Abstract
Guidance concerning tyrosine kinase inhibitors (TKIs) for patients with wild type epidermal growth factor receptor (EGFR) and advanced non–small-cell lung cancer (NSCLC) after first-line treatment is unclear. We assessed the effect of TKIs as second-line therapy and maintenance therapy after first-line chemotherapy in two systematic reviews and meta-analyses, focusing on patients without EGFR mutations. Systematic searches were completed and data extracted from eligible randomized controlled trials. Three analytical approaches were used to maximize available data. Fourteen trials of second-line treatment (4388 patients) were included. Results showed the effect of TKIs on progression-free survival (PFS) depended on EGFR status (interaction hazard ratio [HR], 2.69; P = .004). Chemotherapy benefited patients with wild type EGFR (HR, 1.31; P < .0001), TKIs benefited patients with mutations (HR, 0.34; P = .0002). Based on 12 trials (85% of randomized patients) the benefits of TKIs on PFS decreased with increasing proportions of patients with wild type EGFR (P = .014). Six trials of maintenance therapy (2697 patients) were included. Results showed that although the effect of TKIs on PFS depended on EGFR status (interaction HR, 3.58; P < .0001), all benefited from TKIs (wild type EGFR: HR, 0.82; P = .01; mutated EGFR: HR, 0.24; P < .0001). There was a suggestion that benefits of TKIs on PFS decreased with increasing proportions of patients with wild type EGFR (P = .11). Chemotherapy should be standard second-line treatment for patients with advanced NSCLC and wild type EGFR. TKIs might be unsuitable for unselected patients. TKIs appear to benefit all patients compared with no active treatment as maintenance treatment, however, direct comparisons with chemotherapy are needed.
Keywords: Chemotherapy, Meta-analysis, NSCLC, RCT, TKI
Introduction
After reports of clinical trials1-5 and clinical guidelines (National Institute for Health and Care Excellence [NICE] TA258 and National Comprehensive Cancer Network [NCCN] Non-Small-Cell Lung-17), the use of the tyrosine kinase inhibitors (TKIs), erlotinib and gefitinib, is now common practice for first-line treatment of patients with non–small-cell lung cancer (NSCLC) with sensitizing epidermal growth factor receptor (EGFR) mutations. Beyond first-line treatment, in particular for patients with wild type EGFR who have received first-line chemotherapy, recommendations regarding the potential benefits of TKIs are less clear (NICE TA162, NCCN Guidelines for Non–Small-Cell Lung Cancer).6,7
With the exception of a few modern trials that only recruited patients with wild type disease,8,9 most evaluations of these drugs have been in unselected patients. Many of these trials did not test for EGFR mutations systematically, therefore, their results are reported either for all randomized patients, ignoring EGFR mutation status, or for the subset of patients in whom the status was known. Results of these trials are mixed and interpretation difficult.
Similarly, attempts have been made to synthesize existing data, to inform clinical guidelines and practice. Some have pooled all available results irrespective of mutation status or treatment line.10,11 This approach gives results based on large numbers of randomized patients, but results are difficult to interpret. Others have attempted to summarize the results of trials within subgroups defined according to EGFR status.12,13 Although this seems sensible and offers a more meaningful interpretation of the results, there are clear drawbacks. For example, trials that did not report their results according to mutation status were excluded, as were patients for whom no EGFR testing was carried out. When only a subset of the randomized patients within a given trial were tested, pooling results might introduce bias,14 not least because we cannot be certain that these patients were representative.
Despite these difficulties, there is a need to obtain effective and reliable summaries of the effects of TKIs in patients with and without sensitizing EGFR mutations after first-line treatment. Therefore, we set out to take into account the potential issues and biases. Rather than relying on one analytical approach, we planned a number of separate analyses. In addition to pooled analyses of all patients from all trials and of patient subgroups defined according to EGFR status, we aimed to carry out appropriate tests of interaction between treatment effects and patient characteristics, namely mutation status, to ascertain whether effects differed between patient groups. Finally, by assuming the likely ratios of patients with wild type and mutated EGFR in trials based on geographical location (East Asia 60:40/rest of world 90:10),15 we could assess the effect of increasing proportions of wild type patients on the overall treatment estimates using metaregression. Interpretation of the results was not based on any one of the individual results from the 3 approaches but on the combined results of all 3 approaches, such that we could feel more confident in our interpretation when results from all 3 analyses were complementary. Analyses were carried out in trials of second-line treatment, when TKIs were compared with standard chemotherapy regimens and in the maintenance therapy setting, compared with no active treatment.
Materials and Methods
All methods were prespecified in 2 registered protocols (CRD42013006449 and CRD42013006251).
Included trials should have randomized patients with advanced NSCLC irrespective of sex, age, histology, ethnicity, smoking history, or EGFR mutational status. Patients should not have received previous TKIs. For the systematic review of second-line treatment, trials should have compared a TKI (erlotinib or gefitinib) versus chemotherapy after first-line chemotherapy. For maintenance treatment, trials should have compared a TKI (erlotinib or gefitinib) versus no TKI after first-line chemotherapy.
Systematic searches16 were conducted in MedLine, EMBASE, Cochrane CENTRAL, clinical trials registers (PDQ, ClinicalTrials.gov), and relevant conference proceedings. We also searched reference lists of relevant randomized controlled trials (RCTs) and clinical reviews.
The risk of bias of individual trials was assessed16,17 with a low risk of bias being desirable for sequence generation, allocation concealment, and completeness of outcome data reporting. Trials in the maintenance setting should have also been at low risk of bias for blinding.
Progression-free survival (PFS) was the primary outcome, allowing assessment of the effects of immediate TKI versus no immediate TKI without interference from the use of TKIs on progression. Overall survival (OS) was the secondary outcome, accepting this limitation. Data on patient characteristics, including histology, ethnicity, EGFR mutational and smoking status, interventions, and outcomes were extracted from trial reports. When EGFR mutational status of patients was not reported it was estimated based on trial characteristics.
Statistical Analysis
Because trials did not necessarily test all patients for EGFR mutations and/or report results according to EGFR mutation status, we used 3 analytical approaches to make maximum use of the data available. Results were assessed for consistency and whether, taken together, they established with greater certainty, the effects of TKIs in patients with wild type EGFR. All interpretation was based on the balance of the results across these 3 approaches and not on any of the individual approaches in isolation.
Estimating the Interaction Between Treatment Effect and EGFR Mutation Status
When possible, hazard ratio (HR) estimates of effect and associated statistics were either extracted or estimated from the reported analyses18-20 according to EGFR mutation status for each trial. These were used to estimate the interaction between treatment effect and EGFR mutational status, calculated as the ratio of the estimated HRs within the EGFR wild type subgroup and the EGFR mutation subgroup for each trial.14 Interaction HRs were combined across trials using the fixed-effects inverse-variance model. Heterogeneity was assessed using χ2 test and I2 statistic.21 Such interactions are based on a comparison of the treatment effects of the TKIs for patients with wild type and mutated EGFR within trials. Therefore, the interactions might only be calculated for trials in which patients were tested for EGFR mutation status and the HR estimates for the wild type and mutation populations were reported. They cannot be calculated for trials that recruited patients with wild type EGFR exclusively.
Estimating the Effects of Treatment in Patients With Wild Type and Mutated EGFR
To estimate the effect of TKI outcomes for patients with wild type and mutated EGFR, using the maximum available data, the trial HRs and associated statistics were combined across trials using the fixed-effects inverse-variance model. Again, we were restricted to patients tested for EGFR mutation status, but could include trials that exclusively recruited patients with wild type EGFR. Heterogeneity was assessed using the χ2 test and I2 statistic21 and where identified, the random effects model was applied. The absolute effect on median PFS was calculated by applying the relevant HR to the average control group median PFS, assuming proportional hazards.
