Abstract
Functional imaging research has yielded evidence of changes in poor readers after instructional intervention. Although it is well established that within the group of children with poor reading there are differences in behavioral response to intervention, little is know about the functional correlates of responsiveness. Therefore, we acquired functional magnetic resonance imaging (MRI) data from children identified as “at risk for reading disability” who responded differently to a reading intervention (5 responders; 5 nonresponders; 4 controls). Groups differed in activation level of the left hemisphere posterior superior temporal and the middle temporal gyri, suggesting that future imaging studies should consider responders and nonresponders separately.
An extensive research base shows that explicit code-based instruction helps most young children who are at-risk for word-level reading disability (RD) achieve decoding and word recognition skills in the average range with effects maintained over time (e.g., Torgesen, 2000; Vellutino et al., 1996). However, large individual differences exist among children at-risk for RD in their response to evidence-based interventions. In fact, a small percentage of children fail to show any significant behavioral response to intervention (Vellutino et al., 1996). This is the basis for the response to intervention (RTI) approach, which became part of the federal educational policy with the reauthorization of the Individuals with Disabilities Education Act (IDEA, 2004). RTI is a promising method for preventing, identifying, and ameliorating RD.
Despite RTI’s popularity and promise, many questions about how to implement it effectively and efficiently remain unanswered. RTI is typically operationalized as a 3-tier prevention system (Bradley, Danielson, & Hallahan, 2002; Donovan & Cross, 2002; Fuchs, Fuchs, & Speece, 2002). All students participate in Tier 1 (primary prevention), which involves the core reading program in their general education classrooms. Students who do not respond to classroom instruction enter Tier 2, where secondary prevention is characterized by the use of a more intensive, researchbased standard treatment protocol delivered in small groups. Continued unresponsiveness to Tier 2 is interpreted as reflecting a disability and a need for the child to move to tertiary intervention (Tier 3 or special education) where more intensive instruction is designed to meet an individual student’s needs. The current overriding assumption is that children’s failure to respond to the tertiary intervention is attributable to a neurobiological origin. However, few studies have modeled the correlations between behavioral diagnoses using the RTI approach and children’s functional profiles. Differences in brain activation as related to behavioral measures may provide insight into the neurobiological phenotypes of intervention responders and non-responders.
A specific area of interest for study by functional imaging is the temporo-parietal cortex (TPC). The TPC is a large network of brain regions, including the angular gyrus, supramarginal gyrus, and the posterior and superior temporal sulci (Black & Behrmann, 1994; Bookheimer, 2002; Gabrieli, Poldrack, & Desmond, 1998). This network of brain regions is part of the dorsal processing stream and responsible for letter (orthographic) to sound (phonological) analyses of words (Shaywitz et al., 2004). Correspondingly, novice readers learning to read and also skilled readers decoding unfamiliar words activate this region in the left hemisphere. Part of the neural signature of brain function in individuals with RD is decreased activity in the left TPC (Brunswick, McCrory, Price, Frith, & Frith, 1999; Horwitz, Rumsey, & Donohue, 1998; Rumsey et al., 1997) and increased activity in the right TPC (Shaywitz et al., 2003). Furthermore, children who respond to intervention exhibit significantly increased activation of left hemisphere TPC and decreased activation of right hemisphere TPC (Aylward et al., 2003; Shaywitz et al., 2004; Simos et al., 2002; Simos et al., 2005; 2006). Such differences in functional activation serve to further elucidate the neurobiological phenotype for responsiveness to intervention.
Until recently most imaging studies compared children with RD to controls, failing to consider differences in responsiveness to intervention within the RD group. Simos et al. (2006) were the first to provide clear evidence that individual variability exists in the brains susceptibility to intervention once level of responsiveness to intervention is considered. Individual reading ability correlated with increased neurophysiological activation during a nonword task in regions within the left hemisphere TPC, including the middle temporal and posterior superior temporal gyri (pSTG) (Simos et al., 2006). Odegard, Ring, Smith, Biggan, and Black (2008), also compared treatment responders and nonresponders in their imaging study with 10–14-year-old children. They found that treatment nonresponders had greater activation in the right middle temporal lobe during a letter–sound task, yet they found no group differences in activation of the left superior temporal lobe. A potential reason that Odegard et al. (2008) did not find the differences within the pSTG as reported by Simos et al. (2006) was that they acquired functional data a year after participants’ had received instruction, at a time when treatment responders and nonresponders had similar scores on a phonological awareness task. The pSTG may be sensitive to individual differences early in reading development when phonological processing is the main focus. However, later in development, other brain regions such as the occipital-temporal cortex may assume greater responsibility during word reading tasks (see review in Price et al., 2003).
The purpose of the current study was to explore the functional activation of the TPC in a group of children at-risk for RD, considering their level of response to intervention. Unlike Simos et al. (2006) and Odegard et al. (2008) participants in our study are all of the same age and all received the same experimental intervention in a controlled environment. However, similar to the Simos et al. (2006) study, our scanning sessions occurred immediately after the 17-week reading intervention. The findings contribute to the existing literature by linking current knowledge in the behavioral field about individual variability in children’s response to reading instruction with knowledge of functional circuits. This information will provide insight into lingering questions about the effectiveness of the RTI approach to select children who are at high risk of developing RD due primarily to neurobiological dysfunction.
