INTRODUCTION
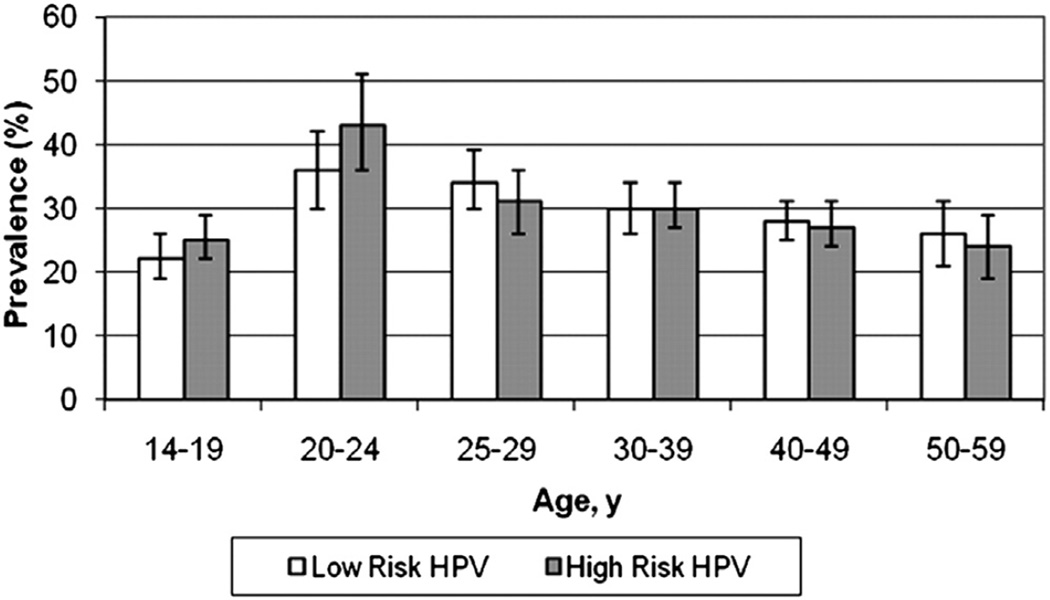
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States with an estimated 20 million currently infected, and 6.2 million becoming newly infected annually.1 A large national study that looked at more than 4000 women found that the overall prevalence of HPV infection among females aged 14 to 59 years was 42.5%. It also demonstrated that HPV infection significantly increased after age 14 to 19 years and peaked at age 20 to 24 years, consistent with the hypothesis that HPV is acquired shortly after onset of sexual activity (Fig. 1).2
Figure 1.
Prevalence of low-risk and high-risk HPV among female respondents 14 to 59 years of age. (Data from Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis 2011;204(4):566–73.)
HPV has been linked to various benign and malignant lesions occurring in mucosal and skin epithelia. Currently, there are 148 recognized HPV types, classified into 33 species.3 Forty HPV types infect the mucosal epithelium with a subset of approximately 12 high-risk subtypes that are etiologically linked to cervical cancer and its immediate premalignant precursors.4 Two of the most important high-risk subtypes, HPV 16 and 18, account for 70% of cervical cancer cases.5 Low-risk subtypes cause low-grade cervical dysplasia, genital warts, respiratory papillomas, and nasopharyngeal papillomas.6 HPV 6 and 11 account for 90% of genital warts found in males and females. In the United States, an analysis of cancer registry data estimated that the total burden of all other HPV-related cancers (excluding cervical cancer) equals that of the cervical cancer burden. According to the National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) databases, HPV-associated cancers accounted for 3.3% of all cancer cases among women and 2.0% of the total cancer cases among men diagnosed in 2009.7 Jemal and colleagues7 reported an increase in incidence rates of HPV-associated cancer of the oropharynx among white men and women, anal cancer among white and black men and women, and vulvar cancer among white and black women from 2000 to 2009. The increases in incidence rates for these cancers were more notable among persons aged 55 to 64 years compared with the younger age groups.7 Sexually active men and women have more than a 50% lifetime risk of becoming infected with HPV, and it is estimated that 80% of women will contract an HPV-related disease by age 50 years.8 Women aged 25 years and younger are at greatest risk of HPV infection, with a second peak of infection occurring after age 55 years.9
Cervical cancer is the second most commonly occurring cancer in women worldwide.10 Most of the global burden (>85%) of cervical cancer occurs in developing countries.11 In 1942, George Papanicolaou published a report on the use of cytology to screen for cervical cancer.12 Since then, cytologic screening has dramatically reduced the prevalence of cervical cancer in developed countries with well-established screening programs. However, millions of women worldwide harbor chronic persistent HPV infections that increase their risk of developing cervical cancer. In 2008, 530,000 womenwere newly diagnosed with cervical cancer and 280,000 died of the disease.11 It is projected that by 2030, 10 to 14 million women will be newly diagnosed with cervical cancer and 5 to 8 million will die of the disease unless preventive efforts are improved, particularly in developing countries where there is still significant disease burden.13
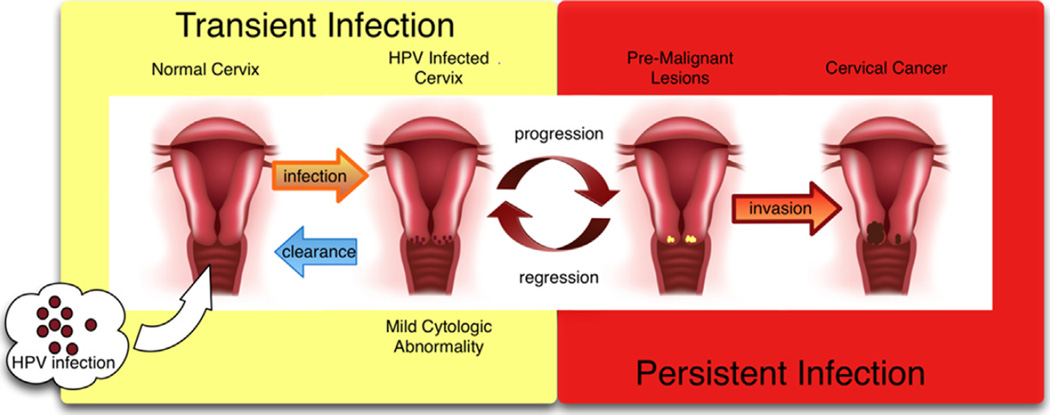
Cancer of the cervix is the end result of a long process that begins with infection with HPV.14 Once a cell is infected with an oncogenic HPV strain, there are typically 4 steps that lead to cervical cancer: HPV transmission, viral persistence, progression of a clone of persistently infected cells to a precancerous lesion, and finally invasion. Forward progression in this process is not always the case. Clearance of HPV infection and regression of precancerous lesions can also occur.15 With a competent immune system, most cervical HPV infections are cleared by cell-mediated immunity within 1 to 2 years of exposure (Fig. 2).16
Figure 2.
Pathogenesis of HPV infection.
The human papillomavirus consists of 8000-basepair long circular DNA molecules wrapped in a protein shell. This protein shell contains 2 molecules, L1 and L2. The HPV genome is composed of 6 early genes, 2 late genes, and a noncoding region. Once the virus infects a cell, the early proteins (E1, E2, E4, E5, E6, E7) are transcribed first, followed by the late proteins (L1 and L2), each of which are necessary for viral replication and assembly of newly formed virus particles in infected cells.17 Both of the currently available vaccines target the L1 protein on different HPV types, but investigators are currently developing new vaccines against the L2 protein.