Estimating the Effects of Treatment According to the Proportion of Patients With Wild Type EGFR
Using metaregression we investigated how increasing proportions of patients with wild type EGFR (considered as a continuous factor) affected treatment effects. All trials reporting overall estimates of effect were included. Estimates of the effect of TKIs on PFS for all randomized patients were extracted or estimated from the reported analyses.18-20 When only a small proportion of patients had been tested for mutation status, we assumed that the proportion of patients with wild type EGFR would remain consistent across the whole trial. When no testing of mutation status had been carried out, or reported in a trial, we estimated the proportions of randomized patients having wild type EGFR to be 90% in western trials and 60% in trials of East Asian patients. We then estimated the change in treatment effect with increasing proportions of patients with wild type EGFR.
We also explored whether the TKI used (erlotinib or gefitinib) or the chemotherapy regimen used (docetaxel alone, pemetrexed alone, or docetaxel with pemetrexed) affected the effect of TKIs. Characteristics found to be important were adjusted for in the metaregression and explored using the F ratio.22
Results
We identified 25 potentially eligible RCTs, of TKIs as second-line treatment (n = 18) and maintenance treatment (n = 7; Figure 1).
Figure 1.
Randomized Controlled Trial (RCT) Identification, Screening, and Inclusion
Abbreviation: NSCLC = non–small-cell lung cancer.
Tyrosine Kinase Inhibitor Versus Chemotherapy in the Second-Line Setting
Of 18 potentially eligible trials, randomizing 4456 patients, 1 trial reported no results,23 2 trials24 and NCT00536107 were not published, and 1 randomized phase II feasibility trial never reached the phase III stage.25 Results were based on the 14 remaining eligible trials (4388 patients, 98% of total randomized, Table 1).8,9,26-37 Trials compared TKIs with either docetaxel or pemetrexed chemotherapy and were conducted between 2003 and 2012. Six trials were carried out in predominantly Asian populations. Randomized patients had good performance status (0-2) and median age ranged from 54.5 to 67.5 years (range, 20-88 years). Most were men and either current or former smokers. One trial33 included considerably more women (85%) and only never-smokers. Three trials randomized patients with wild type EGFR exclusively.8,9,37 Five trials evaluated EGFR mutation status using a range of methods (including DAKO EGFR Pharma DX and Eppendorf Piezo-electric microdissector). Mutation status was not evaluated in 5 trials. Twelve trials (3963 patients, 90% of total) reported PFS and 14 trials (4355 patients, 99% of total) reported OS (Tables 1 and 2).
Table 1.
Trial and Patient Characteristics (Based on All Randomized Patients)
| Trial | Accrual Period | Patient n | TKI | Control | Median Age (Range) | Sex (% Female) | PS (% 0/1) | Ethnicity | Smoking History (% Never) | Histology (% Adenocarinoma) | Patients With Known EGFR Status (% of Total Randomized) | EGFR Mutation, n (% of Total With Known Status) | EGFR Wild Type, n (% of Total With Known Status) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trials of Second-Line Treatment | |||||||||||||
| SIGN26 | 2003-2004 | 141 | Gefitinib | Docetaxel | 61 (29-85) | 30 | 67 | Western | 25 | Unknown | NR | NR | NR |
| V-15-3227 | 2003-2006 | 489 (387a) | Gefitinib | Docetaxel | Unknown | 38 | 96 | Asian | 32 | 78 | 57 (12) | 31 (55) | 26 (45) |
| Herbst et al28 | 2004-2005 | 79 | Erlotinib | Docetaxel or pemetrexed with bevacizumab | 65.5 (40-88) | 49 | 100 | Western | 13 | 78 | 30 (38) | 1 (3) | 29 (97) |
| INTEREST29 | 2004-2006 | 1466 (1316a) | Gefitinib | Docetaxel | 60.5 (20-84) | 35 | 88 | Western | 20 | 54 | 267 (18) | 38 (14) | 229 (86) |
| ISTANA30 | 2005-2006 | 161 | Gefitinib | Docetaxel | 57.5 (20-74) | 38 | 93 | Asian | 41 | 68 | NR | NR | NR |
| Li et al36 | 2006-2008 | 98 | Gefitinib | Docetaxel | Unknown | Unknown | Unknown | Asian | Unknown | Unknown | NR | NR | NR |
| TITAN31 | 2006-2010 | 424 | Erlotinib | Docetaxel or pemetrexed | 59 (22-79) | 24 | 80 | Western | 17 | 50 | 160 (38) | 11 (7) | 149 (93) |
| HORG32 | 2006-2010 | 332 | Erlotinib | Pemetrexed | 65.5 (37-86) | 18 | 85 | Western | 16 | 77 (non-sq) | NR | NR | NR |
| CTONG 08069,b | 2009-2012 | 157 | Gefitinib | Pemetrexed | 56.5 (24-78) | 36 | 100 | Asian | 49 | 96 | 157 (100) | Only WT patients | 157 (100) |
| TAILOR8,b | 2007-2012 | 219 | Erlotinib | Docetaxel | 66.5 (35-83) | 31 | 91 | Western | 22 | 68 (greater % in TKI arm) |
219 (100) | Only WT patients | 219 (100) |
| KCSG-LU08-0133 | 2008-2010 | 135 | Gefitinib | Pemetrexed | 61 (30-78) (younger in TKI arm) |
85 | 91 | Western | 100 | 100 | 71 (53) | 33 (46) | 38 (54) |
| PROSE34 | 2008-2012 | 263 | Erlotinib | Docetaxel or pemetrexed | 65 (33-85) | 27 | 94 | Western | 14 | 88 (non-sq) | 177 (67) | 14 (8) | 163 (92) |
| DELTA35 | 2009-2012 | 301 | Erlotinib | Docetaxel | 67.5 (31-85) | 29 | 96 | Asian | 25 | 69 | 255 | 51 (20) | 199 (78) |
| Li et al37,b | 2008-2014 | 123 | Erlotinib | Pemetrexed | 54.5 (30-75) | 36 | 94 | Asian | 26 | 100 | 123 (100) | Only WT patients | 123 (100) |
| Total | 4388 (4136) | 1516 (35) | 179 (12) | 1332 (88) | |||||||||
| Trials of Maintenance Treatment | |||||||||||||
| SATURN38 | 2005-2008 | 889 | Erlotinib | Placebo | 60 (30-83) | 26 | 100% | Western | 17 | 45 | 368 (41) | 40 (11) | 328 (89) |
| IFCT-GFPC 0502 (NCT00300586)39 | 2006-2009 | 310c | Erlotinib | Observation | 58 (36-72) | 27 | 100% | Western | 9 | 65 | 114 (37) | 8 (7) | 106 (93) |
| EORTC 0802140 | 2004-2009 | 173 | Gefitinib | Placebo | 61 (28-80) | 23 | 94% | Western | 22 | 51 | NR | NR | NR |
| INFORM41 | 2008-2009 | 296 | Gefitinib | Placebo | 55 (20-75) | 41 | 98% | Asian | 54 | 71 | 79 (27) | 30 (38) | 49 (62) |
| SWOG S002342 | 2001-2005 | 261 | Gefitinib | Placebo | 61 (24-81) | 37 | 96% | Western | Unknown | 31 | NR | NR | NR |
| ATLAS43,d | 2005-2008 | 768 | Erlotinib | Placebo | 64 (range unknown) | 48 | 100% | Western | 16 | 81 | 347 (45)e | 52 (15) | 295 (85) |
| Total | 2697 | 908 (34) | 130 (14) | 778 (86) |
Abbreviations: ATLAS = Avastin Tarceva Lung Adenocarcinoma Study; CTONG = Chinese Thoracic Oncology Group; DELTA = Docetaxel and Erlotinib Lung Cancer Trial; EGFR = epidermal growth factor receptor; EORTC = European Organisation for Research and Treatment of Cancer; HORG = Hellenic Oncology Research Group; IFCT-GFPC = Partenariat Intergroupe Francophone de Cancérologie Thoracique-Groupe Français de Pneumo-Cancérologie; INFORM = Iressa in NSCLC FOR Maintenance; INTEREST = IRESSA Non-small-cell lung cancer Trial Evaluating REsponse and Survival against Taxotere; ISTANA = Iressa as Second-line Therapy in Advanced NSCLC; KCSG = Korean Cancer Study Group; non-sq = Non-Squamous; PROSE = Predicting Response to Second-Line Therapy Using Erlotinib; PS = performance status; SATURN = Sequential Tarceva in Unresectable NSCLC; SIGN = Second-line Indication of Gefitinib in NSCLC; SWOG = South West Oncology Group; TAILOR = Tarceva Italian Lung Optimization Trial; TITAN = Tarceva In Treatment of Advanced NSCLC; TKI = tyrosine kinase inhibitor; WT = wild type.
