Abstract
Development of chilling and freezing tolerance is complex and can be affected by photoperiod, temperature and photosynthetic performance; however, there has been limited research on the interaction of these three factors. We evaluated 108 recombinant inbred lines of Boechera stricta, derived from a cross between lines originating from Idaho and Colorado, under controlled Long-Day (LD), Short-Day (SD) and in an Outdoor Environment (OE). We measured maximum quantum yield of photosystem II, lethal temperature for 50% survival and electrolyte leakage of leaves. Our results revealed significant variation for chilling and freezing tolerance and photosynthetic performance in different environments. Using both single and multi-trait analyses, three main-effect Quantitative Trait Loci (QTL) were identified. QTL on LG3 were SD-specific, whereas QTL on LG4 were found under both LD and SD. Under all conditions, QTL on LG7 were identified, but were particularly predictive for the Outdoor Experiment. The co-localization of photosynthetic performance and freezing tolerance effects supports these traits being co-regulated. Finally, the major QTL on LG7 is syntenic to the Arabidopsis CBF locus, known regulators of chilling and freezing responses in A. thaliana and other species.
Keywords: cold-tolerance, photoperiod, natural variation, Boechera, Brassicaceae
INTRODUCTION
Genetic variation for freezing tolerance is important for understanding both adaptation of species to natural environments and for improving crop performance in stressful conditions. Research over the last decades has demonstrated that complex physiological and biochemical changes occur in a wide range of plant species during cold acclimation, including both freezing-tolerant and freezing-sensitive plants (Leinonen, 1996; Hannah et al. 2006; Carvallo et al. 2011). The level of tolerance to freezing in plants is influenced by membrane and cell composition, the accumulation of carbohydrates and adjustments to the photosynthetic apparatus (Sandve et al. 2011). Some low-temperature differentially-regulated genes can be associated with these important changes and can be used for understanding the mechanisms of chilling and freezing tolerance (Thomashow, 2010).
QTL (Quantitative Trait Locus) studied in Arabidopsis thaiana and temperate Triticeae species of the grass family have shown QTL regions containing CBF (C-repeat binding factor) genes are associated with their freezing tolerance. Previously, it has been established that three members of the CBF family, CBF1, CBF2 and CBF3, play a key role in the regulation of the transcriptome during cold acclimation (Maruyama et al. 2004; Xu et al. 2011). These CBF genes have been isolated from several herbaceous and woody plant species and different studies have demonstrated their significant role for development of freezing tolerance (Zhang et al. 2004; Skinner et al. 2005; Gamboa et al. 2007; Welling and Palva, 2008; He et al. 2012). CBFs have therefore been used to develop strategies to enhance freezing tolerance in cultivated crops and to understand adaptation to cold environments in native species. However, variation in CBF loci does not explain all the quantitative natural variation for freezing tolerance (Gery et al. 2011; Meissner et al. 2013). In addition, the ecological context of Arabidopsis, a model species, is often unclear due to their growth in highly disturbed environments.
The genus Boechera is closely related to Arabidopsis and is being developed as an additional model system to understand plant adaptation. Boechera stricta is a genetically tractable, short-lived, overwintering, perennial species. It grows mostly in undisturbed habitats of Western North America, with habitats widely varying in abiotic and biotic conditions. For example, populations can be found across a 2000 m elevation gradient, and this is expected to have an effect on the genetic variation of genes controlling ecologically important traits (Schranz et al. 2009). Previously, a number of genomic resources and a genetic map have been developed for identifying ecologically relevant QTL in B. stricta, allowing extensive comparative analyses with Arabidopsis (Schranz et al. 2007a; Anderson et al. 2011; Anderson et al. 2014). Here, we utilized these resources to study freezing tolerance in B. stricta grown under both controlled and outdoor conditions. In addition, photosynthetic performance can be also associated with freezing tolerance because photosynthesis may be a critical factor for freezing or frost tolerance. However, there has been little research conducted on the genetic regulation of freezing tolerance and photosynthetic performance. Hence, we also analyzed photosynthetic performance by measuring the maximum quantum yield of photosystem II.
In this study, we found significant variation on freezing tolerance and photosynthetic performance to freezing stress conditions in B. stricta and also observe that photosynthetic performance may be genetically associated with freezing tolerance. Within the QTL analysis, we have identified three major QTLs, which will be useful for clarifying underlying ecologically important questions on freezing tolerance.
MATERIALS AND METHODS
Plant materials
For all experiments we used 108 selected RIL lines and parental genotypes from a population derived from a cross between two highly inbred lines of B. stricta (Graham) Al-Shehbaz. The maternal line ‘SAD12’ was collected from Colorado (elevation: 2530m) and the paternal line ‘LTM’ was collected in Montana (elevation: 2390m). The parental sites in Montana and Colorado differ in rainfall, temperature, day length and ecological community (Schranz et al. 2007b). The F7 RILs have previously been genotyped and used in mapping and QTL experiments (Anderson et al. 2011).
Plant growth
Seeds were germinated in petri dishes, and then transferred onto pots with the soil mixture of No.1 and No.3 soil (Jongkind Ground BV) in a 1:2 proportion. Plants were established for three to four weeks, and then used to perform three different freezing stress experiments: two controlled climate chamber experiments differing in photoperiod, Long Day (LD) and Short Day (SD), and an Outdoor Environment (OE). Day-lengths in outdoor environment were slightly shorter in Amsterdam than the native field site in Montana
Controlled LD and SD freezing stress experiments
RIL and parental lines were grown at 20°C in growth chambers under LD and SD photoperiod regimes. The LD photoperiod was 14 hours light/10 hours darkness, while the SD photoperiod was 10 hours light/14 hours darkness. Details of Experimental Methods are given below, but are alluded to here in relation to plant growth. One-month-old plants were acclimated to cold by growing at 6°C for 3 weeks. A freezing treatment was done in darkness at −8°C for 24 hours. Ice crysatilization was induced using ice chips. Plants and the soil were totally frozen. Plants were then returned to 6°C. Relative freezing tolerance in RILs and their parental lines was measured before and after 3 weeks of cold acclimation, and the electrolyte leakage was screened immediately after a freezing treatment of 24 hours, and 1 day after they were returned to 6°C to investigate their actual responses to selected freezing temperature. Maximum quantum yield of Photosystem II (Fv/Fm) was measured before and after cold acclimation, and 1 day after freezing treatment, and 1 day after they were returned to 6°C to observe the changes in the photosynthetic performance. To minimum a gradient in damage by harvesting samples, we conducted these experiments dividing into 6 sets, which consisted with 16~20 RILs per group. In addition, plants were randomly placed for the experiment.
Outdoor environment (OE) experiment
The freezing stress experiment in Outdoor Environment (OE) was conducted in the winter and early spring of 2011–2012. Responses during and post freezing, and frost damage were evaluated in the same 108 RILs. Plants were germinated and transplanted in mid-October, 2011. Three weeks after transplanting, three individuals/line from 108 RILs plus 3 individuals of each parental line (n= 330 individuals total) were randomized in a research plot area at the University of Amsterdam, The Netherlands and were maintained until the end of experiment. Figure 1 shows the minimum and maximum temperatures during the critical experimental period (January and February). It was a relatively warm early winter. The first sub-zero freezing event occurred on 16th January 2012 with a minimum temperature of −4°C. In February there was a much colder and prolonged period of freezing. The minimum air temperature was −17°C on 4th February 2012, the lowest temperature of the winter. Temperatures remained sub-zero until 13th February 2012. Responses of selected RILs and parental lines to outdoor freezing conditions were screened on both 17th January and 18th February 2012 by measuring Fv/Fm. Plants were frozen during the first and second cold spells, and were not covered with snow. Temperature was measured in the field with use of HOBO® data loggers for outdoor use. The logger was placed at plant height. Overall frost damage from the winter was scored in the middle of March 2012.
