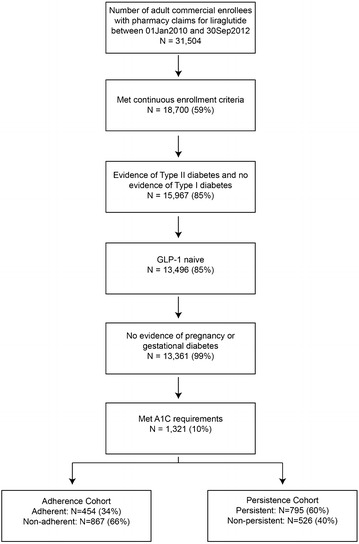
Sample selection and attrition. GLP-1 glucagon-like peptide-1, A1C glycated hemoglobin
Table 1.
Patient demographics and baseline characteristics
| Total | Adherence group | Persistence group | |||||
|---|---|---|---|---|---|---|---|
| Adherent | Non-adherent | p value | Persistent | Non-persistent | p value | ||
| Total, N | 1321 | 454 | 867 | 795 | 526 | ||
| Age, mean (SD) | 52.95 (8.55) | 53.86 (8.03) | 52.48 (8.77) | 0.004b | 53.41 (8.32) | 52.26 (8.85) | 0.016a |
| Age, N (%) | |||||||
| 18–44 | 227 (17) | 58 (13) | 169 (19) | 0.002b | 121 (15) | 106 (20) | 0.020a |
| 45–64 | 1030 (78) | 373 (82) | 657 (76) | 0.008b | 638 (80) | 392 (75) | 0.014a |
| 65+ | 64 (5) | 23 (5) | 41 (5) | 0.786 | 36 (5) | 28 (5) | 0.510 |
| Gender, N (%) | |||||||
| Male | 677 (51) | 253 (56) | 424 (49) | 0.018a | 424 (53) | 253 (48) | 0.062 |
| Female | 644 (49) | 201 (44) | 443 (51) | 371 (47) | 273 (52) | ||
| Region, N (%) | |||||||
| Northeast | 79 (6) | 32 (7) | 47 (5) | 0.236 | 49 (6) | 30 (6) | 0.730 |
| Midwest | 119 (9) | 37 (8) | 82 (9) | 0.430 | 66 (8) | 53 (10) | 0.270 |
| South | 960 (73) | 328 (72) | 632 (73) | 0.802 | 578 (73) | 382 (73) | 0.974 |
| West | 163 (12) | 57 (13) | 106 (12) | 0.863 | 102 (13) | 61 (12) | 0.505 |
| DCSI, mean (SD) | 0.59 (1.05) | 0.57 (1.04) | 0.59 (1.06) | 0.783 | 0.61 (1.07) | 0.55 (1.02) | 0.358 |
| Baseline A1C (%), mean (SD) | 8.22 (1.71) | 8.08 (1.64) | 8.29 (1.75) | 0.033a | 8.18 (1.66) | 8.28 (1.80) | 0.351 |
| Baseline medication use, N (%) | |||||||
| Metformin | 920 (70) | 334 (74) | 586 (68) | 0.025a | 577 (73) | 343 (65) | 0.004b |
| Sulfonylureas | 473 (36) | 168 (37) | 305 (35) | 0.511 | 290 (36) | 183 (35) | 0.531 |
| TZDs | 354 (27) | 145 (32) | 209 (24) | 0.002b | 239 (30) | 115 (22) | <0.001b |
| DPP-4 inhibitors | 435 (33) | 184 (41) | 251 (29) | <0.001b | 290 (36) | 145 (28) | <0.001b |
| α-Glucosidase inhibitors | 5 (0) | 1 (0) | 4 (0) | 0.498 | 3 (0) | 2 (0) | 0.993 |
| Meglitinide derivatives | 27 (2) | 21 (5) | 6 (1) | <0.001b | 22 (3) | 5 (1) | 0.022a |
| Insulin (any) | 271 (21) | 103 (23) | 168 (19) | 0.157 | 172 (22) | 99 (19) | 0.215 |
| Number of baseline OAD medications, mean (SD) | 1.68 (1.10) | 1.88 (1.12) | 1.57 (1.08) | <0.001b | 1.79 (1.10) | 1.51 (1.09) | <0.001b |
| Comorbidities of interest, N (%) | |||||||
| Dyslipidemia | 1074 (81) | 381 (84) | 693 (80) | 0.077 | 665 (84) | 409 (78) | 0.007b |
| Hypertension | 981 (74) | 344 (76) | 637 (73) | 0.364 | 587 (74) | 394 (75) | 0.664 |
| Renal disease | 111 (8) | 37 (8) | 74 (9) | 0.810 | 68 (9) | 43 (8) | 0.808 |
| Non-alcohol fatty liver disease | 52 (4) | 19 (4) | 33 (4) | 0.737 | 40 (5) | 12 (2) | 0.012a |
| Baseline diabetes-related costs, mean | |||||||
| Medical costs | $1517 | $1517 | $1516 | 0.998 | $1321 | $1813 | 0.205 |
| Pharmacy costs | $985 | $1279 | $821 | <0.001b | $1110 | $796 | <0.001b |
| Total costs | $2502 | $2816 | $2338 | 0.161 | $2431 | $2609 | 0.654 |