CURRENT HPV VACCINES
Given the well-established etiologic relationship between HPV infection and cervical cancer, a vaccine to immunize against HPV may reduce the burden of cervical caner and other HPV-related diseases.18 In 2006, the US Food and Drug Administration (FDA) approved the first vaccine against HPV by Merck & Co, Inc. Gardasil or HPV4 is a quadrivalent vaccine consisting of 4 different types of virallike particles from the 4 serotypes of HPV (types 6, 11, 16, and 18). In 2009, a second bivalent HPV vaccine (HPV2; Cervarix by GlaxoSmithKline) targeting HPV types 16 and 18 was also approved by the FDA. These were the first vaccines developed specifically to prevent a sexually transmitted infection and the second (after the hepatitis B vaccine) designed to prevent cancer.19 Gardasil is adjuvated with simple aluminum salt and is produced in yeast.19 Cervarix contains the adjuvant ASO4, which includes monophosphoryl lipid A and an aluminum salt, and it is produced in insect cells.19 Both vaccines are delivered via intramuscular injection in 3 doses over 6 months.
The Gardasil vaccine was initially licensed for use in females aged 9 to 26 years of age. In March 2007, the Centers for Disease Control Advisory Committee on Immunization Practices (ACIP) recommended routine vaccination with HPV4 for all girls aged 11 to 12 years old. The vaccine can be administered as early as 9 years of age and catch-up vaccination is recommended for those aged 13 to 26 years old who have not been vaccinated previously.20,21 Cervarix was initially approved for use in females from 10 to 25 years of age in October 2009. The ACIP also approved its routine use in girls aged 11 to 12 years, starting as early as 10 years of age and catch-up vaccination from 13 to 25 years of age (Table 1).22
Table 1.
FDA-approved HPV vaccines
| Gardasil | Cervarix | |
|---|---|---|
| Proper name | HPV quadrivalent (types 6, 11, 16, 18) vaccine, recombinant | HPV bivalent (types 16 and 18) vaccine, recombinant |
| Manufacturer | Merck & Co. | GlaxoSmithKline Biologicals |
| VLP types | 6, 11, 16, 18 | 16, 18 |
| Adjuvant | 225 µg aluminum hydroxyphosphate sulfate | 500 µg aluminum hydroxide, 50 µg3-O-deacylated-4′-monophosphoryl lipid A |
| Schedule | 0, 2, 6 mo | 0, 1, 6 mo |
| Indications | Vaccination in females between 9 and 26 y of age for prevention of diseases caused by HPV 6/11/16/18: Cervical cancer Genital warts Cervical adenocarcinoma in situ (AIS) Cervical intraepithelial neoplasia (CIN) grades 1, 2, and 3 Vulvar intraepithelial neoplasia (VIN) grade 2 and grade 3 Vaginal intraepithelial neoplasia (VaIN) grade 2 and grade 3 Prevention of vulvar and vaginal cancer Vaccination in boys and men between 9 and 26 y of age for the prevention of genital warts caused by HPV 6/11 Vaccination in people aged 9 to 26 y for the prevention of anal cancer and associated precancerous lesions caused by HPV 6/11/16/18 |
Vaccination in females between 9 and 25 y of age for the prevention of disease caused by HPV 16/18: Cervical cancer CIN grades 1, 2, and 3 AIS |
Data from FDA-approved indications for Gardasil. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042. Accessed December 10, 2012; and FDA-approved indications for Cervarix. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042. Accessed December 10, 2012.
NEW INDICATIONS
HPV vaccination efforts have focused heavily on women and young adolescent girls. However, more than 25% of all HPV-related cancers occur in men and an estimated 250,000 cases of genital warts that occur each year in the United States affect sexually active men.23 HPV-related malignancies affecting men include anal, penile, and oropharyngeal cancers, which are caused primarily by HPV 16.24–26 There has been an increased incidence of oropharyngeal and anal cancer in men.24,26 In October 2009, the FDA approved the use of Gardasil in males aged 9 to 26 years of age.27 The ACIP subsequently approved permissive guidance to vaccinate males aged 9 to 26 years of age to decrease their risk of acquiring genital warts, although it was not recommended for routine use in males at that time.28
Infection with HPV 16 and 18 accounts for 90% of anal cancers and the incidence of anal cancers has been steadily increasing for the past 3 decades. Anal carcinoma is still relatively rare when compared with cervical cancer, with 5820 estimated new cases and 770 deaths in the United States in 2011. According to a populationbased review of the SEER cancer registry, there has been a 2.8-fold increase in the incidence of anal cancer from 1973 to 1998,29 and there has been a similar increased trend in mortality over the past few years.30 Anal cancer is more prevalent in individuals infected with human immunodeficiency virus (HIV) and men who have sex with men, even those without HIV infection.29,31 However, there is a higher disease burden in women. In 2003, the Centers for Disease Control and Prevention (CDC) reported that anal cancer was more common in women (1.6 per 100,000 women) than in men (1.3 per 100,000 men).32 They estimate 1600 new cases of HPV-related anal cancers in women compared with only 900 new cases in men in the United States.32 In 2010, the FDA included prevention of anal cancer in men and women as an additional indication for use of the HPV4 vaccine.33
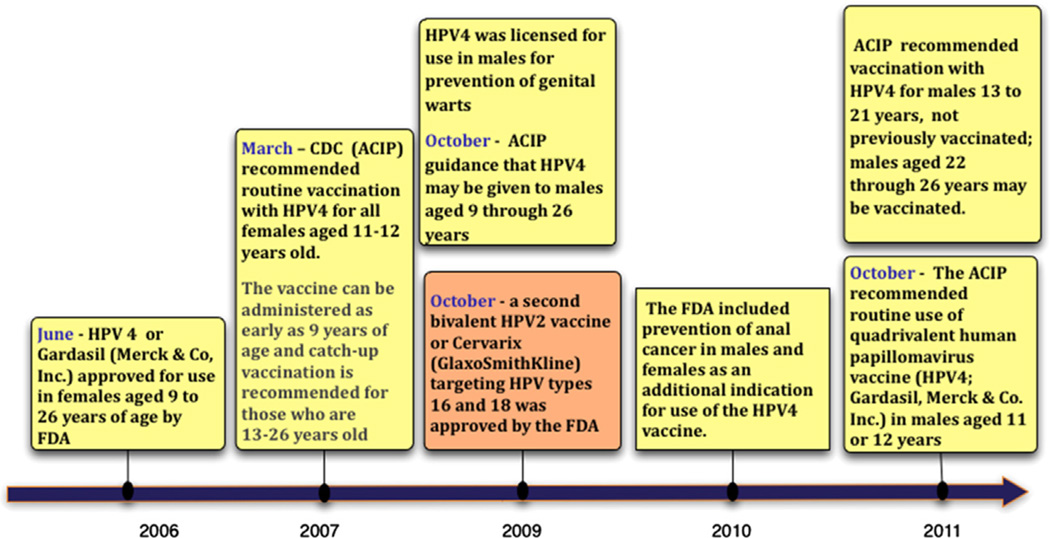
Guiliano and colleagues34 demonstrated that the quadrivalent vaccine was effective in preventing the development of anal intraepithelial neoplasia in men. In this study, the vaccine had an 89.3% efficacy in the prevention of HPV 6/11/16/18–related genital warts in the per protocol population. A substudy of the phase III efficacy trial was performed and included 598 men who have sex with men aged 16 to 26 years.35 The subanalysis demonstrated an 88.1% per protocol efficacy in preventing HPV 6/11/ 16/18–related genital warts. The per protocol efficacy for prevention of HPV 6/11/ 16/18–related anal intraepithelial neoplasia (AIN) grades 1 to 3 was 77.5% and AIN lesions grades 2 to 3 was 74.9%. In October 2011, the ACIP extended their recommendations to include routine vaccination for all boys aged 11 to 12 years. It can be administered as early as 9 years and catch-up vaccination is recommended for boys aged 13 to 21 years. Men aged 22 to 26 years may be vaccinated. For special populations such as immunocompromised men and men who have sex with men, vaccination is recommended up to 26 years of age (Fig. 3).35
Figure 3.