Figure 1.
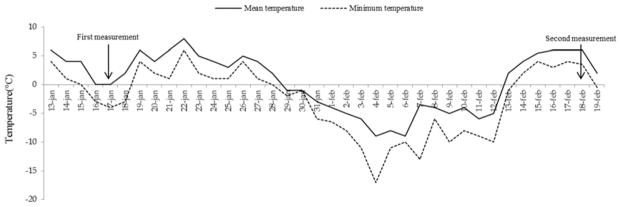
Maximum and Minimum daily temperatures for Outdoor Environment (OE) experienced by Bochera stricta Recombinant Inbred Lines (RILs) during January and February of 2012 in Amsterdam, The Netherlands. Responses to freezing-stress were assessed during a mild freezing event (January 18) and after a prolonged severe-freezing event (February 18). Three-week old plants were initially established outdoors in November 2011.
Trait measurements
Measurement of relative freezing tolerance before and after cold acclimation
Relative plant freezing tolerance was assayed as described previously (Murray et al. 1989), with some modifications. Three uniform leaf disks per plant per line were cut (diameter size of 0.5cm) from non-acclimated or cold-acclimated plants with the use of a leaf disc borer and placed into test tubes containing 100μl de-ionized water. Test tubes were subsequently placed in a completely randomized design in a −1°C Cooling Thermostats containing anti-freeze (Lauda Ecoline RE 312, Lauda Germany) for 1h after which ice crystals were added to nucleate freezing. After an additional 2h of equilibration at −1°C the samples were gradually cooled in increments of −2°C/h. A replicate sample from each genotype was removed every 2 h starting at −3°C until the last samples were removed at −13°C. Once removed the samples were stored on ice until all samples had been collected and left to thaw overnight in the cold room at 4°C. After thawing, all samples, including the unfrozen controls kept at 4°C (ELunfrozen), were incubated in 1ml de-ionized water with gentle shaking (125 motions/min) at room temperature for 2h. Electrolyte leakage from the leaves was measured using a conductivity meter (B-173 compact conductivity meter, Horiba Scientific). The samples were then placed for 1h in a −80°C freezer, thawed for 30min in a 57°C stove, and shaken gently for an additional 2h before the conductivity of the resulting solution was measured to obtain a value for 100% electrolyte leakage (EL100). The percentage of electrolyte leakage from frozen leaves was calculated according to the equation as described by Webb et al. (1994): %EL = (ELfrozen–ELunfrozen) / (EL100–ELunfrozen) * 100. Response curves were developed for each plant and each LT50 was determined based on the sigmoidal response equation by the statistic program R (R Development Core Team. 2008). Two replicate measurements for each photoperiod/temperature combination were performed, and total 5280 individuals were used for this experiment (two temperature conditions × two photoperiods × six time samplings using 6 individuals × two replicates × 108 RILs plus two parental lines ; n= 5280 individual total).
Measurement of maximum quantum yield of photosystem II (Fv/Fm)
Fv/Fm was determined with a PAM-2000 chlorophyll fluorometer system (Heinz Walz, Effeltrich, Germany) using standard instrument settings (e.g. saturating pulse of 12,000 mmol m−2 s−1 for 0.8s). Fluorescence data were recorded and computed with windows software for PAM fluorometers (2.133 version, Heinz Walz, Effeltrich, Germany). For determination of Fv/Fm, one uniform leaf disc from middle part of the sixth leaf was removed and placed into black 96-well plates containing 200μl de-ionized water. Leaves were dark adapted for 20 min prior to determination of Fv/Fm. In dark-adapted plants, F0 and also levels of fluorescence measured at a very low photosynthetic photon flux density (PPFD) of, 1 mmolm−2 s−1 and during a short light-saturating pulse (Fm) were measured and used to estimate the maximum quantum yield of PSII when fully oxidized (Fv/Fm= Fm – F0/Fm). Three replicate measurements were performed for measuring Fv/Fm, and total 2640 individuals were used for this experiment (four temperature conditions × two photoperiods × three replicates × 108 RIL plus two parental lines ; n= 2640 individual total).
Measurement of electrolyte leakage during and post freezing
Plant responses during and after freezing were measured by another type of electrolyte leakage assay (Scarpeci et al. 2008), with the protocol slightly modified. Three uniform leaf discs (Ø 0.5cm) from rosette leaves in the same plant being used for measurement of Fv/Fm were sampled with a leaf disc borer and immediately placed into 15ml Greiner tube containing 10ml de-ionized water. The discs were washed for 30min on a shaking platform to remove soil attached during cutting of the disc. Leaf discs were then placed in 12-well plates filled with 3ml of 0.01% Silwet 77 solution and maintained at 25°C for 1hr 30min on a shaking platform. Next, initial leakage was determined by measuring the electrical conductivity of the well plate solution, using a conductivity meter (B-173 compact conductivity meter, Horiba Scientific) with data expressed as mScm−1. The samples were then placed for 2 × 20 sec in microwave (600W) and shaken gently for an additional 4 h before the conductivity of the resulting solution was measured to obtain a value for electrolyte leakage of the heat treated cells. Results were expressed as percentage of total conductivity ‘initial leakage / final leakage × 100’. Measurements were carried out in triplicate.
Assessment of winter survival
Winter survival was visually scored on a scale of 0–10 using three individuals among families (0 : plant was killed, 10 : no apparent frost damage).
Quantitative trait loci analysis
Single-trait, multi-trait linkage and mixed model multi-environment QTL analyses were performed with GenStat software (15th edition, VSN International, United Kingdom). To normalize results, all QTL analysis was carried out with log10 transformed-adjusted means of each trait. For single-trait QTL analysis, the data was firstly used for the preliminary single environment analysis. Subsequently calculations of the genetic predictors were conducted with a step size of 2cM, and then an initial Genome Scan produced candidate QTL positions by Simple Interval Mapping (SIM). SIM results were then used as cofactors in a subsequent Genome Scan by Composite Interval Mapping (CIM), which was allowed to detect candidate QTLs. The final selection of significant QTL was obtained after a scan with backward elimination of putative QTL. LT50, EL, and Fv/Fm results were subjected to a multi-trait linkage QTL analysis. To arrive at a multi-QTL model, the significant markers, or putative QTL, of the single marker analyses were used as the starting input set of predictor variables. After completion of this step, genome wide QTL scans by SIM and CIM were performed and then a multi-QTL model after backward selection was fitted to estimate QTL locations and effects (Pastina et al. 2012). For Fv/Fm, mixed model multi-environment QTL analysis was additionally applied. The best suitable variance–covariance model was detected after environment exploratory analysis. Genetic predictors were computed for a step size of 2cM. The genetic model was built using the suggested candidate QTL as main or QTL × Environment (QTLxE) interactions. CIM was used for selecting the final QTL model. QTL significance levels and effects were determined by a final backward selection step at a significance level of 0.05. A significant QTL effect at particular genome positions was associated with a low P value, which was graphically shown on a –log10 scale to resemble the typical LOD profile plot (Pastina et al. 2012). The additive effects, standard errors, high value alleles, the Percentage of Explained Variances (PVE) and positions of the QTLs were estimated and used to determine what traits were affected by the specific QTL.
