Abstract
PURPOSE
Evaluate the relationship between survivorship care planning (SCP) and survivorship care and health outcomes reported by long-term lung and colorectal cancer survivors.
METHODS
Participants (n=832) were diagnosed and enrolled during 2003-2005. In 2012, patient-reported outcomes (survivorship care and health outcomes) and two patient-reported SCP measures (receipt of written summary of cancer treatment and receipt of instructions on who to see for routine cancer follow-up) were collected. Analyses controlled for SCP predictors collected from medical records and an interview 1 year after diagnosis.
RESULTS
One-in-four survivors reported receiving both SCP elements. Those receiving both were more certain which doctor was in charge (OR 7.0; 95% CI 3.9-12.5), more likely to report follow-up check-ups (OR 5.1; 95% CI 3.3-8.0) and had an MRI/PET/CT scan in the past 2 years (OR 2.8; 95% CI 1.7-4.7) compared to those receiving neither. Physician communication experiences were significantly more positive and having physical exams (OR 2.0; 95% CI 1.2-3.4) and meeting exercise guidelines (OR 1.6; 95% CI 1.004-2.4) more likely. Physical health (p=0.012) and good-to-excellent self-perceived health status (OR 2.2; 95% CI 1.3-3.9) were better for those receiving both elements.
CONCLUSION
SCP may lead to better cancer follow-up care, long-term physical health, and physician/patient communication experiences.
IMPLICATIONS FOR CANCER SURVIVORS
The positive association between outcomes and SCP suggest that efforts to implement SCP should be fruitful.
Keywords: Cohort Studies, Colorectal Neoplasms, Lung Neoplasms, Survivors, Quality of Life
Introduction
In 2006, the Institute of Medicine (IOM) recommended that cancer patients receive a survivorship care plan (SCP) to help them make the transition from the period of active treatment to post-treatment survivorship [1]. Core elements of a SCP include a treatment summary and a plan for follow-up care. Population-based research has identified several deficiencies in care that could be addressed by SCPs [2], but randomized controlled trials have failed to show an effect for SCP use on such measures as psychological well-being, treatment satisfaction, health-related quality of life (HRQOL), or survival [3-5].
It is possible that results from existing randomized trials do not generalize to the kinds of patients who would benefit most from SCPs. For example, the three RCTs conducted so far have been conducted at either university-affiliated or tertiary care hospitals that may provide more comprehensive care in general. The effect of SCPs in the broad population remains to be assessed. It is also unclear how survivorship care planning affects care coordination, health behaviors, or usage of health care services [6]. This is a critical area to explore, because these behaviors are likely to provide the mechanism through which SCPs could improve patient outcomes.
For this study, we analyzed data from long-term disease-free survivors of lung and colorectal cancer in the Cancer Care Outcomes Research and Surveillance Consortium [7] (CanCORS) study. We examined 1) patient characteristics associated with self-reported receipt of two core SCP elements (receipt of a written summary of cancer treatment and instructions on who to see for routine cancer follow-up) and 2) the relationship between receiving survivorship care planning and subsequent HRQOL, as well as patient-reported physician communication, use of cancer follow-up services, and meeting exercise and preventive service guidelines.
Materials and Methods
Study Population
Participants diagnosed with colorectal or lung cancer during 2003-2005 were prospectively enrolled approximately 4 months after diagnosis in the 7 year CanCORS cohort study. CanCORS sites recruited participants 21 years of age or older who were recruited though a number of population-based cancer registries, health maintenance organizations and Veterans Health Administration hospitals from across the country.
We conducted baseline and 1-year follow-up telephone interviews with all study participants. A second follow-up interview was conducted in 2012, approximately 7 years after diagnosis for survivors considered to be disease free. Patient medical records were abstracted to cover a period from 3 months prior to diagnosis to 15 months after diagnosis.
We included only those participants who survived and completed the disease-free follow-up interview in these analyses (N=832; 210 lung and 622 colorectal cancer survivors). This cohort was comparable to the characteristics of the overall CanCORS participants [8] in terms of sex distribution (44% and 47% female, respectively) and race. Survivors, however, had been diagnosed at an earlier stage and younger mean age than the overall cohort.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Measures
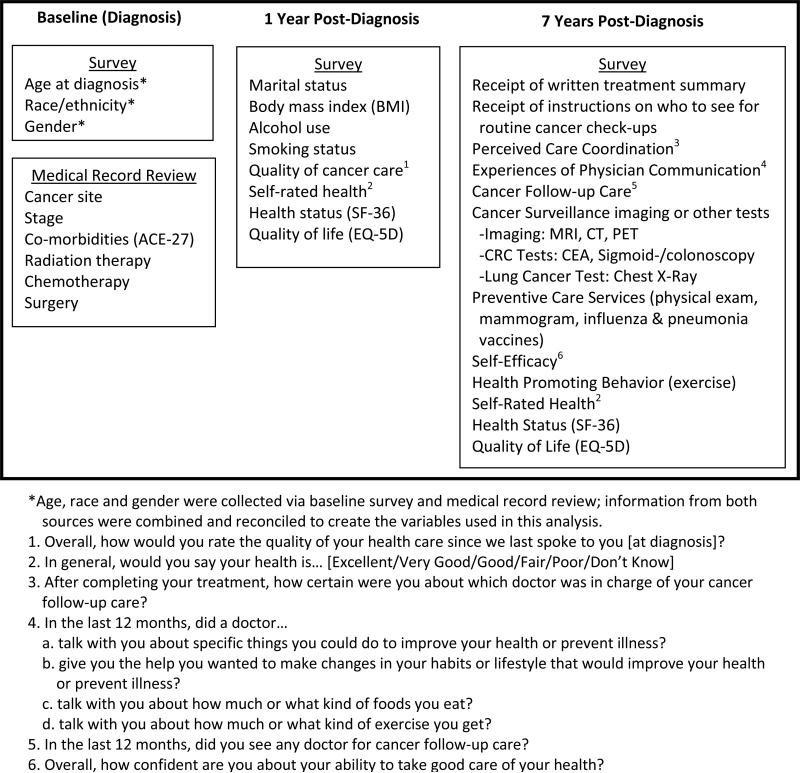
Analytical variables were obtained from surveys at all 3 time points and from medical record data at baseline (Figure 1). Variables constructed using the baseline survey or medical records included age at diagnosis, sex, race/ethnicity, a summary comorbidity index (ACE-27) [9, 10], cancer site and stage, and cancer treatments.
