Since the discovery in the 1800s that the addition of carbon dioxide to water makes it effervescent and pleasant, carbonation has gained popularity for its enjoyable taste and has become an important ingredient of sparkling drinks such as sodas. In recent years, there has been an exponential rise in the consumption of sweetened, carbonated drinks, which has been linked to increasing rates of obesity and increasing prevalence of metabolic diseases.1 Surprisingly, the consumption of diet sodas, which have reduced or zero calories because of the noncaloric sweeteners, is also linked to the risk of obesity and poor health outcomes.2 The question arising then is: What is the culprit for the carbonated drinks-related health issues–the carbonation itself, the sweeteners, or both? In the present issue of Gastroenterology, Di Salle et al3 have used functional magnetic resonance imaging (fMRI) in humans to examine the brain regions activated in response to naturally or artificially sweetened carbonated beverages to determine the effect of carbonation on the perception of sweetness and whether carbonation differentially affects the perception of natural and artificial sweeteners. This is a critical question to begin to understand where and how chemosensory detection of sweetened drinks affects food intake. Studies relying on fMRI approaches have shown that the brain is capable of distinguishing natural and artificial sweeteners4 and that distinct responses to different sweeteners are influenced by the level of consumption of diet soda.5 The Di Salle group went further and asked whether carbonation directly affects sweetness perception. Carbonation activates different sensory systems, including the gustatory system, and the pathway through which carbonation is tasted and how it differs from other tastes have been recently discovered.6
How Does “Taste” Work?
The sense of taste serves as the gatekeeper by controlling access to food consumption, regulating feeding behavior and guiding in the selection of palatable food or drink, while avoiding toxins and poisons.7–9 Humans perceive 5 distinct basic tastes: sweet, umami (savory taste or amino acids), bitter, sour, and salty, although other taste modalities should be added to these basic taste qualities, including carbonation. Taste reception is orchestrated by distinct populations of selectively tuned cells clustered to form taste buds in the tongue and mouth, namely type I, II, and III cells, which express specific receptors for different gustatory stimuli (Figure 1). Sweet, umami, and bitter tastes are detected by 2 distinct families of G-protein–coupled receptors (GPCRs) localized on type II cells or receptor cells: The T1R taste receptor family is composed of 3 distinct members that heterodimerize to sense sweetness (T1R2 and T1R3) and amino acids (T1R1 and T1R3); the T2R taste receptor families include numerous divergent GPCRs that act as narrowly or broadly tuned bitter sensors to detect a myriad of bitter substances. By contrast, sour and salty tastes are sensed by ion channels. The polycystic kidney disease channel has been proposed as the acid-sensing machinery detecting the sour (acid) taste expressed by type III cells or presynaptic cells.7–9 CO2 is also detected by sour-sensing cells; however, the taste of carbonation is separated from acid detection because it is mediated by carbonic anhydrase 4, a glycosylphosphatidyl inositol-anchored enzyme tethered on these cells' surface. This enzyme serves as the principal sensor of CO2 by catalyzing the conversion of CO2 into bicarbonates and protons, with the protons being the relevant signal.6 Thus, carbonation does not taste sour despite being detected by sour-sensing cells. Finally, the salty taste is likely to be mediated by the epithelial sodium channel expressed by type I cells or glia-like supporting cells.7–9
Figure 1.
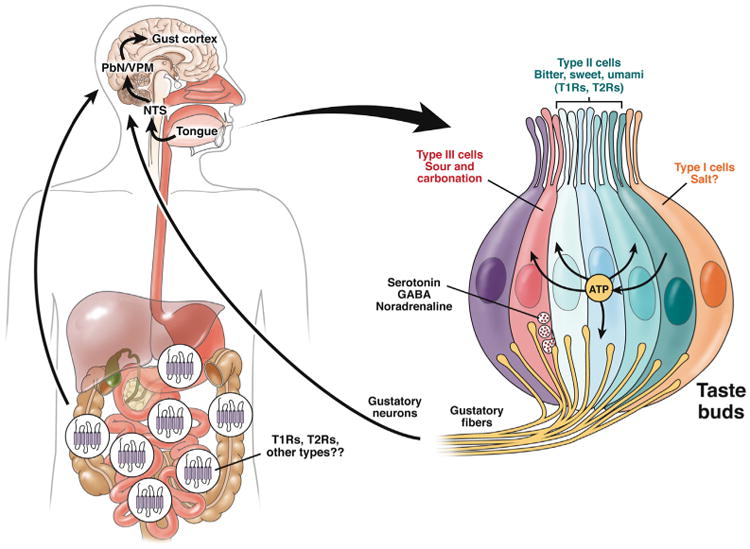
Taste buds and taste transmission. Taste buds are made up of clusters of cells tasting different tastes. These include 3 major types of cells, type I or glia-like cells, which are likely to detect salt; type II or receptor cells, which detect sweet, umami, and bitter tastes; and type III or presynaptic cells, which detect sour and carbonation. Each taste is detected by specialized sensors expressed on these cells: sweet and umami are detected by T1Rs and bitter by T2R receptor families; sour, carbonation, and salt by ion channels (see paragraph, How Does “Taste” Work?). Type II cells, when activated, release adenosine triphosphate (ATP) that in turn activates other type II cells and type III cells to release transmitters as well as gustatory fibers. Type III cells release different transmitters: serotonin, GABA, and noradrenaline. It is not known whether type I cells release any transmitters. Each cell communicates with afferent fibers that are intermingled in the gustatory nerves, which transport the information generated in the taste buds to the gustatory cortex through different neuronal stations, including the nucleus of the solitary tract (NTS), the parabrachial nucleus (PbN), and the ventral posteromedial nucleus (VPM) of the thalamus. Information is processed in the brain where each taste activates distinct clusters of neurons. GPCRs detecting sweet and umami (T1Rs) and bitter (T2Rs) tastes are also found in the gastrointestinal mucosa and their activation results in the release of hormones/signaling molecules that induce a variety of functions, including motility and secretion and activate afferent neurons communicating with the brain.