Statistical analysis
All further statistical tests were also performed using the GenStat software package (15th edition, VSN International, United Kingdom). To examine the correlation among traits measured across environmental conditions, correlation coefficients (Pearson’s) were calculated. A principal component analysis (PCA) of Fv/Fm values under different conditions was also performed to visualize which environmental factors affected the Fv/Fm ratio of RILs most.
RESULTS
Relative freezing tolerance (RFT) under SD and LD conditions
Boechera stricta RILs and parental lines were grown under two photoperiods (SD and LD). The temperature at which 50% plants are killed by freezing (LT50) was estimated from fitted response curves. The average values, ranges and differences among RILs are presented in Table 1. Non-acclimated RILs and parental lines showed small differences in LT50 under either photoperiod conditions. After cold acclimation with SD grown plants, the LT50 of the parental lines LTM and SAD12 were −11.3°C and −8.6°C, respectively. Under LD growth conditions, the LT50 of the LTM and SAD12 were −10.4°C and −8.1°C, respectively (delta LTM to SAD = −2.3°C). In both conditions, there was significantly difference of freezing tolerance (p<0.05). LTM shows more freezing tolerance, but also more pronounced difference in SD relative to LD (delta of LTM SD-LD = −0.9°C; delta of SAD12 SD-LD = −0.5°C). In both light regimes, most RILs had intermediate LT50 values to the parents. Similar to results with parental lines, the LD grown RILs on average were less freezing tolerant than SD grown RILs.
Table 1.
LT50, Fv/Fm and EL in the RIL population (LTM × SAD12) under control and stressed conditions
| LTM | SAD12 | Range in RIL population | Mean in RIL population | |
|---|---|---|---|---|
|
| ||||
| LT50(SD_CA) | −11.10 | −8.60 | −5.10 ~ −14.20 | −10.07 |
| LT50(SD_NA) | −6.40 | −5.70 | −4.60 ~ −7.20 | −6.21 |
| Fv/Fm(SD_NA) | 0.80 | 0.80 | 0.78 ~ 0.81 | 0.80 |
| Fv/Fm(SD_CA) | 0.77 | 0.77 | 0.75 ~ 0.79 | 0.77 |
| Fv/Fm(SD_FT) | 0.71 | 0.65 | 0.54 ~ 0.77 | 0.68 |
| EL(SD_FT) | 26.90 | 35.30 | 17.80 ~ 57.10 | 28.94 |
| Fv/Fm(SD_1DPF) | 0.71 | 0.58 | 0.21 ~ 0.77 | 0.63 |
| EL(SD_1DPF) | 24.80 | 44.60 | 16.20 ~ 74.10 | 32.69 |
| LT50(LD_CA) | −10.40 | −8.10 | −4.70 ~ −14.20 | −9.33 |
| LT50(LD_NA) | −6.30 | −5.80 | −4.40 ~ −7.10 | −6.00 |
| Fv/Fm(LD_NA) | 0.81 | 0.80 | 0.79 ~ 0.82 | 0.80 |
| Fv/Fm(LD_CA) | 0.77 | 0.76 | 0.74 ~ 0.78 | 0.76 |
| Fv/Fm(LD_FT) | 0.68 | 0.60 | 0.55 ~ 0.75 | 0.66 |
| EL(LD_FT) | 29.10 | 41.00 | 20.20 ~ 60.40 | 32.12 |
| Fv/Fm(LD_1DPF) | 0.67 | 0.42 | 0.16 ~ 0.77 | 0.57 |
| EL(LD_1DPF) | 28.80 | 52.50 | 18.20 ~ 80.10 | 39.00 |
| Fv/Fm(OE_MF) | 0.66 | 0.57 | 0.41 ~ 0.72 | 0.63 |
| Fv/Fm(OE_SF) | 0.38 | 0.37 | 0.02 ~ 0.64 | 0.38 |
| OWFD | 3.30 | 4.00 | 0.307.30 | 3.32 |
SD ; short day, LD ; long day, OE : outdoor environment, CA : cold acclimation, FT : freezing treatment, 1DF : 1 day post freezing, MF : mild freezing (Jan., 2012), SF : severe freezing (Feb., 2012)
Electrolyte leakage (EL) and maximum quantum yield of photosystem II (Fv/Fm)
We quantified EL of leaves from plants exposed to freezing temperatures. EL increased during or after freezing treatment, and genotypic responses were highly associated with LT50 values. The maximum quantum yield of photosystem II, Fv/Fm, was measured to examine its relationship with cold acclimation ability and actual freezing tolerance. During cold acclimation, Fv/Fm decreased slightly in most of RILs. Although differences on Fv/Fm were detected among RILs, the variation in Fv/Fm was small and no significant correlation between LT50 and Fv/Fm was observed (Table S1). The freezing treatment in darkness for 24 h led to a significant reduction in Fv/Fm. Different responses of the Fv/Fm ratio and EL were observed among RILs and parental lines during and after freezing treatment (Table 1). Interestingly, Fv/Fm was highly correlated with electrolyte leakage assays (Table 2). Plants responded differently to freezing treatment, depending on the photoperiod during cold acclimation. Large variation among RILs was detected 1-day after freezing treatment among experimental time points in both photoperiod conditions (Table 1, see SD_1DFT and LD_1DFT). SAD12, which showed less freezing tolerance, also had lower Fv/Fm than LTM. Similarly, Fv/Fm was negatively affected by freezing, but the decrease of Fv/Fm in freezing tolerant RILs was much less than in freezing sensitive RILs (data not shown). These results indicate that components of the photosynthetic apparatus likely are damaged in freezing sensitive RILs, while freezing tolerant RILs were less affected.
Table 2.
Phenotypic correlations among traits under controlled environmental conditions
| SD | LT50_CA | EL_FT | Fv/Fm_FT | EL_1DPF | FvFm_1DPF |
|---|---|---|---|---|---|
|
| |||||
| LT50_CA | 1 | ||||
| EL_FT | .861** | 1 | |||
| Fv/Fm_FT | −.912** | −.926** | 1 | ||
| EL_1DPF | .874** | .910** | −.892** | 1 | |
| FvFm_1DPF | −.825** | −.867** | .829** | −.953** | 1 |
|
| |||||
| LD | LT50_CA | EL_FT | Fv/Fm_FT | EL_1DPF | FvFm_1DPF |
|
| |||||
| LT50_CA | 1 | ||||
| EL_FT | .805** | 1 | |||
| Fv/Fm_FT | −.796** | −.836** | 1 | ||
| EL_1DPF | .858** | .839** | −.804** | 1 | |
| FvFm_1DPF | −.809** | −.804** | .780** | −.969** | 1 |
means correlation is significant at the 0.01 level (2-tailed) and * means correlation is significant at the 0.05 level (2-tailed).