Figure 1.
Timing and source of data elements included in analyses (exact wording of survey questions provided for selected elements).
In order to assess patient status at a time closer to their transition to follow-up care, we included the following variables from the 1-year follow-up survey (conducted approximately 15 months post diagnosis): marital status, body mass index, alcohol use, smoking and patients’ overall ratings of quality of cancer care. Both the 1-year and 7-year follow up surveys queried patients on their general health and HRQOL. This included a single item self-rating of general health, the mental and physical summary scores from the SF-12 [11] and the preference-weighted health status index from the EQ-5D [12].
The SCP indicators of interest were assessed by two items in the 7-year follow-up survey: 1) “After completing your cancer treatment, did any doctor, nurse, or other health professional ever give you a written summary of the cancer treatments that you received” and 2) “After completing your cancer treatment, did you ever receive instructions from a doctor, nurse, or other health professional about where you should return or who you should see for routine cancer check-ups after completing your cancer treatments?” Responses to the two questions were used to create a three-category summary measure of survivorship care planning: did not receive a written summary or follow-up instructions, received either a written summary or follow-up instructions, or received both a written summary and follow-up instructions.
Also from the 7-year follow-up survey, we obtained variables related to perceived care coordination (level of certainty about doctor in charge), physician communication in the preceding 12 months, cancer surveillance imaging in the past 2 years, preventive services, health-promoting behavior (4 items queried whether respondents exercised regularly and for how long at 2 levels, moderate and vigorous), patient self-efficacy about taking care of their health, and health status and HRQOL measures.
Statistical Analysis
Polytomous logistic regression methods were used to examine characteristics associated with the three-category SCP summary variable. Adjusted percents (with their 95% confidence intervals) were generated to assess the magnitude of differences for categorical variables, using Graubard and Korn's extension to polytomous responses [13]. We assessed whether the SCP summary variable was associated with self-reported long-term outcomes in logistic regression models for each dichotomous outcome. Hypothesized potential mediating roles of self-efficacy and certainty about which doctor was in charge of follow-up care were examined by comparing the adjusted odds ratios (ORs) and 95% confidence intervals in models with and without these variables. Continuous HRQOL scores were modeled with general linear model methods and the magnitude of differences was assessed with least square means. Analyses were run in SAS 9.3 and statistical significance was defined as p-values ≤ 0.05.
Results
Table 1 displays characteristics of patients according to whether or not they reported receiving one or both of the elements of a SCP. Of the 832 survivors, 210 (25%) reported receiving a written summary of their treatment and instructions on who to see for routine care; 391 (47%) indicated they received either a written summary of their treatment or instructions on who to see for routine care (but not both); and 231 (28%) received neither SCP element. Of the same 832 patients, 247 (30%) reported receiving a written summary of their treatment and 564 (68%) reported instructions on who to see for routine care. Of the 391 survivors who received only one of the two SCP elements, 37 (9%) received a written summary and 354 (91%) received follow-up instructions.
Table 1.
Description of baseline and 1-year characteristics associated with reported receipt of survivorship care planning by long-term (7-years) survivors.
