Abstract
Large-scale studies are needed to increase our understanding of how large-scale conservation threats, such as climate change and deforestation, are impacting diverse tropical ecosystems. These types of studies rely fundamentally on access to extensive and representative datasets (i.e., “big data”). In this study, I asses the availability of plant species occurrence records through the Global Biodiversity Information Facility (GBIF) and the distribution of networked vegetation census plots in tropical South America. I analyze how the amount of available data has changed through time and the consequent changes in taxonomic, spatial, habitat, and climatic representativeness. I show that there are large and growing amounts of data available for tropical South America. Specifically, there are almost 2,000,000 unique geo-referenced collection records representing more than 50,000 species of plants in tropical South America and over 1,500 census plots. However, there is still a gaping “data void” such that many species and many habitats remain so poorly represented in either of the databases as to be functionally invisible for most studies. It is important that we support efforts to increase the availability of data, and the representativeness of these data, so that we can better predict and mitigate the impacts of anthropogenic disturbances.
Introduction
Big problems call for big ecology. Big ecology needs big data.
There is a rapidly increasing need for large-scale studies in order to predict and mitigate the effects of large-scale conservation threats, such as deforestation and climate change [1,2]. For example, various studies have used massive collections of natural history records, range maps, and census plot data to estimate patterns of biodiversity across continental-scale areas and to predict how diversity will be impacted under different scenarios of climate change and habitat loss [3–10]. These large-scale studies use “big data”—data that is generally beyond the scope of what can be collected by individual researchers or through individual projects [2]. As such, these studies often depend heavily on extensive and expansive collations of datasets that are standardized and made available through collaborative networks or data clearinghouses.
One of the most important clearinghouses for biogeographic and natural history data is the Global Biodiversity Information Facility (GBIF; http://www.gbif.org/). Indeed, since it contains copious amounts of data, is easy to use, is compatible with popular biogeographic methods (e.g., species distribution modeling) and is entirely open access, GBIF has rapidly become one of the most widely-used and important resources in ecology, biogeography, and conservation biology since its launch in 1997. According to their own statistics, GBIF data has been used in nearly 900 peer-reviewed scientific publications to date.
While species occurrence databases, such as those linked through GBIF, are clearly a powerful and important resource, many studies have pointed out its potential limitations for biogeographic and ecological studies [11]. These limitations can be due to problem with data quality. For example, collections data are prone to taxonomic and georeferencing errors [12,13] and may suffer from biases in the taxonomic and spatial representativeness of the samples [14–18] due in part to the understandable tendency of collectors to focus their efforts in accessible areas and areas with well-established logistical and intellectual infrastructures [19–21].
Another potential limitation of occurrence databases is simply insufficient data quantity [14]. In 2011, Feeley and Silman, reported on the extreme paucity of collections data in GBIF (and a similar database for Brazil named SpeciesLink; http://splink.cria.org.br/) for tropical plant species. Specifically, using data downloaded in 2009 they estimated that only about 65% of tropical plant species were represented by any available geo-referenced collections and that of the represented species, only about 8% or 0.5% (approx. 5% or 0.3%, respectively, of all tropical plant species) had enough available records to be used in species distribution models or other analyses requiring 20 or 100 minimum samples, respectively [22]. Given the dominant role that GBIF (and at the time, SpeciesLink) plays in distributing natural history records, this lack of data from the tropics was considered a major constraint on studies of tropical species and diversity. Perhaps more troubling, the lack of available records from the tropics was considered symptomatic of a more general lack of knowledge about the distribution and ecology of most tropical species as well as a lack of knowledge about the composition and structure of vast expanses of the tropics. Feeley and Silman referred to this lack of knowledge as the “data void” [22].
Since Feeley and Silman published their study in 2011, GBIF has continued to grow and the amount of data available from all regions, including the tropics, has greatly increased. This growth has been due to ongoing collection efforts, the digitization of additional pre-existing records, and inclusion of new datasets into GBIF. There have also been laudable efforts at data standardization and cleaning (e.g., the Taxonomic Name Resolution Service, TNRS; http://tnrs.iplantcollaborative.org/) which affects the number of species represented in the dataset (in most cases decreasing the number of species through the elimination of synonyms and false species created by spelling errors) and the number of records available per species (generally increasing the number of records per species through the combination of records formerly assigned to different species names).
While clearly important, natural history and occurrence records such as those provided by GBIF are inherently limited in their utility. For example, the geo-referenced data contained in natural history records can be used to map species ranges in relation to large climatic gradients, but they provide no information about local patterns of occurrence, species abundances, alpha diversity, or community composition. These types of patterns are better assessed through analyses of intensive plot inventories or censuses. Over the past several years there have been notable attempts to collate and standardize tropical forest inventory data (i.e., plot data) through collaborative networks. For example, the Amazon Tree Diversity Network (ATDN; http://web.science.uu.nl/Amazon/atdn/) and the RAINFOR Amazon Forest Inventory Network (http://www.rainfor.org/) have each compiled data from hundreds of pre-existing forest inventory plots in the Amazon basin supplemented with subsequent installations of new plots in targeted areas. These and other networks of plot census data are being used to look at large-scale patterns in forest structure and composition across the Amazon [5,6,23–26].
Here, I asses the availability of occurrence and census data from the tropics and examine how this availability has varied through time as well as how it varies through space. Specifically, I quantify the amount of occurrence data available through GBIF for plant species and from different habitats in tropical South America and examine how data availability has changed through time. I also analyze the spatial and habitat distribution of South American forest census plots as represented in several of the most prominent plot networks. The goal of this study is to characterize the state of data availability for tropical species and habitats of South America and to evaluate the rate that we are, or are not, filling the data void. By understanding where our data limitations are, we can better assess the generalizability of the results emerging from analyses of the existing data and direct future studies to help reduce these data limitations.
Methods
Data
All records for plant species (kingdom plantae) occurring in the tropical latitudes of South America were downloaded from the GBIF data portal on February 1st 2014 (see S1 File for list of contributing databases and herbaria). The records were screened to exclude those without geo-referencing information or with obvious errors in geo-referencing data (i.e., flagged by GBIF for data quality issues or with coordinates occurring in ocean or in areas outside of South America). All of the species names listed with the collection records were then verified using the online Taxonomic Name Resolution Service (TNRS) to remove synonyms and correct for spelling errors. Duplicate records were removed by screening for records with identical species names and collection coordinates.
I collated the locations of inventory plots as published online and in published articles for five of the largest and most prominent South American census plot networks: RAINFOR, ATDN, Forestplots.net (https://www.forestplots.net/; [6]), the Smithsonian Institute’s Center for Tropical Forest Science (CTFS; http://www.ctfs.si.edu/), and the Red de Bosques (http://www.condesan.org/redbosques/). Many individual census plots are members of multiple networks so all duplicate records were identified and removed.
