Abstract
Objective
We sought to delineate the predictor of saphenous vein graft (SVG) failure and to evaluate the impact of sequential grafting of SVG on graft flow as the significant predictor of patency.
Methods
Angiograms and clinical records of 439 patients who underwent coronary artery bypass grafting with aortocoronary SVG were reviewed. Of these, 708 distal anastomoses were created by 480 SVGs. Of 349 patients who underwent isolated coronary artery bypass grafting, operation was performed with an off-pump technique in 347 patients (99%). For 90 patients, a combined procedure on cardiopulmonary bypass was performed. A postoperative angiography was performed in 230 SVGs for clinical reasons. Insufficient flow (IF) was defined as a graft flow of 20 mL/min or less, measured by transit-time Doppler flowmetry during operation.
Results
In 480 SVGs, 44 (9.2%) presented IF, and 24 SVGs presented partial or total occlusion. Six of the nine failed individual SVG had IF, whereas none of the failed sequential SVG was associated with IF. Univariate and multivariate logistic regression analyses demonstrated that IF (P = 0.002; odds ratio, 6.63) and sequential grafting (P = 0.004; odds ratio, 2.51) were significantly correlated with a failure of the SVG. The patency rate of sequential SVG to the most distal target was 78/93 (83.9%), which was significantly lower than 9/139 (93.5%) of the individual SVG (P = 0.02) and 7/113 (93.8%) of the sequential SVG to proximal targets (P = 0.02).
Conclusions
When both targets seem to have sufficient demand, avoidance of sequential grafting would be reasonable. Moreover, the important target should be grafted by individual grafting or sequential proximal anastomosis.
Key Words: Coronary artery bypass graft, Saphenous vein graft, Graft flow, Angiography
In coronary artery bypass grafting (CABG), complete revascularization has been considered to be associated with a lower incidence of adverse cardiac events in the postoperative period.1,2 For multivessel disease, multiple arterial bypass grafts would be reasonable to achieve durable completeness.3 However, it has been generally considered that the use of arterial grafts has an increased risk for perioperative complications.4 Consequently, most of the patients underwent CABG with one or more venous grafts, even in the current era. Improvement of patency of venous grafts is still an important issue.
Graft flow during an operation is reported to be useful for detecting technical failure and predicting graft occlusion. Early venous graft failure is caused by technical failure, thrombosis, and intimal hyperplasia. This is the initial process of atherosclerosis, which is the main cause of late graft failure.5
We examined the impacts of intraoperative graft flow in sequential and individual venous grafts on angiographic patency and sought to delineate the risks of graft failure in sequential and individual grafts.
PATIENTS AND METHODS
Angiograms and clinical records of 439 patients, who underwent CABG with saphenous vein graft (SVG) between May 2007 and March 2013, were reviewed. In total, 1430 distal anastomoses, including 708 distal anastomoses of 480 SVGs, were assessed. This study was approved by our institutional review board. Ninety patients had a combined procedure necessitating cardiopulmonary bypass (valvular, 81; others, 9), and 349 patients had isolated CABG. Isolated CABG was performed with an off-pump technique in 347/349 patients (99%) and with on-pump in 2/349 patients (1%) (Table 1).
TABLE 1.