METHODS
Participants
The data presented here represent findings from a study investigating the neural correlates of responsiveness within a RTI model. Imaging data were acquired on sixteen children with a mean age of 7.5 years (SD = .43). All participants were recruited from a sample (N = 298) of first graders in Nashville participating in a federally funded randomized control trial (RCT). The RCT explores the effectiveness of response to intervention (RTI) as a means of identifying and preventing RD. All participants, including control participants, were screened and determined to be at-risk for reading difficulties at the beginning of first grade. Children with brain injury, other physical disabilities, severe emotional problems, uncorrected sensory disorders, attention deficit hyperactivity disorder (ADHD), or an IQ < 80, all of which may interfere with the specificity of the brain activation patterns, were excluded during recruitment for this neuroimaging portion of the project. No child who was defined as having limited proficiency in English participated in the imaging study. No restriction was made for gender, ethnicity, or socioeconomic status. For the imaging study, children were screened for claustrophobia and possible contra-indicators such as dental braces.
Of the 20 participants who reported for scanning appointments, three children refused to enter the scanner. We acquired imaging data from the remaining 17 participants. Of these, one child completed the structural imaging but did not complete the functional session. Another participant’s functional data were not included due to severe head motion that rendered the data unreliable. Additionally, imaging data from one participant were discarded during analysis due to excessive functional magnetic resonance imaging (fMRI) signal change. The remaining 14 participants were categorized into three groups based upon intervention eligibility and response to intervention. Intervention eligibility refers to whether children as part of the RCT qualified for small group Tier 2 intervention after 6 weeks of instruction in the general education classroom (Tier 1). “Response to intervention” in this study corresponds to the degree to which children benefited from general classroom instruction (Tier 1) and small group intervention (Tier 2). As such, classroom controls (C; n = 4) were defined as children who were initially identified as at risk in the fall of first grade but benefited from Tier 1 instruction and therefore did not qualify for small group Tier 2 reading intervention. Treatment responders (R; n = 5) were defined as children who did not benefit from Tier 1 instruction, were eligible for small group Tier 2 reading intervention, and attained growth rates above the median in our sample indicating a relative response to intervention. Treatment non-responders (NR; n = 5) were defined as children who did not benefit from Tier 1 instruction, were eligible for small group Tier 2 reading intervention, and exhibited growth rates below the median in our sample indicating a lesser response to intervention.
Behavioral Measures and Responsiveness
Within the RCT, children found by screening measures to be potentially at risk for RD (n = 218) were monitored for reading progress by weekly administration of a measure of word identification fluency (WIF). WIF is a form of curriculum-based measure that has been found to be sensitive to first-grade children’s response to both classroom Tier 1 instruction and Tier 2 small group instruction (see Fuchs, Fuchs, & Compton, 2004). Children are presented weekly with two parallel WIF forms consisting of 100 words presented in list form randomly sampled from high-frequency words on the Dolch pre-primer, primer, and first-grade level lists. Children’s growth in reading, expressed as level and slope of WIF performance over time, was used as a proxy of children’s response to the instruction. Compton, Fuchs, Fuchs, and Bryant (2006) reported that growth (i.e., slope) on WIF correlated strongly with end of first-grade word identification (r = 0.79), passage reading fluency (r = 0.85), and passage comprehension (r = 0.66). In addition, first-grade growth in WIF has been shown to be a significant predictor of RD status at the end of second grade (Compton, Fuchs, Fuchs, & Bryant, 2006).
Growth on WIF was used to make decisions regarding children’s response to general education instruction (i.e., who was eligible for Tier 2 small group instruction) and whether children who received small group Tier 2 intervention were responsive. Growth modeling of WIF over 6 weeks at the beginning of first grade indicated each child’s responsiveness to the general classroom instruction prior to intervention. Children identified as unresponsive to general classroom instruction were assigned to small group intervention (Tier 2). In Tier 2, trained research assistants provided a scripted reading intervention for 45 minutes each session, 3 days/wk, for 17 weeks. The intervention comprised sight word reading (10 min), letter sound practice (5 min), decoding (15 min), and story reading fluency (15 min). For decoding, a tutoring system derived from the Peer-Assisted Learning Strategies (PALS) was used (Fuchs & Fuchs, 2005). For fluency, children engaged in repeated reading of story passages, which were based on Samuels’s (l979; 1994) fluency-building strategies and has been shown to promote fluency among first-grade struggling readers (Denton, Fletcher, Anthony, & Francis, 2006).
Weekly progress monitoring using WIF continued throughout the course of intervention. Upon conclusion of the intervention, responsiveness to intervention was determined for participants in this portion of the study (n = 116) using WIF intercept and slope. For the imaging study, limitations in participant number necessitated ranking participants by WIF intercept and slope and dividing participants to designate equal groups of responders and non-responders (Table 1). We sorted participants first by their WIF intercept scores from high to low and then secondarily by their slope scores, also from high to low. We used a median split to separate the groups, in which the top ranked participants were designated responders and the bottom ranked participants were designated as nonresponders. Rank ordering and partitioning of growth data is a heuristic that has been used to divide participants in the absence of a true definition for delineation (Vellutino et al., 1996). These designations of responders and non-responders were used in all subsequent analyses.