In order to assess the representation of different habitats in the herbarium and plot datasets, I classified the habitats of Tropical South America according to WWF ecoregions (http://www.worldwildlife.org/publications/terrestrial-ecoregions-of-the-world; [27]). In addition, I divided tropical South America into discrete climatic zones on the basis of mean annual temperature (MAT) and total annual precipitation (TAP). Estimates of MAT and TAP were based on “current” (mean of 1960–1990) conditions according to the WorldClim database (http://www.worldclim.org/ [28]). Climatic zones were characterized as those areas having different combinations of MAT = 0–2°C, 2–4°C, 4–6°C, 6–8°C, 8–10°C, 10–12°C, 12–14°C, 14–16°C,16–18°C, 18–20°C, 20–22°C, 22–24°C, 24–26°C, 26–28°C, 28–30°C; and TAP = 0–500mm, 1000–1500mm, 1500–2000mm, 2000–3000mm, 3000–4000mm, 4000–6000mm, and >6000mm.
Analyses
To calculate the change in data availability in GBIF through time, I tallied the number of records available in each year from 2007 (when GBIF launched) to the end of 2013. I then tallied the number of records available per species in each year. I calculated and mapped the average density of records (no. of records per km2) per 0.5 x 0.5 longitude/latitude degree cell and how this record density has changed through time. Using the collated list of plot locations, I measured the straight line distance of all possible 30 arc second grid cell centers in tropical South America to the closest census plot. Finally, I calculated and mapped the average density of collections and census plots within each of the WWF ecoregions and within each of the climatic zones as defined above.
Results
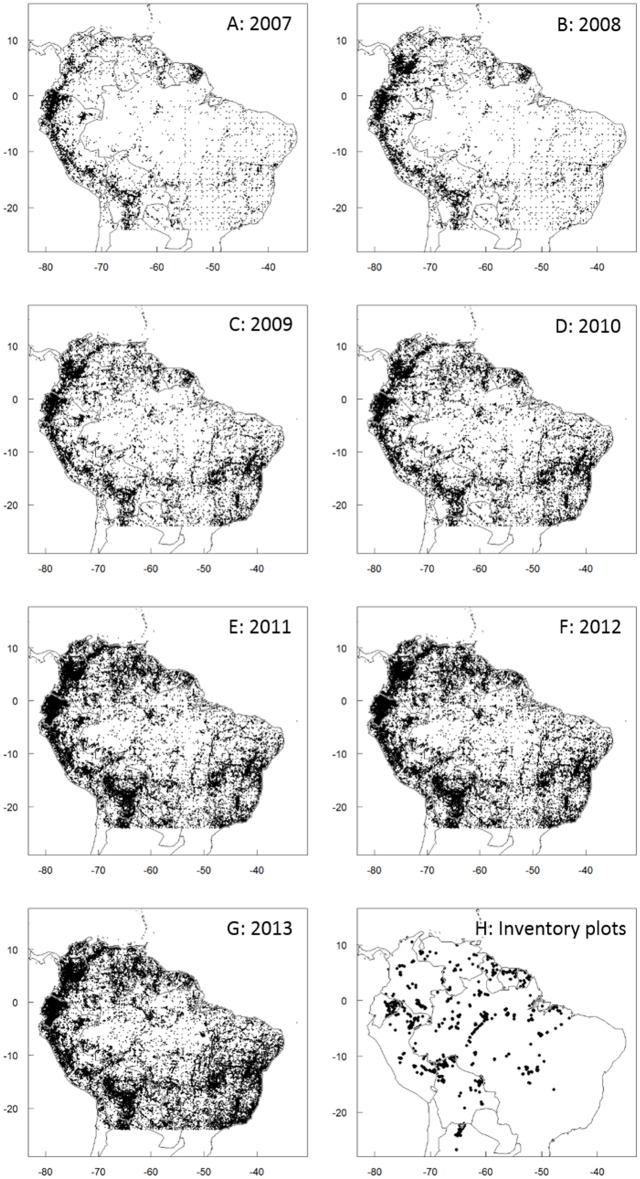
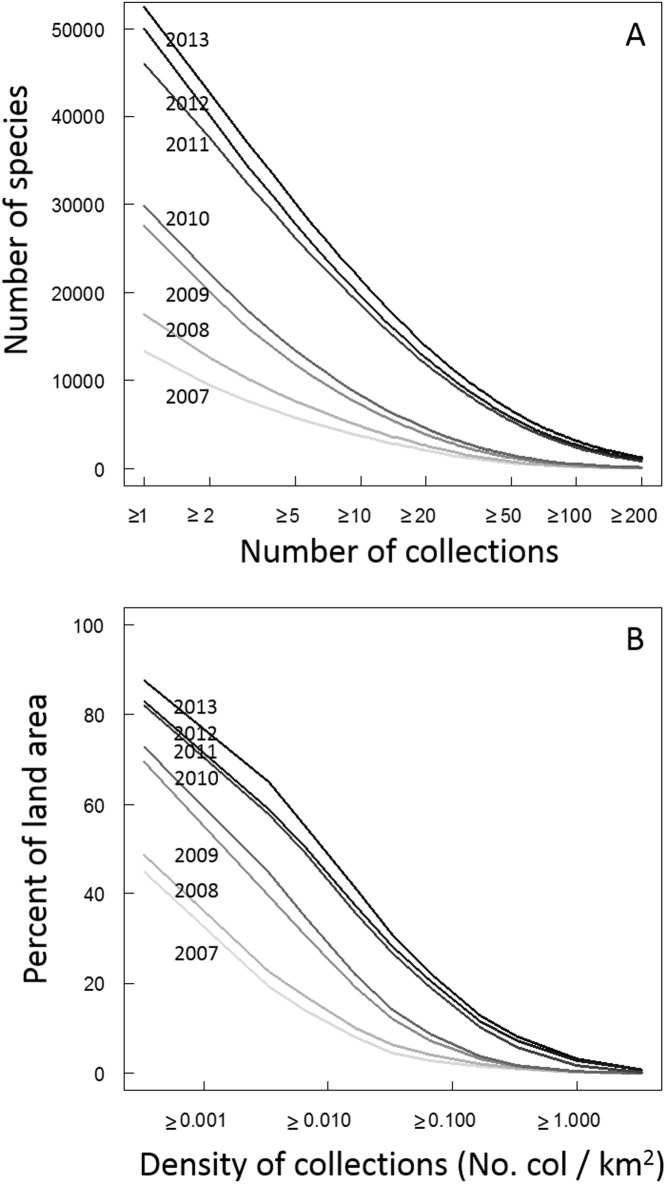
The number of georeferenced plant records available through GBIF for tropical South America has increased rapidly since 2007 (Table 1 and Fig 1a–1g), as has the number of represented species and the number of collections available per species (Table 1 and Fig 2a). Of note is the fact that most of this increase is due to the inclusion of additional pre-existing records rather than new collections. For example, of the 177,925 records added to GBIF in 2013, only 2,910 (1.5%) were of collections that were actually made in 2013. The largest increase in data availability came between 2010 and 2011 when the number of records increased by nearly 300%; this increase was driven in large part by the incorporation of SpeciesLink data into GBIF.