Patients’ Baseline Characteristics
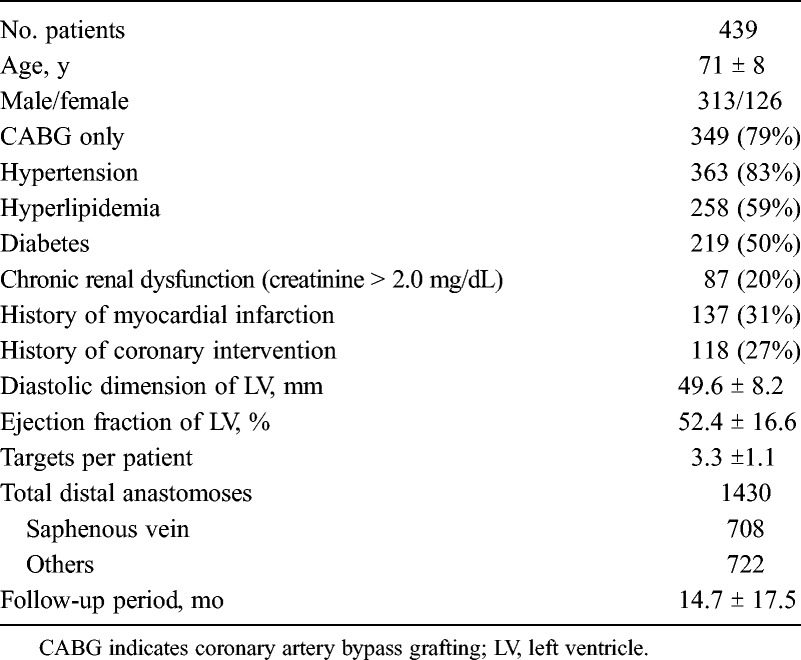
Our standard strategy has been off-pump revascularization with multiple arterial grafts without aortic manipulation. Saphenous vein graft was preferably chosen, when the target branch in the left circumflex and right coronary artery territories had moderate stenosis and patients had considerable operative risks from multiple arterial graftings, such as those with insulin-dependent diabetes, severe chronic occlusive pulmonary disease, or older than 80 years. All SVGs were used as an aortocoronary bypass. When the patient had two or more targets of SVG, sequential grafting was considered first. The target of the distal end was selected so that the length of the SVG would be minimal. Characteristics of SVG were summarized in Tables 2 and 3. Calcification of the ascending aorta was evaluated by preoperative computed tomography, and when calcification was significant, partial clamping was avoided, and a sealing device such as Enclose II (Novare Surgical Systems, Cupertino, CA USA), Heartstring (MAQUET, San Jose, CA USA), or an anastomotic device such as PAS-Port (Cardia Inc, Redwood City, CA USA) was used for proximal anastomosis. The graft flow was measured at the proximal portion by a transit-time Doppler flowmetry after completion of anastomoses or a cardiopulmonary bypass at approximately 100 to 120 mm Hg of systolic arterial pressure. In our institution, early postoperative catheterization or computed tomography angiography was performed routinely, except for patients with a considerable risk of angiography, such as renal failure, calcification of the aorta, history of stroke, postoperative complications, or those older than 80 years. Computed tomography was first considered at the outpatient clinic for patients without renal disease. During the follow-up period, angiography was performed for clinical reasons, such as chest pain or ischemic finding on electrocardiogram or scintigraphy.
TABLE 2.
Characteristics and Angiographic Results of Saphenous Vein Grafts
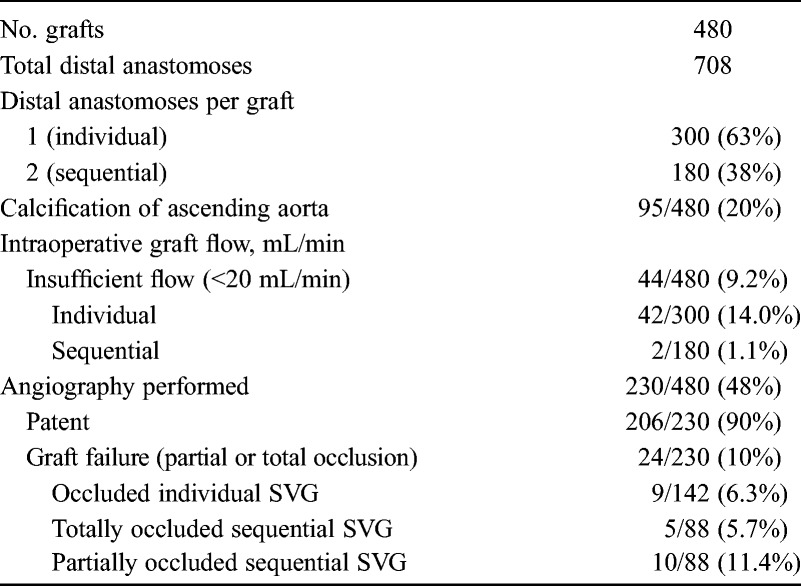
TABLE 3.