TABLE 1.
Intervention Response as Determined by Word Identification Fluency (WIF) Final Intercept and Slope During the 17-Week Intervention
| Participant | Intercepta | Slopeb | Response Status |
|---|---|---|---|
| 510 | 15.79 | −0.06 | Nonresponder |
| 514 | 18.47 | 0.42 | Nonresponder |
| 489 | 22.21 | 1.03 | Nonresponder |
| 497 | 23.30 | 0.62 | Nonresponder |
| 582 | 31.27 | 0.37 | Nonresponder |
| 482 | 30.75 | 1.03 | Responder |
| 487 | 32.82 | 0.99 | Responder |
| 493 | 38.26 | 1.21 | Responder |
| 585 | 52.61 | 0.83 | Responder |
| 500 | 59.08 | 1.93 | Responder |
Progress of control participants was not monitored during intervention.
Words read correctly.
Growth in words read correctly/week.
Pre- and post-test behavioral measures were administered to all participants receiving Tier 2 intervention. Pre-test was administered in November of first grade prior to intervention. Post-test was administered in April after the conclusion of intervention. Pre- and post-test measures included word identification, decoding, passage comprehension, sight word efficiency, and decoding efficiency. It is important to note that the WIF growth was used to identify groups, and we used the pre- and post-test measures to validate group assignment. Summary statistics for the behavioral measures are given in Table 2, which shows raw scores. Standard scores allow comparisons across the tests, but in this age group they are less sensitive to group differences than the raw scores, and very different raw scores can equal the same standard score.
TABLE 2.
Mean and Standard Deviation on Pre-Test and Post-Test Behavioral Measures by Condition With Associated Effect Sizes
| Control (C) | Responders (R) | Nonresponders (NR) | Effect Size and Significancec | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | Pre | Post | Pre | Post | Pre | Post | C vs. R | C vs. NR | R vs. NR |
| WIF-levela | 30.1 (5.7) | 15.1 (2.7) | 11.8 (7.4) | 3.36* | 2.77* | 0.59 | |||
| WIF-slopea | 2.1 (1.1) | 1.1 (0.8) | 0.1 (0.8) | 1.04 | 2.05* | 1.23 | |||
| Word IDb | 42.8 (6.1) | 26.2 (8.0) | 38.5 (7.6) | 16.0 (5.4) | 26.0 (5.9) | 0.63 | 2.80* | 1.84* | |
| Word Attackb | 17.8 (6.3) | 10.0 (6.0) | 13.0 (7.8) | 2.4 (1.5) | 3.6 (4.1) | 0.67 | 2.67* | 1.51* | |
| Passage Compb | 20.0 (4.1) | 16.5 (1.3) | 12.0 (1.9) | 1.15 | 2.50* | 2.76* | |||
| Word Efficiencyb | 39.5 (4.8) | 17.2 (3.3) | 32.3 (7.4) | 12.8 (4.3) | 20.8 (5.9) | 1.15 | 3.48* | 1.72* | |
| Decoding Efficiencyb | 12.8 (4.5) | 7.0 (1.2) | 13.5 (2.7) | 1.2 (2.7) | 4.2 (3.0) | −0.19 | 2.25* | 3.26* | |
Standard deviations are in parentheses. WIF=word identification fluency; Word ID=Word Identification subtest from the Woodcock Reading Mastery Test–R/NU; Word Attack=Word Attack subtest from the Woodcock Reading Mastery Test–R/NU; Passage Comp=Passage Comprehension subtest from the Woodcock Reading Mastery Test–R/NU; Word Efficiency=Sight Word Efficiency subtest from the Test of Sight Word Reading Efficiency; Decoding Efficiency=Phonemic Decoding Efficiency subtest from the Test of Sight Word Reading Efficiency
Effect sizes based on pre-test performance.
Effect sizes based on post-test performance.
Statistical significance based on ANOVA.
p < .05.
Measures
Progress Monitoring: Word Identification Fluency (WIF)
WIF consists of single-page lists of 100 high-frequency words randomly sampled from the Dolch pre-primer, primer, and first-grade level lists (Fuchs et al., 2004). Participants read as many of the 100 words as they can in 1 minute.
Untimed word identification skill
The Woodcock Reading Mastery Test–R/NU: Word Identification (WRMT–R: WID, Woodcock, 1998) is a norm-referenced test that requires participants to read individual words ordered in difficulty. Participants read until reaching a ceiling of six sequential incorrect responses.
Untimed decoding skill
The Woodcock Reading Mastery Test–R/NU: Word Attack (WRMT–R: WAT, Woodcock, 1998) is a norm-referenced test that requires participants to pronounce decodable pseudowords presented in ordered difficulty until a ceiling of six sequential incorrect responses is reached.
Reading comprehension
Reading comprehension was assessed with the Woodcock Reading Mastery Test–R/NU: Passage Comprehension (WRMT–R: PC) subtest (Woodcock, 1998). This is a norm-referenced, modified cloze procedure. Items are presented in increasing difficulty. Initial items require the participant to point to a rebus and more advanced items require the participant to read a sentence and verbally supply the missing word.