Timeline of HPV VLP vaccine licensure and approved indications for use.
In 2011, the Costa Rica Vaccine Trial, 1 of the phase III efficacy studies performed on the HPV 2 vaccine, reported that the vaccine may be as effective in preventing anal HPV infection.36 The bivalent vaccine demonstrated 62% efficacy against prevalent HPV 16/18 infection in the anus. In the HPV-naive cohort, vaccine efficacy against anal HPV 16/18 infection was 83.6%, which was similar to its efficacy in preventing cervical HPV 16/18 infection (87.9%). However, the bivalent vaccine has not yet been approved for use for the prevention of anal HPV infection.
HPV VACCINE EFFICACY TRIALS
Quadrivalent HPV Vaccine: FUTURE I and II Trials
There have been several randomized clinical trials conducted to investigate the efficacy of the quadrivalent vaccine. A phase II randomized placebo-controlled trial enrolled 1158 female participants, aged 16 to 23 years who were randomized to receive placebo or the quadrivalent vaccine. In this study, there was a 90% reduction in the incidence of HPV 6/11/16/18–related cervical or genital diseases in the vaccinated group.37 The interim analyses performed in this study helped establish the dose of the vaccine used in phase III studies.
The phase III efficacy trials for the HPV4 vaccine were primarily designed to demonstrate its efficacy in preventing new-onset vaccine-related HPV infection as well as precancerous lesions that result from persistent infection. The FUTURE I trial also evaluated its participants for the development of HPV 6/11/16/18–associated cervical intraepithelial neoplasia grade 1 and higher (CIN 1+) and external genital lesions, including genital warts and vulvar/vaginal intraepithelial neoplasia.38 The primary end points for the FUTURE II study included premalignant precursors such as high-grade dysplasia (CIN 2 or 3), adenocarcinoma in situ (AIS), and cervical cancer associated with HPV 16 and 18.39 Both trials collectively enrolled and randomized 17,622 women and were designed to have at least 4 years of follow-up (Table 2).
Table 2.
Phase III efficacy trials on HPV vaccines
| Trial | FUTURE I Trial | FUTURE II Trial | PApilloma TRial Against Cancer In Young Adults (PATRICIA) |
|---|---|---|---|
| Vaccine | Quadrivalent vaccine against HPV 6/11/16/18 | Quadrivalent vaccine against HPV 6/11/16/18 | Bivalent vaccine against HPV 16/18 |
| Study type | Phase III double-blinded randomized controlled trial | Phase III double-blinded randomized controlled trial | Phase III double-blinded randomized controlled trial |
| Length of follow-up (y) | 4 | 4 | 4 |
| Age of participants (y) | 16–24 | 15–26 | 15–25 |
| Lifetime no. of sexual partners | ≤4 | ≤4 | ≤6 |
| Exclusion criteria | Pregnancy, history of abnormal Pap smear or genital warts History of severe allergic reaction; known immune disorders; receipt of attenuated or live vaccines within 14–21 d of enrollment |
Pregnancy, breastfeeding, history of colposcopy, autoimmume disease, or immunodeficiency | |
| Primary end points | Incident HPV 6/11/16/18-associated genital warts, CIN 1–3, VIN 1–3, VaIN 1–3, AIS and cervical, vaginal, or vulvar cancer | Incident HPV 16/18-associated CIN 2–3, AIS, or cervical cancer | Incident HPV 16/18-associated CIN 2+ |
| Outcome | 96% efficacy against CIN 1 in unexposed Protection from high-grade cervical dysplasia: 86.3% in placebo vs 20.1% in vaccinated 98% efficacy in high-grade disease related to HPV 16/18 in unexposed 44% efficacy in high-grade disease related to HPV 16/18 in exposed Protection from noncervical low-grade disease: 81.5% in placebo vs 21.7% in vaccinated 100% efficacy against VIN 1 and VaIN 1 in unexposed 99% efficacy against condyloma in unexposed |
92% efficacy against ≥CIN 2 related to HPV 16/18 54% efficacy against ≥CIN 2 related to nonvaccine HPV types 100% efficacy against ≥CIN 3 related to HPV 16/18 90% efficacy against ≥CIN 3 overall 100% efficacy against adenocarcinoma in situ in those negative for HPV 77% efficacy against AIS in all Reduction in high-grade disease caused by other nonvaccine HPV types |
|
The end-of-study analyses for both trials showed promising results in the prevention of vaccine-related HPV genital and cervical disease. There was 100% efficacy in the prevention of HPV 6/11/16/18–related CIN 3 in the cohort of women with no baseline cervical cytologic abnormality or prevalent infection of any HPV types (14 types were tested) and serologic evidence of previous exposure to the vaccine-related HPV types. Women in the intention-to-treat group (ITT) had lower efficacy, 45.1%, because this group included women with previous exposure to HPV at enrollment.39,40 There was greater than 95% efficacy in the prevention of HPV 6/11/16/18–related vulvar intraepithelial neoplasia grade 2 and 3 (VIN 2/3) in both the HPV-naive group and the ITT group. The HPV4 vaccine also demonstrated greater than 75% efficacy in the prevention of vaginal intraepithelial neoplasia grade 2 and 3 (VaIN 2/3) and genital warts in the same cohort.40 In addition, there was high efficacy in prevention of VIN 2/3, VaIN 2/3, and genital warts caused by non–vaccine-related HPV types.41
Bivalent HPV Vaccine: PATRICIA Trial
The Papilloma Trial against Cancer In young Adults (PATRICIA) study was a randomized double-blind study conducted in 14 countries to study the efficacy of the bivalent HPV vaccine against HPV types 16 and 18. Women were randomized to receive either the bivalent HPV vaccine or a control hepatitis A vaccine. More than 18,000 women were enrolled and a total of 17,106 women completed the vaccine series. The primary end point was to evaluate vaccine efficacy against CIN 2+ that was associated with HPV 16 or HPV 18 in women who were seronegative at baseline, and DNA negative at baseline and at month 6.42 The average duration of follow-up was 35 months after completion of the vaccine series. The analysis was done according to the protocol cohort, the total vaccinated cohort (TVC, which included all women receiving at least 1 vaccine dose, regardless of baseline HPV status and including those who were sexually active), and TVC-naive (women with no evidence of oncogenic HPV infection at baseline.)