SD standard deviation, DCSI Diabetes Complications Severity Index, TZD thiazolidinedione, DPP-4 dipeptidyl peptidase-4, OAD oral antidiabetic, A1C glycated hemoglobin
a p value <0.05
b p value <0.01
The mean (SD) PDC was 0.59 (0.31); 454 patients (34%) were classified as adherent by way of a PDC of at least 80%. Sixty percent of patients were persistent for the entire 365-day follow-up period, and the mean (SD) length of persistence was 263 (136) days. Adherent patients were slightly older and more frequently male, while the mean DCSI was similar between adherent and non-adherent patients (0.57 vs. 0.59, p = 0.783, Table 1), as was the prevalence of comorbid conditions. Adherent patients had lower mean baseline A1C (8.08% vs. 8.29%, p = 0.033). Differences between persistent and non-persistent patients mirrored those of the adherent vs. non-adherent cohorts for several characteristics, including age (persistent patients were slightly older) and mean DCSI (no difference between persistent and non-persistent patients). However, there was no significant difference in gender or baseline mean A1C by persistence groups, and a higher percentage of persistent patients had dyslipidemia (84% vs. 78%, p = 0.007) and non-alcoholic fatty liver disease (5% vs. 2%, p = 0.012) than did non-persistent patients (Table 1).
Metformin was the most common antidiabetic medication used during baseline, and was significantly more common in adherent patients (74%) than non-adherent patients (68%, p = 0.025). Other significant differences in baseline medication use between adherent and non-adherent patients included thiazolidinediones (32% vs. 24%, p = 0.002), DPP-4 inhibitors (41% vs. 29%, p < 0.001), and meglitinides (5% vs. 1%, p < 0.001). There was no significant difference in the percent of patients who used sulfonylureas or insulin between adherence cohorts. The index dose of liraglutide was fairly evenly split between 1.2 and 1.8 mg, but adherent patients were more likely to have an index dose of 1.2 mg compared with non-adherent patients (53% vs. 46%, p < 0.001). Comparisons of baseline medication use between persistent and non-persistent patients were similar to those for adherent and non-adherent patients (Table 1).
A1C Outcomes
Unadjusted A1C outcomes are shown in Table 2. The reduction in mean A1C from baseline to follow-up was greater in adherent patients compared with non-adherent patients (0.81% vs. 0.42%, p < 0.001) and in persistent patients than in non-persistent patients (0.78% vs. 0.21%, p < 0.001). Additionally, when compared with the non-adherent cohort, those in the adherent cohort were more likely to achieve A1C goals of <7.0% (50% vs. 39%, p < 0.001) and ≤6.5% (35% vs. 26%, p = 0.001), and were more likely to have at least a 1.0% reduction in their A1C (38% vs. 32%, p = 0.022). Similar results were seen when persistence cohorts were compared; A1C levels <7.0% and ≤6.5%, as well as A1C reductions of at least 1.0%, were more often attained by persistent patients than non-persistent patients (p < 0.001 for all three outcomes).
Table 2.