Sight word reading efficiency
The Test of Sight Word Reading Efficiency (TOWRE: SWE, Torgesen, Wagner, & Rashotte, 1997) is a norm-referenced measure of sight word reading accuracy and fluency that requires participants to read a list of words of increasing difficulty for 45 sec.
Phonemic decoding efficiency
The Test of Phonemic Decoding Efficiency (TOWRE: PDE, Torgesen et al., 1997) is a norm-referenced measure of decoding accuracy and fluency that requires participants to read a list of decodable pseudowords of increasing difficulty for 45 sec.
Intake Procedure
In May, letters were sent to the parents of children who had successfully completed the RCT research protocol (including R, NR, and C participants) and met our recruitment criteria. All participants attended a single imaging session, in which each child was acclimated to the lab and received a child-oriented explanation of the study procedures. A play tunnel and a mock scanner were used to practice the tasks and prepare the child for the scanning environment. All tasks and trials were practiced (using exactly the same stimuli) in both the intake room and the mock scanner to ensure familiarity with the tasks.
fMRI Paradigm
Children performed a letter–sound matching task and a control task. Letter/letter sound matching (LSM) measured the ability to match letters with their corresponding sounds (Adams, 1990). During this task, children heard a letter sound (/b/) and simultaneously saw an uppercase letter on the screen (B). They were instructed to push the “correct” button if the sound and letter matched and the “incorrect” button if the sound and letter did not match. Each of the letter stimuli contained their speech sounds at the beginning of their letter names (Treiman & Keissler, 2003). In addition, two vowels (/a/ and /i/), which have distinctive acoustic properties from each other, were used. The control task consisted of visual presentation of easily recognized images (e.g., sun, triangle). Participants received auditory stimuli that either matched all of the visual images or did not match all visual images. This control task matched the LSM task in attentional, motor, and auditory demands. Stimuli presentation for the two tasks was controlled by a computer running E-Prime software (Psychology Software Tools, Inc). Three 5 min runs of randomized task blocks were administered during the functional acquisition. For each stimulus event, the imaging sequence included a period of 0.5 sec during which images were not acquired, providing a gap in scanning acquisition noise for the auditory stimulus presentation. All trials were varied such that the location of the correct response was counterbalanced across trials. During functional imaging scanning, participants viewed visual stimuli projected onto a screen attached to the back of the head coil and responded using a button box to select “correct” or “incorrect.”
Imaging and Analysis
All imaging was performed on a 100% research-dedicated Philips Achieva 3T MR scanner.
Structural imaging
High resolution 3D T1-weighted anatomical images were acquired (in a sagittal orientation) in just under 6 min. An inversion-prepared turbo field echo sequence (IR-TFE), with TI = 916 msec, TR = 7.9 msec, TE = 3.6 msec, a SENSE acceleration factor of 2, matrix size 256×256×170, and FOV 256 × 256 × 170 mm3 for isotropic 1 mm3 resolution. These images were used for subsequent scan prescription, as anatomic underlay for the functional data, and for inter-subject spatial normalization.
Functional imaging
Functional images were prescribed parallel to the AC-PC line. They were acquired using a gradient echo planar imaging sequence, with 28 slices, each 3 mm thick. Other relevant imaging parameters for the functional images are FOV = 240 mm, TE = 35 msec (for optimal BOLD contrast at 3T), TR = 2 sec, matrix size 80 × 80 reconstructed to 128 × 128 pixels, and a SENSE factor of 1.8.
Analysis
All functional data were analyzed using Brain Voyager (Brain Innovations BV, Maastricht, NL). Images were first motion-corrected, and all data that exceeded motion thresholds (>3 mm translational displacement, 3° rotation) were discarded. Data were processed via linear interpolation and were spatially smoothed with a 6 mm FWHM Gaussian filter. The functional data for each participant were aligned to the 3D anatomical image. Single-subject analysis was performed by creating a regression model with estimated hemodynamic response (HRF) for each condition and six motion parameters (x, y, z translational; x, y, z rotational). From these, contrast maps were calculated between task within a participant and between groups based on averaged and normalized group activation maps. Each participant’s activation maps were normalized to a common reference space (Talairach space). At this point, individual participants were screened for outlier time courses. A single control participant had signal change greater than 2% on the LSM task and appeared to be an outlier. All data for this participant were removed from the analyses discussed in this article. Behaviorally this participant was similar to the other control participants. Finally, contrast maps were calculated using a random effects design with a cluster threshold of 10 contiguous significant voxels and a significance value of p < .001. Five a priori regions of interest were specified within the left hemisphere temporoparietal cortex based on previous research and analyzed using the Talairach-Tournoux Atlas (TTatlas+tlrc) dataset from AFNI (Cox, 1996). These regions were the: (1) anterior superior temporal gyrus (BA 41); (2) superior temporal gyrus (BA 22); (3) anterior superior/middle temporal gyrus (BA 21); (4) posterior middle temporal gyrus/angular gyrus (BA 39); and supramarginal gyrus (BA 40). Group differences in level of activation of these regions were explored with a one-way ANOVA, in which the relationship between percentage of voxels activated in each ROI and the between-subjects factor of Group was computed (Control, Responder, Nonresponder).