Progression-free survival analyses for patient number in parentheses, but patient characteristics reported for all patients.
Only randomized patients with wild type EGFR.
Three-arm trial including 464 randomized patients but only 2 arms included here.
Includes bevacizumab in both arms.
Total for progression-free survival, total for overall survival is 345.
Table 2.
Results for Overall Survival
| Trial, n | Patient, n | Fixed Effect |
Random Effect |
Interaction HRa (95% CI) P | Interaction Heterogeneity, P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||||
| Second-Line Treatment | ||||||||||
| EGFR wild type | 9 | 1400 | 1.06 | 0.93-1.22 | .37 | 1.06 | 0.93-1.20 | .37 | 1.15 (0.60-2.18) .68 | .37 |
| EGFR mutations | 4 | 97 | 0.90 | 0.49-1.64 | .72 | 0.90 | 0.49-1.64 | .72 | ||
| Maintenance Treatment | ||||||||||
| EGFR wild type | 3 | 707 | 0.85 | 0.72-1.02 | .06 | 0.87 | 0.70-1.07 | .70 | 1.40 (0.76-2.57) .28 | .49 |
| EGFR mutations | 3 | 120 | 0.59 | 0.33-1.05 | .07 | 0.59 | 0.33-1.05 | .07 | ||
Abbreviations: EGFR = epidermal growth factor receptor; HR = hazard ratio; TKI = tyrosine kinase inhibitor.
Interaction HR > 1 shows greater TKI benefit for mutated EGFR.
Assessment of Risk of Bias
One trial,36 published in Chinese language, was judged to be unclear for all domains. The remaining 13 trials were all at low risk of bias regarding incomplete outcome data. Missing data on EGFR mutational status largely resulted from unavailable tumor samples or because the trials were conducted before widespread testing. All were judged to be at low risk of bias for sequence generation. For allocation concealment, 10 trials were judged to be at low risk of bias and 3 were judged as unclear risk. No trials were judged to be at high risk for any of the domains assessed (see Supplemental Table 1 in the online version).8,9,26-37 When information could not be obtained from the publications, we contacted the authors.44
Progression-Free Survival
Interaction Between Treatment Effect and EGFR Mutation Status
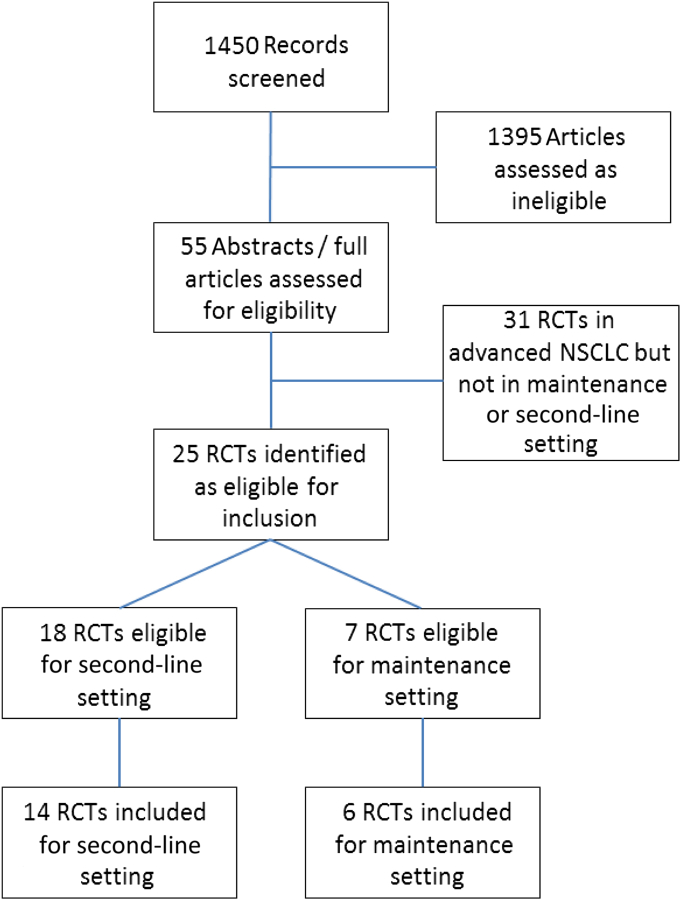
Data on the effects of TKIs compared with chemotherapy on PFS within groups of patients with EGFR mutations and wild type EGFR were available from 4 trials, including 442 patients with wild type EGFR and 113 with EGFR mutations (13% of the total randomized in all trials). There was strong evidence of an interaction between the effect of TKIs and EGFR mutational status (interaction HR, 2.69; 95% confidence interval [CI], 1.37-5.29; P = .004; Figure 2A),8,9,27,29,31,33,35,37 with the benefit of treatment of TKIs evident only among patients with EGFR mutations. This was consistent across trials (heterogeneity P = .179; I2, 39%).
Figure 2.
(A) Tyrosine Kinase Inhibitor (TKI) Versus Chemotherapy in the Second-Line Setting: Interaction Between Treatment Effect and Epidermal Growth Factor Receptor (EGFR) Mutation Status for Progression-Free Survival. The Circles Represent (Fixed Effect) Meta-Analysis of the Hazard Ratios (HRs) Representing the Interaction Between the Effect of Treatment (TKI) in Wild Type EGFR Compared With Mutated EGFR; the Horizontal Lines Show the 95% CI. (B) TKI Versus Chemotherapy in the Second-Line Setting: Effect of Treatment in 1302 Patients With Wild Type EGFR on Progression-Free Survival. Each Square Denotes the HR for That Trial With the Horizontal Lines Showing the 95% CI. The Size of the Square Is Directly Proportional to the Amount of Information Contributed by That Trial. The Diamond Gives the Pooled HR From the Fixed Effect Model; the Center of the Diamond Denotes the HR and the Extremities, the 95% CI. (C) TKI Versus Chemotherapy in the Second-Line Setting: Effect of Treatment in 113 Patients With Mutated EGFR on Progression-Free Survival
Abbreviations: CTONG = Chinese Thoracic Oncology Group; DELTA = Docetaxel and Erlotinib Lung Cancer Trial; INTEREST = IRESSA Non-small-cell lung cancer Trial Evaluating REsponse and Survival against Taxotere; KCSG = Korean Cancer Study Group; PROSE = Predicting Response to Second-Line Therapy Using Erlotinib; TAILOR = Tarceva Italian Lung Optimization Trial; TITAN = Tarceva In Treatment of Advanced NSCLC.