| Characteristic | n (%) | Reported receipt of a written summary of cancer treatment and/or instructions on who to see for routine cancer fol ow-ups (N=832) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Percent (95% Confidence Interval)a | Adjusted Percent (95% Confidence Interval)c | ||||||||
| Received Neither (n=231) | Received Only One (n=391) | Received Both (n=210) | p-valueb | Received Neither (n=231) | Received Only One (n=391) | Received Both (n=210) | p-valued | ||
| Age in years | <0.0001 | 0.005 | |||||||
| ≤ 54 | 164 (19.7) | 20 (14-26) | 46 (39-54) | 34 (26-41) | 23 (17-30) | 44 (36-52) | 33 (25-40) | ||
| 55-64 | 252 (30.3) | 22 (17-27) | 50 (44-57) | 28 (22-33) | 22 (17-27) | 50 (43-56) | 28 (22-34) | ||
| 65-74 | 258 (31.0) | 28 (22-33) | 49 (43-55) | 23 (18-28) | 27 (21-32) | 49 (43-55) | 24 (19-29) | ||
| ≥ 75 | 158 (19.0) | 45 (37-53) | 39 (31-46) | 16 (11-22) | 41 (33-48) | 41 (33-48) | 19(13-25) | ||
| Gender | 0.833 | 0.775 | |||||||
| Male | 441 (53.0) | 28 (24-32) | 46 (41-51) | 26 (22-30) | 29 (25-33) | 46 (41-50) | 26 (22-30) | ||
| Female | 391 (47.0) | 27 (23-32) | 48 (43-53) | 25 (20-29) | 26 (22-31) | 47 (42-52) | 26 (22-31) | ||
| Race/ethnicity | 0.094 | 0.079 | |||||||
| White | 633 (76.2) | 28 (25-32) | 49 (45-53) | 23 (20-26) | 28 (24-31) | 49 (45-53) | 24 (20-27) | ||
| Hispanic | 22 (2.7) | 36 (16-56) | 36 (16-56) | 27 (9-46) | 33 (12-54) | 34 (13-55) | 33 (12-54) | ||
| Black | 105 (12.6) | 26 (17-34) | 38 (29-47) | 36 (27-45) | 30 (21-39) | 34 (25-43) | 37 (27-46) | ||
| Other | 71 (8.5) | 24 (14-34) | 46 (35-58) | 30 (19-40) | 22 (12-32) | 49 (37-61) | 29 (18-40) | ||
| Marital Status at 1 year | 0.345 | 0.771 | |||||||
| Married/living with partner | 582 (70.0) | 26 (23-30) | 48 (44-52) | 26 (22-29) | 27 (23-31) | 47 (43-51) | 26 (22-29) | ||
| Not married | 250 (30.0) | 31 (25-37) | 44 (38-51) | 24 (19-30) | 29 (23-35) | 45 (38-51) | 26 (21-32) | ||
| BMI at 1 year | 0.090 | 0.249 | |||||||
| Underweight/Normal | 210 (25.4) | 34 (28-41) | 43 (37-50) | 22 (17-28) | 33 (26-39) | 42 (35-49) | 26 (20-32) | ||
| Pre-obese | 316 (38.2) | 28 (23-33) | 47 (41-52) | 25 (21-30) | 28 (23-33) | 46 (40-51) | 26 (21-31) | ||
| Obese | 301 (36.4) | 23 (18-28) | 50 (44-55) | 27 (22-32) | 23 (18-28) | 50 (45-56) | 26 (21-31) | ||
| Alcohol Use (drinks per week)- 1-year survey | 0.632 | 0.512 | |||||||
| None | 367 (44.3) | 29 (25-34) | 46 (41-51) | 25 (20-29) | 31 (26-36) | 45 (40-50) | 24 (20-29) | ||
| >0 – < 7 | 324 (39.1) | 24 (20-29) | 50 (45-55) | 26 (21-30) | 24 (19-29) | 49 (44-55) | 26 (22-31) | ||
| 7-10.5 | 109 (13.1) | 31 (23-40) | 43 (34-52) | 26 (17-34) | 27 (18-35) | 43 (34-53) | 30 (22-39) | ||
| ≥ 17.5 | 29 (3.5) | 34 (17-52) | 38 (20-56) | 28 (11-44) | 30 (13-47) | 41 (23-60) | 28 (11-45) | ||
| Smoking Status at 1 year | 0.094 | 0.162 | |||||||
| Never | 281 (33.8) | 23 (19-28) | 51 (45-56) | 26 (21-31) | 24 (19-29) | 50 (44-56) | 26 (21-31) | ||
| Former | 410 (49.3) | 28 (24-33) | 47 (42-52) | 25 (21-29) | 28 (23-32) | 45 (41-50) | 27 (22-31) | ||
| Current | 55 (6.6) | 25 (14-37) | 44 (31-57) | 31 (19-43) | 27 (15-39) | 47 (34-61) | 26 (14-38) | ||
| Unknown | 86 (10.3) | 41 (30-51) | 38 (28-49) | 21 (12-30) | 40 (29-51) | 36 (25-47) | 24 (14-33) | ||
| Comorbidity Status at Diagnosis | 0.744 | 0.568 | |||||||
| None | 251 (30.2) | 26 (21-32) | 51 (45-57) | 23 (18-28) | 28 (23-34) | 50 (44-56) | 22 (16-27) | ||
| Mild | 351 (42.2) | 28 (24-33) | 44 (39-50) | 27 (23-32) | 28 (23-33) | 44 (38-49) | 29 (24-33) | ||
| Moderate | 148 (17.8) | 28 (20-35) | 46 (38-54) | 26 (19-33) | 26 (19-34) | 45 (36-53) | 29 (22-37) | ||
| Severe | 82 (9.9) | 30 (21-40) | 48 (37-58) | 22 (13-31) | 27 (17-37) | 49 (39-60) | 24 (14-33) | ||
| Cancer Type | 0.138 | 0.039 | |||||||
| Lung | 210 (25.2) | 28 (22-34) | 52 (45-59) | 20 (15-26) | 24 (18-30) | 56 (49-63) | 20 (15-26) | ||
| CRC | 622 (74.8) | 28 (24-31) | 45 (41-49) | 27 (23-30) | 29 (25-33) | 43 (39-47) | 28 (24-32) | ||
| Cancer Stage | 0.554 | 0.162 | |||||||
| Stage I | 367 (44.8) | 30 (26-35) | 45 (40-50) | 25 (21-30) | 29 (24-34) | 41 (36-46) | 30 (26-35) | ||
| Stage II | 206 (25.2) | 26 (20-32) | 48 (41-54) | 26 (20-32) | 24 (18-30) | 49 (42-56) | 26 (20-33) | ||