RESULTS
Behavioral
Intake measures
Participants’ performances on the post-test measures by group were compared with Analysis of Variance (ANOVA). The F test and significance levels from this analysis are presented in Table 2 along with effect sizes. The magnitude of the difference between the effect sizes was estimated with Cohen’s d. These estimators provide an indication of a standardized difference between two groups. These analyses indicate that the present sample of treatment responders and nonresponders did not differ at the beginning of the RCT study on theWIF intercept or slope. However, similar to the findings reported in Simos et al. (2006) and in Odegard et al. (2008), we found that children in the R and C groups scored comparably on the Word ID, Word Attack, PDE and TOWRE, whereas children in the NR group scored significantly lower than the C on all measures.
fMRI task performance
Participants’ in-magnet task performance was highly accurate with all participants scoring above 75% correct (Table 3). Noticeable trends in the data included the more accurate and quicker in-magnet responses of the C and R groups relative to the NR group. These group differences were not statistically significant.
TABLE 3.
In-Magnet Performance and Signal Change by Condition
| Mean Values | Contrast t-Values | |||||
|---|---|---|---|---|---|---|
| In-Magnet Performance | Controls (C) | Responders (R) | Nonresponders (NR) | C vs. R | C vs. NR | R vs. NR |
| Control Task Correcta | 0.93 (0.1) | 0.93 (0.1) | 0.80 (0.1) | 0.00 | 1.87 | 2.03 |
| Letter Sound Correcta | 0.80 (0.2) | 0.83 (0.1) | 0.76 (0.1) | 0.32 | 0.43 | 0.08 |
| Control Task Responseb | 1344 (143) | 1411 (242) | 1434 (140) | 0.48 | 0.94 | 0.17 |
| Letter Sound Responseb | 1280 (54) | 1360 (336) | 1394 (95) | 0.47 | 2.10 | 0.22 |
| Mean Signal Change | ||||||
| LH STG (BA22) | −0.08 (0.1) | 0.23 (0.3) | −0.36 (0.2) | −1.98 | 3.03* | 4.06* |
| LH MTG/Angular Gyrus (BA 39) | −0.39 (0.2) | 0.21 (0.5) | −0.15 (0.3) | −2.41* | −2.26 | 1.60 |
Standard deviations are in parentheses.
Expressed as percent correct.
Expressed in msec.
p < .05.
fMRI Activation
A second ANOVA was run to determine whether a significant difference exists in variability of the mean activation of the three groups, within the five ROIs. Results from this analysis identified two regions for further analysis: the left hemisphere STG (BA22; F (2) = 8.17, p = .01) and the left hemisphere MTG/Angular Gyrus (BA 39; F (2) = 3.39, p = .07). Mean activations for these two regions are provided in Table 3 under the column heading Mean Signal Change. All remaining contrasts, including those in the right hemisphere brain regions, did not reach statistical significance and are not discussed further. However, this null result needs to be explored further in imaging studies with larger sample sizes.
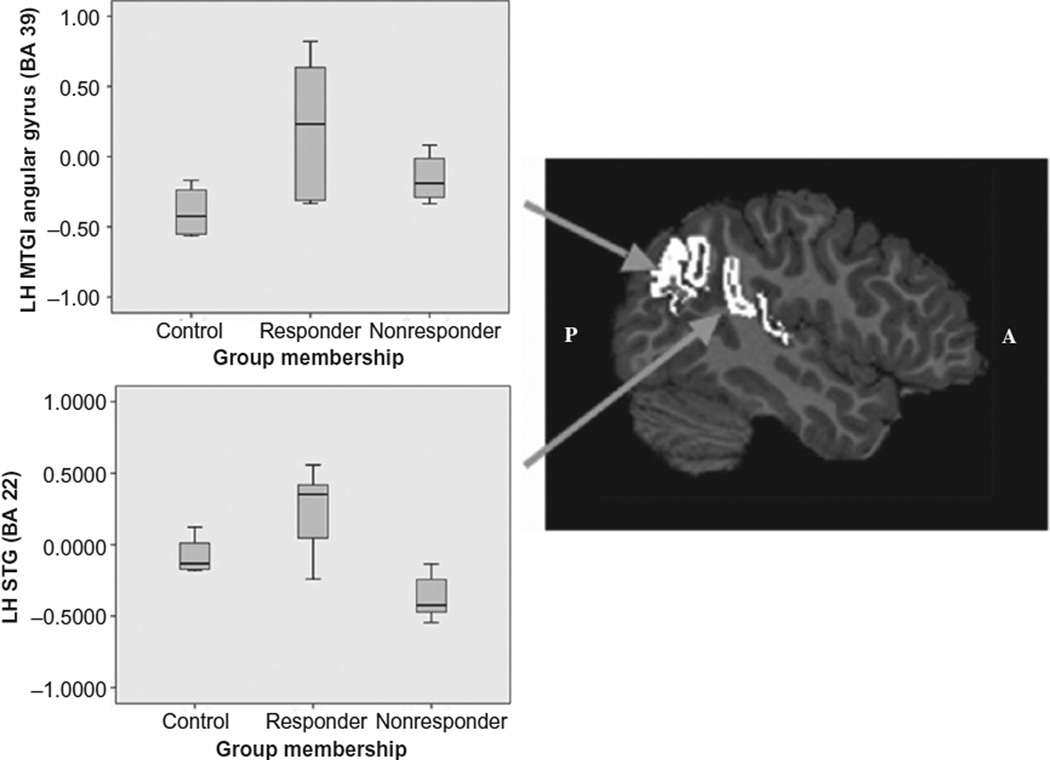
We performed independent sample t-tests to determine whether the C, NR, and R groups’ mean activation within the LH STG and MTG/Angular gyrus regions differed from one another. Results from these tests are shown in Table 3. Group differences between R and NR groups reached a statistically significant level in the STG region (t (8) = 4.06, p < .005, uncorrected). In this same region, statistically significant group differences were found between NR and C groups (t (7) = 3.03, p < .02, uncorrected). R and the C groups differed significantly in activation the left hemisphere middle temporal gyrus/angular gyrus region (BA 39; t (7) = −2.41, p = .05, uncorrected). Within this region, the contrast between C and NR groups was close to significant (t (7) = −2.26, p = .06). Bar graphs of functional activation for these two left hemisphere regions are shown in Figure 1.