Unadjusted glycated hemoglobin (A1C) outcomes by adherence and persistence
| Adherence group | Persistence group | |||||
|---|---|---|---|---|---|---|
| Adherent | Non-adherent | p value | Persistent | Non-persistent | p value | |
| Total, N | 454 | 867 | 795 | 526 | ||
| Change in A1C from baseline, mean (SD) | 0.81 (1.54) | 0.42 (1.70) | <0.001b | 0.78 (1.56) | 0.21 (1.74) | <0.001b |
| A1C goal attainment (<7.0%) | 50 (%) | 39 (%) | <0.001b | 49 (%) | 35 (%) | <0.001b |
| A1C goal attainment (≤6.5%) | 35 (%) | 26 (%) | 0.001b | 33 (%) | 24 (%) | <0.001b |
| Reduction in A1C ≥1.0% | 38 (%) | 32 (%) | 0.022a | 39 (%) | 27 (%) | <0.001b |
SD standard deviation
a p value <0.05
b p value <0.01
Adjusted, multivariate analysis of the A1C outcomes confirmed the unadjusted results (Fig. 2a, b). Specifically, adherent patients had a significantly larger decrease in A1C and were more likely to achieve at least a 1.0% reduction in A1C than their non-adherent counterparts (odds ratio [OR] = 1.86, p < 0.001). Additionally, adherent patients were more likely to achieve an A1C <7.0% (OR = 1.84, p < 0.001) and an A1C ≤6.5% (OR = 1.70, p < 0.001) than non-adherent patients. Persistent patients were more than twice as likely to achieve A1C goal levels and to experience at least a 1.0% decrease in their A1C than non-persistent patients (OR for A1C <7.0% = 2.37; OR for A1C ≤6.5% = 2.01; OR for A1C reduction ≥1.0% = 2.34; all p values <0.001). Similar results were produced when analyses were repeated after stratifying patients into adherence cohorts based on MPR (data not shown).
Fig. 2.
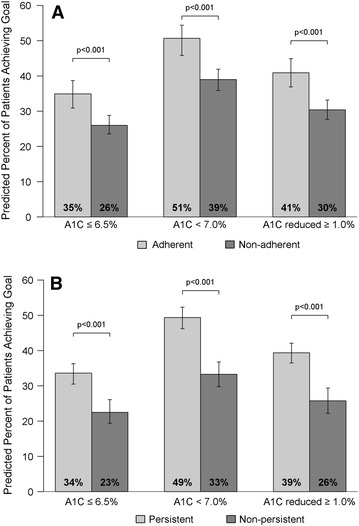
Adjusted glycated hemoglobin (A1C) outcomes by a adherence and b persistence
Diabetes-Related Healthcare Costs and Resource Use
Overall, unadjusted diabetes-related mean (SD) total healthcare costs per patient were $8186 ($12,209) for the final sample. Compared with non-adherent patients, adherent patients had lower levels of diabetes-related utilization for ER (13 vs. 18 visits per 100 patients, p = 0.030) and IP (6 vs. 9 visits per 100 patients, p = 0.051) services, resulting in lower unadjusted diabetes-related medical costs ($2743 vs. $4149, p = 0.018; Table 3), on average (median diabetes-related medical costs were similar between groups). However, adherent patients had significantly higher mean pharmacy costs than non-adherent patients ($6338 vs. $3568, p < 0.001; Table 3). When medical costs (which include costs for ambulatory visits, ER services, IP services, and other services) and pharmacy costs were summed, the result was a higher total healthcare cost among adherent patients ($9081 vs. $7717, p = 0.028; Table 3), on average. After adjustment, total diabetes-related healthcare costs remained significantly higher for the adherent group (p = 0.005), with a predicted (95% confidence interval) cost of $9419 ($8574–$10,308) versus $7667 ($6903–$8573) for non-adherent patients (Fig. 3a).
Table 3.