| Stage III | 218 (26.6) | 26 (20-31) | 49 (42-55) | 26 (20-31) | 29 (23-35) | 50 (44-57) | 21 (15-26) | ||
| Stage IV | 28 (3.4) | 14 (1-27) | 61 (43-79) | 25 (9-41) | 17 (3-31) | 64 (46-82) | 19 (4-34) | ||
| Had Radiation Treatment | 0.645 | 0.501 | |||||||
| Yes | 118 (14.2) | 29 (21-37) | 43 (34-52) | 28 (20-36) | 33 (29-36) | 43 (39-46) | 24 (21-28) | ||
| No | 714 (85.8) | 28 (24-31) | 48 (44-51) | 25 (22-28) | 27 (19-35) | 47 (38-56) | 26 (18-34) | ||
| Had Chemotherapy | 0.003 | 0.029 | |||||||
| Yes | 374 (45.0) | 23 (18-27) | 48 (43-53) | 30 (25-34) | 24 (20-28) | 44 (39-49) | 32 (28-37) | ||
| No | 458 (55.0) | 32 (28-36) | 47 (42-51) | 22 (18-25) | 30 (26-35) | 49 (43-54) | 21 (17-25) | ||
| Had Surgery | 0.819 | 0.395 | |||||||
| Yes | 778 (93.5) | 28 (24-31) | 47 (44-51) | 25 (22-28) | 28 (15-41) | 47 (32-61) | 26 (13-38) | ||
| No | 54 (6.5) | 31 (19-44) | 44 (31-58) | 24 (13-35) | 25 (22-28) | 39 (36-43) | 35 (32-39) | ||
| Quality of Cancer Care at 1 year | 0.012 | 0.215 | |||||||
| Excellent or Very Good | 677 (82.1) | 27 (24-30) | 47 (43-51) | 26 (23-30) | 27 (24-30) | 46 (42-50) | 27 (24-30) | ||
| Good | 112 (13.6) | 26 (18-34) | 52 (43-61) | 22 (15-30) | 25 (17-34) | 52 (43-62) | 22 (14-30) | ||
| Fair or poor | 36 (4.4) | 53 (36-69) | 28 (13-42) | 19 (7-32) | 43 (26-60) | 38 (22-54) | 19 (6-33) | ||
| Self-rated Health at 1 year | 0.023 | 0.170 | |||||||
| Excellent/Very Good | 419 (50.5) | 25 (21-29) | 50 (45-55) | 25 (21-29) | 26 (21-30) | 49 (45-54) | 25 (21-29) | ||
| Good | 278 (33.5) | 26 (21-31) | 49 (43-54) | 25 (20-30) | 27 (22-33) | 47 (41-53) | 26 (21-31) | ||
| Fair/Poor | 132 (15.9) | 38 (30-46) | 35 (27-43) | 27 (20-35) | 34 (26-42) | 35 (27-44) | 31 (23-39) | ||
Wald Confidence intervals;
Unadjusted Pearson chi-square value;
Extension of Graubard and Korn [13];
Wald chi-square p-value. Adjusted for study site, age, gender, race, marital status at 1 year, BMI at 1 year, drinks per week at 1 year, history of smoking at 1 year, cancer type, cancer stage, surgery, radiation, chemotherapy, quality of care at 1 year, self-rated health at 1 year.
Older people and lung cancer survivors were significantly less likely to report receiving survivorship care planning, while those who received chemotherapy were more likely. Survivorship care planning also varied by participating study site. None of the other baseline or 1-year measured characteristics were significantly associated with the SCP indicators in the multivariable model.
The adjusted associations between receiving survivorship care planning and perceived care coordination, experiences of physician communication, cancer follow-up care, receipt of preventive care services, self-efficacy, health promoting behavior, and general health are illustrated in Table 2. Receipt of survivorship care planning was significantly associated with all four measures of physician communication about health promotion, with patients who received both SCP elements being the most likely to have talked with their physician about these issues. Patients who received survivorship care planning were much more likely to be very certain about which doctor was in charge of their cancer follow-up care and have more positive self-efficacy. They were also significantly more likely to have seen a physician for cancer follow-up care, and to have an MRI, PET, or CT scan in the two years prior to the 7-year follow-up survey. However, they were no more likely to see a primary care provider in the past 12 months. Having a physical exam was the only preventive service associated with having received survivorship care planning. Survivorship care planning was significantly associated with achieving exercise guidelines. In general, associations between survivorship care planning and outcomes were stronger among those who received both elements compared to those who only received one, particularly in perceived care coordination and seeing a doctor for follow-up care.
Table 2.
Relationship of receiving survivorship care planning with perceived coordination of care, physician-patient communication, use of health care services, self-efficacy, health (exercise) behavior, and self-rated health.