SD ; Short-day, LD ; Long-day, CA ; cold acclimation, FT ; freezing treatment, 1DF : 1 day post freezing treatment
Maximum quantum yield and winter frost damage of outdoor environment (OE) freezing conditions
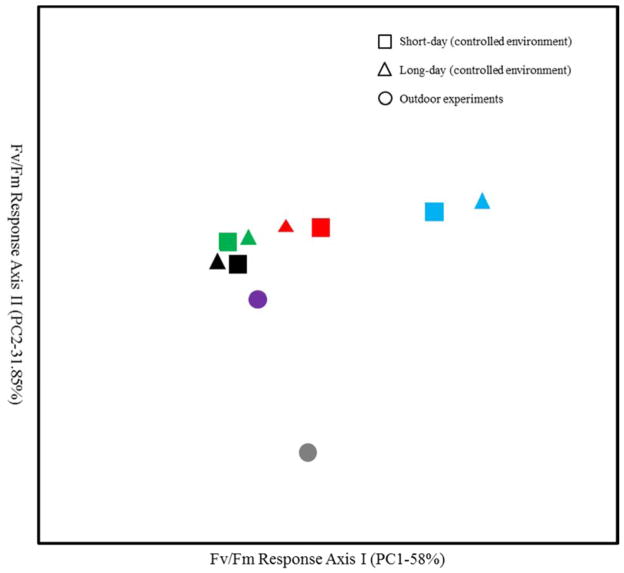
In Outdoor Environment (OE) conditions, we measured Fv/Fm to evaluate freezing injury after two freezing periods during the winter of 2011–2012 (Table 1). The first measurement was carried out during a mild freezing event (MF) in mid-January, the first freezing event of the year. Both parental lines had relatively low Fv/Fm values (LTM 0.66, SAD12 0.57), showing both lines suffered from the freezing event. The RILs showed a greater variation in values (0.41–0.72). The second measurement was performed after a prolonged severe freezing (SF) event in mid-February. Significant freezing damage was observed with Fv/Fm values for parental lines and RILs (LTM=0.38, SAD12=0.37, RILs=0.02–0.64). Thus, while the difference between the two parents was minimal in the severe cold period, more variation was observed in the RILs. Interestingly, genotypic responses to two outdoor freezing stress conditions did not correlate well with those under controlled freezing stress conditions (Fig. 2 and Table S1). The extreme freezing events of late winter caused the death of individual plants in some RILs. However, it was sometimes observed that overall winter damage scores for some RILs with low Fv/Fm had less final frost damage due to successful re-growth or recovery of the plants during warm temperatures in early spring.
Figure 2.
First two principal component axes of Fv/Fm responses of 108 Recombinant Inbred Lines of Boechera stricta grown under short-day/controlled environment (squares) long-day/controlled environment (triangles), and outdoor environment (circles) and measured after various temperature regimes (black = non-acclimated at 20°C; green = cold at 6°C for 3 weeks; red = freezing at −8°C for 24 hour;, blue = 1 day post-freezing; purple = outdoor mild-freezing January, 2012; gray = outdoor severe-freezing February, 2012).
QTL analysis
Complex traits such as freezing tolerance need to be measured for collections of genotypes across multiple environments, because the relative performance of genotypes can change between environments, a well-known phenomenon called genotype by environment interaction. To identify constitutive and inducible QTLs controlling freezing tolerance in B. stricta efficiently, we performed multiple trait QTL analysis to test for pleiotropy or close linkage of QTLs, as well as mixed model based multi-environment single trait QTL analysis to detect QTLxEnvironment (QTLxE) interaction loci. Three QTLs were identified by traditional single trait analysis, i.e., on LG 3, 4 and 7 for Fv/Fm, EL and LT50, and the QTL of traits tended to be overlapping (Table 3). Multiple traits QTL analysis revealed that Fv/Fm was genetically associated with freezing tolerance-related traits. Multi-environment single trait QTL analysis using Fv/Fm identified three QTLs on LG 3, 4 and 7 again, QTL on LG 7 showed significant QTLxE interactions (Table 4).
Table 3.
Summary of putative QTLs controlling freezing tolerant and photosynthetic performance related traits in the single trait QTL model
| Condition | Trait | Linkage group | Locus name | -log10(p) | % Expl. Var. | Add.eff | High value allele |
|---|---|---|---|---|---|---|---|
| Cold acclimation | LT50_SD | 3 | A10 | 3.95 | 11.69 | 0.03 | LTM |
| LT50_SD | 4 | At2g36390 | 3.94 | 12.21 | 0.03 | LTM | |
| LT50_LD | 4 | At2g36390 | 3.17 | 11.01 | 0.02 | LTM | |
| Fv/Fm_SD | 7 | Bst001405 | 5.42 | 20.95 | 0.00 | LTM | |
| Fv/Fm_LD | 7 | Bst001405 | 5.63 | 21.76 | 0.00 | LTM | |
|
| |||||||
| Freezing treatment | EL_SD | 4 | At2g36390 | 4.01 | 14.21 | 0.04 | SAD12 |
| EL_LD | 4 | At2g36390 | 3.12 | 10.83 | 0.03 | SAD12 | |
| Fv/Fm_SD | 3 | A10 | 4.48 | 13.86 | 0.01 | LTM | |
| Fv/Fm_SD | 4 | At2g36390 | 3.01 | 8.89 | 0.01 | LTM | |
| Fv/Fm_LD | 4 | At2g36390 | 3.97 | 14.07 | 0.01 | LTM | |
|
| |||||||
| 1 day post freezing treatment | EL_SD | 4 | At2g36390 | 4.90 | 17.52 | 0.07 | SAD12 |
| EL_LD | 4 | At2g36390 | 5.07 | 18.16 | 0.07 | SAD12 | |
| Fv/Fm_SD | 4 | At2g36390 | 3.48 | 12.18 | 0.03 | LTM | |
| Fv/Fm_LD | 4 | At2g36390 | 3.93 | 13.91 | 0.05 | LTM | |
|
| |||||||
| Outdoor environment | Fv/Fm_MF | 7 | Bst001405 | 3.95 | 15.06 | 0.01 | LTM |
| Fv/Fm_SF | 7 | Bst001405 | 3.98 | 15.18 | 0.09 | LTM | |
SD ; short day, LD ; long day, MF : mild freezing (Jan., 2012), SF : severe freezing (Feb., 2012)
Table 4.