Unadjusted diabetes-related health care costs during follow-up by adherence and persistence
| Adherence group | Persistence group | |||||
|---|---|---|---|---|---|---|
| Adherent | Non-adherent | p value | Persistent | Non-persistent | p value | |
| Total, N | 454 | 867 | 795 | 526 | ||
| Medical costs | ||||||
| Mean (SD) | $2743 ($8065) | $4149 ($13,383) | 0.018a | $3103 ($10,124) | $4516 ($14,017) | 0.047a |
| Median | $683 | $687 | $682 | $699 | ||
| Inpatient costs | ||||||
| Mean (SD) | $1134 ($7157) | $1805 ($9410) | 0.148 | $1402 ($9198) | $1835 ($7903) | 0.363 |
| Median | $0 | $0 | $0 | $0 | ||
| Ambulatory costs | ||||||
| Mean (SD) | $1377 ($3140) | $1917 ($5164) | 0.019a | $1470 ($3643) | $2127 ($5685) | 0.019a |
| Median | $535 | $539 | $526 | $550 | ||
| Emergency room costs | ||||||
| Mean (SD) | $50 ($238) | $106 ($367) | <0.001b | $59 ($268) | $129 ($401) | <0.001b |
| Median | $0 | $0 | $0 | $0 | ||
| Other medical costs | ||||||
| Mean (SD) | $182 ($487) | $320 ($4118) | 0.330 | $172 ($457) | $426 ($5276) | 0.272 |
| Median | $89 | $81 | $86 | $80 | ||
| Pharmacy costs | ||||||
| Mean (SD) | $6338 ($2639) | $3568 ($2439) | <0.001b | $5571 ($2658) | $2931 ($2298) | <0.001b |
| Median | $5606 | $3074 | $5039 | $2341 | ||
| Total costs | ||||||
| Mean (SD) | $9081 ($8685) | $7717 ($13,679) | 0.028a | $8675 ($10,611) | $7447 ($14,270) | 0.092 |
| Median | $6797 | $4647 | $6180 | $3864 | ||
SD standard deviation
a p value <0.05
b p value <0.01
Fig. 3.
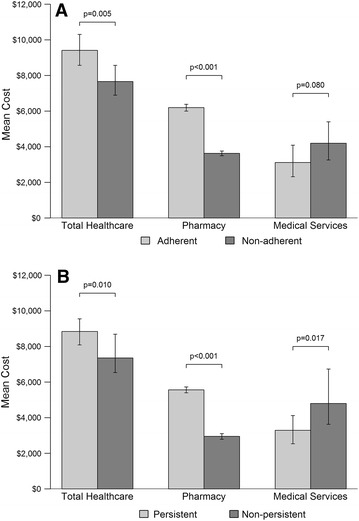
Adjusted follow-up diabetes-related cost by a adherence and b persistence
Diabetes-related medical services costs were lower for persistent patients (adjusted model p value = 0.017), with a predicted cost difference of about $1500 ($3298 vs. $4805; Fig. 3b) compared to patients who discontinued liraglutide. Total diabetes-related healthcare costs, however, were higher for persistent patients than for non-persistent patients driven by higher pharmacy costs (Table 3; Fig. 3b; adjusted model p value = 0.010).
Discussion
Liraglutide patients who were adherent had better A1C outcomes and higher total diabetes-related healthcare costs than their non-adherent counterparts—driven by higher pharmacy costs. Similar results were observed when comparing patients split into either persistent or non-persistent groups. The fact that those classified as adherent and/or persistent had higher pharmacy costs is not unexpected, given that these cohorts include patients who consistently adhered to their medication and continued to fill their prescriptions as directed, while those in the non-adherent and non-persistent cohorts did not. It is likely that adherent and persistent patients experienced better A1C outcomes as a direct result of their consistent medication use, demonstrating that the additional cost comes with improved clinical outcomes. However, adherence to other medications was not directly assessed. Those in the adherent and persistent groups had a significantly higher number of baseline OAD medications on average, which could indicate a higher degree of familiarity with medications in general or with adhering to prescribed medications. Additionally, while non-adherent and non-persistent patients had lower pharmacy costs, mean diabetes-related medical costs were significantly higher in non-persistent patients and trended towards being significantly higher (p = 0.082) in non-adherent patients. It is possible that the additional medical costs among these patients may have been due, at least in part, to how well their diabetes was managed (as evidenced by worse A1C outcomes), and may have been lower had they been more adherent or persistent to their medication. Further, since the median diabetes-related medical costs were similar between adherent and non-adherent patients (and between persistent and non-persistent patients), there is likely a subset of non-adherent (and non-persistent) patients who contributed very large diabetes-related medical costs, perhaps as a result of their non-adherence and/or non-persistence.