| Outcome Variable | Received Summary and/or Instructionsa | Adjustedb OR (95% CI) |
|---|---|---|
| Perceived Care Coordination | ||
| Very certain about which doctor was in charge of cancer follow-up care | Both | 7.0 (3.9-12.5) |
| One | 2.2 (1.5-3.3) | |
| Experiences of Physician Communication | ||
| MD talked about things to improve health or prevent illness | Both | 2.6 (1.6-4.0) |
| One | 1.6 (1.1 -2.4) | |
| MD gave help wanted to change lifestyle to improve health | Both | 2.8 (1.8-4.4) |
| One | 1.2 (0.8-1.8) | |
| MD talked about how much/what kind of foods to eat | Both | 3.6 (2.2-5.9) |
| One | 1.6 (1.02-2.5) | |
| MD talked about how much/what kind of exercise to get | Both | 3.3 (2.2-5.1) |
| One | 1.6 (1.1-2.4) | |
| Cancer Follow-up Care | ||
| Saw any doctor for cancer f/up care in the past 12 months | Both | 5.1 (3.3-8.0) |
| One | 2.8 (1.9-4.1) | |
| Saw any cancer specialists in the past 12 months | Both | 4.0 (2.5-6.3) |
| One | 2.1 (1.4-3.2) | |
| Saw a primary care provider in the past 12 months | Both | 0.9 (0.3-2.6) |
| One | 0.5 (0.2-1.2) | |
| Had an MRI, PET or CT in the past 2 years | Both | 2.8 (1.7-4.7) |
| One | 2.0 (1.3-3.2) | |
| Receipt of Preventive Care Services | ||
| Had a physical exam in the past 12 months | Both | 2.0 (1.2-3.4) |
| One | 1.3 (0.8-2.0) | |
| Had a mammogram within past 2 years (females only) | Both | 1.3 (0.6-3.0) |
| One | 1.6 (0.8-3.4) | |
| Had a pap test within past 2 years (females only) | Both | 1.7 (0.8-3.4) |
| One | 1.5 (0.8-2.8) | |
| Had cholesterol checked in the past 12 months | Both | 1.3 (0.8.-2.4) |
| One | 1.2 (0.7-2.0) | |
| Ever received a pneumonia vaccine | Both | 1.5 (0.95-2.4) |
| One | 1.1 (0.7-1.7) | |
| Had an influenza vaccine in the past 12 months | Both | 1.0 (0.6-1.6) |
| One | 1.1 (0.7-1.7) | |
| Self-efficacy | ||
| Completely or very confident about ability to take good care of health | Both | 1.8 (1.04-3.1) |
| One | 1.0 (0.6-1.6) | |
| Health Promoting Behavior | ||
| Exercised regularly in last 4 weeks | Both | 1.4 (0.8-2.5) |
| One | 1.0 (0.6-1.7) | |
| Met or exceeded exercise guidelines (150 minutes moderate or 75 minutes vigorous activity per week) | Both | 1.6 (1.004-2.4) |
| One | 1.3 (0.9-1.9) | |
| General health | ||
| Excellent/very good/good vs. fair/poor | Both | 2.2 (1.3-3.9) |
| One | 1.8 (1.1-2.9) | |
Reference category is those who received neither a written treatment summary nor instructions about who to see for cancer follow-up care.
Adjusted for study site, age, gender, race, marital status at 1 year, BMI at 1 year, drinks per week at 1 year, history of smoking at 1 year, cancer type, cancer stage, surgery, radiation, chemotherapy, quality of care at 1 year, and self-rated health at 1 year. Significant values are indicated in bold.
HRQOL outcomes in relation to reporting receipt of survivorship care planning are presented in Table 2 (general self-perceived health) and Table 3 (SF-12 scales and EQ-5D index). After adjusting for variables shown to be significant in Table 1, patients who received survivorship care planning were significantly more likely to report good or better health status and had significantly higher SF-12 physical health component scores. There were no significant differences in mental health or EQ-5D health index rating.
Table 3.
Relationship of receiving survivorship care planning with health status and quality of life outcomes.
| Reported receipt of a written summary of cancer treatment and/or instructions on who to see for routine cancer check-ups | Outcome Measure Adjusteda Mean Score (Standard Error) | ||
|---|---|---|---|
| SF-12 Mental Health | SF-12 Physical Health | EQ-5D Index | |
| Received both | 51.8 (1.4) | 40.1 (1.4) | 0.80 (0.02) |
| Received one | 52.1 (1.4) | 40.0 (1.4) | 0.81 (0.02) |
| Received neither | 53.3 (1.4) | 37.8 (1.4) | 0.78 (0.02) |
| p-value | 0.221 | 0.012 | 0.180 |
Adjusted for participating site, age, gender, race, marital status at 1 year, BMI at 1 year, drinks per week at 1 year, history of smoking at 1 year, cancer type, cancer stage, surgery, radiation, chemotherapy, quality of care at 1 year, and score on the outcome measure at 1 year.
A potential mediating role of self-efficacy and certainty about who was in charge of cancer follow-up care was evaluated by adding these variables to the other outcome models in Tables 2 and 3 (data not shown). The odds ratios for survivorship care planning did not change appreciably (i.e., the odds ratios decreased by only 11% or less) for the physician communication and cancer follow-up care outcomes, however there was evidence of a modest mediating role (odds ratio decreased by 25%) for receiving a physical exam. The findings for the HRQOL outcomes were also unchanged.
Discussion
Recognizing that many cancer patients lack adequate support to successfully transition from being a patient to a survivor, the IOM recommended in 2006 that every cancer patient be provided a survivorship care plan (SCP) that includes a treatment summary and a plan for future care. The IOM believed this was a “common sense” intervention that should be immediately implemented even though there was little evidence for its effectiveness at the time. Although a major US initiative has ensued to aggressively implement the IOM recommendation [1, 14, 15], randomized controlled trials have failed to show a consistent benefit of using SCPs [3-5].