FIGURE 1.
Mean-values in the left hemisphere MTG/Angular gyrus (BA39) and the posterior STG (BA22) for the in-magnet letter sound task are shown in a bar graph with lines representing upper and lower limits of the data range.
DISCUSSION
The aim of the current study was to investigate the functional activation differences in a group of children at-risk for reading disability while considering variability in their responsiveness to a reading instruction. We focused specifically on functional activation of the TPC, because the TPC supports neurophysiological processes for sound–letter correspondences (Breier et al., 2003), which is an early developing skill that is foundational to reading growth. An additional reason that we chose this brain region was because of its strong associations with RD. Individuals with RD exhibit decreased activation in left hemisphere TPC and increased right hemisphere TPC during word reading tasks (Paulesu et al., 2001; Rumsey et al., 1992; Shaywitz et al., 1998; Shaywitz et al., 2002; Simos et al., 2000; 2002). Several subregions compose the TPC, and each associates with different aspects of reading (Bookheimer, Zeffiro, Blaxton, Gaillard, & Theodore, 1995; Price, Moore, & Frackowiak, 1996). For this reason, we divided the TPC into five functionally distinct regions and performed a region of interest (ROI) analysis. We found group differences in the functional activation of each region; the largest difference occurred in a posterior region of the STG (BA 22). These findings are strengthened by the fact that participants had similar performances on the in-magnet task, indicating that group differences in functional activation are likely due to differences in recruitment of cognitive processes to perform the task.
Although differences in functional activation were seen between the control group and the two treatment groups, the comparison of most interest was the treatment nonresponders versus the treatment responders. These children received the same quantity and quality of treatment at the same point in time. From these contrasts, we found that children who had limited reading growth relative to their peers, those within the NR group, exhibited a functional profile typical for individuals with RD. That is, they had relative under activation in their left hemisphere cortices during a letter–sound matching task. As expected, compared to the NR group the R group exhibited greater left hemisphere activation in the STG (Aylward et al., 2003; Shaywitz et al., 2004; Simos et al., 2002; 2005; 2006). Our largest group difference was found in a posterior region of the STG (BA 22). This region of the ST lobe is involved in processes related to speech perception and speech production (Hickock & Poeppel 2000; Indefrey & Levelt, 2000). In particular, this region is believed to be involved in constructing the phonological representations of speech sounds, which are critical for word reading. Collectively, the functional evidence in our study suggests a relationship between responsiveness to treatment and the ability to construct sound based representations of speech.
Analysis of the post-test behavioral data corresponded with the functional data: The children in the NR group were relatively poor readers. This outcome is consistent with previous studies indicating that children with dyslexia have a deficit in phonological representations, which disrupts their phonological processing skills and word reading development. Altogether, the functional and behavioral data are consistent with the RTI philosophy that treatment nonresponse is indicative of a neurobiologically based reading disability. A limitation in the current study is it lacks an fMRI scan prior to receipt of intervention. Future studies would benefit from the acquisition of imaging data before and after intervention to investigate whether the group differences between treatment responders and nonresponders is a cause or result of children’s responsiveness to instruction. Related to this, a noteworthy finding from the behavioral component of the current study was significant group differences between R and NR groups in the behavioral measures administered at pre-test, thus providing a means to predict children’s treatment response.