Table 1. The availability of plant collections data through the Global Biodiversity and Information Facility (GBIF).
| Year | No. of collections | No. of species | Mean no. of collections per species* | Median no. of collections per species* | Mean no. col / km2 | Median no. col / km2 | % of area with zero collections |
|---|---|---|---|---|---|---|---|
| 2007 | 164214 | 13339 | 3.15 (12.40) | 0 (3) | 0.01 | 0.00 | 54.81 |
| 2008 | 206218 | 17454 | 3.95 (12.93) | 0 (3) | 0.02 | 0.00 | 51.25 |
| 2009 | 331856 | 27532 | 6.36 (12.15) | 1 (3) | 0.02 | 0.00 | 30.91 |
| 2010 | 386042 | 29824 | 7.40 (13.04) | 1 (4) | 0.03 | 0.00 | 27.50 |
| 2011 | 1098386 | 46056 | 21.05(23.98) | 4 (6) | 0.08 | 0.01 | 18.50 |
| 2012 | 1638600 | 50026 | 31.40 (32.92) | 5 (6) | 0.12 | 0.01 | 17.73 |
| 2013 | 1816525 | 52432 | 34.81(34.81) | 6 (6) | 0.13 | 0.01 | 13.14 |
* the values in parentheses indicate the mean or median number of collections per species if only the species represented in that year are included.
Fig 1. Locations of occurrence records and census plots in tropical South America.
Maps showing the location of plant occurrence records available through GBIF each year from 2007 to 2013 (Panels A-G, respectively) and the location of the networked vegetation census plots included in the analyses (Panel H).
Fig 2. Number of occurrence records per species and density of collection in tropical South America.
Panel A shows the cumulative number of occurrence records per plant species of tropical South America available through GBIF in 2007–2013. Panel B shows the cumulative mean density of occurrence records (no. per km2) available through GBIF for tropical South America in 2007–2013.
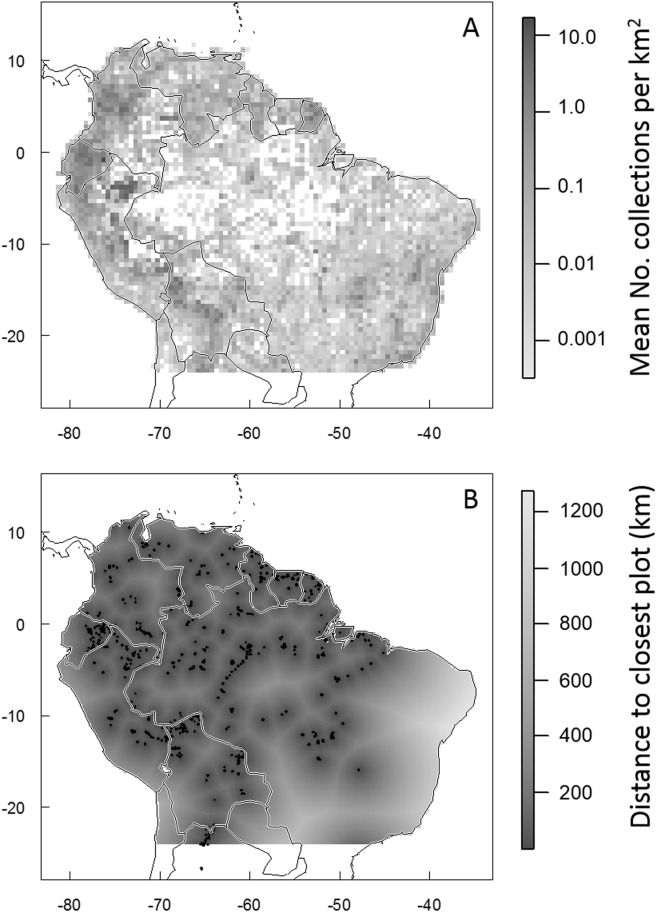
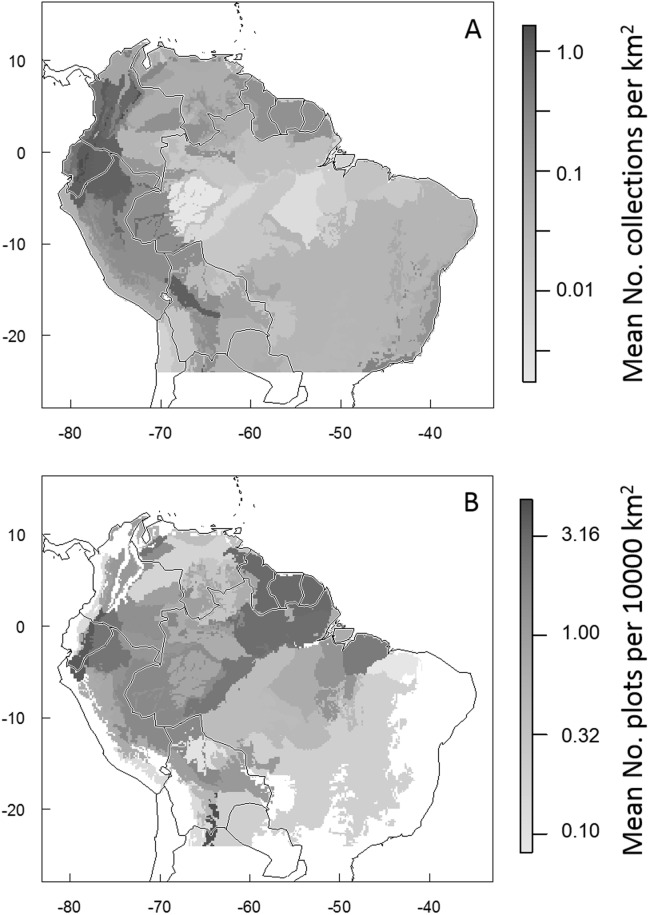
In terms of spatial representation, the average and median density of collections has increased by an order of magnitude since 2007 (Table 1 and Fig 2b). In 2007, the majority of tropical South America was unrepresented by any GBIF herbarium collections (when collections are aggregated at a spatial scale of 0.5° latitude/longitude); by 2013, more than 85% of tropical South America was represented by at least one GBIF collection (Table 1 and Fig 3a). The density of collections varies greatly across space (Fig 3a) and between ecoregions (Table 2 and Fig 4a) and climatic zones (Tables 3 and 4). The greatest density of collections comes from the Northern Andean Paramo ecoregion. This ecoregion is relatively small (approximately 25000 km2) but is represented by more than 40000 unique plant collections (Table 2). The 2nd through 5th best collected ecoregions are also Andean (High Monte, Northwestern Andean Montane Forests, Eastern Cordillera Real Montane Forests, and Bolivian Yungas). Accordingly, the greatest density of collections come from cool, wet habitats such as those occurring in the montane Paramo. More generally, dryer areas and areas with hot (>20°C) or very cold (<10°C) mean annual temperatures are underrepresented in the GBIF database (Table 4).
Fig 3. Density of occurrence records and distance to census plots in tropical South America.
Panel A maps the mean density of plant occurrence records available in 2013 for tropical South America. Density is mapped at a spatial resolution of 0.5 x 0.5°, white pixels are areas where there are no available occurrence records. Panel B maps the distance from points in tropical South America to the closest vegetation census plot (mapped at a spatial resolution on 30 arc seconds).