We examined the survivorship care planning experience among 7-year disease-free survivors from the multi-center, population- and health system-based CanCORS cohort. This is the first large study to report on experiences of survivorship care planning in community practice among long-term lung and colorectal cancer survivors, and the associations reported here lend support for the major initiative underway to widely implement survivorship care plans. Although only one-fourth of patients received both written treatment summary and instruction on who to see for routine cancer follow-ups, those who did had better outcomes. Specifically, those who reported receiving both of these core elements were more likely to report ongoing checkups with a doctor for follow-up cancer care and to have had cancer surveillance imaging. Health-promoting activities including having physical exams and meeting exercise guidelines were also more likely. Physical health scores were significantly better for those who reported survivorship care planning, though there was no difference in overall HRQOL or mental health. Patients who received both SCP components were more likely to be confident in their ability to take care of their own health. These community-based findings are more positive than results of randomized trials of SCPs.
The first large randomized trial [3] compared breast cancer survivors who received a SCP to survivors who received a discharge evaluation along with a discharge letter sent to the follow-up PCP. Results showed no differences between the two groups in terms of distress, patient satisfaction, health status, or continuity of care. However, the relevance of the comparison group for US patients has been criticized [16]. Another randomized trial [4] involved a treatment group which met with a nurse practitioner to review a personalized SCP based on the template developed by ASCO [14] and also received a survivorship manual [17]; the control group received the manual alone. No differences were found among measures of treatment satisfaction, cancer impact, physical well-being, or quality of life except that the SCP intervention reduced health worry. A third study examined the experiences of gynecologic cancer survivors whose physicians were randomly assigned to SCP versus No SCP groups [5]. They found no differences on survivors’ perceived quality of care, satisfaction with health services, or rated helpfulness of written materials.
Whereas randomized trials have not found improvements in continuity of care or health status, we found improvements in both. One reason for this difference may be that randomized trials primarily include relatively high functioning people. For example, the cohort studied by Grunfeld et al. had low scores of distress at baseline [3, 18], and these people may be less likely to benefit from a SCP. Indeed, patients with lower mental well-being have reported the greatest need for health information. This effect is especially pronounced for those who had low confidence in their ability to obtain information [19]. It is also possible that the settings of existing randomized trials, university-based or tertiary care hospitals, generally provide more comprehensive care to their patients. The follow-up periods for the trials were also much shorter than that in our study.
Comparatively, there is little research on the effect that SCPs have on potential mediating factors such as coordination of care, physician-patient communication, health behaviors, or usage of health care services. This is a critical area to explore, because these behaviors are likely to provide the mechanism through which SCPs may potentially improve patient symptoms and HRQOL. Parry et al. developed a model that portrays care plans working within an infrastructure to affect the coordination of care among patients and all of their providers [6]. In this model, effective communication and coordination leads to better short-term outcomes (e.g., effective use of health care resources) that, in turn, lead to better long-term outcomes (e.g., improved HRQOL). In support of the model, we found associations of SCPs with markers of care coordination, patient experiences of physician communication, and cancer follow-up care and health promotion. Others have shown that patients report high satisfaction with treatment plans, and they also believe it helps doctor/patient and doctor/doctor communication [20-23]. Another study has shown that treatment summaries are associated with more accurate survivor knowledge about breast and colorectal cancer diagnoses and the treatment they received [24]. Our results agree with other findings [25] that SCPs are associated with feelings of self-efficacy. We were unable to demonstrate compelling evidence of a mediating effect of self-efficacy on outcomes, however.
Similarly, primary care providers report that SCPs increase their confidence in their ability to care for cancer survivors’ follow-up needs, and they also report that SCPs lead to better coordination of care and improved communication among physicians [26]. We found effects of SCPs on cancer surveillance imaging and visits to cancer physicians, but little impact on preventive service use. One reason may be that the two SCP questions focused on cancer treatments and who to see for cancer follow-up care and this may not correlate with providing advice with preventive services. One of the few studies to examine the effect of SCPs on use of health resources evaluated a random sample of Hodgkin lymphoma patients who had not had a recommended screening mammogram or echocardiogram within the prior two years [27]. Six months after a short SCP with recommendations for surveillance was mailed to the survivors and their physicians, 41% of the participants reported having a mammogram and 20% reported having an echocardiogram. This suggests that SCPs can encourage appropriate follow-up care.
We found evidence of potential disparities in receipt of survivorship care planning. Patients were less likely to have received both elements of care planning if they were lung cancer survivors or over 65 at the time of diagnosis. These may reflect patient preferences for less information or lower perceived need for full survivorship care planning by physicians, either due to less aggressive initial treatment or lower expectations of longevity. It is possible that not all patients require the same degree of survivorship care planning. However, because of the number of patients affected (one-fourth of long-term disease free survivors were lung cancer patients and half were 65 years of age or older), these findings require future attention to ensure that survivorship care is delivered to all patients who need and want it.
Potential Limitations
The CanCORS study enrolled a cohort of patients with lung and colorectal cancer diagnosed during 2003-2005 who would have been entering follow-up care about the time that the 2006 IOM report was published. Our focus, therefore, was not on any specific, contemporary SCP template. Rather, our focus has been on two specific elements that are core components of survivorship care planning: treatment summaries and instructions for follow-up. We found that 247 (30%) patients reported being given a written treatment summary and 564 (68%) patients reported receiving instructions on who to see for follow-up care.
In contrast, a recent nationally representative survey of oncologists found that less than 5% reported providing written SCPs to their patients, and 32% reported discussing who patients should see for follow-up care [28]. Although these metrics are conceptually different than our patient-reported measures, these data do suggest that patients experience survivorship care even if their oncologists do not often provide it formally. It is not clear what actually constituted a treatment summary as far as the patients were concerned. It could have been anything from a roughly sketched outline to a more complete summary. Also, we did not ask who provided the instructions about who to see and it could be that nurses or primary care providers are a frequent source of this information. Our findings essentially reflect patient perceptions of the two SCP components that were assessed by the surveys.