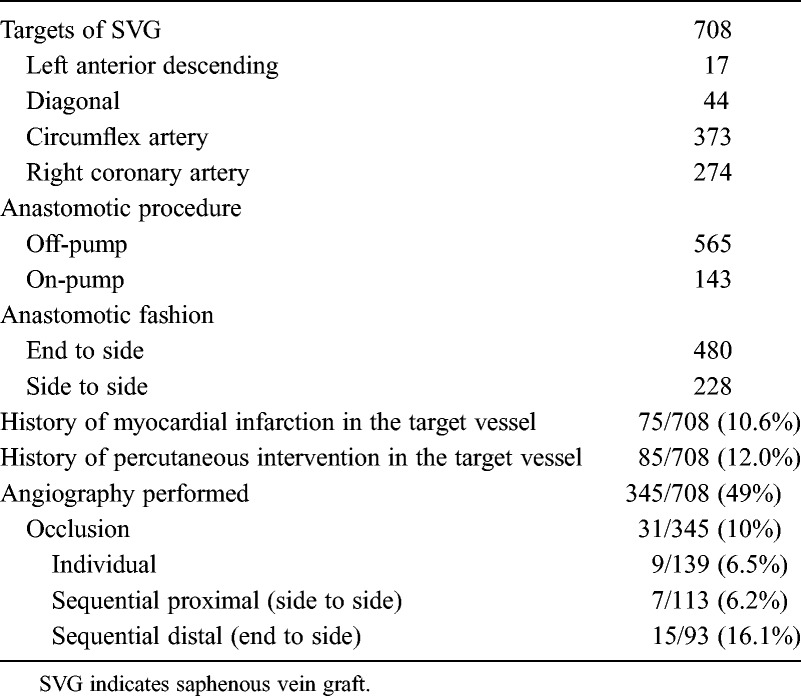
Characteristics and Angiographic Results of SVG
We examined the correlations between detailed preoperative and operative factors, which included hypertension, hyperlipidemia, diabetes, chronic kidney disease (which was defined as creatinine > 2.0 mg/dL), history of myocardial infarction in the target region, percutaneous coronary intervention in the target region, calcification of ascending aorta, the number of distal anastomoses for an SVG in sequential grafting, the number of grafted territories (one-vessel, two-vessel, and three-vessel territories), the use of cardiopulmonary bypass, as well as postoperative angiographic findings and intraoperative graft flow. The mean (SD) number of distal anastomoses was 3.3 (1.1), and the mean follow-up period was 14.7 (17.5) months. The mean (SD) interval period between CABG and angiographic assessment with computed tomography or catheterization was 3.4 (9.4) months. Insufficient flow (IF) was defined as an intraoperative graft flow of 20 mL/min or less. Our definition was not to aim at detection of technical failure but to assess the increased risk of graft failure. The value was considered feasible because IF significantly predicted future graft nonfunction in our previous study.6 Graft failure was defined as partial or total occlusion of sequential and individual SVG. Patent was defined as luminal continuity from the aorta to the target branch.
Statistical Analyses
The continuous variables are expressed as mean (SD). The cumulative rate was determined by the Kaplan-Meier method, and the data of two independent groups were compared by a χ2 test. Logistic regression analysis was used to examine the significance of the clinical, angiographic, and operative variables in predicting graft failure. Longitudinal data were estimated by the Kaplan-Meier method. Statistical analyses were performed using SPSS 18.0 software (SPSS Inc, Chicago, IL USA). The differences in the outcomes were considered statistically significant when the P value was less than 0.05.
RESULTS
There were 44/480 cases of IF (9.2%) at the intraoperative flowmetry. The rate of IF of individual SVG was 14.0% (42/300), which was significantly higher than that of sequential SVG (1.1%, 2/180) (P < .0001) (Table 2).
A postoperative catheterization or computed tomography angiography was performed for 230 SVGs, which included 142 individual SVGs and 88 sequential SVGs (Table 2). Of these, 24/230 (10.4%) presented failure of SVG. The rate of failure of sequential SVG was 17.0% (15/88), which was significantly higher than that of individual SVG (6.3%, 9/142) (P = .01).
The patency rate of sequential SVG to the most distal target was 78/93 (83.9%), which was significantly lower than 9/139 (93.5%) of the individual SVG (P = 0.02) and 7/113 (93.8%) of the SVG to sequential proximal targets (P = 0.02) (Table 3).
Graft Failure and Intraoperative Flow in Sequential and Individual Grafting
There were 24/230 (10.4%) failed SVGs. Of the 24 failed SVGs, 9 were found in the individual grafting and 6/9 (66.7%) presented IF during the operation. Of the 15 failed sequential SVGs, none was associated with IF (Fig. 1). Five were totally occluded, whereas only the distal portion occluded in 10 patients.
FIGURE 1.