In the current study, we found no significant group differences in the left hemisphere MTG (BA 21) and anterior STG (BA 41). Although previous studies have found a relation between these regions and reading ability, the relation may be due to semantic rather than phonological processing. Our findings are consistent with research using cortical stimulation to research speech perception, in which perception is interrupted within the posterior STG, but not the anterior or middle temporal gyri. Stimuli in our LSM task had no semantic meaning and should not have elicited activations in brain regions related to semantics. To confirm this, we used a control task with semantic demands so that we could subtract out any unintentionally elicited semantic activations. This imaging design may explain our lack of findings within the MTG. Related to this, though, the significant findings within the angular gyrus (BA 39) were unexpected. Although several studies have found decreased activation in angular gyrus related to RD, the angular gyrus is related to word reading. In particular, it is associated with the orthographic processing of a string of letters that make up a word rather than the phonological processing of these letters. We verified that our results in the angular gyrus were not due to increased activation in the control task, and our use of a stringent significance threshold limits the possibility of a false positive. Nevertheless, interpretation of these findings is limited by the lack of a word reading task in the current study. Future imaging studies using both letter sound and word reading tasks should further investigate the angular gyrus activation to determine its relation to responsiveness to intervention.
Our findings are in contrast with those of Odegard et al. (2008), in which a group difference in activation was found between treatment responders and nonresponders in the right middle temporal lobe. In the Odegard et al. (2008) study treatment nonresponders had greater activation compared to the treatment responders and controls (coordinates 46, −32, −3). A failure to replicate this result could be due to differences in study design (participants, tasks, and analysis methods). With regard to the participants, not only was children’s response to intervention defined differently in the two studies, but perhaps more consequential was a difference in the time lapse between the intervention administration and the scans. In the Odegard study, participants completed the intervention a year prior to the scan event; whereas the current study scanned children immediately following the intervention. It is possible that follow up of children in the current study would lead to similar results, and a longitudinal design is warranted to answer that question. With regard to the tasks, the control tasks used in the two studies were very different. The control task in the current study intentionally activated semantic regions in order to subtract out any unintentional semantically related activations in the letter–letter sound task. In contrast, the control task in the Odegard study was a tone task with no semantic demands. Despite their differences, both studies indicate that a functional difference does exist between treatment responders and non-responders.
Our findings demonstrate the importance of considering responsiveness to instruction in future studies of RD. In particular, this study provides evidence that brain activation can be significantly different after considering children’s responsiveness to reading instruction. Furthermore, our outcomes advance the field’s knowledge of RD in general and contribute to the field’s understanding of early behaviors and neurological markers that are valid and reliable predictors of later RD. Finding a behavioral measure that correlates strongly with neurological measures may be a predictor of children’s responsiveness to intervention. The detection of a neurobiological marker of responsiveness could potentially refine the current RD phenotype, clarifying the underlying behavioral markers that define RD and improving identification and treatment of RD. Our study took into account participants’ intercept and slope of response to intervention, which is a somewhat novel concept in RTI research. Within the current study, the intercept and slope of response to intervention may be a better predictor of brain-based differences related to responsiveness than standardized measures of reading ability. Consequently, similar measures deserve consideration in future studies on responsiveness to intervention. Nevertheless, our groups were arbitrarily defined by median split. Future studies need to validate group designations by the use of multimodal types of information. For example, rank-ordering functional activation patterns along with the intercept and slope of behavioral response to intervention may be particularly revealing in terms of neurobiological phenotype. In addition, future studies should be longitudinal in design, administering a scan prior to intervention, in order to investigate whether the differences between Tier2 responders and nonresponders was present before intervention. Furthermore, the small sample size is an issue and replication of the study with a larger sample size is necessary.
Acknowledgments
This work was supported in part by the Vanderbilt Kennedy Center Hobbs Discovery Award, Grant R324G060036 from the U. S. Department of Education, Institute of Education Sciences, Grant NS049096 from the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health through the Biomedical MRI and MRS Training Program T32EB001628.
Footnotes
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://www.tandfonline.com/page/terms-and-conditions
Contributor Information
Nicole Davis, Vanderbilt University Institute of Imaging Science, Nashville, Tennessee Department of Radiology and Radiological Sciences, Vanderbilt University School of Medicine, Nashville, Tennessee.
Laura Barquero, Department of Special Education, Vanderbilt Peabody School of Education, Nashville, Tennessee.
Donald L. Compton, Department of Special Education, Vanderbilt Peabody School of Education, Nashville, Tennessee
Lynn S. Fuchs, Department of Special Education, Vanderbilt Peabody School of Education, Nashville, Tennessee
Douglas Fuchs, Department of Special Education, Vanderbilt Peabody School of Education, Nashville, Tennessee.