It is possible that other factors associated with SCP use are responsible for the improved outcomes we observed. Highly engaged patients, for example, may encourage physicians to provide documentation of their treatment plans, and these patients may also be more diligent about getting appropriate follow-up care, exercising, and communicating with their care team. Similarly, physicians who provide SCP elements may be more effective in encouraging patients in these same behaviors. Well-designed RCTs are generally not vulnerable to such limitations, and this illustrates the need for such prospective trials in community settings with diverse patient populations.
This study only included those who were disease free survivors at 7 years post-diagnosis and findings may not generalize to those who survived the initial treatment phase but then experienced recurrence and/or death prior to 7 years. If receipt of SCP and the outcomes of interest were both associated with surviving disease-free to the 7 year assessment then this could introduce bias in the estimated associations between SCPs and the outcomes of interest.
Indicators for receiving written treatment summary or instructions on who to see for routine cancer check-ups were based on patient recall. Blinder et al. [20] reported high (>90%) short-term recall of receiving a treatment plan or summary. However, our recall period was much longer. It is possible that the relationships we found are a result of better generalized recall of health-related issues. However, the specificity observed argues against this, such as the strong relationship with certainty about who to see for follow-up care, the smaller association with self-efficacy for taking care of health, and the much stronger relationships with cancer follow-up care than with preventive care and behavior. In addition, the greater frequency of care planning among patients who received chemotherapy (obtained from the medical record) could not be explained by recall bias. Patient recall of survivorship care planning also correlated with assessments of quality of cancer care that they made 6 years previously. This is a time when they were most likely to receive a SCP, although we don't have information on when patients actually received the SCP elements described here.
Conclusion
Since the IOM report was published in 2006 [1], studies have revealed specific areas of survivorship care that could be addressed through the use of SCPs. Arora et al., for example, found that over 60% of post-treatment survivors in their study reported that they did not get the help they needed to improve their health once their treatment ended, and they also didn't get support to make healthy lifestyle changes [2]. The same number of participants reported that their physician did not understand how their cancer had affected their quality of life.
Many organizations responded to this need and the IOM recommendation by developing SCP templates [14, 29, 30], and by 2015, cancer programs must employ SCPs in order to maintain accreditation from the American College of Surgeons Commission on Cancer [15]. Recent calls for better evaluation and attention to the processes involved in survivorship care planning are likely to result in improved models for delivering transitional and follow-up care. Our findings of positive relationships between survivorship care planning, health outcomes and patients’ ability to navigate their care and health needs, suggest that these efforts will be fruitful.
Acknowledgements
The work of the CanCORS Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network U01 CA093332, Harvard Medical School/Northern California Cancer Center U01 CA093324, RAND/UCLA U01 CA093348, University of Alabama at Birmingham U01 CA093329, University of Iowa U01 CA093339, University of North Carolina U01 CA093326) and by a Department of Veterans Affairs grant to the Durham VA Medical Center CRS 02-164. This project was also supported in part by the University of Iowa Holden Comprehensive Cancer Center (HCCC) Population Research Core, funded in part by P30 CA086862, as well as Institutional Research Grant IRG-77-004-34 from the American Cancer Society to the HCCC.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Elizabeth A. Chrischilles, S424 CPHB, Department of Epidemiology University of Iowa, Iowa City, Iowa 52242
Bradley D. McDowell, 5240 MERF, Holden Comprehensive Cancer Center University of Iowa Iowa City, IA 52242
Linda Rubenstein, S415 CPHB, Department of Epidemiology University of Iowa Iowa City, IA 52242.
Mary Charlton, S453 CPHB, Department of Epidemiology University of Iowa Iowa City, IA 52242.
Jane Pendergast, N314 CPHB, Department of Biostatistics University of Iowa Iowa City, IA 52242.
Grelda Yazmin Juarez, 2230 WL, Center for Public Health Statistics University of Iowa Iowa City, IA 52242.
Neeraj K. Arora, 9609 Medical Center Dr. Room 2E532 MSC 9760 National Cancer Institute Bethesda, MD 20892
References
- 1.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. National Academies Press; Washington, D.C.: 2006. [Google Scholar]
- 2.Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor's perspective. J Clin Oncol. 2011;29(10):1280–9. doi: 10.1200/JCO.2010.32.1554. doi:10.1200/JCO.2010.32.1554; 10.1200/JCO.2010.32.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011;29(36):4755–62. doi: 10.1200/JCO.2011.36.8373. doi:10.1200/JCO.2011.36.8373; 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- 4.Hershman DL, Greenlee H, Awad D, Kalinsky K, Maurer M, Kranwinkel G, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795–806. doi: 10.1007/s10549-013-2486-1. doi:10.1007/s10549-013-2486-1. [DOI] [PubMed] [Google Scholar]
- 5.Brothers BM, Easley A, Salani R, Andersen BL. Do survivorship care plans impact patients' evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecol Oncol. 2013;129(3):554–8. doi: 10.1016/j.ygyno.2013.02.037. doi:10.1016/j.ygyno.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parry C, Kent EE, Forsythe LP, Alfano CM, Rowland JH. Can't See the Forest for the Care Plan: A Call to Revisit the Context of Care Planning. J Clin Oncol. 2013;31(21):2651–3. doi: 10.1200/JCO.2012.48.4618. doi:10.1200/JCO.2012.48.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayanian JZ, Chrischilles EA, Fletcher RH, Fouad MN, Harrington DP, Kahn KL, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22(15):2992–6. doi: 10.1200/JCO.2004.06.020. doi:10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Catalano PJ, Ayanian JZ, Weeks JC, Kahn KL, Landrum MB, Zaslavsky AM, et al. Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the surveillance, epidemiology, and end results program. Med Care. 2013;51(2):e9–15. doi: 10.1097/MLR.0b013e318222a711. doi:10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccirillo J, Costas I, Claybour P, Borah A, Grove L, Jeffe D. The measurement of comorbidity by cancer registries. J Registry Manag. 2003;30:8–14. [Google Scholar]
- 10.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of Comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–7. doi: 10.1001/jama.291.20.2441. doi:10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy. 1997;2(1):14–8. doi: 10.1177/135581969700200105. [DOI] [PubMed] [Google Scholar]
- 12.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 13.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 14.ASCO Cancer Treatment Summaries [July 15 2014];American Society of Clinical Oncology. 2013 http://www.cancer.net/survivorship/follow-care-after-cancer-treatment/asco-cancer-treatment-summaries.