Interaction of gustatory stimuli with taste receptors initiates distinct signal transduction cascades leading to the secretion of different transmitters. Signals generated by taste receptor activation are transmitted to gustatory fibers intermingled within gustatory afferent nerve bundles and carry taste information to different regions of the brain, including the nucleus of the tractus solitarius in the brainstem, the parabrachial nucleus, the thalamus, and the primary gustatory cortex, the insula.9,10 Even though different tastes act on different sets of taste cells, there is significant cell–cell communication within taste buds and transmitters released in response to gustatory stimuli act on adjacent cells. For instance, adenosine triphosphate (ATP), the major transmitter released by type II receptor cells in response to sweet, bitter, and umami tastes stimulates both afferent fibers and adjacent type III presynaptic cells as well as other type II cells, thus stimulating them to release transmitters (eg, serotonin, GABA, and noradrenaline from type III cells and ATP from type II cells)8 (Figure 1). This results in the integration of gustatory information from different taste cells.
How Is the Taste of Carbonation Processed in the Presence of Natural or Artificial Sweetener?
The gustatory system has the responsibility to distinguish between palatable or nutritional and non-palatable or harmful substances, which is essential for survival and nutrition. Taste responses are genetically coded, but feeding behavior is influenced by many factors, including individual variations, habituation to certain bitter, acid, or sour tastes, and adaptive behavior dictated by environmental changes.11–13 Sweet, umami, and lowsalt tastes are considered “good” tastes because they signal the presence of carbohydrates and amino acids, which are sources of energy and proteins, and elicit food acceptance and consumption. By contrast, bitter, sour, and high-salt tastes are “bad” tastes in that they alert the organism against toxins and dietary acids, spoiled food, or too much salt, evoking aversion and rejection. Because the taste of carbonation is enjoyable and favors drink consumption, carbonation might be considered a “good” taste. However, the definition of “good taste” is relative in that excessive amount of any nutrients resulting in overeating and excessive energy intake versus energy consumption is unhealthy and leads to diet-related disorders. Furthermore, the combination of different tastes in food and drink might affect the way each taste is processed and perceived, which might in turn affect eating habits and weight. For instance, increased consumption of sweetened carbonated beverages is associated with increased obesity and metabolic syndromes, independent of whether they contain caloric or noncaloric sweeteners, which is counterintuitive. Why should drinks with no calories cause weight gain or metabolic disease? The findings of Di Salle et al,3 together with previous observations from other groups4,5 are providing new insights into this enigma. Di Salle et al have used fMRI to identify the brain regions in healthy volunteers activated in response to carbonated or noncarbonated solutions sweetened with sucrose or artificial sweetener (aspartame and acesulfame) in the mouth and then swallowed. The authors found a reduction in brain activity in the gustatory regions of the brain such as the anterior-insula, orbitofrontal cortex, and posterior pons in response to carbonation independently of what sweetener was used, although the effect of carbonation on sucrose was stronger compared with the effect on artificial sweetener.3 The imaging data are supported by behavioral data (presented in the supplemental material) showing that CO2 reduces sweetness perception and the differentiation between natural and artificial sweeteners. The authors also showed that carbonation and sourness, which are sensed by the same cells in the taste buds, but with different mechanisms,6 activate different brain regions within the anterior insula, although there is some overlap, providing evidence that the effect of carbonation on sweetness perception did not involve co-activation of sour receptors. The finding of neural separation of different tastes is in line with the recent knowledge that, contrary to the previous belief of broadly tuned neurons processing gustatory stimuli, there is a topographic representation of the distinct tastes in the brain and each taste activates different clusters of neurons in the gustatory cortex.10
This study provides compelling evidence that carbonation modulates sweetness perception of both natural and artificial sweeteners. Making the perception of noncaloric sweetener similar to the caloric sweetener, carbonation might then favor the consumption of low-calorie, diet beverages. However, the reduced sweetness perception due to carbonation might be a double-edged sword in that it could also stimulate sucrose and food consumption because the brain perceives less sugar intake, and because energy balance is impaired.14 Interestingly, individuals who regularly consume artificially sweetened beverages seem to process the sweet taste of nutritive or non-nutritive sweetener during hedonic evaluation differently compared with non-diet soda drinkers and show alteration in the reward processing of sweet taste.5 Because the reward system plays a role in the modulation of energy intake, the increased activation of reward regions of the brain in diet soda drinkers might impact eating behaviors. The modulation of sweetness perception by the taste of carbonation might also be affected by the physical properties of natural and artificial sweeteners in the mouth, such as texture and bulking, that vary between different sweeteners and activate the primary taste cortex.15,16