Descriptive summary results from multi-environment QTL model for Fv/Fm
| Linkage group | Locus name | Position | -log10(p) | Environment | Add.eff. | High value allele | % Expl. Var. | P | s.e. |
|---|---|---|---|---|---|---|---|---|---|
| 3 | A10 | 92.74 | 6.57 | SD_NA | 0.00 | SAD12 | 0.10 | 0.74 | 0.00 |
| LD_NA | 0.00 | SAD12 | 0.00 | 0.90 | 0.00 | ||||
| SD_CA | 0.00 | LTM | 0.00 | 0.82 | 0.00 | ||||
| LD_CA | 0.00 | SAD12 | 0.00 | 0.93 | 0.00 | ||||
| SD_FT | 0.02 | LTM | 12.80 | 0.00 | 0.01 | ||||
| LD_FT | 0.00 | LTM | 0.00 | 0.98 | 0.00 | ||||
| SD_1DPF | 0.02 | LTM | 2.10 | 0.11 | 0.01 | ||||
| LD_1DPF | 0.02 | LTM | 1.00 | 0.26 | 0.01 | ||||
| OE_MF | 0.01 | LTM | 0.60 | 0.39 | 0.01 | ||||
| OE_SF | 0.01 | LTM | 0.90 | 0.32 | 0.01 | ||||
|
| |||||||||
| 4 | At2g36390 | 83.33 | 4.21 | SD_NA | 0.00 | LTM | 5.00 | 0.02 | 0.00 |
| LD_NA | 0.00 | LTM | 0.00 | 0.89 | 0.00 | ||||
| SD_CA | 0.00 | LTM | 2.60 | 0.08 | 0.00 | ||||
| LD_CA | 0.00 | LTM | 5.40 | 0.01 | 0.00 | ||||
| SD_FT | 0.02 | LTM | 9.40 | 0.00 | 0.01 | ||||
| LD_FT | 0.02 | LTM | 15.20 | 0.00 | 0.01 | ||||
| SD_1DPF | 0.04 | LTM | 13.40 | 0.00 | 0.01 | ||||
| LD_1DPF | 0.06 | LTM | 17.20 | 0.00 | 0.01 | ||||
| OE_MF | 0.00 | LTM | 0.10 | 0.70 | 0.01 | ||||
| OE_SF | 0.01 | SAD12 | 0.50 | 0.45 | 0.01 | ||||
|
| |||||||||
| 7 | Bst001405 | 83.33 | 12.45 | SD_NA | 0.00 | LTM | 4.60 | 0.04 | 0.00 |
| LD_NA | 0.00 | SAD12 | 0.20 | 0.65 | 0.00 | ||||
| SD_CA | 0.00 | LTM | 21.40 | 0.00 | 0.00 | ||||
| LD_CA | 0.00 | LTM | 22.30 | 0.00 | 0.00 | ||||
| SD_FT | 0.01 | LTM | 2.50 | 0.08 | 0.01 | ||||
| LD_FT | 0.01 | LTM | 4.40 | 0.03 | 0.01 | ||||
| SD_1DPF | 0.02 | LTM | 3.00 | 0.07 | 0.01 | ||||
| LD_1DPF | 0.03 | LTM | 3.60 | 0.04 | 0.01 | ||||
| OE_MF | 0.02 | LTM | 13.90 | 0.00 | 0.01 | ||||
| OE_SF | 0.05 | LTM | 12.90 | 0.00 | 0.01 | ||||
SD ; short day, LD ; long day, OE : outdoor environment, CA ; cold acclimation, FT ; freezing treatment, 1DF : 1 day post freezing treatment, MF : mild freezing (Jan., 2012), SF : severe freezing (Feb., 2012)
Single trait QTL analysis
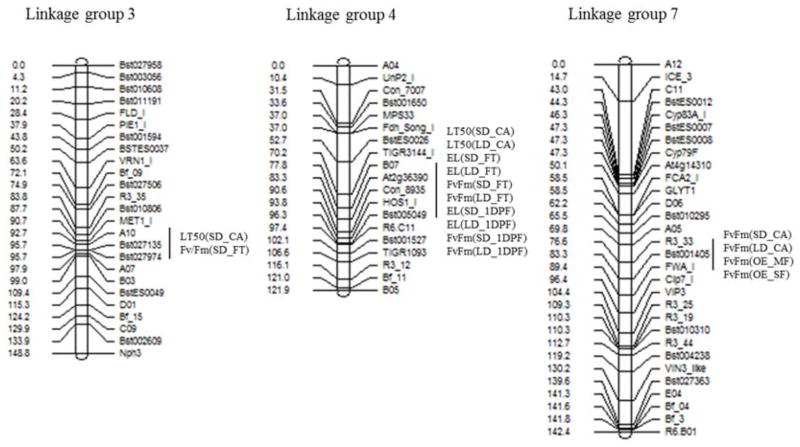
For LT50 values of non-acclimated RILs in both LD and SD controlled conditions no significant QTLs were identified, likely due to the relative lack of trait variance among RILs. Two LT50 QTL regions for cold acclimated plants were identified, one that was SD specific (LG 3, marker A10) and one common to LD and SD (LG 4, marker At2g36390) (Fig. 3 and Table 3). For SD conditions, the accumulated total phenotypic variation explained by the two QTLs was 23.9% (11.7% by LG 3 and 12.2% by LG 4). The single QTL for LT50 was found only for long-photoperiod, with -log10(p) = 3.18, and phenotypic variation of this QTL was 11.0%. All superior alleles for freezing tolerance originated from the LTM parent from Montana, regardless of photoperiod.
Figure 3.
Quantitative Trait Loci (QTL) identified for several traits under variable conditions on the three of the seven Linkage Groups (LG) of this study. SD; short day, LD ; long day, OE : outdoor environment, CA : cold acclimation, FT : freezing treatment, 1DF : 1 day post freezing, MF : mild freezing (Jan., 2012), SF : severe freezing (Feb., 2012).
For EL and Fv/Fm measurements from different photoperiods and experimental time points, CIM mapping identified a total of 11 QTLs. One putative QTL for Fv/Fm in acclimated plants grown under SD and LD was identified on linkage group 7. The QTLs on linkage group 7 explained 20.9% and 21.8% for short and long photoperiod, respectively. One QTL for Fv/Fm and EL was detected for the freezing treatment and the 1st day after freezing stress in both photoperiods, co-located on linkage group 4. We also identified other QTLs under different time points. One interesting QTL was identified on linkage group 3 for Fv/Fm at freezing treatment under short photoperiod, overlapping with the locus for LT50 under short photoperiod.
Under outdoor freezing stress conditions, no putative QTL was observed for overall winter damage. Fv/Fm measurements during winter, however, revealed one QTL region on linkage group 7 that exceeded the significance threshold across stress conditions.
Multi-trait QTL analysis and multi-environment single trait QTL analysis
One QTL for EL collocated with a QTL for Fv/Fm from single trait QTL analysis under controlled environments. In order to confirm if freezing tolerant-related traits were associated with photosynthetic performance-related traits, a multi-trait QTL mapping approach was applied, in which variation for several traits was analyzed simultaneously. The results presented in Table S2 showed that most QTLs identified by single-trait analyses were still significant in the multi-trait QTL model. The QTL for EL and Fv/Fm detected on linkage group 4 post freezing was also identified in the multi-trait QTL analysis and the QTL for EL was strongly associated with Fv/Fm. This result suggests that these traits may be co-regulated. Multi-trait QTL analysis revealed exceptions for the short day/cold acclimated (SDCA) and short day/freezing treated (SDFT) conditions. The LT50 QTL on linkage group 3 in SDCA was no longer significant in multi-trait model, reflecting a lack of association with Fv/Fm. By contrast, multivariate analysis detected an additional QTL on linkage group 2 which was not identified with the single-QTL model for SDFT.