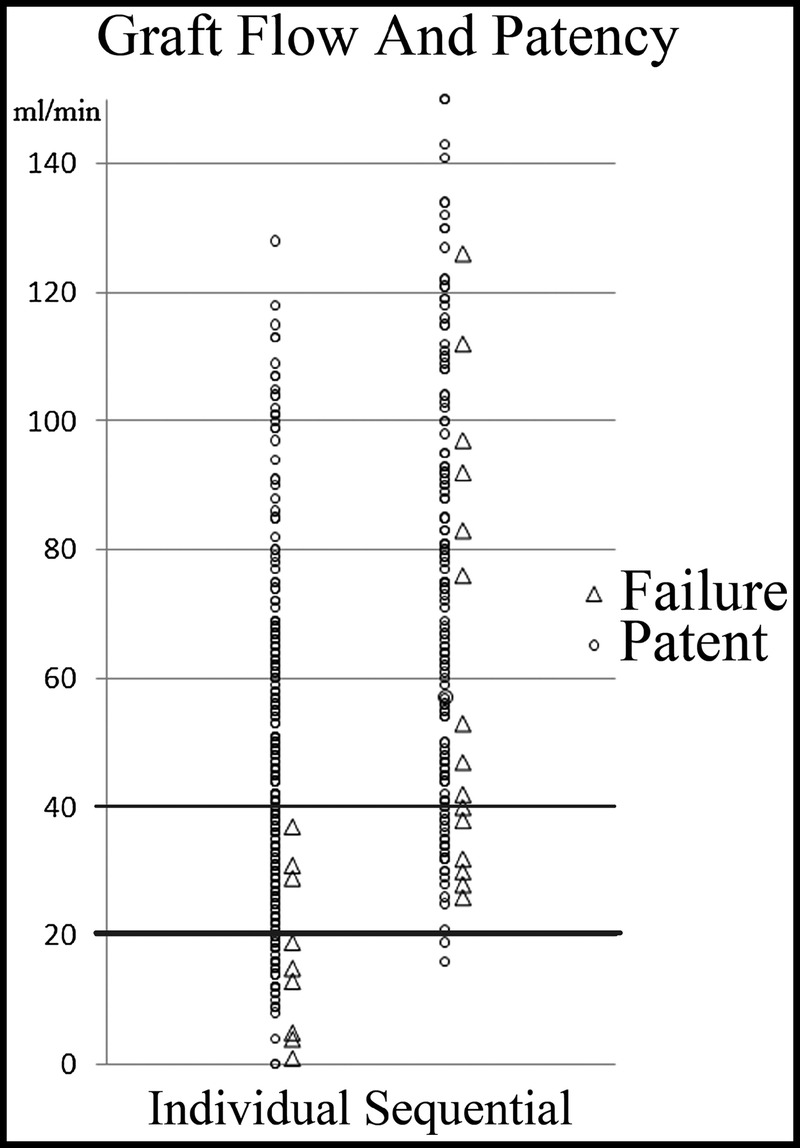
For individual grafting, insufficient flow during operation was significantly correlated with the occurrence of postoperative graft failure. Inversely, graft failure was occasionally found, even for the sequential graft with the abundant flow.
Predictors of Graft Failure: Analyses for 230 SVGs
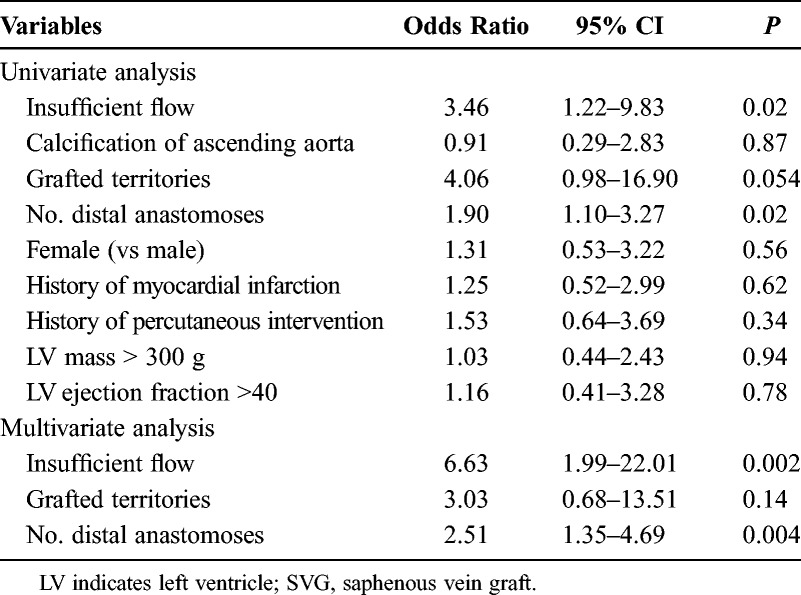
Univariate and multivariate logistic regression analyses for 230 SVGs demonstrated that IF (P = 0.002; odds ratio, 6.63) and the number of distal anastomoses in a sequential fashion (P = 0.004; odds ratio, 2.51) significantly were correlated with failure (Table 4).