Effect of Treatment in Patients With Wild-Type and Mutated EGFR
Results for patients with wild type EGFR were available for 9 trials and 1302 patients (30% of the total randomized in all trials). There was evidence of a detriment with TKIs compared with chemotherapy (HR, 1.31; 95% CI, 1.16-1.48; P < .0001; Figure 2B), with some evidence of variation between the trial results (heterogeneity P = .09; I2, 41%). However, the effect was fairly similar with a random-effects model (HR, 1.27; 95% CI, 1.08-1.51; P = .005). Assuming a median baseline PFS of 13 weeks, based on the average time of PFS in the control arms of included trials; HR, 1.31 translates to a 3-week absolute reduction in median PFS (from 13 weeks to 10 weeks).
Four trials reported PFS for patients with EGFR mutations. Based on these 113 patients (2% of the total randomized in all trials), there was evidence of a benefit with TKIs compared with chemotherapy (HR, 0.34; 95% CI, 0.20-0.60; P = .0002) and no evidence of variation between the trial results (heterogeneity P = .26; I2, 26%; Figure 2C). Again, assuming a median PFS of 13 weeks, this translates to a 25-week increase in the absolute median PFS (from 13 weeks to 38 weeks).
Effect of Treatment According to Proportion of Patients With Wild Type EGFR
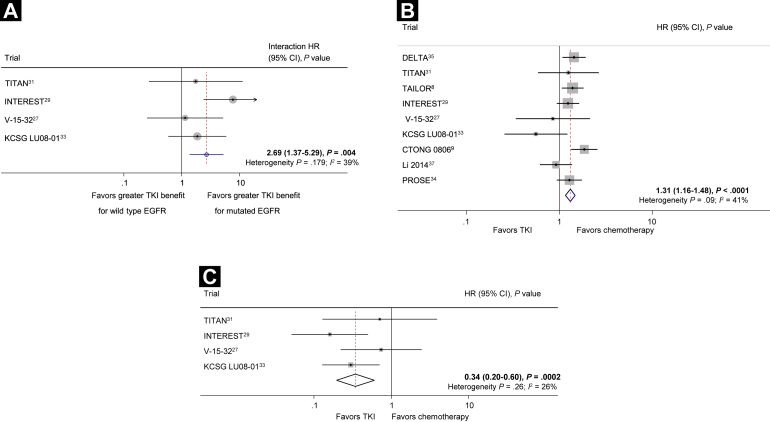
Twelve trials including 3963 patients reported PFS for all patients, irrespective of EGFR status. Metaregression suggested a decreasing effect of TKIs with increasing proportions of wild type patients (P = .014). The treatment effect predicted by the model when 100% of patients had wild type EGFR favors chemotherapy (HR, 1.28; 95% CI, 1.08-1.53; P = .005), whereas when 100% of patients had EGFR mutations, the model predicted a benefit of TKIs (HR, 0.45; 95% CI, 0.25-0.80; P = .007; Figure 3).8,9,26-29,31,33-35,37
Figure 3.
Tyrosine Kinase Inhibitor (TKI) Versus Chemotherapy in the Second-Line Setting: Effect of Treatment According to the Proportion of Patients With Wild-Type Epidermal Growth Factor Receptor (EGFR) on Progression-Free Survival
Abbreviations: CTONG = Chinese Thoracic Oncology Group; DELTA = Docetaxel and Erlotinib Lung Cancer Trial; INTEREST = IRESSA Non-small-cell lung cancer Trial Evaluating REsponse and Survival against Taxotere; ISTANA = Iressa as Second-line Therapy in Advanced NSCLC; KCSG = Korean Cancer Study Group; PROSE = Predicting Response to Second-Line Therapy Using Erlotinib; SIGN = Second-line Indication of Gefitinib in NSCLC; TAILOR = Tarceva Italian Lung Optimization Trial; TITAN = Tarceva In Treatment of Advanced NSCLC.
Assessing Whether the Treatment Effect Varies With the TKI or Chemotherapy Used
No differences in the treatment effects of TKIs versus chemotherapy were observed when trials were subdivided according to chemotherapy used: docetaxel alone, pemetrexed alone, or docetaxel and pemetrexed (test for between-subgroup heterogeneity P = .30). There was a difference in the treatment effect according to the TKI used in all randomized patients (test for between-subgroup heterogeneity P = .008). However, when the analysis was adjusted to account for substantial heterogeneity within the group of trials using gefitinib (P < .0001; I2, 82%), there was no longer evidence of this difference between the TKIs (metaregression P = .24; F ratio P = .18). Additionally, when the TKI type was taken into account in the metaregression, there was still evidence of a decreasing effect of TKIs with increasing proportions of patients with wild type EGFR (P = .043).
Overall Survival
Data on the effects of TKIs on OS within groups of patients with EGFR mutations and wild type EGFR were available from 4 trials, including 540 patients with wild type EGFR and 97 with EGFR mutations (15% of the total randomized in all trials). Based on the available data, there was no evidence of an interaction between the effect of TKIs on OS and EGFR mutational status (interaction HR, 1.15; 95% CI, 0.60-2.18; P = .68; Table 2). This relationship appeared consistent across trials (heterogeneity P = .37; I2, 4%).
Maintenance TKI Versus No Active Treatment
We identified 7 eligible trials. No results were available for 1 ongoing trial (NCT00153803), therefore, 6 trials38-43 were included (2697 randomized patients, 100% of total; Table 1). Trials were conducted between 2001 and 2009 and compared TKIs with placebo38,40-43 or observation.39 Five trials randomized predominantly western patients38-40,42,43 and 1 trial randomized only Chinese patients.41 Overall, randomized patients had good performance status (0-2); with median age from 55 to 64 years (range, 20-83 years). They were mostly men and either current or former smokers, except for 1 trial,41 in which more than half of the included patients had never smoked. Three trials evaluated EGFR mutation status using a range of methods (EGFR mutation detection kit [DxS, Manchester, UK], and sequencing of polymerase chain reaction products from exons 18 to 21 of the EGFR gene). Mutation stssatus was not evaluated in the remaining trials.
Assessment of Risk of Bias
Five trials were judged to be at low risk of bias for allocation concealment, sequence generation, and blinding.38-41,43 One trial was at low risk of bias for all domains except for sequence generation and allocation concealment, which were unclear.42 No trials were identified as being at high risk of bias. Missing data on EGFR mutational status largely resulted from unavailable tumor samples or because the trials were conducted before widespread testing (see Supplemental Table 1 in the online version).