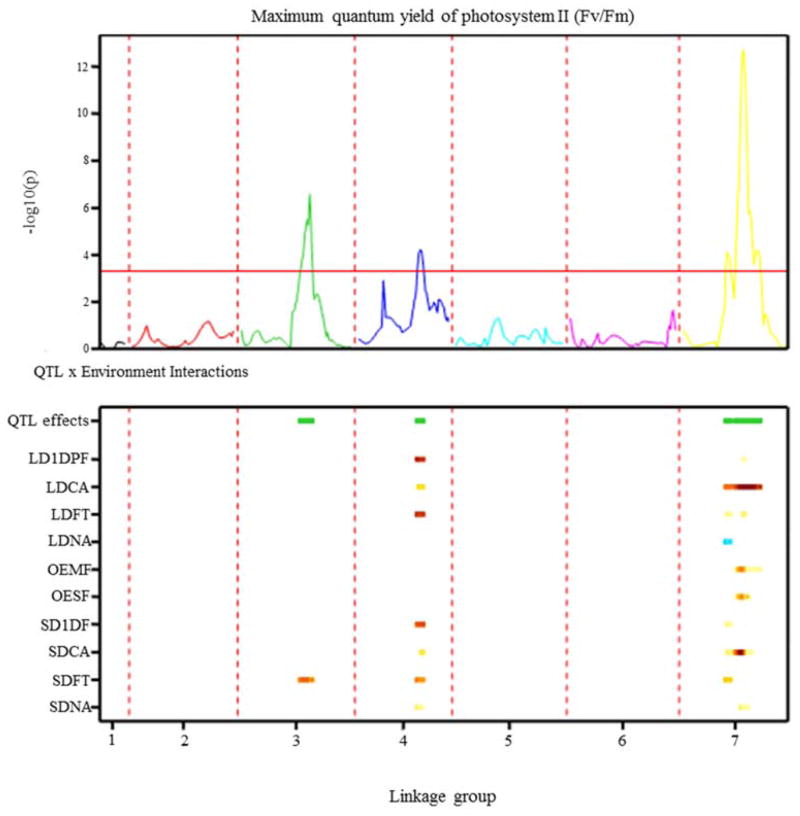
Multi-environment, single trait QTL analysis for Fv/Fm was performed to evaluate QTL by environment interaction. Three QTL × E interaction loci were found, on linkage group 3, 4 and 7 (Fig. 4 and Table 4). The QTL of linkage group 3 was shown to be highly significant (-log10(p) = 6.57), mainly due to a substantial effect from the specific environment (SDFT). Linkage group 4 contained a QTL (-log10(p) = 4.21), which had relatively high effects on Fv/Fm in controlled environments, but it had little effect in outdoor environments. The most significant QTL × E effect (-log10(p) = 12.45) in the multi-environment QTL model was detected on linkage group 7. Percentage of total phenotypic variation explained was between 0.2 and 22.3%, depending on environmental conditions. This locus had little effect on Fv/Fm in controlled non-acclimated and freezing treated conditions, but it was consistently present in cold or freezing stress conditions, with the superior LTM allele active in the most stressful conditions (Table 4). Our results suggest that QTLs on linkage group 7 would have important roles for freezing tolerance in all environments, while QTLs on linkage group 3 and 4 would be more environment-specific.
Figure 4.
QTL profiles of LTM × SAD12 population, showing chromosomal regions with significant QTLs (upper panel) and their additive effect across environments. SD ; short day, LD ; long day, OE : outdoor environment, CA : cold acclimation, FT : freezing treatment, 1DF : 1 day post freezing, MF : mild freezing (Jan., 2012), SF : severe freezing (Feb., 2012).
DISCUSSION
Boechera stricta provides an excellent model system to study ecologically important traits such as freezing tolerance. There have often been observed differences in QTLs found under control versus outdoor conditions (Weinig et al. 2002; Martin et al. 2006). For example, a QTL study of flowering time in B. stricta identified different loci under different growth conditions (Anderson et al. 2011). Development of freezing tolerance can also be highly influenced by environmental factors such as temperature and photoperiod conditions. Thus, we conducted our QTL study under varying day-length conditions in a controlled environment, and also under outdoor conditions where plants experienced two periods of outdoor freezing stress to provide further information on freezing tolerance. We have found significant variation for freezing tolerance and photosynthetic performance to freezing stress conditions in B. stricta, and also show that photosynthetic performance-related trait may be genetically associated with freezing tolerance. Below, we discuss our findings and identify a potential candidate locus.
Effect of photoperiod on freezing tolerance
Developing freezing tolerance is a complex phenomenon. Freezing tolerance is highly influenced by environmental factors such as duration of cold exposure, conditions of photoperiod, and developmental stage. Transcript profiling studies have shown that hundreds of genes are up-regulated in A. thaliana and they can be differently regulated with responses to environmental conditions in many species (Limin et al. 1997; Skinner et al. 2005; Vagujfalvi et al. 2005; Miller et al. 2006; Baga et al. 2007). Photoperiod condition is an important factors controlling freezing tolerance, but the genetic mechanism is still poorly understood.
In the climate chamber, we determined relative freezing tolerance (LT50) using one-month old RILs and their parents before and after cold acclimation for 3 weeks in different photoperiods. Electrolyte leakage (EL) was also quantified to assess the damage of cellular membranes during and after the freezing treatment. Our genetic analysis indicated that several regulatory factors may influence freezing tolerance in both photoperiods. A total of six LT50- and EL-related QTLs identified across experimental conditions showed intermediate effects. The most significant QTL for freezing tolerance was located on linkage group 4. This QTL was identified across experimental conditions (both LD and SD) and with two types of electrolyte leakage methods, indicating that it may contain genes important for freezing tolerance by protecting membrane integrity. Another interesting QTL was identified on linkage group 3. Although the effect of this QTL was small, it was detected by LT50 measurements only after cold acclimation grown in the short photoperiod condition. This result indicates that short day can be contributed to the increase of freezing tolerance in B. stricta. It has been reported that freezing tolerance in Gaura coccinea is enhanced with cold treatment when they are exposed to short-day photoperiods (Pietsch et al. 2009). Similarly, cold acclimation ability in barley is enhanced by a short photoperiod condition in combination with low acclimating temperatures, which is associated with a short photoperiod induced delay in the transition from vegetative to reproductive stage (Flower et al. 2001). In Arabidopsis, two accessions also showed different levels of freezing tolerance in response to photoperiods, and phenotypic expression was traced to different QTLs (Alonso-Blanco et al. 2005). Although the role and mechanism for photoperiod on freezing tolerance in herbaceous species is unclear unlike woody plants (Li et al. 2003; Welling and Palva, 2006), this result suggests that that photoperiod conditions affecting freezing tolerance in B. stricta can also be under genetic control and lead to differential physiological, metabolic, and transcriptional adjustments during cold acclimation. The molecular mechanism and underlying gene(s) for the SD-specific QTL could not be ellucidated in the current study.
Although remarkable progress has been made in understanding cold acclimation, the molecular mechanisms underlying how plants perceive low temperature and short photoperiod conditions during cold acclimation is still unclear. Further detailed study of the genetic variation present in B. stricta can uncover the adaptive mechanism for these differential freezing tolerances and photoperiod responses.