Direct comparisons of our results to previous studies require careful consideration, since most of the current literature on diabetic medication adherence involves OADs or insulin. However, our mean adherence rate of 59% is within the range of 36–87% reported by Lee et al. [10], although the authors measured adherence by MPR and included OADs and insulin. In a 2006 article, Ho et al. [12] reported that 79% of patients had PDC of at least 80%, which is noticeably higher than in our study, where only 34% had a PDC ≥80%. However, the PDC in the article by Ho et al. was a summary measure potentially of multiple medications, and referred to oral hypoglycemic agents. A study by Rhee et al. [14] reported an adherence of >75% in only 8% of patients, but the study years were 1991 through 2001 and adherence was measured as the percentage of visits in which self-reported diabetic medication use was as recommended at the preceding visit. In other studies offering a direct comparison of our findings of adherence to liraglutide, patients using liraglutide between 2010 and 2011 had a mean MPR of 70%, with 46% of patients being at least 80% adherent [26]. In another, more recent study involving GLP-1 receptor agonist use, 14,211 liraglutide patients had a median unadjusted PDC of 0.72 [27]. In our study, the average adherence rate was 0.65 using PDC and 72% using MPR with 45% of patients having an MPR of at least 80%, indicating a very similar level of adherence to these studies.
Although our study suggests that better adherence and persistence are associated with better A1C outcomes, there are potential barriers to adherence that need to be addressed. Patient attitudes, beliefs, and knowledge about diabetes, as well as culture, language capabilities, financial resources, comorbidities, and social support, have all been cited as potential barriers [28]. Other challenges for patients may include paying for medications, remembering doses, reading prescription labels, and obtaining refills [29]. More frequent administrations may also be linked to adherence, as taking more than two doses of diabetes medication daily has been associated with higher A1C levels [29], and in a comparison of injectable GLP-1 receptor agonists, patients using once-daily liraglutide were more adherent than those using twice-daily exenatide [26].
Claims database analysis allows for estimation of real-world treatment patterns, and the strength of our analysis derives from the large, geographically diverse population studied. The plans used for analysis include a wide geographic distribution across the US, and therefore provide the capability for generalization to managed care populations on a national level, although such generalizations should be made with caution since those selected for this analysis may represent a select patient population. All retrospective database analyses are subject to certain limitations, however, and the results of this study must be interpreted with appropriate consideration of these limitations. Claims data are collected primarily for payment purposes, not research, and are subject to coding errors. The presence of a claim for a filled prescription does not necessarily indicate that the medication was consumed or that it was taken as prescribed. Additionally, the data have no information on patient weight, body mass index, or blood pressure. Lab results were only available for a subset of patients (those without lab data were excluded), and only include those performed by a lab during a patient visit; therefore, they may not completely represent A1C outcomes in T2D patients treated with liraglutide. Data used for this study came from a commercial managed care population with 18 months of continuous health plan enrollment who also had multiple A1C results during a specific time frame around GLP-1 therapy initiation. Therefore, results of this analysis are primarily applicable to T2D patients on similar therapies in stable managed care settings receiving frequent care from their providers. Adherence to medications other than liraglutide was not assessed or controlled for, and if differences in adherence to other medications existed, this could potentially have impacted the results. Finally, it is possible that positive feedback improves adherence; therefore, observable improvements in A1C could have induced patients to be more adherent.
Conclusion
In this study, we sought to examine how adherence and persistence to once-daily liraglutide impact glycemic control and healthcare costs in a real-world T2D population. Patients included in this study who were adherent or persistent to liraglutide had better adjusted A1C outcomes than those who were not. Persistent patients had significantly lower diabetes-related medical costs compared to patients discontinuing liraglutide. However, total diabetes-related costs were higher in adherent and persistent patients than in their non-adherent and non-persistent counterparts, which were largely driven by their higher pharmacy costs. This study highlights the importance of adherence and persistence on diabetes control and associated healthcare costs. These results will assist payers and policy makers in making informed decisions by highlighting the impact and importance of medication adherence to liraglutide on clinical and economic outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Funding for this study and article processing charges were provided by Novo Nordisk, Inc., Plainsboro, New Jersey, USA. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors would like to acknowledge Randall Gerdes and Feng Cao of Optum, who assisted in the preparation of the data for analysis. Editorial assistance in the preparation of this manuscript was provided by Craig Solid, Solid Research Group LLC, St. Paul, Minnesota, USA; support for this assistance was provided by Novo Nordisk, Inc.