TABLE 4.
Predictors of Failure: Analyses of 230 SVGs
DISCUSSION
Arterial grafts have been considered as the reliable conduit in CABG. In fact, even for the right coronary artery, previous reports have shown that the use of an internal thoracic or gastroepiploic artery provided improved clinical outcomes.3 Recently, limitations of arterial grafts have also been recognized.7 First, the use of an arterial graft increases the risk of perioperative complications, such as mediastinitis. Second, patency of the arterial graft to the moderately stenotic target was not favorable,8 especially for the in situ gastroepiploic artery.9 Saphenous vein grafts from the ascending aorta are advantageous, in terms of capacity of high intraluminal pressure and absence of critical complications associated with harvesting the graft. Jeong et al10 have described that moderate stenosis was predictive of graft failure in arterial grafts, but not in SVG. There was no significant difference in the clinical outcomes and graft patency in the gastroepiploic artery and SVG for the right coronary artery, but the rate of functioning graft was significantly higher in SVG.9 Furthermore, Guru et al7 have reported that there was no obvious advantage in the use of three arterial grafts versus two arterial grafts. When an arterial graft may not be advantageous, SVG can be a reasonable option.
Several studies described that, for a venous graft, blood flow is one of the important parameters for predicting graft patency. As reported by Di Giammarco et al,11 reduced graft flow during operation was a significant predictor of venous graft failure within a year. In addition, Tokuda et al12 have reported a negative impact of reduced intraoperative flow on midterm patency of bypass grafts, which were angiographically patent, immediately after an operation.
The rationale for sequential grafting may be the important issue with SVG. Sequential grafting is obviously advantageous in terms of saving SVG length, minimizing aortic manipulation, and shortness of procedural time because of simple operative maneuvers. In addition, Kim et al13 have shown that sequential grafting provided higher graft flow and subsequently a higher graft patency rate than did individual grafting, whereas Dion et al14 have reported that the patency rate at 7.5 years of sequential SVG was comparable with that of arterial graft and was significantly higher than that of individual SVG. It has been widely considered that a small or poor run-off target should be anastomosed with proximal portion, whereas the target of the distal end should have good run-off.5,15,16 On the contrary, PREVENT IV trial has demonstrated inferior graft patency and clinical outcomes, as compared with individual grafting.17 For multiple targets, multiple individual grafting was recommended.
The results of this study suggest that the mechanism of early failure in sequential grafting may be different from that of individual grafting. For individual grafting, IF had been seen in 67% of the failed SVG, whereas there were no failed grafts when the flow was more than 40 mL/min. Inversely, for sequential grafting, IF and subsequent graft failure at the proximal portion could be efficiently avoided. However, occlusion was commonly found at the distal segment of sequential SVG, and irrespective of graft flow with more than 40 mL/min, graft failure was occasionally found. Therefore, we have presumed two possible explanations for the failure of sequential SVG. First, the distal segment of the SVG might have IF, even when graft flow at the proximal segment was sufficient. In this setting, if individual grafting was chosen, the risk of occlusion might increase because longer SVG was necessary. Second, it might be caused by the presence of proximal target or a technical error in sequential grafting, although most of isolated CABG in our patients was performed by an off-pump technique. Individual grafting would be a reasonable option of choice.
The implications of these results are as follows. For the two targets, which seem to have sufficient flow demand, two individual grafts should be considered first. On the other hand, to achieve patency of a bypass graft to the target, which is relatively small or seems to have poor flow demand, sequential grafting at proximal portion is recommended.
The present study revealed that the patency rate of the distal end in sequential grafting was significantly lower than that of the proximal portion and individual grafting. Previous studies also reported that the graft patency rate of the proximal target was significantly higher than that of the target of the distal end.14,18,19 We would suggest that sequential grafting with a proximal for an important target and the distal for a less important target may be rationalized. This is because it may be reasonable to sacrifice a bypass of the distal end to assure patency to the important target. Not only the run-off of the target but also the importance should be taken into account.