Table 2. Density of collections data and census plots in different ecoregions of tropical South America (ecoregions defined according to the World Wildlife Fund; http://www.worldwildlife.org/publications/terrestrial-ecoregions-of-the-world; [23]).
| Ecoregion name | Area (km2)* | No. collections | No. col / km2 | No. plots | No. plots / 10000 km2 |
|---|---|---|---|---|---|
| Cerrado | 1914593 | 69234 | 0.036 | 39 | 0.204 |
| Southwest Amazon moist forests | 760847 | 196813 | 0.259 | 175 | 2.300 |
| Caatinga | 737482 | 27906 | 0.038 | 0 | 0.000 |
| Madeira-Tapajos moist forests | 721309 | 10059 | 0.014 | 33 | 0.458 |
| Guianan moist forests | 482415 | 97136 | 0.201 | 233 | 4.830 |
| Uatuma-Trombetas moist forests | 470092 | 10173 | 0.022 | 203 | 4.318 |
| Mato Grosso seasonal forests | 410995 | 14911 | 0.036 | 20 | 0.487 |
| Llanos | 380653 | 20805 | 0.055 | 6 | 0.158 |
| Dry Chaco | 347572 | 16802 | 0.048 | 7 | 0.201 |
| Tapajos-Xingu moist forests | 338782 | 1259 | 0.004 | 27 | 0.797 |
| Alto Parana Atlantic forests | 310138 | 10593 | 0.034 | 0 | 0.000 |
| Japura-Solimoes-Negro moist forests | 271443 | 3714 | 0.014 | 22 | 0.810 |
| Xingu-Tocantins-Araguaia moist forests | 267363 | 2276 | 0.009 | 45 | 1.683 |
| Napo moist forests | 258925 | 250810 | 0.969 | 96 | 3.708 |
| Jurua-Purus moist forests | 244283 | 431 | 0.002 | 25 | 1.023 |
| Bahia interior forests | 230463 | 12721 | 0.055 | 0 | 0.000 |
| Chiquitano dry forests | 230418 | 20922 | 0.091 | 35 | 1.519 |
| Guianan piedmont and lowland moist forests | 229187 | 13029 | 0.057 | 7 | 0.305 |
| Central Andean dry puna | 211583 | 3388 | 0.016 | 0 | 0.000 |
| Negro-Branco moist forests | 204934 | 43609 | 0.213 | 25 | 1.220 |
| Caqueta moist forests | 196159 | 7201 | 0.037 | 39 | 1.988 |
| Tocantins/Pindare moist forests | 194526 | 3441 | 0.018 | 65 | 3.341 |
| Peruvian Yungas | 186496 | 43757 | 0.235 | 16 | 0.858 |
| Sechura desert | 185253 | 8100 | 0.044 | 0 | 0.000 |
| Solimoes-Japura moist forests | 178847 | 30188 | 0.169 | 29 | 1.621 |
| Purus-Madeira moist forests | 174201 | 765 | 0.004 | 62 | 3.559 |
| Pantanal | 170444 | 3111 | 0.018 | 0 | 0.000 |
| Central Andean puna | 157648 | 16545 | 0.105 | 2 | 0.127 |
| Purus varzea | 150857 | 2929 | 0.019 | 32 | 2.121 |
| Guianan Highlands moist forests | 144118 | 15749 | 0.109 | 15 | 1.041 |
| Maranhao Babatu forests | 143813 | 1800 | 0.013 | 1 | 0.070 |
| Beni savanna | 123015 | 3492 | 0.028 | 1 | 0.081 |
| Central Andean wet puna | 122709 | 16060 | 0.131 | 0 | 0.000 |
| Ucayali moist forests | 116663 | 38358 | 0.329 | 11 | 0.943 |
| Atlantic dry forests | 112157 | 8017 | 0.071 | 0 | 0.000 |
| Bahia coastal forests | 112007 | 21895 | 0.195 | 0 | 0.000 |
| Magdalena Valley montane forests | 108222 | 90259 | 0.834 | 17 | 1.571 |
| Eastern Cordillera real montane forests | 105832 | 110215 | 1.041 | 67 | 6.331 |
| Guianan savanna | 105492 | 5861 | 0.056 | 8 | 0.758 |
| Iquitos varzea | 103580 | 66528 | 0.642 | 18 | 1.738 |
| Rio Negro campinarana | 94963 | 2722 | 0.029 | 24 | 2.527 |
| Bolivian Yungas | 92087 | 95328 | 1.035 | 12 | 1.303 |
| Marajo varzea | 87750 | 527 | 0.006 | 7 | 0.798 |
| Northwestern Andean montane forests | 83136 | 91144 | 1.096 | 1 | 0.120 |
| Atacama desert | 81276 | 529 | 0.007 | 0 | 0.000 |
| Magdalena-Uraba moist forests | 76294 | 6196 | 0.081 | 0 | 0.000 |
| Bolivian montane dry forests | 73599 | 17495 | 0.238 | 4 | 0.543 |
| Cordillera Oriental montane forests | 69768 | 22947 | 0.329 | 0 | 0.000 |
| Monte Alegre varzea | 69280 | 654 | 0.009 | 24 | 3.464 |
| La Costa xeric shrublands | 68871 | 4332 | 0.063 | 3 | 0.436 |
| Apure-Villavicencio dry forests | 68637 | 6859 | 0.100 | 14 | 2.040 |
| Choco-Darian moist forests | 60304 | 36148 | 0.599 | 0 | 0.000 |
| Humid Chaco | 57333 | 3395 | 0.059 | 0 | 0.000 |
| Serra do Mar coastal forests | 55436 | 19440 | 0.351 | 0 | 0.000 |
| Pantepui | 48698 | 8866 | 0.182 | 3 | 0.616 |
| Southern Andean Yungas | 44794 | 11735 | 0.262 | 35 | 7.813 |
| Tumbes-Piura dry forests | 42161 | 1977 | 0.047 | 0 | 0.000 |
| Cauca Valley montane forests | 34628 | 30424 | 0.879 | 4 | 1.155 |
| Western Ecuador moist forests | 33726 | 22367 | 0.663 | 0 | 0.000 |
| Amazon-Orinoco-Southern Caribbean mangroves | 33167 | 5505 | 0.166 | 2 | 0.603 |
| Maracaibo dry forests | 30841 | 1485 | 0.048 | 2 | 0.648 |