Close relationship between photosynthetic performance and freezing tolerance
Low temperature stress can lead to a reduced rate of photosynthetic electron transport by strongly decreasing CO2 fixation and increase relative excess energy, which can eventually lead to the production of potentially dangerous reactive oxygen species (ROS) and photo-inhibition of photosystem (Baker, 1994). Because production of ROS and occurrence of photo-inhibition may result in the disruptions of cold-induced physiological and biochemical changes necessary for cellular stability against freezing stress, overwintering species must increase their photosynthetic capacities to regulate such a balance in response to freezing condition during cold acclimation (Hüner et al. 1998). It has been demonstrated that the status of PSII is positively associated with freezing tolerance (Rizza et al. 2001; Pocock et al. 2001; Rapacz and Wolniczka, 2009). In our study, freezing tolerant and photoinhibition-related traits were evaluated, and QTL regions controlling these traits were compared. Like in previous studies, we found significant correlations between freezing tolerance and photoinhibition-related traits during freezing or post-freezing. Freezing treatments caused photoinhibition in the RILs, and the decrease in Fv/Fm was much higher in freezing sensitive RILs than in freezing tolerant RILs. These results indicated that components of PSII can be significantly damaged in freezing sensitive RILs. Results of our QTL analysis supported that these traits are mechanically or genetically associated, suggesting that key genes involved in the freezing tolerance can also control photo-inhibition related traits. This implies that a study of genes regulating reduced inhibition of photosynthesis in freezing tolerant genotypes would provide new insights on the complexity of freezing tolerance in plants.
From a methodological point of view we found that chlorophyll fluorescence can be used as a reliable high-throughput phenotyping proxy for measuring freezing tolerance. It is a quick and inexpensive method widely used for analyzing the status of photosynthetic apparatus and understanding the mechanism by which a range of environmental factors alter photosynthetic activity (Baker, 2008). Among various chlorophyll fluorescence parameters, maximum quantum yield of photosystem II (Fv/Fm) has been applied for evaluating photosynthetic performance because it is the most easily measured and reflects a progressive inactivation of PSII-mediated electron transport. It was already suggested as one of promising methods to evaluate freezing tolerance of plants (Ehlert and Hincha, 2008), but few studies tested if it was a reliable high-throughput phenotyping method for measuring freezing tolerance. Chlorophyll fluorescence and electrolyte leakage methods were applied for our study. We studied the correlation between a direct method to measure freezing tolerance in plants and chlorophyll fluorescence. Our results demonstrated that there were significantly correlations between two methods. Routine frost damage assay such as electrolyte leakage is accurate, but laborious. Winter survival test is also relatively easy and can provide a reliable result, but experimental variability due to all kinds of uncontrolled factors accumulating in long term experiments can cause noisy result, and several years of replicated experiments are needed. They have disadvantages that are not easily applicable for large-scale screening purposes (Ehlert and Hincha, 2008). Our extensive comparative studies, therefore, support that chlorophyll fluorescence is a powerful tool for studying freezing tolerance in plants.
Outdoor environment QTL and potential candidate gene
The genetic basis of research for understanding freezing tolerance is often obstructed by the difficulty in mimicking realistic outdoor environments under controlled conditions and in facing unpredictable environmental events or physiological damages in natural conditions. Significant and high correlations between Fv/Fm and two types of electrolyte leakage assessments were found during freezing and post freezing treatments in controlled environment conditions, indicating that Fv/Fm can be routinely used as a reliable phenotypic assessment for freezing tolerance in B. stricta for QTL study. Therefore, this approach was also applied for evaluating freezing tolerance in outdoor conditions. No QTL was detected for overall winter damage, which might be due to high levels of experimental noise under less controlled conditions outdoors. By contrast, data from Fv/Fm measured during winter identified one QTL. Our QTL analyses in more complex freezing conditions and different developmental stages thus identified one genomic region on linkage group 7 with strong effects on freezing tolerance in B. stricta.
Data analyses of Fv/Fm also provide the chance to study phenotypic plasticity of related factors. Although the QTL was not identified under controlled freezing conditions, the locus identified in nature environments also has relatively high and consistent effects in controlled stress conditions. This result suggests that it can be a form of phenotypic plasticity that is a key trait affecting fitness of B. stricta in variable environments. The locus at linkage group 7 is syntenic with a genomic region in A. thaliana that contains DREB1/CBF-type transcription factors. In A. thaliana, DREB1/CBF transcription factors have been suggested as a master regulator of morphological, physiological and biochemical adjustments for increasing freezing tolerance (Thomashow, 1999). They play an important role in the protection and stabilization of cellular membranes by inducing cryoprotectant solutes and cryoprotective proteins and, leading to improvement of anti-oxidative mechanisms in A. thaliana (Jaglo-Ottosen et al. 1998, Kasuga et al. 1999). These genes are highly conserved in cold adaptable plants, and their importance for freezing tolerance has been reported in a number of species (Zhang et al. 2004; Skinner et al. 2005). Given that in B. stricta the LG 7 QTL shows consistent results in both controlled and outdoor conditions, this suggests that DREB1/CBF-type transcription factors may be promising candidates for unraveling molecular mechanism and fitness of freezing tolerance in this species.
Supplementary Material
Acknowledgments
This research was supported by funding from Netherlands Science Organization (NWO), the National Science Foundation (EF-0723447 to T.M.-O.) and the National Institutes of Health (R01 GM086496 to T.M.-O.).