The PATRICIA trial demonstrated 100% efficacy in the prevention of CIN 3+ caused by HPV 16/18 in the TVC-naive group. As expected, the efficacy in the TVC group was lower at 45.7%. There was a 93.2% efficacy in the prevention of CIN 3 in the TVC-naive group irrespective of HPV type.43,44 In addition, there was also 100% efficacy in the prevention of AIS in the TVC-naive group and 76.9% in the TVC group. The vaccine had a 90% efficacy in preventing all CIN 3+ lesions in all age groups in the TVC-naive cohort. Among the TVC group, there was a progressive decline in vaccine efficacy against all HPV 16/18–associated CIN 3+ among different age groups. The highest efficacy (65.5%) was seen in women aged 15 to 17 years of age; 33.1% efficacy was seen in the group aged 18 to 20 years and 19.5% in those aged 21 to 25 years. The investigators attributed this decline in efficacy among the older age group to the higher exposure to HPV at baseline among older women (see Table 2).
The bivalent vaccine has also been shown to reduce the risk of high-grade cervical disease caused by nonvaccine HPV types, including HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.45
Comparison of Quadrivalent and Bivalent HPV Vaccines
The cross-protective effects of both available vaccines have become a focus of interest, particularly among public health figures in countries that are in the midst of deciding which vaccine to use in their national programs. However, because of differences in participant characteristics and the prevalence of HPV infections among trial participants, comparisons between the 2 vaccines have been difficult. Malagon and colleagues46 performed a systematic review and meta-analysis comparing the cross-protective effects of the quadrivalent vaccine versus the bivalent vaccine. To minimize differences in study design and participants, they focused the analysis on HPV-naive populations. In this comparison, it was demonstrated that both the quadrivalent and bivalent vaccines offer cross-protection against disease caused by nonvaccine HPV types. Pooled data from PATRICIA and FUTURE I/II showed that cross-protective efficacy was greater with the bivalent vaccine compared with the quadrivalent vaccine. The quadrivalent vaccine was noted to be efficacious against HPV 31 whereas the bivalent vaccine showed greater efficacy against HPV 31, 33, and 45 for persistent infection and CIN 2+ disease. Both vaccines showed little evidence of cross-protection against HPV 52 and 58. Prolonged follow-up in the bivalent trials showed decreasing efficacy against HPV 31 and 45 suggesting waning of crossprotection over time.46
IMMUNOGENICITY TRIALS
The 2 currently available HPV viruslike particle (VLP) vaccines have been developed using innovative recombinant molecular technology and consist only of the major viral capsid protein L1. VLP vaccines confer their protective effect by inducing high concentrations of type-specific virion neutralizing antibodies and they have demonstrated remarkable efficacy against genital diseases caused by the vaccine HPV types.47 Clinical trials have shown that both vaccines are highly immunogenic demonstrating 100% seroconversion, and showing peak geometric antibody titers (GMTs) to be 10-fold to 100-fold higher compared with GMTs after natural infection.48,49 Studies measuring the antibody responses of both vaccines used different assays, which make it impossible to make quantitative comparisons based on published studies; Gardasil was evaluated with a Luminex-based assay whereas Cervarix was evaluated with a VLP-based enzyme-linked assay (ELISA).50 Nevertheless, titers for both vaccines peaked 1 month after the third dose and declined over the next year but remained relatively stable for the duration of follow-up (4 years for Cervarix and 4.5 years for Gardasil).51,52 Women vaccinated with Cervarix showed titers greater than the GMT observed after natural infection during the plateau stage. However, almost one-third of those vaccinated with Gardasil experienced a drop in HPV 18 titers to less than the level of detection. This may be due to the intrinsic immunogenicity of HPV 18 VLPs or merely confounded by the monoclonal antibody assay used to detect the HPV 18 antibodies.
Immunogenic studies on adolescent boys and girls not only demonstrated vaccine safety in this age group but also exhibited a noninferior antibody response to the vaccine by boys compared with girls.53,54 In addition, the GMT of VLP-specific antibodies in boys and girls were 2-fold higher than that found in young women.55,56
A phase III randomized study was conducted to compare the immunogenicity and safety of the bivalent vaccine and the quadrivalent vaccine in 1106 healthy women aged 18 to 45 years (they were stratified by age groups: 18–26 years, 27–35 years, and 36–45 years).57 At month 7 after first vaccination, all women in the according-to-protocol cohort who were seronegative/DNA negative before vaccination had seroconverted for HPV 16 and HPV 18 serum neutralizing antibodies except for 2 women aged 27 to 35 years in the Gardasil group who did not seroconvert for HPV 18 (98%). However, geometric mean titers of serum neutralizing antibodies ranged from 2.3-fold to 4.8-fold higher for HPV 16 and 6.8-fold to 9.1-fold higher for HPV 18 after vaccination with Cervarix compared with Gardasil, across all age groups. At month 24, 100% of the women who received the HPV 16/18 vaccine and 97.5% to 100% of those who received the HPV 6/11/16/18 vaccine remained seropositive for HPV 16; 99% to 100% of the women who received the bivalent vaccine and 72.3% to 84.4% of the women who received the quadrivalent vaccine remained seropositive for HPV 18.58 Higher antibody levels among the HPV 16/18 group compared with the HPV 6/11/16/18 group were similarly noted at month 24. In accordance with other immunogenicity studies, the geometric mean titer ratios of anti-HPV 16 and 18 antibodies between the 2 vaccine groups were greater in the younger age group (18–26 years and 27–36 years) compared with the older age group (35–45 years) from month 7 to month 24.
Other immunobridging studies have been implemented to generate data that can support the use and efficacy of the vaccine outside the recommended guidelines. Romanowski and colleagues59 conducted a partially blinded randomized controlled trial evaluating a 2-dose vaccine schedule versus a 3-dose schedule of the HPV 16/18 vaccine in healthy young females aged 9 to 25 years. The study participants were stratified into age groups (9–14 years, 15–19 years, and 20–25 years) and randomized to receive 2 or 3 doses of 20 µg of HPV 16 and 18 L1 VLP or 2 higher doses of 40 µg of HPV 16 and 18 L1 VLP. Results showed that the 2-dose schedule of HPV 16/18 in the age group 9 to 14 years was noninferior to the 3-dose schedule in women aged 15 to 25 years at 1 month after the last dose and this was maintained at month 24 after completion of the vaccine series. The safety profiles were similar in all 3 groups and this study supports that a 2-dose schedule may infer adequate immunogenic response in younger females.
In a subanalysis of the Costa Rica Vaccine Trial, the investigators also aimed to analyze the efficacy of less than 3 vaccine doses of the bivalent vaccine.60 Excluding women who had no follow-up or who were HPV 16 or 18 DNA positive at enrollment, they compared 5967 women who received all 3 vaccines, 802 women who received 2 doses, and 384 women who only received 1 dose. The efficacy of the vaccine against incident HPV 16 or 18 infection that persisted at least 1 year was 80.9% for those who received 3 doses, 84.1% for those who received 2 doses, and 100% for those who received 1 dose only. This subanalysis, although obtained from a non-randomized sample, contributes further evidence that less than 3 doses of the vaccines may confer adequate immunoprotection.
Currently, ongoing studies are also investigating the safety and immunogenic effect of the HPV vaccine in individuals infected with HIV. Women infected with HIV have been shown to have a higher prevalence of HPV coinfection and HPV-related diseases.61 Linkage studies also demonstrate a 2-fold to 22-fold increase in cervical cancer in HIV-positive women compared with the general population.62 In addition to safety and efficacy studies, investigators are also looking to determine if there is any difference in antibody titers between HIV-negative and HIV-infected individuals and to determine the difference in antibody titers among HIV-infected individuals on antiretroviral therapy versus those who are not.63 Preliminary results of a phase II trial of the bivalent vaccine performed in South Africa showed that both HIV-negative and HIV-infected women seroconverted to HPV 16 and 18 regardless of baseline HPV status.51 However, women with HIV had significantly lower geometric mean titers compared with their HIV-negative counterparts. There are many other studies looking at adolescents, young women, and men infected with HIV. Several of these studies are being conducted in countries where both HIV and HPV pose a significant public health problem.52,64 Administering the HPV vaccine to this population, who are at a higher risk for HPV-related morbidity, provides reasonable rationale for disease prevention and infection control.