| Venezuelan Andes montane forests | 29935 | 8765 | 0.293 | 12 | 4.009 |
| Guajira-Barranquilla xeric scrub | 28053 | 2168 | 0.077 | 4 | 1.426 |
| Orinoco Delta swamp forests | 27895 | 1227 | 0.044 | 1 | 0.358 |
| Campos Rupestres montane savanna | 26940 | 8806 | 0.327 | 0 | 0.000 |
| Sin· Valley dry forests | 26434 | 2133 | 0.081 | 0 | 0.000 |
| Northern Andean paramo | 24388 | 40023 | 1.641 | 2 | 0.820 |
| Ecuadorian dry forests | 22366 | 6670 | 0.298 | 0 | 0.000 |
| Pernambuco interior forests | 21750 | 1138 | 0.052 | 0 | 0.000 |
| Catatumbo moist forests | 21075 | 1490 | 0.071 | 0 | 0.000 |
| Magdalena Valley dry forests | 19231 | 7324 | 0.381 | 0 | 0.000 |
| Pernambuco coastal forests | 17689 | 1343 | 0.076 | 0 | 0.000 |
| Lara-Falcon dry forests | 17577 | 935 | 0.053 | 0 | 0.000 |
| Paraguana xeric scrub | 15553 | 204 | 0.013 | 0 | 0.000 |
| Cordillera La Costa montane forests | 15243 | 4418 | 0.290 | 0 | 0.000 |
| Maranon dry forests | 12314 | 3797 | 0.308 | 0 | 0.000 |
| Cordillera Central paramo | 11595 | 1500 | 0.129 | 0 | 0.000 |
| Northeastern Brazil restingas | 11002 | 81 | 0.007 | 0 | 0.000 |
| Gurupa varzea | 9633 | 107 | 0.011 | 0 | 0.000 |
| Araucaria moist forests | 7556 | 309 | 0.041 | 0 | 0.000 |
| Guianan freshwater swamp forests | 6850 | 90 | 0.013 | 0 | 0.000 |
| South American Pacific mangroves | 6534 | 1405 | 0.215 | 0 | 0.000 |
| Araya and Paria xeric scrub | 6090 | 482 | 0.079 | 0 | 0.000 |
| Southern Atlantic mangroves | 6019 | 918 | 0.153 | 0 | 0.000 |
| Orinoco wetlands | 5432 | 21 | 0.004 | 0 | 0.000 |
| Santa Marta montane forests | 5072 | 2568 | 0.506 | 0 | 0.000 |
| Cauca Valley dry forests | 4803 | 3138 | 0.653 | 0 | 0.000 |
| Atlantic Coast restingas | 3299 | 424 | 0.129 | 0 | 0.000 |
| Guayaquil flooded grasslands | 3096 | 1263 | 0.408 | 0 | 0.000 |
| Caatinga Enclaves moist forests | 3089 | 98 | 0.032 | 0 | 0.000 |
| Cordillera de Merida paramo | 2721 | 827 | 0.304 | 0 | 0.000 |
| Chilean matorral | 2211 | 18 | 0.008 | 0 | 0.000 |
| Patia Valley dry forests | 2064 | 97 | 0.047 | 0 | 0.000 |
| Santa Marta paramo | 1352 | 378 | 0.280 | 0 | 0.000 |
| High Monte | 1261 | 1397 | 1.108 | 0 | 0.000 |
| Eastern Panamanian montane forests | 341 | 26 | 0.076 | 0 | 0.000 |
* area only within tropical South America; some ecoregions may extend beyond this region.
Fig 4. Density of occurrence records and census plots in ecoregions of tropical South America.
Maps showing the mean density of (A) occurrence records and (B) vegetation census plots in each of tropical South American ecoregions.
Table 3. The extent of land area (km2) under different climatic conditions as defined by current Total Annual Precipitation (TAP) and Mean Annual Temperature (MAT).
| TAP (mm) MAT (°C) | 0–500 | 500–1000 | 1000–1500 | 1500–2000 | 2000–3000 | 3000–4000 | 4000–6000 | >6000 | >0 |
|---|---|---|---|---|---|---|---|---|---|
| 0–2 | 7781 | 15654 | 0 | 0 | 0 | 0 | 0 | 0 | 23435 |
| 2–4 | 36708 | 54744 | 0 | 0 | 342 | 0 | 0 | 0 | 91452 |
| 4–6 | 49923 | 63869 | 3383 | 342 | 1019 | 0 | 0 | 0 | 118536 |
| 6–8 | 75382 | 73829 | 7499 | 2400 | 1717 | 0 | 0 | 0 | 160827 |
| 8–10 | 104778 | 65755 | 13303 | 6523 | 1019 | 0 | 0 | 0 | 191378 |
| 10–12 | 44748 | 35271 | 23235 | 8590 | 2734 | 0 | 0 | 0 | 114578 |
| 12–14 | 44012 | 46770 | 19396 | 7882 | 4451 | 0 | 0 | 0 | 122511 |
| 14–16 | 51591 | 43582 | 23777 | 19147 | 11294 | 342 | 0 | 0 | 149732 |
| 16–18 | 53790 | 45071 | 29256 | 32344 | 25799 | 3776 | 0 | 0 | 190035 |
| 18–20 | 46792 | 42818 | 123692 | 82946 | 28958 | 9591 | 677 | 0 | 335475 |
| 20–22 | 11238 | 162000 | 365341 | 119160 | 72751 | 27076 | 4109 | 0 | 761676 |
| 22–24 | 85953 | 460572 | 782311 | 466536 | 126930 | 43135 | 12599 | 685 | 1978721 |
| 24–26 | 52874 | 550604 | 800039 | 1322593 | 1805551 | 420056 | 22959 | 13373 | 4988049 |
| 26–28 | 5399 | 201323 | 500020 | 1038270 | 2339775 | 220335 | 9575 | 14750 | 4329448 |
| 28–30 | 5056 | 9459 | 15610 | 9520 | 35546 | 3409 | 1705 | 0 | 80305 |
| >0 | 676025 | 1871321 | 2706862 | 3116252 | 4457545 | 727720 | 51625 | 28808 | 13636159 |
Table 4. The density of collection records (No. col / km2) available for areas under different climatic conditions as defined by current Total Annual Precipitation (TAP) and Mean Annual Temperature (MAT).