Progression-Free Survival
Interaction Between Treatment Effect and EGFR Mutation Status
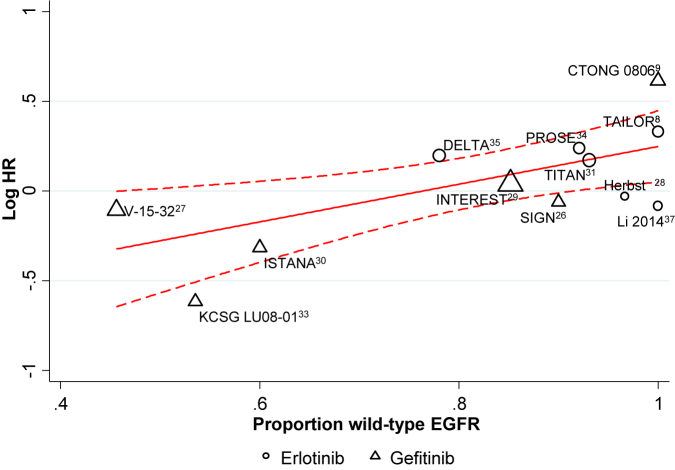
Progression-free survival results were reported separately in 4 trials for wild type patients and EGFR mutation-positive patients, 908 patients (34% of the total randomized in these trials; Table 1). There was strong evidence of an interaction between the effect of TKIs on PFS and EGFR mutational status, with the larger effect being observed in patients with EGFR mutations (interaction HR, 3.58; 95% CI, 2.19-5.85; P < .0001; Figure 4A).38,39,41,43 There was some evidence of inconsistency in the effect between trials (heterogeneity P = .12; I2, 48%). However, the effect was fairly similar with a random effects model (HR, 3.83; 95% CI, 1.85-7.95; P = .0003).
Figure 4.
(A) Maintenance Tyrosine Kinase Inhibitor (TKI) Versus No Active Treatment: Interaction Between Treatment Effect and Epidermal Growth Factor Receptor (EGFR) Mutation Status for Progression-Free Survival. (B) Maintenance TKI Versus No Active Treatment: Effect of Treatment in 778 Patients With Wild Type EGFR on Progression-Free Survival. (C) Maintenance TKI Versus No Active Treatment: Effect of Treatment in 130 Patients With Mutated EGFR on Progression-Free Survival
Abbreviations: ATLAS = Avastin Tarceva Lung Adenocarcinoma Study; IFCT GFPC = Partenariat Intergroupe Francophone de Cancérologie Thoracique-Groupe Français de Pneumo-Cancérologie; INFORM = Iressa in NSCLC FOR Maintenance; SATURN = Sequential Tarceva in Unresectable NSCLC.
Effects of Treatment in Patients With Wild Type and Mutated EGFR
Progression-free survival results for patients with wild type EGFR were available from 4 trials and 778 patients. There was evidence of a PFS benefit with TKIs in patients with wild type EGFR (HR, 0.82; 95% CI, 0.71-0.96; P = .01; Figure 4B) and no evidence of variation between the trial results (heterogeneity P = .90; I2, 0%). Assuming a median PFS in the control group of 13 weeks, this translates to an absolute improvement in median PFS of approximately 3 weeks (from 13 weeks to 16 weeks).
For patients with EGFR mutations, data were available from 4 trials but only 130 patients. Although the data available for this analysis were very limited, there was a large PFS benefit with TKIs (HR, 0.24; 95% CI, 0.15-0.37; P < .0001; Figure 4C) but with clear evidence of variation between the trial results (heterogeneity P = .06; I2, 58%). However, the results were similar when a random effects model was used (HR, 0.22; 95% CI, 0.10-0.46; P < .0001). This translated to an absolute improvement in median PFS of approximately 10 months (from 13 weeks to 13 months).
Effect of Treatment According to the Proportion of Patients With Wild Type EGFR
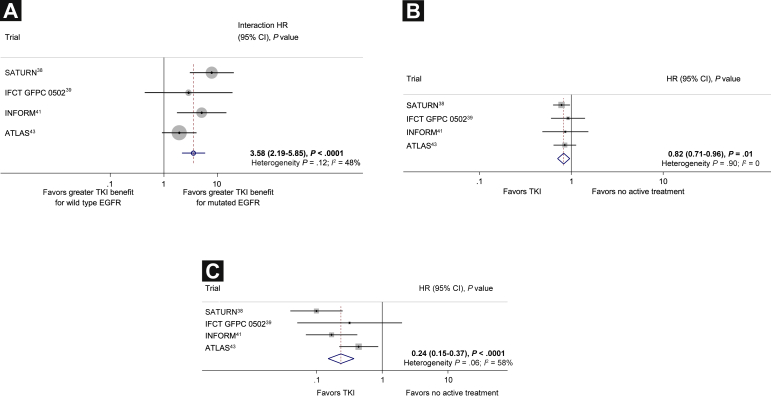
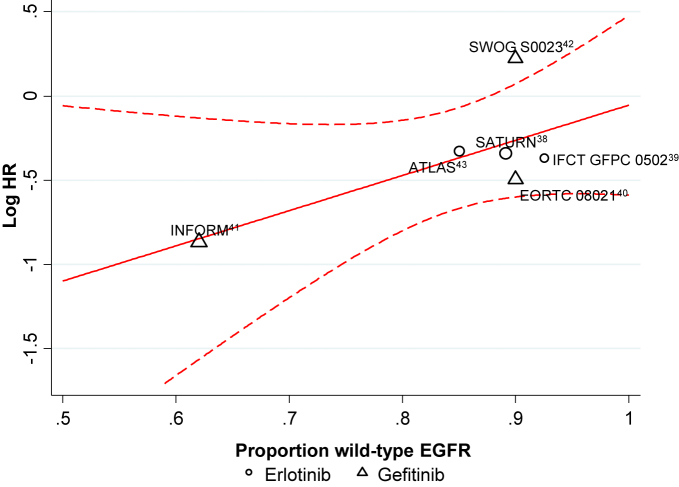
Six trials (2672 patients; 99% of total randomized) reported PFS for all patients irrespective of EGFR mutation status. The metaregression suggested that treatment effect varied according to the proportion of patients with wild type EGFR (P = .11). When 100% of patients had wild type EGFR, the model suggested that there is no difference in PFS with TKIs compared with no active treatment (HR, 0.95; 95% CI, 0.65-1.38; P = .78), whereas when 100% of patients had EGFR mutations, a large benefit of TKIs was indicated (HR, 0.12; 95% CI, 0.02-0.66; P = .015; Figure 5).38-43 However, the metaregression was based on only 6 trials and was clearly limited.
Figure 5.
Maintenance Tyrosine Kinase Inhibitor Versus No Active Treatment: Effect of Treatment According to the Proportion of Patients With Wild Type Epidermal Growth Factor Receptor (EGFR) on Progression-Free Survival
Abbreviations: ATLAS = Avastin Tarceva Lung Adenocarcinoma Study; EORTC = European Organisation for Research and Treatment of Cancer; IFCT GFPC = Partenariat Intergroupe Francophone de Cancérologie Thoracique-Groupe Français de Pneumo-Cancérologie; INFORM = Iressa in NSCLC FOR Maintenance; SATURN = Sequential Tarceva in Unresectable NSCLC; SWOG = South West Oncology Group.
Interaction Between Treatment Effect and Histology in Patients With Wild Type EGFR
We conducted an exploratory analysis to assess whether the benefit of TKIs in patients with wild type EGFR was related to histological type (adenocarcinoma/squamous cell carcinoma). Data were available for 4 trials and 2129 patients (1430 adenocarcinoma; 699 squamous/other nonadenocarcinoma). There was a significant difference in effect between the 2 subgroups (interaction HR, 1.41; 95% CI, 1.11-1.80; P = .004) with little suggestion of variation between trials (heterogeneity P = .347; I2, 3.8%). However, benefits of TKI were observed for patients with squamous (HR, 0.77; 95% CI, 0.64-0.92; P = .004; I2, 0%; heterogeneity P = .89) and adenocarcinoma (HR, 0.59; 95% CI, 0.52-0.66; P < .0001; I2, 79%; heterogeneity P = .002).