HPV VACCINE UPTAKE
Given the positive results from multiple efficacy studies on the 2 currently available HPV vaccines, widespread uptake and administration before sexual debut can potentially reduce the public health burdens brought about by HPV-related infections. However, there are impediments to widespread vaccine acceptability and barriers in achieving high vaccination rates among the preferred target age group. In adolescents, disparities in HPV vaccination use have been demonstrated by age, race, insurance, and poverty level.65 Several studies among women, adolescents, and parents have documented concerns regarding vaccine safety, efficacy, and cost.56,66,67 Among parents, there is concern regarding the potential influence of early vaccination on adolescent sexual behavior.68–70
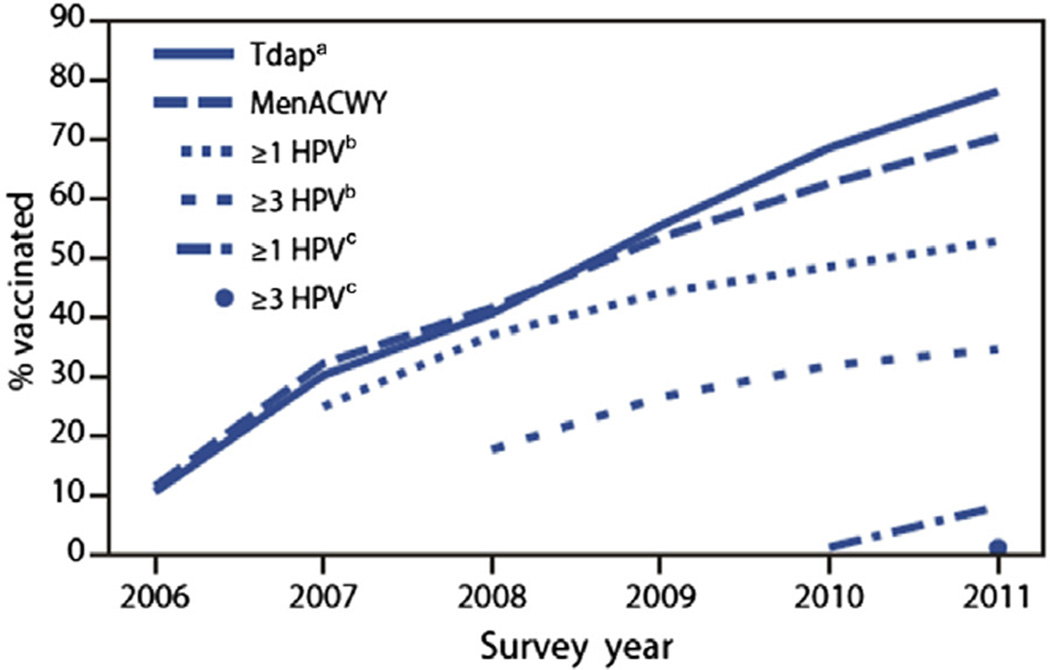
The CDC tracks vaccination coverage among adolescents aged 13 to 17 years through the National Immunization Survey–Teen (NIS-Teen).65 An analysis of the NIS-Teen data for 2010 was performed to provide updated vaccination coverage estimates (Fig. 4). The 2010 data demonstrated that (among females in this age group) those receiving 1 or more dose of HPV increased from 44.3% to 48.7%, and 26.7% to 32.0% received 3 more doses of HPV compared with 2009 estimates. Among females who initiated the HPV series, 94.3% met the minimum period needed to complete the series and 69.6% received 3 or more doses. Among adolescent males, 1.4% (confidence interval 1.1–1.8) received 1 or more doses of HPV.
Figure 4.
National immunization survey-Teen, United States, 2006–2011. Estimated vaccination coverage with selected vaccines and doses among adolescents aged 13 to 17 years, by survey year. a On or after age 10 years. b Among females. c Among males. Adolescent vaccination coverage increased from 2006 to 2011, although the rate of increase differed by vaccine. MenACWY, meningococcal conjugate vaccine; Tdap, tetanus toxoid, diphtheria toxoid, acellular pertussis vaccine. (Data from Morbidity and Mortality Weekly Report (MMWR). National and state vaccination coverage among adolescents aged 13–17 years — United States, 2011. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6134a3.htm?s_cid5mm6134a3_e%0d%0a#fig. Accessed February 28, 2013.)
HPV vaccine initiation was observed to be lower among the white population than among Hispanic and American Indian/Alaskan Native populations. However, the racial disparities in vaccine completion showed that completion of the 3 doses was lower among the black and Hispanic populations than among the white population. There was no difference observed by poverty status for initiation of the vaccine. However, completion of the series among adolescents living below the poverty line was lower compared with those living at or above the poverty line. The price for the complete 3-dose series of Gardasil is about $399 and $386 for the Cervarix vaccine.71 Most insurance covers the cost of the ACIP recommended vaccines, and the federally funded Vaccine for Children program has also helped to alleviate the financial barrier to children who may not otherwise have access because of lack of insurance coverage. Having insurance coverage has been associated with higher rates of vaccination (both initiation and completion) among adolescent females. Completion of the vaccine series has also varied by type of insurance coverage; higher rates of completion were observed within the private insurance sector.72
There is also a paucity of information on the use of the HPV vaccine among young adult women.64 Women aged 19 to 26 years of age, perhaps, have the highest rates of HPV infection and HPV-related diseases.73,74 Seventy-four percent of the new HPV infections annually occur among women aged 15 to 24 years.75 However, reports by the CDC 2 to 5 months after HPV vaccination approval showed that only 10% of women aged 18 to 26 years had initiated the vaccine series.76 Another study by Dempsey and colleagues64 showed that the rates of HPV vaccination between 2007 and 2009 among women aged 19 to 26 years continued to be low with only 18% initiating the series and only 10% completing all 3 vaccine doses within a period of 30 months. National Health Interview Survey (NHIS) data in 2010 showed that 20.7% of women aged 19 to 26 years received at least 1 dose of HPV vaccine, which was a significant increase from 17.1% in 2009 and 10.5% in 2008. Less than 1% of men aged 19 to 26 years received at least 1 dose of the vaccine.77
Disparities in vaccine series initiation by race and insurance type were also found to worsen over time. African Americans in this study, as in other studies of younger age groups,78 were shown to have decreased rates of completing the vaccine series. Minority women are at increased risk of HPV-related disease and mortality. The persistently low rates of vaccination initiation and completion continue to be a barrier in eradicating HPV-related disease and may further inflate the racial disparities in rates of cervical cancer and other HPV-related diseases.
HPV VACCINE UPTAKE WORLDWIDE
By 2008, more than 100 countries worldwide had approved the use of either one or both vaccines, and developed countries such as the United States, New Zealand, Australia, and Canada were first to initiate national vaccination programs.34,79 In addition, most of the 27 European Union (EU) member states have incorporated HPV vaccination into their national immunization programs, and several countries such as Italy, Luxembourg, Norway, and the United Kingdom have started offering the vaccine free of charge to young adolescent girls. Other countries have provided reimbursement for vaccine administration to young adolescent girls.