Conflict of interest
Erin Buysman is an employee of Optum, who received funding from Novo Nordisk to perform this research. Fang Liu is an employee of Optum, who received funding from Novo Nordisk to perform this research. Mette Hammer is an employee of Novo Nordisk and shareholder of Novo Nordisk. Jakob Langer is an employee of Novo Nordisk and shareholder of Novo Nordisk.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Appendix 1: Algorithm (with associated codes) for identifying patients with T2D ≥1
Medical claim for T2D (ICD-9-CM diagnosis code of 250.x0 or 250.x2) and no claims for type 1 diabetes mellitus (T1DM), identified with ICD-9-CM diagnosis codes 250.x1 or 250.x3. Diagnosis codes in any position were used; OR ≥1 claim for an OAD including sulfonylureas, metformin, thiazolidinediones, α-glucosidase inhibitors, meglitinide derivatives, DPP-4 inhibitors, SGLT2 inhibitors, bromocriptine 0.8 mg, or combination medications and no claims for T1D.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. In: Department of Health and Human Services, editor. Atlanta, GA. 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed 15 Nov 2015.
- 2.American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes care. 2013;36(4):1033–46. [DOI] [PMC free article] [PubMed]
- 3.Miser WF. The management of type 2 diabetes mellitus focus on quality. Prim Care. 2007;34(1):1–38. doi: 10.1016/j.pop.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. [PubMed]
- 5.American Diabetes A. Standards of medical care in diabetes—2012. Diabetes care. 2012;35 (Suppl 1):S11–S63. [DOI] [PMC free article] [PubMed]
- 6.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122(5):443–453. doi: 10.1016/j.amjmed.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract: Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2011;17(Suppl 2):1–53. doi: 10.4158/EP.17.S2.1. [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health: J Int Soc Pharmacoecon Outcomes Res. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31–41. [PubMed] [Google Scholar]
- 11.Currie CJ, Peyrot M, Morgan CL, Poole CD, Jenkins-Jones S, Rubin RR, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35(6):1279–1284. doi: 10.2337/dc11-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 13.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee MK, Slocum W, Ziemer DC, Culler SD, Cook CB, El-Kebbi IM, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240–250. doi: 10.1177/0145721705274927. [DOI] [PubMed] [Google Scholar]
- 15.Wild H. The economic rationale for adherence in the treatment of type 2 diabetes mellitus. Am J Manag Care. 2012;18(3 Suppl):S43–S48. [PubMed] [Google Scholar]
- 16.Nagrebetsky A, Griffin S, Kinmonth AL, Sutton S, Craven A, Farmer A. Predictors of suboptimal glycaemic control in type 2 diabetes patients: the role of medication adherence and body mass index in the relationship between glycaemia and age. Diabetes Res Clin Pract. 2012;96(2):119–128. doi: 10.1016/j.diabres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Stuart BC, Simoni-Wastila L, Zhao L, Lloyd JT, Doshi JA. Increased persistency in medication use by U.S. Medicare beneficiaries with diabetes is associated with lower hospitalization rates and cost savings. Diabetes Care. 2009;32(4):647–649. doi: 10.2337/dc08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pharmacy Quality Alliance. PQA performance measures. 2014. http://pqaalliance.org/measures/default.asp. Accessed 10 Oct 2014.
- 19.Centers for Medicare and Medicaid Services. Medicare Health and Drug Plan Quality and Performance Ratings 2013 Part C and Part D Technical Notes. 2013. http://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/Technical-Notes-2013-.pdf. Accessed 10 Oct 2014.
- 20.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health: J Int Soc Pharmacoecon Outcomes Res. 2007;10(1):3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 21.HCUP CCS. Healthcare Cost and Utilization Project: Agency for Healthcare Research and Quality, Rockville, MD. 2014. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 15 Nov 2014.
- 22.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Predicting costs with Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18(4):213–219. [PubMed] [Google Scholar]
- 24.US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Medical Care. Series ID: CUUR0000SAM Washington, DC: US Department of Labor, Bureau of Labor Statistics. 2012. http://data.bls.gov/cgi-bin/surveymost?su. Accessed 15 Nov 2014.
- 25.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. doi: 10.1016/S0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 26.Malmenas M, Bouchard JR, Langer J. Retrospective real-world adherence in patients with type 2 diabetes initiating once-daily liraglutide 1.8 mg or twice-daily exenatide 10 mug. Clin Ther. 2013;35(6):795–807. doi: 10.1016/j.clinthera.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SS, Nguyen H, Felber E, Cappell K, Nelson JK, Chu BC, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31(11):1119–1133. doi: 10.1007/s12325-014-0166-0. [DOI] [PubMed] [Google Scholar]
- 28.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93(1):1–9. doi: 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Odegard PS, Gray SL. Barriers to medication adherence in poorly controlled diabetes mellitus. Diabetes Educ. 2008;34(4):692–697. doi: 10.1177/0145721708320558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