| TAP (mm) MAT (°C) | 0–500 | 500–1000 | 1000–1500 | 1500–2000 | 2000–3000 | 3000–4000 | 4000–6000 | >6000 | >0 |
|---|---|---|---|---|---|---|---|---|---|
| 0–2 | 0.018 | 0.014 | - | - | - | - | - | - | 0.009 |
| 2–4 | 0.010 | 0.053 | - | - | 6.376 | - | - | - | 0.032 |
| 4–6 | 0.019 | 0.115 | 0.844 | 0.056 | 3.553 | - | - | - | 0.117 |
| 6–8 | 0.069 | 0.162 | 1.440 | 0.767 | 0.474 | - | - | - | 0.158 |
| 8–10 | 0.078 | 0.205 | 0.890 | 1.586 | 0.692 | - | - | - | 0.190 |
| 10–12 | 0.045 | 0.642 | 1.334 | 0.847 | 0.520 | - | - | - | 0.544 |
| 12–14 | 0.079 | 0.513 | 0.859 | 0.740 | 1.240 | - | - | - | 0.425 |
| 14–16 | 0.022 | 0.481 | 0.791 | 0.628 | 0.898 | 0.056 | - | - | 0.414 |
| 16–18 | 0.022 | 0.349 | 1.007 | 0.450 | 0.762 | 0.753 | - | - | 0.433 |
| 18–20 | 0.063 | 0.446 | 0.271 | 0.336 | 0.602 | 0.851 | 0.095 | - | 0.317 |
| 20–22 | 0.022 | 0.162 | 0.121 | 0.256 | 0.430 | 0.457 | 1.875 | - | 0.200 |
| 22–24 | 0.029 | 0.048 | 0.067 | 0.067 | 0.183 | 0.541 | 0.966 | 0.026 | 0.083 |
| 24–26 | 0.056 | 0.030 | 0.049 | 0.069 | 0.125 | 0.365 | 0.186 | 0.803 | 0.109 |
| 26–28 | 0.009 | 0.012 | 0.030 | 0.091 | 0.131 | 0.158 | 0.420 | 1.181 | 0.110 |
| 28–30 | 0.003 | 0.025 | 0.025 | 0.052 | 0.096 | 0.102 | 0.056 | - | 0.062 |
| >0 | 0.046 | 0.110 | 0.113 | 0.105 | 0.146 | 0.323 | 0.549 | 0.978 | 0.131 |
Combined, the included plot networks represent 1535 census plots distributed throughout tropical South America (Table 2 and Fig 1h). Any given point in tropical South America is an average of 235.7 km from the closest census plot (median distance = 149.2 km; Fig 3b). 1.3% of tropical South America is within 10 km of the closet census plot; 14.9% is within 50 km of a plot; 33.8% is within 100 km of a plot; and 86.7% is within 500 km of a plot. The greatest distance from any point in tropical South America to its closest of the included plots is 1273.3 km. As with the collections data, the greatest concentration of plots are in the tropical montane ecoregions (the Southern Andean Yungas, Eastern Cordillera Real Montane Forests, and Venezuelan Andes Montane Forests are the best, second-best, and fifth-best represented ecoregions, respectively; Table 2 and Fig 4b). The best-represented climatic zones, by far, are the areas with 4000–6000 mm rainfall and mean annual temperatures of 20–22°C, and areas with 3000–4000 mm rainfall and mean annual temperatures of 16–18°C. The other climatic zones have markedly lower densities of plots (Table 5).
Table 5. The density of census plots (No. plots / 10000 km2) in areas under different climatic conditions as defined by current Total Annual Precipitation (TAP) and Mean Annual Temperature (MAT).
| TAP (mm) MAT (°C) | 0–500 | 500–1000 | 1000–1500 | 1500–2000 | 2000–3000 | 3000–4000 | 4000–6000 | >6000 | >0 |
|---|---|---|---|---|---|---|---|---|---|
| 0–2 | 0.000 | 0.000 | - | - | - | - | - | - | 0.000 |
| 2–4 | 0.000 | 0.000 | - | - | 0.000 | - | - | - | 0.000 |
| 4–6 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - | - | - | 0.000 |
| 6–8 | 0.000 | 0.000 | 2.667 | 0.000 | 0.000 | - | - | - | 0.124 |
| 8–10 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - | - | - | 0.000 |
| 10–12 | 0.000 | 0.567 | 0.000 | 2.328 | 0.000 | - | - | - | 0.349 |
| 12–14 | 0.454 | 0.000 | 0.000 | 17.762 | 4.494 | - | - | - | 1.469 |
| 14–16 | 0.000 | 1.147 | 0.841 | 0.000 | 1.771 | 0.000 | - | - | 0.601 |
| 16–18 | 0.000 | 1.331 | 6.153 | 0.000 | 2.713 | 42.375 | - | - | 2.473 |
| 18–20 | 0.000 | 1.868 | 0.081 | 0.241 | 0.000 | 8.341 | 0.000 | - | 0.566 |
| 20–22 | 0.000 | 0.370 | 0.849 | 0.755 | 0.825 | 0.369 | 55.968 | - | 0.998 |
| 22–24 | 0.000 | 0.000 | 0.179 | 0.514 | 0.551 | 2.550 | 0.794 | 0.000 | 0.288 |
| 24–26 | 0.000 | 0.054 | 0.525 | 0.832 | 1.313 | 2.643 | 0.000 | 0.000 | 1.008 |
| 26–28 | 0.000 | 0.248 | 0.220 | 0.905 | 2.740 | 2.088 | 0.000 | 0.000 | 1.841 |
| 28–30 | 0.000 | 0.000 | 0.000 | 1.050 | 0.563 | 0.000 | 0.000 | - | 0.374 |
| >0 | 0.030 | 0.187 | 0.447 | 0.821 | 2.028 | 2.652 | 4.649 | 0.000 | 1.126 |
Discussion
In order to advance conservation science we need to overcome the Wallacean and Linnean shortfalls [29–31]. In other words, we need to know what species are out there and where they occur [32]. One tool that can help us to bypass these shortfalls is the rapidly expanding availability of natural history and collections data. For tropical South America, the amount of collections data that is available online has skyrocketed over the past 2 decades (Fig 1a–1g). Indeed, since the launch of GBIF in 2007, the number of records available from tropical South America has increased by nearly 60% annually.
The rapid increase in available collection data has led to a marked decrease in the “data void”; however, the data void still exists and in some regards remains unacceptably large. This is because the majority of newly-added collections have gone towards increasing the number of species represented but relatively few collections go towards augmenting the sample size of records available for the already-collected species. In other words, the records now include many more species than previously. For example, the number of species represented in the available data rose from <15,000 species in 2007 to >52,000 species in 2013. However, most species remain so poorly represented that they are functionally invisible to ecological studies since they have too small of sample sizes to be included in most modelling exercises or conservation assessments (e.g., in 2013, <14,000 species [26%] had 20 or more available records while >30,000 species [57%] are represented by fewer than 10 records and nearly 10,000 species [19%] are represented by just a single record; Fig 2a). It is also highly likely that there are many more species that remain unnamed or that are not represented by the online databases [31,33]. Indeed, many species will probably never be represented in herbaria or online databases due to their rarity as well as the difficulty of collecting flowers and fruits which are often needed to accurately identify the collections to species. The vast majority of specimens gathered for ecological studies in the tropics are sterile; therefore many collections are not identified to species or are identified incorrectly [6].
Likewise, from a spatial perspective, the data void has shrunk but still remains distressingly large. To date, more than 10% of tropical South America is still represented by no collection records at all and an additional 15% remains with a density of less than 0.0005 available records per km2—or in other words, with just one collection for every 2000 km2 (Fig 3a). Indeed, the overall collection density across the region is approximately 1 collection record for every 10 km2. Making matters worse, the density of collection records is not evenly distributed amongst habitat types or climatic zones. Many ecoregions are very poorly represented in the GBIF collections database. For example, the Cerrado is one of the South America’s largest, most diverse, and most threatened ecoregions [4,34] but it is represented by an average of just one record for approximately every 30 km2. The Caatinga Dry Forest ecoregion of northern Brazil is represented by an approximately equal density of collection records while other ecoregions such as the Madeira-Tapajos Moist Forests are represented by even lower densities of records (1 collection for every 70 km2). In contrast, several of the Andean ecoregions are relatively well-represented with densities of more than 1 collection record per km2 (Table 2). In accord with these patterns, there are also large disparities between climatic zones in their collection intensities. Hot, dry habitats are the least-represented, potentially due to lower diversities and densities of plants in these area. However, there are other very large and relatively-hospitable climate zones which are also very poorly represented. For example, large parts of tropical South America have mean annual rainfalls of between 1500–2000mm and mean annual temperatures of 24–28°C, but these areas are not well-represented in the collections database (Table 4). This may be due to a lack of access (due to physical or bureaucratic impediments) or infrastructure in these areas.