In low-income and middle-income countries, uptake of HPV vaccine into national vaccination programs has not been implemented as easily because of financial constraints, lack of infrastructure, and competing health priorities.80 However, in 2008, the Global Alliance for Vaccines and Immunization (GAVI), an organization that subsidizes vaccines for poor countries, pledged to secure funds to make the HPV vaccine available in underdeveloped countries. Recently, the GAVI Alliance announced a price of US$5 per dose for HPV vaccine, which makes the vaccine more affordable for low-resource countries that are eligible for subsidized vaccine purchase and increases the likelihood of introducing the vaccine.81 The GAVI hopes to make funds available to eligible countries interested in HPV vaccine implementation between 2010 and 2020. Panama and Mexico were the first middle-income countries to introduce HPV vaccination; Rwanda and Bhutan have also started national vaccination campaigns after receiving adequate donations to fund their programs.82 From 2006 to 2010, PATH, a global nongovernmental health organization, collaborated with the governments of India, Peru, Uganda, and Vietnam to gather evidence to support decisions on whether and how to introduce HPV vaccines.83 The objective was to determine what level of complete HPV vaccination coverage in the target population could be achieved for each country with a specific strategy for vaccine distribution. High HPV vaccination coverage was achieved where school-based delivery strategies were used. The coverage achieved through school-based programs was 82.6% in Peru and 88.9% in 2009 in Uganda. In Vietnam, there was an improvement in coverage in the second year of the program with rates increasing from 83.0% to 96.1%. A combination of school-based and health center–based delivery was used in India. The coverage achieved by the program ranged from 77.2% to 87.8%.
The positive impact of widespread HPV vaccination has been recently demonstrated in Australia. A significant decline in the incidence of genital warts as well as histologically confirmed high-grade cervical lesions has been observed since the implementation of free universal HPV vaccination in 2007.84–86 Australia’s vaccination program has resulted in complete vaccination of 70.8% of females aged 12 to 26 years. As a result, they observed a 39% reduction in the incidence of genital warts in nonvaccinated heterosexual men of the same age range, which is probable evidence of the herd immunity effect of the HPV vaccine.84
NEW VACCINES
The results of vaccine trials worldwide will provide important information that can be used in implementing national policies on vaccine administration and perhaps extend coverage to include secondary populations such as older women and individuals infected with HIV. However, the 2 currently available vaccines have a limited efficacy on other high-risk HPV types and the cost of the vaccine limits wide-spread access, especially in low-income countries. In addition, storage requires refrigeration, and administration requires multiple doses and intramuscular injection. Second-generation vaccines are currently being developed to address these shortcomings. The objectives in developing second-generation vaccines will be to (1) broaden coverage to include protection against other genital oncogenic HPV types; (2) induce long-term protection; (3) make the vaccines more affordable and easily stored; (4) allow vaccines to be administered easily via noninjectable methods; and (5) potentially provide therapeutic efficacy. Multiple companies are investigating new vaccines targeting various immunogens in the HPV virus. The nonavalent vaccine being investigated by Merck is the closest to approval for use (Table 3).87
Table 3.
HPV vaccines in development
| Vaccine Type | Immunogen | Target | Sponsor |
|---|---|---|---|
| Prophylactic Vaccines | |||
| Octavalent vaccine | L1 virus like particle of HPV types 6, 11, 16,18, 31, 45, 52, 58 | Cervical, Anal, Vaginal, Vulvar dysplasia and cancer | Merck |
| V504 (administered with Gardasil) | L1 virus like particles of HPV types 31, 33, 45, 52, 58 | Cervical, Anal, Vaginal, Vulvar dysplasia and cancer | Merck |
| MEDI-517 | Virus like particles of HPV types 16, 18 | Cervical dysplasia and cancer | Glaxo Smith Kline |
| 9-valent vaccine (V503) | L1 virus like particles of HPV types 6, 11, 16, 18, 31, 33, 45, 52, 58 | Cervical, Anal, Vaginal, Vulvar dysplasia and cancer Genital Warts | Merck |
| VGX-3100 | Plasmids targeting E6 and E7 proteins of HPV types 16, 18 | Cervical dysplasia and cancer | Inovio Pharmaceuticals |
| Therapeutic Vaccines | |||
| pNGVL-4a-CRT/E7 (detox) DNA vaccine | E7 DNA of HPV16 | Cervical dysplasia Head and neck cancers | Sidney Kimmel Comprehensive Cancer Center |
| HPV-16 vaccine | 4 HPV 16 E6 proteins | Cervical dysplasia | University of Arkansas |
| TA-HPV | E6 and E7 proteins of HPV 16/18 | Cervical cancer | European Organization for Research and Treatment of Cancer |
| VGX-3100 | Plasmids targeting E6 and E7 proteins of HPV 16/18 | Cervical dysplasia and cancer | Inovio Pharmaceuticals |
| HPV 16 E6 and E7 vaccine | E6 and E7 proteins HPV 16 | Cervical cancer, anal cancer, esophageal cancer, head and neck cancer, penile cancer, vulvar cancer | National Cancer Institute |
| MAGE-A3-HPV 16 | Trojan peptide complexes containing MAGE-A3 and HPV 16 | Head and neck cancer | University of Maryland |
| ADXS11-001 | E7 protein of HPV 16 | Cervical dysplasia and cancer, anal cancer, oropharyngeal cancer | Advaxis |
| Hsp-E7 | Recombinant DNA fusion of heat shock protein 65 and E7 protein of HPV type 16 | Cervical dysplasia | National Cancer Institute, Dana-Farber/Brigham and Women’s Cancer Center |
| Alternating E7 vaccine and dendritic cells presenting E7 | E7 protein of HPV 16 vaccine and dendritic cells presenting E7 protein | Recurrent or persistent cervical cancer | Steward St. Elizabeth’s Medical Center of Boston, National Cancer Institute |
| Gp100: 209–217 and HPV 16 E7: 12–20 peptide | Amino acids 209–217 of glycoprotein 100 melanoma antigen and amino acids 12–20 of E7 protein of HPV 16 | Melanoma | Providence Cancer Center, Earle A. Chiles Research Institute, National Cancer Institute |
Data from Search term “HPV vaccine.” Available at: www.clinicaltrials.gov. Accessed February 28, 2013.
L2-Based Vaccine
The amino terminus of the L2 capsid protein of HPV contains a major cross-neutralizing epitope. This has been the basis of investigations into L2-based vaccines.88 Research done using mouse monoclonal antibodies shows that there are at least 2 cross-neutralization episodes in the amino acid 56 to 75 region that show neutralization against oncogenic HPV strains.89 Studies on mice and rabbits show that immunization with VLPs expressing the L2 protein of HPV 16 and 31 induced robust antibody titers against various HPV types.90 Mouse studies show that oral administration of a vaccine targeted at the L2 capsid protein induced significant neutralizing activities against genital infection by HPV 16/18/45/58.91
Nonavalent Vaccine
Studies have shown that both HPV vaccines have moderate cross-protection against other high-risk HPV types.27,28 In addition to HPV 16 and 18, other high-risk subtypes that have been associated with 90% of cervical cancer cases worldwide include types 31, 33, 45, 52, and 58.92 Merck has been studying the efficacy of a nonavalent vaccine containing VLPs of 9 high-risk HPV types (6, 11, 16, 18, 31, 33, 45, 52, and 58).19 They are currently in phase III clinical trials and they anticipate presenting their results to the FDA shortly.87 The nonavalent vaccine has potential to significantly reduce cervical precancerous and cancerous lesions.93
Therapeutic Vaccines
The development of a vaccine with both a therapeutic and prophylactic component should prove to be beneficial for individuals who have already been exposed to HPV. Therapeutic vaccines can prevent reinfection and control reactivation of already acquired HPV types. The goal for therapeutic vaccines is to eliminate preexisting lesions and even malignant tumors by generating cell-mediated immunity against HPV-infected cells.94 Current approaches include live vector–based, peptide-based, protein-based, nucleic acid-based, and cell-based vaccines. The antigens targeted in therapeutic vaccines include the HPV early proteins that are expressed throughout the life cycle of the infected cells and cancer cells. In particular, the HPV-encoded proteins E6 and E7 represent ideal targets for the development of these vaccines. Investigators have primarily focused on stimulating the production and activation of T cells by targeting E6 and/or E7 proteins.