Overall Survival
Three trials reported OS according to mutation status. We found no evidence to suggest a difference in the effect of TKIs in patients with mutations compared with those with wild type disease (interaction HR, 1.40; 95% CI, 0.76-2.57; P = .28; Table 2). This relationship was similar between the trials (heterogeneity P = .49; I2, 0%).
Discussion
Taken together, evidence from 3 distinct analytical approaches suggests a difference in the effect of TKIs on PFS according to EGFR mutation status. For patients with wild type EGFR, TKIs seem to be an ineffective second-line treatment compared with chemotherapy, but might be effective as maintenance treatment, compared with no active treatment. In both settings, TKIs offer PFS benefits to patients with mutated EGFR.
Pooling the estimated interactions between treatment effects and patient characteristics for each trial, using only within-trial information and avoiding ecological bias,14 provides the most reliable estimate of the relationship between the effect of TKIs and EGFR mutation status. Nevertheless, it relies on trials reporting results for patients with wild type EGFR and EGFR mutations separately. Although not all trials tested patients systematically, we have no reason to suspect selective reporting of results or selective testing of patients, which could introduce bias. However, the precision of the estimates of interaction was inevitably limited by the relatively low numbers of included trials reporting results for both mutation subgroups (trials of second line treatment, n = 9, and in the maintenance setting, n = 4).
Incorporating additional data, mainly from trials that recruited only patients with wild type EGFR, enabled us to provide further evidence that TKIs are inferior to chemotherapy as second-line treatment in patients with wild type EGFR, reducing median PFS by approximately 3 weeks. As maintenance treatment, we found that TKIs offer a modest improvement in median PFS compared with no active treatment of approximately 3 weeks for patients with wild type EGFR. We did, however, see some evidence of inconsistency between the trial results in the second-line treatment setting, which might reflect the clinically heterogeneous nature of this large group of patients. There will of course be variation in terms of known prognostic factors such as age, tumor histology, and stage, however, it is also possible that other, as yet undefined characteristics, might further explain this variability. Although pooling results for patient subgroups from trials in this way might introduce bias14 and the meta-analysis is again limited because many trials tested only a relatively small subset of patients for mutation status, or did not test at all, they do provide the best available estimate of the effects of TKIs in patients with wild type and mutated EGFR.
Using metaregression, including data from all randomized patients from all eligible trials, we assessed how the proportion of patients with wild type EGFR modified the effect of TKIs on PFS. The results suggest that the benefit of TKIs relative to chemotherapy on PFS diminishes with an increasing proportion of patients with wild type EGFR. This pattern holds for the maintenance setting, suggesting no evidence of a benefit of TKIs when 100% of patients have wild type EGFR. These metaregressions relied in part on assumptions about the EGFR mutation status of trial populations. Furthermore, the relationship between the effect of TKIs and the proportion of patients with wild type EGFR might not be representative of the true relationship between the effect of TKIs and the mutation status of individual patients.14,45 There are clearly limitations to this approach, not only that we had to make assumptions or estimates of the proportions of patients with wild type EGFR for a number of the trials. The relatively small number of data points (second-line setting, n = 12; maintenance setting, n = 6) and that in part because of estimates made, the proportions tend to cluster around either the 60% or 90% positions, mean that the regression model is limited. Nonetheless, this approach has allowed us to maximize the available data to include all relevant trials, and the results do add further support for our other analyses. Although none of the individual approaches is without potential limitations, using results obtained from 3 distinct methods has increased our confidence in interpreting and drawing conclusions regarding the effect of EGFR mutation status on the response to erlotinib and gefitinib in these 2 treatment settings.
These systematic reviews are the first to have estimated the interaction between the effects of TKIs on PFS in patients with wild type EGFR and those with EGFR mutations appropriately and reliably.14 Furthermore, they are the first to have based interpretation on a range of analyses, making the best use of all available data and to assess consistency of results. We have attempted to evaluate the effect of treatment in wild type patients, who form the majority of patients with NSCLC worldwide. Since the importance of activating EGFR mutations in patients' response to TKIs were recognized, research has concentrated on patients carrying such mutations. Moreover, a recent review focused on the effect of TKIs in mutation-positive patients and only used the limited reported data from patients who were tested for EGFR mutations.12 Using or estimating the proportions of patients with wild type EGFR, we included results from all trials in a metaregression, rather than only the minority of patients for whom results according to mutational status were reported. We avoided estimating effects based on all randomized patients irrespective of EGFR status, which are potentially misleading.
Although we provided clear evidence of a difference in the effect of TKIs according to mutation status on PFS, there was no evidence to support a difference in their effect on OS in either treatment setting. However, most of the included trials allowed treatment crossover on progression, inevitably making the OS results more difficult to interpret. The full effect of TKI treatment on OS in patients with advanced NSCLC therefore remains unclear.
In the maintenance setting, our strict eligibility criteria meant that we included only trials in which treatment comparisons were unconfounded. This inevitably limited the number of trials and patients available for this analysis and hence our results must be viewed with particular caution. Furthermore, the comparator in these trials was essentially no active treatment. It is unclear whether the potential benefit of TKIs for patients with wild type EGFR would persist if compared for example, with pemetrexed (for patients with adenocarcinoma) as is recommended in clinical guidelines6-7 and NICE TA162, NCCN. We did not find any trials that directly compared TKIs with chemotherapy in the maintenance setting. One 3-arm trial included in this meta-analysis compared each of gemcitabine and erlotinib with observation alone39 but no comparison between the 2 treatments was reported.
Currently TKIs and chemotherapy are recommended options for second-line treatment for patients with wild type EGFR. Our results bring this into doubt and suggest that for patients with wild type EGFR who are well enough to receive it, chemotherapy should be viewed as the standard of care. Furthermore, particularly outside of East Asia, most unselected patients will have wild type EGFR. Guidelines that recommend TKIs for unselected populations should be reconsidered. Our results highlight the importance of suitable biopsies and reliable EGFR mutation testing to guide optimal clinical treatment.
Our findings regarding maintenance treatment, coupled with our results in the second-line setting might lead us to surmise that, compared with appropriate chemotherapy, patients with wild-type EGFR are unlikely to benefit from TKIs. However, for patients in whom no alternative is recommended, for example, in patients with squamous cell carcinoma, TKIs might be considered. Without direct comparisons of TKIs with chemotherapy in this setting, the best treatment options remain unclear.
Conclusion
There is still uncertainty regarding the best treatment option for the overwhelming majority of advanced NSCLC patients worldwide with wild type EGFR. However, based on these results, TKIs are not an appropriate second-line treatment for patients who are fit to receive chemotherapy, but might offer some scope as maintenance treatment.
Disclosure
The authors have stated that they have no conflicts of interest.
Acknowledgments
This work was supported by the UK Medical Research Council. The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Supplemental Data
Supplemental Table 1.