Natural history and herbarium records are only one form of data. In the past decades there have been multiple independent efforts to collate and standardize census data. However, even when combining these efforts the number of plots pales in comparison to the extent and diversity of tropical South America. Across all of the included networks, there are a total of approximately 1500 plots (Fig 1h) covering roughly 15 km2 of forest in tropical South America. In other words, combining all our efforts we are still only censusing about 0.0001% of the total land area (or <0.0003% of the Amazon). For comparison, this is 1/20th the spatial density of plots in the USA that are maintained by just the US Forest Services’ Forest Inventory and Analysis Program alone (http://fia.fs.fed.us/).
Most places in tropical South America are more than 150 km from the closest census plot (Fig 3b) and most ecoregions in tropical South America have less than 1 plot per 30,000 square km2 (Fig 4b). The relative density of census plots across South American ecoregions is not significantly correlated with the density of collection records (Pearson correlation coefficient R = 0.16). In other words, habitats that are well represented in the plot networks are often only poorly-represented in the collections data and vice versa (Table 2). In some cases, this discrepancy is understandable given the emphasis of the included plot networks on tree census and hence the preclusion of plots from some areas with low forest cover but with high plant diversities and thus many collections (such as the Northern Andean Paramo ecoregion). As such, it is possible that the discrepancy between plot and collection densities could be reduced through the inclusion of additional plots focused on non-forest habitats (for example, the GLobal Observation Research Initiative in Alpine Environments network [GLORIA; http://www.gloria.ac.at/] promotes standardized methodologies to census alpine vegetation around the world, including in the high Andes; the GLORIA network was not included in this analysis because their plot locations and data are not readily available online). In other cases (e.g., high plot density but low collection density in the Monte Alegre Varzea and Southern Andean Yungas ecoregions, and vice versa in the Western Ecuador Moist Forests ecoregion), the discrepancy in plot vs. collection density has no apparent explanation other than differential sampling efforts between habitats and regions. Interestingly, the density of neither plots nor collections appears to be strongly linked to the actual representation of the different habitats in ecological studies. For example, Andean ecosystems are greatly underrepresented in the ecological literature but are fairly well-represented in the two types of databases explored here [35]. Given the high spatial variability in composition, structure and dynamic across the Amazon and Tropical South America, there is a clear need for more and better-distributed census plots, collection campaigns, and research efforts.
From a diversity standpoint, it is hard to assess the representativeness of the census plots. This is because many species remain unidentified [31] and nomenclature and taxonomy has still not been fully standardized between, or even within, separate plot networks [36]. In one recent study [6], the taxonomy and nomenclature of collections from the ATDN, the largest of the tropical plot networks, was standardized. The ATDN’s network of 1170 plots were found to include 4,962 species of trees. This is only 30% of the estimated Amazonian tree diversity and less than 10% of the total tropical South American plant diversity as represented in the herbarium collection database. Furthermore, most species were poorly-represented within and across the plots. 21% of represented species occurred within just a single plot and 13% had only a single individual [6]. In other words, as with the herbarium records, most species remain functionally invisible to ecology and conservation studies due to small sample sizes. Making matters worse, census plot data are not usually open access. Each plot network understandably has its own policies for data distribution and sharing, but in most cases data are provided only upon request for use in specific, pre-approved analyses. In other words, census data are not typically available for data mining, data exploration, preliminary studies, or for general assessments (for this study I only used the published locations of plots and not any actual plot data). Limited access to data can hinder the advancement of large-scale ecological, biogeographic and conservation studies; data should made publically available whenever possible (with proper attribution to the data creators and managers).
The results of this assessment clearly illustrate that while recent expansions of collection databases and census plot networks have greatly increased the amount of data available for tropical South America, the data void remains far from filled. There are many species that are still not adequately represented in either the collection and/or plot databases. There are also huge parts of tropical South America, containing many distinct and diverse habitats and climatic zones, that remain very poorly-represented in either the plot and/or collections databases. The lack of data for particular species, habitats and climate zones limits our ability to predict the impacts of climate change and other large-scale anthropogenic disturbances—especially when these disturbances themselves may disproportionately impact different species and habitats. This limitation is expected to be even more severe in other tropical regions, such as Africa and Southeast Asia, where data availability is generally lower [22].
To conclude, I stress that my goal in this study is not to criticize the workers who are diligently adding to or administrating the natural history databases and plot networks. Quite the opposite, my motivation is to help highlight how valuable these data are, and how important ongoing efforts at increasing sample sizes through the generation of new data and the publishing of existing datasets will be [18,37]. Simply put, we need more data.
Supporting Information
Citations for herbaria and databases contributing information to the Global Biodiversity Information Facility as used in this study (Data was downloaded from GBIF in January and February 2014).
(DOCX)
Acknowledgments
I gratefully acknowledge the thousands of hardworking scientists and workers who contribute to online databases and plot networks worldwide and thus help to make big ecology possible. I thank M. Silman for his contributions to the original “data void” assessment and A. Duque, W. Farfan, and all of the FIU Tropical Ecology and Conservation lab for their comments and suggestions on this manuscript. Publication of this article was funded in part by FIU Library’s Open Access Publishing Fund. This is contribution #299 from the Tropical Biology Program of FIU.
Data Availability
All data used in the analyses are freely available online. Plant collection records data are available from http://www.gbif.org/. Plot census locations data are available from http://web.science.uu.nl/Amazon/atdn/, http://www.rainfor.org/, https://www.forestplots.net/, http://www.ctfs.si.edu/, and http://www.condesan.org/redbosques. Climate data are available from http://www.worldclim.org/. Ecoregion map data are available from http://www.worldwildlife.org/publications/terrestrial-ecoregions-of-the-world.
Funding Statement
The author has no support or funding to report.