Limitations of this study are as follows. First, this study is observational and retrospective. Therefore, we could not measure graft flow under strict physiological conditions, such as heart rate and blood pressure. Second, patients who underwent postoperative angiography may be biased and insufficient. Third, the follow-up period was relatively short. However, to identify the predictors of early graft failure, we focused on the graft flow. We believe that this study is rationalized because technical error, thrombosis, and intimal hyperplasia are considered to be the causes of graft failure within a year. Even in our limited length of follow-up, IF was a significant predictor of occlusion. In a longer follow-up period, biases, which can be associated with progression of atherosclerosis, such as hypertension, diabetes, hyperlipidemia, postoperative medications, as well as renal and cardiac function, will increase remarkably. In addition, graft flow during on-pump CABG was significantly greater than off-pump CABG,20 and in patients with valvular disease, the amount of myocardium and flow demand would usually be increased. In this study, we attempted to examine the use of a cardiopulmonary bypass, myocardial mass, and history of infarction in the grafted territory. However, there was no significant impact on the SVG patency. Moreover, severity of native coronary stenosis, pulsatile index, and diastolic flow would be associated with graft flow. Measurement of flow at the distal segment of sequential graft would be informative. However, we could not analyze these factors in this study.
In conclusion, sequential grafting would have a beneficial effect on patency to the proximal target, in association with an increase of graft flow, and simultaneously may affect patency to the distal end. So it is believed that for the important target, the distal end of sequential grafting should be avoided, and the proximal portion of sequential grafting or individual grafting would be favorable.
CLINICAL PERSPECTIVE
This is an interesting report from Dr Takazawa and his colleagues at Saitama International Medical Center in Japan. They examined angiograms and clinical records from 439 patients who underwent coronary artery bypass grafting with vein grafts. The majority of patients underwent surgery using off-pump techniques. Of the 480 saphenous vein grafts, 9.2% had insufficient flow as defined as a graft flow of 20 mL/min or less. Postoperative angiography was performed in 230 saphenous vein grafts. Univariate and multivariate logistic regression analyses demonstrated that insufficient flow and sequential grafting were significantly correlated with the failure of the vein graft. The authors concluded that avoidance of sequential grafting would be reasonable and that patients with insufficient flow at the time of operation should undergo revision. In individual vein grafts, there were no failed grafts when the flow was more than 40 mL/min. However, intraoperative flow was not a predictive factor in sequential grafts in which graft failure was found regardless of intraoperative flow. This is an important article and suggests that individual grafting should be considered when feasible. If sequential grafts are used, there seems to be a higher likelihood of occlusion of the distal target. They go on to suggest that when doing a sequential grafting, the most important target should be selected for the proximal anastomosis of the sequential graft.
There are numerous limitations to the study. Most importantly, it is observational and retrospective. There was incomplete angiographic followup and it was based only on symptoms. Finally, the follow-up period was relatively short. However, this study does add to the literature and suggests that insufficient flow is a significant predictor of occlusion in individual grafts. This report emphasizes the importance of intraoperative determination of graft flow. It also suggests that sequential grafting may in certain situations jeopardize the distal target vessel.
Footnotes
Presented at the Annual Scientific Meeting of the International Society for Minimally Invasive Cardiothoracic Surgery, June 12–15, 2013, Prague, Czech Republic.
Disclosure: The authors declare no conflicts of interest.