Assessment of Risk of Bias for Included Trials
| Trial | Sequence Generation | Allocation Concealment | Blinding of Participants/Personnel | Blinding of Outcome Assessmenta | Incomplete Outcome Data Addressed | Free of Selective Reporting |
|---|---|---|---|---|---|---|
| Second-Line Treatment | ||||||
| SIGN26 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Randomized 1:1 to 1 of 2 treatment groups | Randomization was performed at the site using sealed randomization envelopes which were allocated sequentially to patients | All randomized patients are included in the analysis | Reports PFS and OS in full | |||
| V-15-9227 Full publication |
Low risk | Unclear | NA | NA | Low risk | Low risk |
| Patients were randomly assigned using stratification factors of sex, PS, histology, and study site | NR | 490 Patients randomized; 1 patient excluded due to protocol violation; 489 patients ITT | Reports OS in ITT patients and PFS for ‘assessable for response’ population | |||
| Herbst et al28 Full publication |
Low risk | Unclear | NA | NA | Low risk | Low risk |
| Patients were randomly assigned on a 1:1:1 basis | NR | The analyses included all treated patients | Reports PFS and OS in full | |||
| INTEREST29 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Patients were randomly assigned with dynamic balancing | Used a centralized registration and randomization center, contacted by telephone, to assign patients to a specific treatment group | 1466 Patients randomized; 1433 patients per protocol population (OS); 1316 patients evaluable for response population (PFS) | Reports PFS in evaluable for response population (89% of randomized patients) and OS in per protocol population (97% of randomized patients) | |||
| ISTANA30 Full publication |
Low risk | Unclear | NA | NA | Low risk | Low risk |
| Eligible patients were randomly assigned to receive gefitinib or docetaxel after stratification for histology, sex, PS, best response to previous therapy, smoking history, and participating center | NR | All randomized patients are included in the analysis | Reports PFS and OS in full | |||
| TITAN31 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Patients who were enrolled into TITAN were randomly assigned (1:1) using an adaptive randomization method (minimization as proposed by Pocock and Simon46) | Randomization and stratification instructions were obtained through a third-party telephone interactive voice response system | All randomized patients are included in the analysis | Reports PFS and OS in full | |||
| HORG32 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Patients were centrally randomized by computer at a 1:1 ratio and stratified according to PS, disease stage, age, and response to first-line treatment | Patients were centrally randomized using a computer | 357 Patients randomized; 332 patients (93%) received treatment and were analyzed | Reports time to progression and OS in full | |||
| CTONG 08069 Abstract and slides |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Patients were randomized to receive gefitinib orally or pemetrexed | Centrally randomized | 161 Patients randomized; 157 (98%) were evaluable | Reports PFS and OS in full | |||
| TAILOR8 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Treatment was randomly allocated in a 1:1 ratio using a minimization algorithm | A customized, Web-based database was set up for registration, randomization, monitoring, local data entry, and central data management | 222 Patients randomized; 219 (99%) patients included in ITT analyses | Reports PFS and OS in full | |||
| KCSG-LU08-0133 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Patient randomization was stratified according to ECOG PS or sex | Patients were consecutively assigned according to a predefined computer-generated randomization scheme | 141 Patients randomized; 135 (96%) patients received treatment and were analyzed | Reports OS and PFS in full | |||
| PROSE34 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Treatment was randomly allocated using a minimization algorithm, which stratified treatment allocation according to smoking history (never, former, or current smokers), ECOG PS (0-1, or 2), proteomic test classification (poor or good), and center | Centrally randomized | 285 Patients randomized; 22 patients excluded for protocol violations or due to not receiving treatment; 263 (92%) patients were analyzed | Reports OS and PFS in full | |||
| DELTA35 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Eligible patients were randomly assigned 1:1 to erlotinib or docetaxel using the minimization method according to sex, PS, histology, and institution | Centrally randomized | All randomized patients are included in the analysis | Reports OS and PFS in full | |||
| Li et al37 Full publication |
Low risk | Low risk | NA | NA | Low risk | Low risk |
| Patients were randomly assigned in a 1:1 ratio; randomization was stratified according to sex, PS, and smoking history using a minimization algorithm | Centrally randomized | All randomized patients are included in the analysis | Reports OS and PFS in full | |||
| *Li et al36 Full publication |
Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Maintenance Treatment | ||||||
| SATURN38 Full publication |
Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Randomization was done using a 1:1 adaptive method using minimization as proposed by Pocock and Simon46 | Via a third-party voice response system | Allocation was not blinded but is unlikely to bias the outcome of PFS | The randomization list was not made available to the study centers, trial monitors, statisticians, or study sponsor | 5 Patients who progressed before randomization were not included in the analysis of PFS (1/438 erlotinib; 4/451 placebo) | Reports PFS and OS in full | |
| EORTC 0802140 Full publication |
Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Minimization with stratification according to stage, PS, best response, and institution | Centralized double blind random assignment | Double-blind | Unblinding was only allowed if the investigator needed the information to determine subsequent therapy | All randomized patients are included in the analysis | Reports PFS and OS in full | |
| IFCT-GFPC-050239 (NCT00300586) Full publication |
Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Minimization adaptive randomization, stratified according to center, sex, histology, smoking status, and response to first-line CT | Randomization was computerized and centrally located | Lack of blinding is unlikely to bias the outcome of PFS | Disease progression was reviewed by a panel of investigators who were blinded to randomization, independently of the treating investigator | All randomized patients were included in the analysis | Reports PFS and OS in full | |
| ATLAS43 Full publication |
Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Patients were randomized (1:1), and stratified according to initial chemotherapy choice, smoking status, and ECOG PS | Centrally randomized via an interactive voice response system | The study sponsor, study investigators, and patients were blinded to treatment | A data safety monitoring committee monitored safety and efficacy | All randomized patients were included | Reports PFS and OS in full | |
| SWOG S002342 Full publication |
Unclear | Unclear | Low risk | Low risk | Low risk | Low risk |
| Randomly assigned | Not reported | No details reported/not blinded but lack of blinding is unlikely to bias the outcome of PFS | No details reported/not blinded but lack of blinding is unlikely to bias the outcome of PFS | 243 Randomly assigned and all included in analyses. An additional 18 patients (7%) who were randomly assigned were found to be ineligible – they were not analyzed | Reports PFS and OS in full | |
| INFORM41 Full publication |
Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| The system generated randomization codes based on dynamic balancing algorithms wrt histology and smoking history | Randomization was done centrally by a third party randomization center that had no other role in the study | Active and placebo drugs were identical in form and packaging to ensure blinding. Further steps to ensure blinding included blinding of AstraZeneca personnel | Investigators and participants were blinded to study treatment until the primary analysis of PFS was complete | All randomized patients appear to have been included in the analyses | Reports PFS and OS in full |
Abbreviations: ATLAS = Avastin Tarceva Lung Adenocarcinoma Study; CT = Chemotherapy; CTONG = Chinese Thoracic Oncology Group; DELTA = Docetaxel and Erlotinib Lung Cancer Trial; ECOG = Eastern Cooperative Oncology Group; EORTC = European Organisation for the Research and Treatment of Cancer; HORG = Hellenic Oncology Research Group; IFCT-GFPC = Partenariat Intergroupe Francophone de Cancérologie Thoracique - Groupe Français de Pneumo-Cancérologie; INFORM = Iressa in NSCLC FOR Maintenance; INTEREST = IRESSA Non-small-cell lung cancer Trial Evaluating REsponse and Survival against Taxotere; ISTANA = Iressa as Second-line Therapy in Advanced NSCLC; ITT = Intention to treat; KCSG = Korean Cancer Study Group; OS = overall survival; PFS = progression-free survival; PROSE =Predicting Response to Second-Line Therapy Using Erlotinib; PS = performance status; SATURN = Sequential Tarceva in Unresectable NSCLC; SIGN = Second-line Indication of Gefitinib in NSCLC; SWOG = South West Oncology Group; TAILOR = Tarceva Italian Lung Optimization Trial; TITAN = Tarceva In Treatment of Advanced NSCLC; wrt = with respect to.
Li trial published in Chinese language.
Blinding only possible for trials comparing TKI with placebo in the maintenance setting.