SUMMARY
HPV is one of the most common sexually transmitted infections affecting both men and women worldwide. The development of prophylactic HPV vaccines is a significant pharmaceutical innovation with the potential to reduce HPV-related morbidity as shown by the positive results of several efficacy trials. However, barriers to the universal use and acceptability of the HPV vaccines continue to exist in both economically privileged and disadvantaged countries. It may be decades before the impact of preventive vaccines on HPV-related diseases will be seen because of the considerable burden of HPV infections. However, collaborative efforts should continue to promote vaccine administration and extension of coverage to other vulnerable populations that may benefit from the vaccines’ prophylactic properties.
KEY POINTS.
Human papillomavirus (HPV) infection is the underlying cause of cervical, vulvar, vaginal, anal and orapharyngeal cancers.
Multiple randomized prospective clinical trials have demonstrated that HPV vaccination prevents HPV-related diseases such as cervical and anal cancer.
Clinical guidelines concerning HPV vaccination have evolved since its introduction in 2006. Current guidelines recommend the vaccination of adolescent and adult males and females aged 9 to 26 years.
New HPV vaccines are currently being developed to protect against more HPV types. Investigators are also developing therapeutic vaccines that can potentially treat HPV-related diseases.
Acknowledgments
Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 2.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 3.Bernard HU, Burk RD, Chen Z, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, de Sanjose S. Chapter 1: human papillomavirus and cervical cancer– burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;(31):3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 6.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) [Accessed May 25, 2011];MMWR Recomm Rep. 2007 Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. [PubMed]
- 7.Jemal A, Simard EP, Dorel C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers ER, McCrory DC, Nanda K, et al. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151(12):1158–1171. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- 9.Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92(6):464–474. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 10.Castellsague X, de Sanjose S, Aguado T, et al. HPV and cervical cancer in the world [Report] Geneva (Switzerland), Barcelona (Spain): WHO, ICO; 2007. [Accessed February 7, 2013]. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Available at: http://www.who.int/hpvcentre/en/. [Google Scholar]
- 11.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 12.Papanicolaou GN. A new procedure for staining vaginal smears. Science. 1942;95(2469):438–439. doi: 10.1126/science.95.2469.438. [DOI] [PubMed] [Google Scholar]
- 13.Sahasrabuddhe V, Sherman ME. Human papillomavirus vaccines for cervical cancer prevention: translating possibility into reality. J Natl Cancer Inst. 2012;104(22):1698–1701. doi: 10.1093/jnci/djs453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman M, Wentzensen N, Wacholder S, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 16.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz N, Castellsagué X, de González AB, et al. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):S3/1–S3/10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 18.Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353(20):2101–2104. doi: 10.1056/NEJMp058171. [DOI] [PubMed] [Google Scholar]
- 19.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-likeparticle vaccine trials. J Infect Dis. 2009;200:166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:RR2. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) FDA licensure of bivalent human papillomavirus vaccine (HPV2 Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(20):626–629. [PubMed] [Google Scholar]
- 22.GlaxoSmithKline. CDC Advisory Committee recommends Cervarix to prevent cervical cancer in girls and young women. [Accessed November 10, 2009]; Available at: http://us.gsk.com/html/media-news/pressreleases/2009/2009_us_pressrelease_10075.htm. [Google Scholar]
- 23.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198:500–507. doi: 10.1016/j.ajog.2008.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer. 2008;113(Suppl 10):2892–2900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraiya M. Presentation before the Advisory Committee on Immunization Practices (ACIP), February 24, 2011. Atlanta (GA): US Department of Health and Human Services, CDC; 2011. [Accessed March 2, 2013]. Burden of HPV-associated cancers in the United States. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb11/11-2-hpv-rela-cancer.pdf. [Google Scholar]
- 27.Food and Drug Administration Center for Biologics Evaluation and Research. Briefing document for the Vaccines and Related Biological Products Advisory Committee. [Accessed September 24, 2009];Subject: Male indication for Gardasil. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM181361.pdf.
- 28.FDA. FDA approves new indication for Gardasil to prevent genital warts in men and boys. [Accessed October 20, 2009];2009 10/16/2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187003.htm.
- 29.Maggard MA, Beanes SR, Ko CY. Anal canal cancer: a population-based reappraisal. Dis Colon Rectum. 2003;46(11):1517–1523. doi: 10.1097/01.DCR.0000093722.63657.B4. [DOI] [PubMed] [Google Scholar]
- 30.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2008. Bethesda (MD): National Cancer Institute; [Accessed December 7, 2012]. [based on November 2010 SEER data submission, posted to the SEER web site, 2011]. Available at: http://seer.cancer.gov/csr/1975_2008/. [Google Scholar]
- 31.Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Human papilloma virus (HPV) associated cancers. [Accessed March 1, 2013]; Available at: http://www.cdc.gov/cancer/hpv/statistics/anal.htm.
- 33.Food and Drug Administration. Highlights of prescribing information. Silver Spring (MD): Food and Drug Administration; 2011. [Accessed December 13, 2012]. Gardasil (human papillomavirus quadrivalent [types 6, 11, 16 and 18]) Available at: http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm111263.pdf. [Google Scholar]
- 34.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) Recommendations for the quadrivalent human papillomavirus vaccine in males – Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 36.Kreimer AR, González P, Katki HA, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011;12(9):862–870. doi: 10.1016/S1470-2045(11)70213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 38.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against the human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 39.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 40.Kjaer SK, Sigurdsson K, Iversen OE, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009;2(10):868–878. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 41.Munoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 42.Paavonen J, Jenkins D, et al. HPV PATRICIA Study Group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 43.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 44.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30S:F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wheeler CM, Castellsagué X, et al. HPV PATRICIA Study Group. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-ofstudy analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 46.Malagón T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 47.Stanley M, Gissman L, Nardelli-Haeflinger D. Immunobiology of human papillomavirus infection and vaccination–implications for second generation vaccines. Vaccine. 2008;26(Suppl 10):K62–K67. doi: 10.1016/j.vaccine.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 48.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 49.Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine. 2006;24(27–28):5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 50.Schiller JT, Castellsague X, Villa L, et al. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trials. Vaccine. 2008;26(Suppl 10):K53–K61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denny L, Hendricks B, Gordon C, et al. Safety and immunogenicity of the HPV-16/18 ASO4-adjuvanted vaccine in HIV positive women in South Africa. Presented at AORTIC 7th International Conference; 2011. Abstract no. 599. [DOI] [PubMed] [Google Scholar]
- 52. [Accessed January 26, 2013]; Available at: http://www.clinicaltrials.gov/ct2/results?term=HPV+and+HIV&pg=1.