References
- 1. Soranno PA, Schimel DS (2014) Macrosystems ecology: big data, big ecology. Frontiers in Ecology and the Environment 12: 3–3. [Google Scholar]
- 2. Hampton SE, Strasser CA, Tewksbury JJ, Gram WK, Budden AE, et al. (2013) Big data and the future of ecology. Frontiers in Ecology and the Environment 11: 156–162. [Google Scholar]
- 3. Feeley KJ, Malhi Y, Zelazowski P, Silman MR (2012) The relative importance of deforestation, precipitation change, and temperature sensitivity in determining the future distributions and diversity of Amazonian plant species. Global Change Biology 18: 2636–2647. [Google Scholar]
- 4. Feeley KJ, Silman MR (2009) Extinction risks of Amazonian plant species. Proceedings of the National Academy of Sciences 106: 12382–12387. 10.1073/pnas.0900698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ter Steege H, Pitman NCA, Phillips OL, Chave J, Sabatier D, et al. (2006) Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443: 444–447. [DOI] [PubMed] [Google Scholar]
- 6. Ter Steege H, Pitman NCA, Sabatier D, Baraloto C, Salomão RP, et al. (2013) Hyperdominance in the Amazonian Tree Flora. Science 342. [DOI] [PubMed] [Google Scholar]
- 7. Ramirez-Villegas J, Cuesta F, Devenish C, Peralvo M, Jarvis A, et al. (2014) Using species distributions models for designing conservation strategies of Tropical Andean biodiversity under climate change. Journal for Nature Conservation 22: 391–404. [Google Scholar]
- 8. Zhu K, Woodall CW, Clark JS (2012) Failure to migrate: lack of tree range expansion in response to climate change. Global Change Biology 18: 1042–1052. [Google Scholar]
- 9. Feeley KJ (2012) Distributional migrations, expansions, and contractions of tropical plant species as revealed in dated herbarium records. Global Change Biology 18: 1335–1341. [Google Scholar]
- 10. Swenson J, Young B, Beck S, Comer P, Cordova J, et al. (2012) Plant and animal endemism in the eastern Andean slope: challenges to conservation. BMC Ecology 12: 1 10.1186/1472-6785-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newbold T (2010) Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Progress in Physical Geography 34: 3–22. [Google Scholar]
- 12. Feeley KJ, Silman MR (2010) Modelling Andean and Amazonian plant species responses to climate change: the effects of geo-referencing errors and the importance of data filtering. Journal of Biogeography 37: 733–740. [Google Scholar]
- 13. Graham CH, Elith J, Hijmans RJ, Guisan A, Peterson AT, et al. (2008) The influence of spatial errors in species occurrence data used in distribution models. Journal of Applied Ecology 45: 239–247. [Google Scholar]
- 14. Nelson BW, Ferreira CAC, da Silva MF, Kawasaki ML (1990) Endemism centres, refugia and botanical collection density in Brazilian Amazonia. Nature 345: 714–716. [Google Scholar]
- 15. Hortal J, Lobo JM, JimÉNez-Valverde A (2007) limitations of biodiversity databases: case study on seed-plant diversity in Tenerife, Canary Islands. Conservation Biology 21: 853–863. [DOI] [PubMed] [Google Scholar]
- 16. Loiselle BA, Jorgensen PM, Consiglio T, Jimenez I, Blake JG, et al. (2008) Predicting species distributions from herbarium collections: does climate bias in collection sampling influence model outcomes? Journal of Biogeography 35: 105–116. [Google Scholar]
- 17. Tobler M, Honorio E, Janovec J, Reynel C (2007) Implications of collection patterns of botanical specimens on their usefulness for conservation planning: an example of two neotropical plant families (Moraceae and Myristicaceae) in Peru. Biodiversity and Conservation 16: 659–677. [Google Scholar]
- 18. Collen B, Ram M, Zamin T, McRae L (2008) The tropical biodiversity data gap: addressing disparity in global monitoring. Tropical Conservation Science 1: 75–88. [Google Scholar]
- 19. Kadmon R, Farber O, Danin A (2004) Effect of roadside bias on the accuracy of predictive maps produced by bioclimatic models. Ecological Applications 14: 401–413. [Google Scholar]
- 20. Moerman DE, Estabrook GF (2006) The botanist effect: counties with maximal species richness tend to be home to universities and botanists. Journal of Biogeography 33: 1969–1974. [Google Scholar]
- 21. Hopkins MJG (2007) Modelling the known and unknown plant biodiversity of the Amazon Basin. Journal of Biogeography 34: 1400–1411. [Google Scholar]
- 22. Feeley KJ, Silman MR (2011) The data void in modeling current and future distributions of tropical species. Global Change Biology 17: 626–630. [Google Scholar]
- 23. Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, et al. (2011) A large and persistent carbon sink in the world’s forests. Science 333: 988–993. 10.1126/science.1201609 [DOI] [PubMed] [Google Scholar]
- 24. Lewis SL, Phillips OL, Baker TR, Lloyd J, Malhi Y, et al. (2004) Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis SL, Malhi Y, Phillips OL (2004) Fingerprinting the impacts of global change on tropical forests. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 437–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson-Teixeira KJ, Davies SJ, Bennett AC, Gonzalez-Akre EB, Muller-Landau HC, et al. (2014) CTFS-ForestGEO: a worldwide network monitoring forests in an era of global change. Global Change Biology. Early View Online. [DOI] [PubMed] [Google Scholar]
- 27. Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, et al. (2001) Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51: 933–938. [Google Scholar]
- 28. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- 29. Lomolino MV, Heaney LR (2004) Frontiers of biogeography: new directions in the geography of nature: Sinauer Associates Sunderland, MA. [Google Scholar]
- 30. Whittaker RJ, Araujo MB, Paul J, Ladle RJ, Watson JEM, et al. (2005) Conservation biogeography: assessment and prospect. Diversity and Distributions 11: 3–23. [Google Scholar]
- 31. Joppa LN, Roberts DL, Pimm SL (2011) How many species of flowering plants are there? Proceedings of the Royal Society B: Biological Sciences 278: 554–559. 10.1098/rspb.2010.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feeley KJ (2015) Moving forward with species distributions. American Journal of Botany 102: 1–3. 10.3732/ajb.102.1.1 [DOI] [PubMed] [Google Scholar]
- 33. Joppa LN, Roberts DL, Myers N, Pimm SL (2011) Biodiversity hotspots house most undiscovered plant species. Proceedings of the National Academy of Sciences 108: 13171–13176. 10.1073/pnas.1109389108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klink CA, Machado RB (2005) Conservation of the Brazilian Cerrado. Conservation Biology 19: 707–713. [Google Scholar]
- 35. Pitman NCA, Widmer J, Jenkins CN, Stocks G, Seales L, et al. (2011) Volume and geographical distribution of ecological research in the Andes and the Amazon, 1995–2008. Tropical Conservation Science 4: 64–81. [Google Scholar]
- 36. Guitet S, Sabatier D, Brunaux O, Hérault B, Aubry-Kientz M, et al. (2014) Estimating tropical tree diversity indices from forestry surveys: A method to integrate taxonomic uncertainty. Forest Ecology and Management 328: 270–281. [Google Scholar]
- 37. Feeley KJ, Silman MR (2011) Keep collecting: accurate species distribution modelling requires more collections than previously thought. Diversity and Distributions 7: 1132–1140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Citations for herbaria and databases contributing information to the Global Biodiversity Information Facility as used in this study (Data was downloaded from GBIF in January and February 2014).
(DOCX)
Data Availability Statement
All data used in the analyses are freely available online. Plant collection records data are available from http://www.gbif.org/. Plot census locations data are available from http://web.science.uu.nl/Amazon/atdn/, http://www.rainfor.org/, https://www.forestplots.net/, http://www.ctfs.si.edu/, and http://www.condesan.org/redbosques. Climate data are available from http://www.worldclim.org/. Ecoregion map data are available from http://www.worldwildlife.org/publications/terrestrial-ecoregions-of-the-world.