An interesting aspect that has been touched upon, but not directly addressed by Di Salle's team in this brief report, is that inputs from the gastrointestinal tract might contribute to the effect of taste and carbonation on dietary behavior and diet-related disorders. Consumption of carbonated beverages induces gastric distension, eliciting a sense of fullness and thus affecting food intake. In addition, the sense of taste in the mouth, together with the sight and the smell of food and drink, initiates physiologic reflexes beyond the oral cavity, such as the secretion of digestive enzymes, hormones, and other signaling molecules from the gastrointestinal tract and its associated glands, which prepare the gut to digest and absorb nutrients or to reject and neutralize potentially dangerous non-nutritive chemicals.3 Furthermore, the gastrointestinal mucosa harbors the same complement of taste receptors as the taste buds, including GPCRs for sweet, umami, and bitter tastes and their downstream signaling molecules.17–20 Therefore, the gut “senses” both nutritive and non-nutritive sweeteners as the taste buds; thus, gut taste receptors may also mediate the effect of ingestion of sweeteners (caloric and noncaloric) on feeding behavior. This could be achieved by the release of glucagon-like peptide-1 and glucose-dependent insulinotropic peptide that regulate nutrient absorption, glucose homeostasis, and satiety–hunger signaling.17 The taste for carbonation has not been identified in the gut, and taste receptors in the gut and other organs might exert nongustatory roles according to the specific region of expression. However, convergence of gustatory and nongustatory stimuli transmitted to the brain as well as the changes occurring in the gut lumen in response to food and drink from the mouth are likely to play a critical, but still not completely understood, function relevant to feeding disorders and diet-related diseases ranging from overeating to diabetes and obesity.
Concluding Remarks
The findings that carbonation reduces the perception of natural and artificial sweetener with a stronger reduction of sucrose processing and the ability to differentiate between the different types of sweeteners are intriguing and provocative. Tricking the brain about the type of sweet could be advantageous to weight loss because it facilitates the consumption of low-calorie drinks because their taste is perceived as pleasant as the sugary, calorie-laden drink. However, there is a downside; the altered energy homeostasis and balance induced by the reduced sweetness perception might stimulate sugar consumption. The latter interpretation might better explain the prevalence of eating disorders, metabolic diseases, and obesity among diet soda drinkers. Future studies combining analysis of carbonation effect on sweetness detection in taste buds and responses elicited by the carbonated sweetened beverages in the gastrointestinal lumen will be required to further elucidate the puzzling link between reduced calorie intake with diet drinks and increased incidence of obesity and metabolic diseases.
See “Effect of carbonation on brain processing of sweet stimuli in humans,” by Di Salle F, Cantone E, Savarese MF, et al, on page 537.
Acknowledgments
NIH-NIDDK grants DK54155 and DK41301.
Footnotes
Conflicts of interest: The author discloses no conflicts.
References
- 1.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler SP, Williams K, Resendez RG, et al. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 3.Di Salle F, Cantone E, Savarese MF, et al. Effect of carbonation on brain processing of sweet stimuli in humans. Gastroenterology. 2013;145:537–539. doi: 10.1053/j.gastro.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Frank GK, Oberndorfer TA, Simmons AN, et al. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–1569. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Green E, Murphy C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol Behav. 2012;107:560–567. doi: 10.1016/j.physbeh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrashekar J, Yarmolinsky D, von Buchholtz L, et al. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roper SD. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 2013;24:71–79. doi: 10.1016/j.semcdb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Gabitto M, Peng Y, et al. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drayna D. Human taste genetics. Annu Rev Genomics Hum Genet. 2005;6:217–235. doi: 10.1146/annurev.genom.6.080604.162340. [DOI] [PubMed] [Google Scholar]
- 12.Shigemura N, Shirosaki S, Sanematsu K, et al. Genetic and molecular basis of individual differences in human umami taste perception. PLoS One. 2009;4:e6717. doi: 10.1371/journal.pone.0006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada-Katsumata A, Silverman J, Schal C. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science. 2013;340:972–975. doi: 10.1126/science.1234854. [DOI] [PubMed] [Google Scholar]
- 14.Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100:55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappes SM, Schmidt SJ, Lee SY. Relationship between physical properties and sensory attributes of carbonated beverages. J Food Sci. 2007;72:S001–S011. doi: 10.1111/j.1750-3841.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 16.Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes (Lond) 2011;35:550–561. doi: 10.1038/ijo.2010.155. [DOI] [PubMed] [Google Scholar]
- 17.Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008;8:78–81. doi: 10.1124/mi.8.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and {alpha}-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 20.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]