- 53.Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(3):201–209. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 54.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen C, Petaja T, Strauss G, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007;40(6):564–571. doi: 10.1016/j.jadohealth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Boehner CW, Howe SR, Bernstein DI, et al. Viral sexually transmitted disease vaccine acceptability among college students. Sex Transm Dis. 2003;30(10):774–778. doi: 10.1097/01.OLQ.0000078823.05041.9E. [DOI] [PubMed] [Google Scholar]
- 57.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5(10):705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 58.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years – Follow up from months 12–24 in a phase III randomized study of healthy women aged 18–45 years. Hum Vaccin. 2011;7(12):1343–1358. doi: 10.4161/hv.7.12.18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romanowski B, Schwarz TF, Ferguson LM, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin. 2011;7(12):1374–1386. doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103(19):1444–1451. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Vuyst H, Lillo F, Broutet N, et al. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008;17:545–554. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 62.Harris TG, Burk RB, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293:1471–1476. doi: 10.1001/jama.293.12.1471. [DOI] [PubMed] [Google Scholar]
- 63.Denny LA, Franceschi S, de Sanjosé S, et al. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30(Suppl 5):F168–F174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 64.Dempsey A, Cohn L, Dalton V, et al. Worsening disparities in HPV vaccine utilization among 19–26 year old women. Vaccine. 2009;29:528–534. doi: 10.1016/j.vaccine.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.CDC. National, state, and local area vaccination coverage among adolescents aged 13–17 years–-United States, 2009. [Accessed February 23, 2013];MMWR Recomm Rep. 2010 59:1018–1023. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6033a1.htm#Tab1. [PubMed] [Google Scholar]
- 66.Dempsey AF, Zimet GD, Davis RL, et al. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117(5):1486–1493. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]
- 67.Wong LP. Young multiethnic women’s attitudes toward the HPV vaccine and HPV vaccination. Int J Gynaecol Obstet. 2008;103(2):131–135. doi: 10.1016/j.ijgo.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Davis K, Dickman ED, Ferris D, et al. Human papillomavirus vaccine acceptability among parents of 10- to 15-year-old adolescents. J Low Genit Tract Dis. 2004;8(3):188–194. doi: 10.1097/00128360-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Zimet GD, Perkins SM, Sturm LA, et al. Predictors of STI vaccine acceptability among parents and their adolescent children. J Adolesc Health. 2005;37(3):179–186. doi: 10.1016/j.jadohealth.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Kahn JA, Ding L, Huang B, et al. Mothers’ intention for their daughters and themselves to receive the human papillomavirus vaccine: a national study of nurses. Pediatrics. 2009;123(6):1439–1445. doi: 10.1542/peds.2008-1536. [DOI] [PubMed] [Google Scholar]
- 71.Stobbe M. Panel recommends a 2nd cervical cancer vaccine as alternative for girls and young women. New York: New York Times; 2009. [Google Scholar]
- 72.Etter DJ, Zimet GD, Vaughn RI. Human papillomavirus vaccine in adolescent women: a 2012 update. Curr Opin Obstet Gynecol. 2012;24:305–310. doi: 10.1097/GCO.0b013e3283567005. [DOI] [PubMed] [Google Scholar]
- 73.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 74.Ault KA. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect Dis Obstet Gynecol. 2006;2006:40470. doi: 10.1155/IDOG/2006/40470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang CM. Human papillomavirus and vaccination. Mayo Clin Proc. 2008;83(6):701–707. doi: 10.4065/83.6.701. [DOI] [PubMed] [Google Scholar]
- 76.Jain N, Euler GL, Shefer A, et al. Human papillomavirus (HPV) awareness and vaccination initiation among women in the United States, national immunization survey-adult 2007. Prev Med. 2009;48(5):426–431. doi: 10.1016/j.ypmed.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 77.Williams WW, Lu PJ, Singleton JA, et al. Adult vaccination coverage — United States, 2010. MMWR Recomm Rep. 2012;61(4):66–70. [PubMed] [Google Scholar]
- 78.Dempsey A, Cohn L, Dalton VA, et al. Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a university based health system. Vaccine. 2010;28(4):989–995. doi: 10.1016/j.vaccine.2009.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barr E, Sings HL. Prophylactic HPV vaccines: new interventions for cancer control. Vaccine. 2008;26:6244–6257. doi: 10.1016/j.vaccine.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 80.Graham J, Mishra A. Global challenges of implementing human papillomavirus vaccines. Int J Equity Health. 2011;10:27. doi: 10.1186/1475-9276-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.GAVI welcomes lower prices for life-saving vaccines. Geneva (Switzerland): GAVI Alliance; 2011. [Accessed August 8, 2011]. Available at: http://www.gavialliance.org/library/news/press-releases/2011/gavi-welcomes-lower-prices-for-life-saving-vaccines/. [Google Scholar]
- 82.Poljak M. Prophylactic human papillomavirus vaccination and primary prevention of cervical cancer: issues and challenges. Clin Microbiol Infect. 2012;18(Suppl 5):64–69. doi: 10.1111/j.1469-0691.2012.03946.x. [DOI] [PubMed] [Google Scholar]
- 83.LaMontagne DS, Barge S, Thi Le N, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011;89:821–830. doi: 10.2471/BLT.11.089862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011;11:39–44. doi: 10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 85.Read TR, Hocking JS, Chen MY, et al. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2011;87:544–547. doi: 10.1136/sextrans-2011-050234. [DOI] [PubMed] [Google Scholar]
- 86.Brotherton JM, Fridman M, May C, et al. Early impact of the HPV vaccination program on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–2092. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 87.Peres J. For cancers caused by HPV, two vaccines were just the beginning. J Natl Cancer Inst. 2011;103(5):360–362. doi: 10.1093/jnci/djr053. [DOI] [PubMed] [Google Scholar]
- 88.Seitz H, Schmitt M, Böhmer G, et al. Natural variants in the major neutralizing epitope of human papillomavirus minor capsid protein L2. Int J Cancer. 2013;1323:E139–E148. doi: 10.1002/ijc.27831. [DOI] [PubMed] [Google Scholar]
- 89.Nakao S, Mori S, Kondo K, et al. Monoclonal antibodies recognizing crossneutralization epitopes in human papillomavirus 16 minor capsid protein L2. Virology. 2012;434(1):110–117. doi: 10.1016/j.virol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Nieto K, Weghofer M, Sehr P, et al. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One. 2012;7(6):e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoon SW, Lee TY, Kim SJ, et al. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine. 2012;30(22):3286–3294. doi: 10.1016/j.vaccine.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Franceschi S, Cuzick J, Herrero R, et al. EUROGIN 2008 roadmap on cervical cancer prevention. Int J Cancer. 2009;125(10):2246–2255. doi: 10.1002/ijc.24634. [DOI] [PubMed] [Google Scholar]
- 93.Van de Velde N, Boily MC, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a modelbased analysis. J Natl Cancer Inst. 2012;104(22):1712–1723. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 94.Hung CF, Ma B, Monie A, et al. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 2008;8(4):421–439. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joura EA, Garland SM, Paavonen J, et al. FUTURE I and II Study Group. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi: 10.1136/bmj.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.FUTURE I/II Study Group. Dillner J, Kjaer SK, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ault KA Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 98.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199(7):926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 99.Paavonen J, Naud P, et al. HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]