References
- 1.Fukuoka M., Wu Y.L., Thongprasert S. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R., Carcereny E., Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Makoto M., Akira I., Kunihiko K. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Caicun Z., Yi-Long W., Gongyan C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T., Morita S., Yatabe Y. Gefitinib versus cisplatin plus docetaxel in patients with non–small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Azzoli C.G., Baker S., Temin S. American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non–Small-Cell Lung Cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters S., Adjei A.A., Gridelli C. Metastatic non–small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii56–vii64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 8.Garassino M.C., Martelli O., Broggini M. Erlotinib versus docetaxel as second-line treatment of patients with advanced non–small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14:981–988. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q., Cheng Y., Zhao M.F. Final results of CTONG 0806: a phase II trial comparing pemetrexed with gefitinib as second-line treatment of advanced non-squamous NSCLC patients with wild-type EGFR. J Thorac Oncol. 2013;8(Suppl 2):S194. (abstract O15.07) [Google Scholar]
- 10.Petrelli F., Borgonovo K., Cabiddu M., Barni S. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non–small-cell lung cancer: a meta-analysis of 13 randomized trials. Clin Lung Cancer. 2012;13:107–114. doi: 10.1016/j.cllc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Gao H., Ding X., Wei D. Efficacy of erlotinib in patients with advanced non–small-cell lung cancer: a pooled analysis of randomized trials. Anticancer Drugs. 2011;22:842–852. doi: 10.1097/CAD.0b013e328349c303. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.K., Brown C., Gralla R.J. Impact of EGFR inhibitor in non–small-cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.K., Hahn S., Kim D.W. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non–small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis. JAMA. 2014;311:1430–1437. doi: 10.1001/jama.2014.3314. [DOI] [PubMed] [Google Scholar]
- 14.Fisher D.J., Copas A.J., Tierney J.F., Parmar M.K. A critical review of methods for the assessment of patient-level interactions in individual patient data (IPD) meta-analysis of randomised trials, and guidance for practitioners. J Clin Epidemiol. 2011;64:949–967. doi: 10.1016/j.jclinepi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Ellison G., Zhu G., Moulis A. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66:79–89. doi: 10.1136/jclinpath-2012-201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Green S.J. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. 2011 Version 5.1.0 [updated March 2011] Available from www.cochrane-handbook.org. [Google Scholar]
- 17.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmar M.K., Torri V., Stewart L.A. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Williamson P.R., Tudur Smith C., Hutton J.L., Marson A.G. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 20.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Collaborative Ovarian Neoplasm (ICON) Group Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphomide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505–515. doi: 10.1016/S0140-6736(02)09738-6. [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar A.R., Singh D.P., Sharma R., Kumbhaj P. Docetaxel versus gefitinib in patients with locally advanced or metastatic NSCLC pre-treated with platinum-based chemotherapy. J Thorac Oncol. 2012;7(Suppl 3):S159. (abstract 102-P12) [Google Scholar]
- 24.Dilts D.M., Adjei A.A., Mandrekar S.J. Impact of trial development time on accruals at CCOPs: the case of the MARVEL trial. J Clin Oncol. 2010;28(15 suppl (May 20 Supplement)):e16505. [Google Scholar]
- 25.Soria J.C., Barlesi F., Besse B. Results of the prospective, randomized, and customized NSCLC adjuvant phase II trial (IFCT-0801, TASTE trial) from the French Collaborative Intergroup (abstract 7505) J Clin Oncol. 2013;31 No 15 suppl (May 20 Supplement) [Google Scholar]
- 26.Cufer T., Vrdoljak E., Gaafar R. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non–small-cell lung cancer. Anticancer Drugs. 2006;17:401–409. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- 27.Maruyama R., Nishiwaki Y., Tamura T. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non–small-cell lung cancer. J Clin Oncol. 2008;26:4244–4252. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 28.Herbst R.S., O'Neill V.J., Fehrenbacher L. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non–small-cell lung cancer. J Clin Oncol. 2007;25:4743–4750. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 29.Douillard J.Y., Shepherd F.A., Hirsh V. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non–small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;2009:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 30.Lee D.H., Park K., Kim J.H. Randomized phase III trial of gefitinib versus docetaxel in non–small-cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16:1307–1314. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 31.Ciuleanu T., Stelmakh L., Cicenas S. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non–small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012;13:300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 32.Karampeazis A., Voutsina A., Souglakos J. Pemetrexed versus erlotinib in pretreated patients with advanced non–small-cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer. 2013;119:2754–2764. doi: 10.1002/cncr.28132. [DOI] [PubMed] [Google Scholar]
- 33.Ahn M.J., Sun J.M., Kim S.W. Randomized phase III trial of gefitinib or pemetrexed as second line treatment in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01) J Thorac Oncol. 2011;6(Suppl 2):s317. (abstract no O10.04) [Google Scholar]
- 34.Gregorc V., Novello S., Lazzari C. Predictive value of a proteomic signature in patients with non–small-cell lung cancer treated with second-line erlotinib or chemotherpay (PROSE): a biomarker-statified, randomised phase 3 trial. Lancet Oncol. 2014;15:713–721. doi: 10.1016/S1470-2045(14)70162-7. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi T., Ando M., Asami K. Randomised phase III trial of erlotinib versus docetaxel as second or third-line therapy in patients with advanced non–small-cell lung cancer: docetaxel and erlotinib lung cancer trial (DELTA) J Clin Oncol. 2014;32:1902–1908. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 36.Li H., Wang X., Hua F. Second-line treatment with gefitinib or docetaxel for advanced non–small-cell lung cancer [in Chinese] Chin J Clin Oncol. 2010;37:16–18. [Google Scholar]
- 37.Li N., Ou W., Yang H. A randomized phase 2 trial of erlotinib versus pemetrexed as second-line therapy in the treatment of patients with advanced EGFR wild-type and EGFR FISH-positive lung adenocarcinoma. Cancer. 2014;120:1379–1386. doi: 10.1002/cncr.28591. [DOI] [PubMed] [Google Scholar]
- 38.Cappuzzo F., Ciuleanu T., Stelmakh L. Erlotinib as maintenance treatment in advanced non–small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 39.Perol M., Chouaid C., Perol D. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non–small-cell lung cancer. J Clin Oncol. 2012;30:3516–3524. doi: 10.1200/JCO.2011.39.9782. [DOI] [PubMed] [Google Scholar]
- 40.Gaafar R.M., Surmont V.F., Scagliotti G.V. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03) Eur J Cancer. 2011;47:2331–2340. doi: 10.1016/j.ejca.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L., Ma S., Song X. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non–small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double blind randomised phase 3 trial. Lancet Oncol. 2012;13:466–475. doi: 10.1016/S1470-2045(12)70117-1. [DOI] [PubMed] [Google Scholar]
- 42.Kelly K., Chansky K., Gaspar L.E. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non–small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 43.Kabbinavar F., Fehrenbacher L., Hainsworth J. Biomarker analyses from a randomized, placebo-controlled, phase IIIb trial comparing bevacizumab with our without erlotinib as maintenance therapy for treatment of advanced non–small-cell lung cancer (ATLAS) J Thorac Oncol. 2014;9:1411–1417. doi: 10.1097/JTO.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 44.Vale C.L., Tierney J.F., Burdett S. Can trial quality be reliably assessed from published reports of cancer trials: evaluation of risk of bias assessments in systematic reviews. BMJ. 2013;346:f1798. doi: 10.1136/bmj.f1798. [DOI] [PubMed] [Google Scholar]
- 45.Berlin J.A., Santanna J., Schmid C.H. Individual patient- versus group-level data meta-regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med. 2002;21:371–387. doi: 10.1002/sim.1023. [DOI] [PubMed] [Google Scholar]
- 46.Pocock S.J., Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]