References
- Alonso-Blanco C, Gomez-Mena C, Llorente F, Koornneef M, Salinas J, Martínez-Zapater JM. Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis. Plant Physiology. 2005;139:1304–1312. doi: 10.1104/pp.105.068510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Lee CR, Mitchell-Olds T. Life-history QTLs and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis. Evolution. 2011;65:771–787. doi: 10.1111/j.1558-5646.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Lee CR, Mitchell-Olds T. Strong selection genome-wide enhances fitness tradeoffs across environments and episodes of selection. Evolution. 2014;68:16–31. doi: 10.1111/evo.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M, Chodaparambil SV, Limin AE, Pecar M, Fowler DB, Chibbar RN. Identification of quantitative trait loci and associated candidate genes for low-temperature tolerance in cold-hardy winter wheat. Functional & Integrative Genomics. 2007;7:53–68. doi: 10.1007/s10142-006-0030-7. [DOI] [PubMed] [Google Scholar]
- Baker NR. Chilling stress and photosynthesis. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 127–154. [Google Scholar]
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Carvallo MA, Pino MT, Jeknic Z, Zou C, Doherty CJ, Shiu SH, Chen THH, Thomashow MF. A comparison of the low temperature transcriptomes and CBF regulons of three plant species that differ in freezing tolerance: Solanum commersonii, Solanum tuberosum and Arabidopsis thaliana. Journal of Experimental Botany. 2011;62:3807–3819. doi: 10.1093/jxb/err066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert B, Hincha D. Chlorophyll fluorescence imaging accurately quantifies freezing damage and cold acclimation responses in Arabidopsis leaves. Plant Methods. 2008;4:12. doi: 10.1186/1746-4811-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DB, Breton G, Limin AE, Mahfoozi S, Sarhan F. Photoperiod and temperature interactions regulate low-temperature-induced gene expression in barley. Plant Physiology. 2001;127:1676–1681. [PMC free article] [PubMed] [Google Scholar]
- Gamboa MC, Rasmussen-Poblete S, Valenzuela PD, Krauskopf E. Isolation and characterization of a cDNA encoding a CBF transcription factor from E. globulus. Plant Physiology and Biochemistry. 2007;45:1–5. doi: 10.1016/j.plaphy.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Gery C, Zuther E, Schulz E, Legoupi J, Chauveau A, McKhann HI, Hincha DK, Teoule E. Natural variation in the freezing tolerance of Arabidopsis thaliana: effects of RNAi-induced CBF depletion and QTL localisation vary among accessions. Plant Science. 2011;180:12–23. doi: 10.1016/j.plantsci.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiology. 2006;142:98–112. doi: 10.1104/pp.106.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LG, Wang HL, Liu DC, Zhao YL, Xu M, Zhu M, Wei GQ, Sun ZH. Isolation and expression of a cold-responsive gene PtCBF in Poncirus trifoliate and isolation of citrus CBF promotors. Biologia Plantarum. 2012;56:484–492. [Google Scholar]
- Hüner NPA, Öquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends in Plant Science. 1998;3:224–230. [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnology. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- Leinonen I. A simulation model for the annual frost hardiness and freeze damage of Scots Pine. Annals of Botany. 1996;78:687–693. [Google Scholar]
- Li C, Junttila O, Ernstsen A, Heino P, Palva ET. Photoperiodic control of growth, cold acclimation and dormancy development in silver birch (Betula pendula) ecotypes. Physiologia Plantarum. 2003;117:206–212. [Google Scholar]
- Limin AE, Danyluk J, Chauvin LP, Fowler DB, Sarhan F. Chromosome mapping of low temperature induced Wcs120 family genes and regulation of cold-tolerance expression in wheat. Molecular and General Genetics. 1997;253:720–727. doi: 10.1007/s004380050376. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: An overview. Archives of Biochemistry and Biophysics. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Martin NH, Bouck AC, Arnold ML. Detecting adaptive trait introgression between Iris fulva and I. brevicaulis in highly selective field conditions. Genetics. 2006;172:2481–2489. doi: 10.1534/genetics.105.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H, Shimada Y, Yoshida S, Shinozahki K, Yamaguchi-Shinozaki K. Identification of cold inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant Journal. 2004;38:982–93. doi: 10.1111/j.1365-313X.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- Meissner M, Orsini E, Ruschhaupt M, Melchinger AE, Hincha DK, Heyer AG. Mapping quantitative trait loci for freezing tolerance in a recombinant inbred line population of Arabidopsis thaliana accessions Tenela and C24 reveals REVEILLE1 as negative regulator of cold acclimation. Plant, Cell & Environment. 2013;36:1256–1267. doi: 10.1111/pce.12054. [DOI] [PubMed] [Google Scholar]
- Miller AK, Galiba G, Dubcovsky J. A cluster of 11 CBF transcription factors is located at the frost tolerance locus Fr-Am2 in Triticum monococcum. Molecular Genetics and Genomics. 2006;275:193–203. doi: 10.1007/s00438-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Murray MB, Cape JN, Fowler D. Quantification of frost damage in plant tissues by rates of electrolyte leakage. New Phytologist. 1989;113:307–311. doi: 10.1111/j.1469-8137.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. URL http://www.R-project.org. [Google Scholar]
- Rapacz M, Wołniczka A. A selection tool for freezing tolerance in common wheat using the fast chlorophyll a fluorescence transcients. Plant Breeding. 2009;128:227–234. [Google Scholar]
- Rizza F, Pagani D, Stanca AM, Cattivelli L. Use of chlorophyll fluorescence to evaluate the cold acclimation and freezing tolerance of winter and spring oats. Plant Breeding. 2001;120:389–396. [Google Scholar]
- Pastina MM, Malosetti M, Gazaffi R, Mollinari M, Margarido GR, Oliveira KM, Pinto LR, Souza AP, van Eeuwijk FA, Garcia AA. A mixed model QTL analysis for sugarcane multiple-harvest-location trial data. Theoretical and Applied Genetics. 2012;124:835–849. doi: 10.1007/s00122-011-1748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietsch GM, Anderson NO, Li PH. Cold tolerance and short day acclimation in perennial Gaura coccinea and G. drummondii. Scientia Horticulturae. 2009;120:418–425. [Google Scholar]
- Pocock TH, Hurry VM, Savitch LV, Huner NPA. Susceptibility to low-temperature photoinhibition and the acquisition of freezing tolerance in winter and spring wheat: the role of growth temperature and irradiance. Physiologia Plantarum. 2001;113:499–506. [Google Scholar]
- Sandve SR, Kosmala A, Rudi H, Fjellheim S, Rapacz M, Yamada T, Rognli OA. Molecular mechanisms underlying frost tolerance in perennial grasses adapted to cold climates. Plant Science. 2011;180:69–77. doi: 10.1016/j.plantsci.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Schranz ME, Song BH, Windsor AJ, Mitchell-Olds T. Comparative genomics in the Brassicaceae: a family-wide perspective. Current Opinion in Plant Biology. 2007a;10:168–175. doi: 10.1016/j.pbi.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Schranz ME, Windsor AJ, Song B, Lawton-Rauh A, Mitchell-Olds T. Comparative genetic mapping in Boechera stricta, a close relative of Arabidopsis. Plant Physiology. 2007b;144:286–298. doi: 10.1104/pp.107.096685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz ME, Manzaneda AJ, Windsor AJ, Clauss MJ, Mitchell-Olds T. Ecological genomics of Boechera stricta: identification of a QTL controlling the allocation of methionine- vs branched-chain amino acid-derived glucosinolates and levels of insect herbivory. Heredity. 2009;102:465–474. doi: 10.1038/hdy.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JS, Zitzewitz J, Szucs P, Marquez-Cedillo L, Filichkin T, Amundsen K, Stockinger EJ, Thomashow MF, Chen THH, Hayes PM. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Molecular Biology. 2005;59:533–551. doi: 10.1007/s11103-005-2498-2. [DOI] [PubMed] [Google Scholar]
- Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Molecular Biology. 2008;66:361–78. doi: 10.1007/s11103-007-9274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Role of cold-responsive genes in plant freezing tolerance. Plant Physiology. 1998;118:1–7. doi: 10.1104/pp.118.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiology. 2010;154:571–577. doi: 10.1104/pp.110.161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vágújfalvi A, Aprile A, Miller A, Dubcovsky J, Delugu G, Galiba G, Cattivelli L. The expression of several CBF genes at the Fr-A2 locus is linked to frost resistance in wheat. Molecular Genetics and Genomics. 2005;274:506–514. doi: 10.1007/s00438-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Weinig C, Ungerer MC, Dorn LA, Kane NC, Toyonaga Y, Halldorsdottir SS, Mackay TF, Purugganan MD, Schmitt J. Novel loci control variation in reproductive timing in Arabidopsis thaliana in natural environments. Genetics. 2002;162:1875–84. doi: 10.1093/genetics/162.4.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling A, Palva ET. Molecular control of cold acclimation in trees. Physiologia Plantarum. 2006;127:167–181. [Google Scholar]
- Xu ZS, Chen M, Li LC, Ma YZ. Functions and application of the AP2/ERF transcription factor family in crop improvement. Journal of Integrative Plant Biology. 2011;53:570–585. doi: 10.1111/j.1744-7909.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Creelman RA, Zhu JK. From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiology. 2004;135:615–21. doi: 10.1104/pp.104.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.