REFERENCES
- 1. Bell MR, Gersh BJ, Schaff HV, et al. Effect of completeness of revascularization on long-term outcome of patients with three-vessel disease undergoing coronary bypass surgery. A report from the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1992; 86: 446– 457. [DOI] [PubMed] [Google Scholar]
- 2. van den Brand MJ, Rensing BJ, Morel MA, et al. The effect of completeness of revascularization on event-free survival at one year in the ARTS Trial. J Am Coll Cardiol. 2002; 39: 559– 564. [DOI] [PubMed] [Google Scholar]
- 3. Glineur D, D’hoore W, Price J, et al. Survival benefit of multiple arterial grafting in a 25-year single-institutional experience: the importance of the third arterial graft. Eur J Cardiothorac Surg. 2012; 42: 284– 290. [DOI] [PubMed] [Google Scholar]
- 4. Taggart DP, Altman DG, Gray AM, et al. ; ART Investigators. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J. 2010; 31: 2470– 2481. [DOI] [PubMed] [Google Scholar]
- 5. Sabik JF., III Understanding saphenous vein graft patency. Circulation. 2011; 124: 273– 275. [DOI] [PubMed] [Google Scholar]
- 6. Nakajima H, Iguchi A, Tabata M, et al. Predictors and prevention of flow insufficiency due to limited flow demand. J Cardiothorac Surg. 2014; 9: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guru V, Fremes SE, Tu JV. How many arterial grafts are enough? A population-based study of midterm results. J Thorac Cardiovasc Surg. 2006; 131: 1021– 1028. [DOI] [PubMed] [Google Scholar]
- 8. Nakajima H, Kobayashi J, Tagusari O, et al. Angiographic flow grading and graft arrangement of arterial conduits. J Thorac Cardiovasc Surg. 2006; 132: 1023– 1029. [DOI] [PubMed] [Google Scholar]
- 9. Glineur D, Hanet C, Poncelet A, et al. Comparison of saphenous vein graft versus right gastroepiploic artery to revascularize the right coronary artery: a prospective randomized clinical, functional, and angiographic midterm evaluation. J Thorac Cardiovasc Surg. 2008; 136: 482– 488. [DOI] [PubMed] [Google Scholar]
- 10. Jeong DS, Kim YH, Lee YT, et al. Revascularization for the right coronary artery territory in off-pump coronary artery bypass surgery. Ann Thorac Surg. 2013; 96: 778– 785. [DOI] [PubMed] [Google Scholar]
- 11. Di Giammarco G, Pano M, Cirmeni S, Pelini P, Vitolla G, Di Mauro M. Predictive value of intraoperative transit-time flow measurement for short-term graft patency in coronary surgery. J Thorac Cardiovasc Surg. 2006; 132: 468– 474. [DOI] [PubMed] [Google Scholar]
- 12. Tokuda Y, Song MH, Oshima H, Usui A, Ueda Y. Predicting midterm coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg. 2008; 86: 532– 536. [DOI] [PubMed] [Google Scholar]
- 13. Kim HJ, Lee TY, Kim JB, et al. The impact of sequential versus single anastomoses on flow characteristics and mid-term patency of saphenous vein grafts in coronary bypass grafting. J Thorac Cardiovasc Surg. 2011; 141: 750– 754. [DOI] [PubMed] [Google Scholar]
- 14. Dion R, Glineur D, Derouck D, et al. Complementary saphenous grafting: long-term follow-up. J Thorac Cardiovasc Surg. 2001; 122: 296– 304. [DOI] [PubMed] [Google Scholar]
- 15. Gao C, Wang M, Wang G, et al. The patency of sequential and individual saphenous vein grafts after off-pump coronary artery bypass grafting. J Card Surg. 2010; 25: 633– 637. [DOI] [PubMed] [Google Scholar]
- 16. Vural KM, Sener E, Tasdemir O. Long-term patency of sequential and individual saphenous vein coronary bypass grafts. Eur J Cardiothorac Surg. 2001; 19: 140– 144. [DOI] [PubMed] [Google Scholar]
- 17. Mehta RH, Ferguson TB, Lopes RD, et al. ; Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) IV Investigators. Saphenous vein grafts with multiple versus single distal targets in patients undergoing coronary artery bypass surgery: one-year graft failure and five-year outcomes from the Project of Ex-Vivo Vein Graft Engineering via Transfection (PREVENT) IV trial. Circulation. 2011; 124: 280– 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Neill MJ, Jr, Wolf PD, O’Neill TK, Montesano RM, Waldhausen JA. A rationale for the use of sequential coronary artery bypass grafts. J Thorac Cardiovasc Surg. 1981; 81: 686– 690. [PubMed] [Google Scholar]
- 19. Grondin CM, Limet R. Sequential anastomoses in coronary artery grafting: technical aspects and early and late angiographic results. Ann Thorac Surg. 1977; 23: 1– 8. [DOI] [PubMed] [Google Scholar]
- 20. Balacumaraswami L, Abu-Omar Y, Selvanayagam J, Pigott D, Taggart DP. The effects of on-pump and off-pump coronary artery bypass grafting on intraoperative graft flow in arterial and venous conduits defined by a flow/pressure ratio. J Thorac Cardiovasc Surg. 2008; 135: 533– 539. [DOI] [PubMed] [Google Scholar]