- 15.Cancer Program Standards [July 15 2014];Ensuring Patient-Centered Care. Commision on Cancer. 2012 http://www.facs.org/cancer/coc/programstandards2012.pdf.
- 16.Stricker CT, Jacobs LA, Palmer SC. Survivorship care plans: an argument for evidence over common sense. J Clin Oncol. 2012;30(12):1392–3. doi: 10.1200/JCO.2011.40.7940. author reply 3-5. doi:10.1200/JCO.2011.40.7940; 10.1200/JCO.2011.40.7940. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute Facing Forward: Life After Cancer Treatment. 2012.
- 18.Jefford M, Schofield P, Emery J. Improving survivorship care. J Clin Oncol. 2012;30(12):1391–2. doi: 10.1200/JCO.2011.40.5886. author reply 3-4. doi:10.1200/JCO.2011.40.5886; 10.1200/JCO.2011.40.5886. [DOI] [PubMed] [Google Scholar]
- 19.Kent EE, Arora NK, Rowland JH, Bellizzi KM, Forsythe LP, Hamilton AS, et al. Health information needs and health-related quality of life in a diverse population of long-term cancer survivors. Patient Educ Couns. 2012;89(2):345–52. doi: 10.1016/j.pec.2012.08.014. doi:10.1016/j.pec.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blinder VS, Norris VW, Peacock NW, Griggs JJ, Harrington DP, Moore A, et al. Patient perspectives on breast cancer treatment plan and summary documents in community oncology care: A pilot program. Cancer. 2013;119(1):164–72. doi: 10.1002/cncr.27856. doi:10.1002/cncr.27856. [DOI] [PubMed] [Google Scholar]
- 21.Kantsiper M, McDonald EL, Geller G, Shockney L, Snyder C, Wolff AC. Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers. J Gen Intern Med. 2009;24(Suppl 2):S459–66. doi: 10.1007/s11606-009-1000-2. doi:10.1007/s11606-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marbach TJ, Griffie J. Patient preferences concerning treatment plans, survivorship care plans, education, and support services. Oncol Nurs Forum. 2011;38(3):335–42. doi: 10.1188/11.ONF.335-342. doi:10.1188/11.onf.335-342. [DOI] [PubMed] [Google Scholar]
- 23.Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25(16):2270–3. doi: 10.1200/JCO.2006.10.0826. doi:10.1200/jco.2006.10.0826. [DOI] [PubMed] [Google Scholar]
- 24.Nissen MJ, Tsai ML, Blaes AH, Swenson KK, Koering S. Effectiveness of treatment summaries in increasing breast and colorectal cancer survivors' knowledge about their diagnosis and treatment. Journal of Cancer Survivorship-Research and Practice. 2013;7(2):211–8. doi: 10.1007/s11764-012-0261-7. doi:10.1007/s11764-012-0261-7. [DOI] [PubMed] [Google Scholar]
- 25.Casillas J, Syrjala KL, Ganz PA, Hammond E, Marcus AC, Moss KM, et al. How confident are young adult cancer survivors in managing their survivorship care? A report from the LIVESTRONG Survivorship Center of Excellence Network. J Cancer Surviv. 2011;5(4):371–81. doi: 10.1007/s11764-011-0199-1. doi:10.1007/s11764-011-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who Provides Psychosocial Follow-Up Care for Post-Treatment Cancer Survivors? A Survey of Medical Oncologists and Primary Care Physicians. J Clin Oncol. 2012;30(23):2897–905. doi: 10.1200/JCO.2011.39.9832. doi:10.1200/JCO.2011.39.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oeffinger KC, Hudson MM, Mertens AC, Smith SM, Mitby PA, Eshelman-Kent DA, et al. Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr Blood Cancer. 2011;56(5):818–24. doi: 10.1002/pbc.22696. doi:10.1002/pbc.22696; 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanch-Hartigan D, Forsythe LP, Alfano CM, Smith T, Nekhlyudov L, Ganz PA, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol. 2014;32(15):1578–85. doi: 10.1200/JCO.2013.51.7540. doi:10.1200/jco.2013.51.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Survivorship Care Plan Builder [July 15 2014];Journey Forward. http://www.journeyforward.org/professionals/survivorship-care-plan-builder.
- 30.LIVESTRONG Care Plan [July 15 2014];Trustees of the University of Pennsylvania. http://www.livestrongcareplan.org/.