John C. Gore, Vanderbilt University Institute of Imaging Science, Nashville, Tennessee Department of Radiology and Radiological Sciences, Vanderbilt University School of Medicine, Nashville, Tennessee, Department of Biomedical Engineering, Vanderbilt University School of Engineering, Nashville, Tennessee
Adam W. Anderson, Vanderbilt University Institute of Imaging Science, Nashville, Tennessee Department of Radiology and Radiological Sciences, Vanderbilt University School of Medicine, Nashville, Tennessee, Department of Biomedical Engineering, Vanderbilt University School of Engineering, Nashville, Tennessee
REFERENCES
- Adams MJ. Beginning to read. Urbana-Champaign: University of Illinois; 1990. [Google Scholar]
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, et al. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Black SE, Behrmann M. Localization in alexia. In: Kertesz A, editor. Localization and neuroimaging in neuropsychology. San Diego: Academic Press; 1994. pp. 331–376. [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual review of neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow during object naming and word reading. Human Brain Mapping. 1995;3:93–106. [Google Scholar]
- Bradley R, Danielson L, R Hallahan R. Identification of learning disabilities: Research to practice. Mahwah, NJ: Erlbaum; 2002. [Google Scholar]
- Breier JI, Simos PG, Fletcher JM, Castillo EM, Zhang W, Papanicolaou AC. Abnormal activation of temporoparietal language areas during phonetic analysis in children with dyslexia. Neuropsychology. 2003;17:610–621. doi: 10.1037/0894-4105.17.4.610. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Compton D, Fuchs D, Fuchs LS, Bryant JD. Selecting at-risk readers in first grade for early intervention: A two-year longitudinal study of decision rules and procedures. Journal of Educational Psychology. 2006;98:394–409. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Denton CA, Fletcher JM, Anthony JL, Francis DJ. An evaluation of intensive intervention for students with persistent reading difficulties. Journal of Learning Disabilities. 2006;39:447–466. doi: 10.1177/00222194060390050601. [DOI] [PubMed] [Google Scholar]
- Donovan MS, Cross CT. Minority students in special education and gifted education. Washington, DC: National Academy Press; 2002. [Google Scholar]
- Fuchs D, Fuchs LS. Peer-Assisted Learning Strategies: Promoting word recognition, fluency, and reading comprehension in young children. The Journal of Special Education. 2005;39:34–44. [Google Scholar]
- Fuchs LS, Fuchs D, Compton DL. Monitoring early reading development in first grade: Word identification fluency versus nonsense word fluency. Exceptional Children. 2004;71:7–21. [Google Scholar]
- Fuchs LS, Fuchs D, Speece DL. Treatment validity as a unified construct for identifying learning disabilities. Learning Disability Quarterly. 2002;25:33–46. [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences, USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The neural correlates of language production. In: Gazzaniga M, editor. The new cognitive neurosciences. 2nd ed. Cambridge, MA: MIT Press; 2000. pp. 845–865. [Google Scholar]
- Individuals With Disabilities Education Act (IDEA), Pub. L. N. 108-446, 118 Stat. 2647. 2004 Dec.:2657–2658. [Google Scholar]
- Odegard TN, Ring J, Smith S, Biggan J, Black J. Differentiating the neural response to intervention in children with developmental dyslexia. Annals of Dyslexia. 2008;58:1–14. doi: 10.1007/s11881-008-0014-5. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, et al. Dyslexia: Cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Price CJ, Gorno-Tempini ML, Graham KS, Biggio N, Mechelli A, Patterson K, et al. Normal and pathological reading: Converging data from lesion and imaging studies. NeuroImage. 2003;20:S30–S41. doi: 10.1016/j.neuroimage.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Frackowiak RS. The effect of varying stimulus rate and duration on brain activity during reading. Neuroimage. 1996;3:40–52. doi: 10.1006/nimg.1996.0005. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, et al. Failure to activate the left temporoparietal cortex in dyslexia. An oxygen 15 positron emission tomographic study. Archives of Neurology. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue BC, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Samuels SJ. The method of repeating readings. The Reading Teacher. 1979;32:403–408. [Google Scholar]
- Samuels SJ. Toward a theory of automatic information processing in reading, revisited. In: Ruddell R, Ruddell MR, Singer H, editors. Theoretical models and processes of reading. 4th ed. Newark, DE: International Reading Association; 1994. pp. 816–837. [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, et al. Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Constable RT, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychology. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable T, et al. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Society of Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Science USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Wheless JW, Maggio WW, Fletcher JM, Castillo EM, et al. Brain mechanisms for reading: The role of the superior temporal gyrus in word and pseudoword naming. Neuroreport. 2000;11:2443–2447. doi: 10.1097/00001756-200008030-00021. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, et al. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Denton C, Sarkari S, Billingsley-Marshall R, Papanicolaou AC. Magnetic source imaging studies of Dyslexia interventions. Developmental Neuropsychology. 2006;30(1):591–611. doi: 10.1207/s15326942dn3001_4. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Francis DJ, Castillo EM, et al. Early development of neurophysiological processes involved in normal reading and reading disability: A magnetic source imaging study. Neuropsychology. 2005;19:787–798. doi: 10.1037/0894-4105.19.6.787. [DOI] [PubMed] [Google Scholar]
- Torgesen JK. Individual differences in response to early interventions in reading: The lingering problem of treatment resisters. Learning Disabilities Research and Practice. 2000;15:55–64. [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency. Austin, TX: Pro-Ed; 1997. [Google Scholar]
- Treiman R, Kessler B. The role of letter names in the acquisition of literacy. In: Kail R, editor. Advances in child development and behavior. Vol. 31. San Diego, CA: Academic Press; 2003. pp. 105–135. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Scanlon DM, Sipay ER, Small SG, Pratt A, Chen R, et al. Cognitive profiles of difficult-to-remediate and readily remediated poor readers: Early intervention as a vehicle for distinguishing between cognitive and experimental deficits as basic causes of specific reading disability. Journal of Educational Psychology. 1996;88:601–638. [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery Test–Revised/Normative Update. Circle Pines, MN: AGS; 1998. [Google Scholar]