Abstract
Purpose
The aim of this study was to investigate feasibility of exercise-based rehabilitation delivered after hospital discharge in patients with intensive care unit–acquired weakness (ICU-AW).
Materials and methods
Twenty adult patients, mechanically ventilated for more than 48 hours, with ICU-AW diagnosis at ICU discharge were included in a pilot feasibility randomized controlled trial receiving a 16-session exercise-based rehabilitation program. Twenty-one patients without ICU-AW participated in a nested observational cohort study. Feasibility, clinical, and patient-centered outcomes were measured at hospital discharge and at 3 months.
Results
Intervention feasibility was demonstrated by high adherence and patient acceptability, and absence of adverse events, but this must be offset by the low proportion of enrolment for those screened. The study was underpowered to detect effectiveness of the intervention. The use of manual muscle testing for the diagnosis of ICU-AW lacked robustness as an eligibility criterion and lacked discrimination for identifying rehabilitation requirements. Process evaluation of the trial identified methodological factors, categorized by “population,” “intervention,” “control group,” and “outcome.”
Conclusions
Important data detailing the design, conduct, and implementation of a multicenter randomized controlled trial of exercise-based rehabilitation for survivors of critical illness after hospital discharge have been reported.
Registration
Clinical Trials Identifier NCT00976807
Keywords: Exercise rehabilitation, Critical illness, Hospital discharge, Intensive care unit–acquired weakness
1. Introduction
Peripheral skeletal muscle wasting and dysfunction are major complications of critical illness, both occurring early and progressing rapidly [1]. The resulting intensive care unit–acquired weakness (ICU-AW) is a major factor contributing to “post–intensive care syndrome” evident in survivors [2]. Although there are data to support the use of exercise therapy to address physical deconditioning within the ICU [3–8] and after transfer to the ward [9–11], the clinical benefit beyond hospital discharge is controversial, and therefore, the delivery of such a service has been inconsistent. In the UK, despite the high profile of this area and publication of guidelines from the National Institutes of Health and Care Excellence [12], we have recently shown that there is lack of available services for post–critical illness patients [13]. Indeed, a paucity of evidence, including randomized controlled trial (RCT) data [14], to underpin these guidelines was identified as a barrier to their implementation [13]. Specifically, 3 recent interventional trials demonstrated little or no clinical benefit [9,15,16]. Interestingly, however, while methodological variation in the delivery of the intervention across these studies could have contributed to these results, none of these trials stratified patients by presence of ICU-AW or peripheral muscle wasting as an inclusion criterion, and thus, the target population may have been less likely to benefit from the intervention. Diagnosis of ICU-AW, measured by manual muscle testing, has previously been shown to demonstrate a causal association with poor clinical outcome [17–24], and therefore, these patients may have greater ongoing rehabilitation requirements.
This pilot trial investigated (1) the feasibility of an exercise-based rehabilitation program (EBRP) delivered after hospital discharge in patients with ICU-AW and (2) the clinical use of ICU-AW diagnosis as an eligibility criterion for enrolment. In addition, a nested observational study of patients without a diagnosis of ICU-AW was incorporated to define the trajectory of recovery of such patients.
2. Materials and methods
2.1. Ethical approval
Ethical approval was obtained from London Westminster Research Ethics Committee (London, UK, 09/H0802/80), and local Research & Development site-specific approval from participating organizations. Written informed consent was obtained from all participants.
2.2. Study design
A pilot feasibility RCT of exercise rehabilitation was conducted in survivors of critical illness with ICU-AW with a nested parallel observational cohort study of post–critical illness patients without ICU-AW. Because of the pilot, feasibility nature of the study, there was no a priori calculated sample size.
2.3. Patients
Patients were recruited from the ICUs of 2 London teaching hospitals within an Academic Health Sciences Centre (60 beds in total). One hospital is a regional trauma and neurosciences center, and the other a tertiary referral center for advanced ventilation, with both having a general medical and surgical case mix.
2.4. Screening
All patients receiving mechanical ventilation (MV) during their ICU admission were screened for potential eligibility. Screening occurred over 6 sessions per week depending on availability of the research team. Participants were approached for recruitment at ICU discharge, and consenting patients were randomized into either standard care or intervention groups if they had evidence of ICU-AW as per definition [18]. If ICU-AW was not present, patients were enrolled into the observational study within 24 hours of ICU discharge.
2.5. Inclusion criteria
Inclusion criteria for enrolment included age 18 years or more, MV for 48 hours or more, Glasgow Coma Scale 15/15, survival to hospital discharge, and sufficient mobility to participate in an EBRP after hospital discharge. Specifically for inclusion into the RCT, participants were required a diagnosis of ICU-AW at ICU discharge. Participants in the observational cohort study did not demonstrate ICU-AW.
2.6. Exclusion criteria
Patients were excluded if they were palliative, had unstable cardiac disease, had limb amputation, neurological diagnoses, had peripheral vascular disease awaiting revascularization, had any musculoskeletal condition or extensive medical comorbidity precluding ability to exercise, had psychiatric illness, had requirement for ongoing renal dialysis, were an extracontractual referral and could not return to the hospital site, or had an existing rehabilitation pathway in place.
2.7. Assessment of ICU-AW
Assessment of ICU-AW was conducted as described previously [17,18,20] by the lead researcher. Adequate conscious level of patients was determined as a score of − 1 (drowsy) to + 1 (restless) on the Richmond Agitation Sedation Scale [25]. Awake patients were then required to demonstrate positive response to a battery of simple one-stage commands including “Open and close eyes” and “Stick out your tongue.” Successful completion of these commands was followed by muscle strength assessment using the Medical Research Council Sum score (MRC-SS), a 6-point grading scale ranging from 0 (no visible contraction) to 5 (normal power) applied to 6 upper and lower limb muscle groups bilaterally (shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension, and ankle dorsiflexion) [26]. The ICU-AW was defined as an MRC-SS of less than 48 out of 60 [17,19,27–29].
2.8. Randomization
Randomization was managed by the Mental Health and Neuroscience Clinical Trials Unit (London, UK) (see Online Supplement Section E1 for further detail). Treatment allocation was undertaken independently of the research team. Once notified of treatment allocation by the Clinical Trials Unit, participants and relevant treating clinicians were informed. Because of the pilot status of the RCT and the nature of the therapy intervention, blinding of participants and the research team was not possible.
2.9. Intervention
Patients randomized to the intervention arm of the RCT completed an EBRP after hospital discharge. Full details of the intervention are detailed in the online supplement (Section E2). In brief, the EBRP is composed of 16 sessions of 40 minutes' duration, including warm-up and cool-down periods and a combination of cardiovascular, upper and lower limb strength, balance, and functional exercises individually tailored for patients. Sessions occurred twice weekly, in an outpatient physiotherapy gymnasium, supervised by members of the research team. Successful completion of the EBRP was defined a priori as attendance at greater than or 50% of sessions, as per local pulmonary rehabilitation practice [30], and participants were required to complete acceptability questionnaires at completion. The EBRP was delivered over a 3-month period to allow for other clinical commitments or factors resulting in patient nonattendance. Patients were strongly encouraged to undertake 1 independent exercise session per week using an accompanying exercise manual to guide and record this. The EBRP contained an informal education component where participants were invited to attend sessions covering breathlessness management, benefits of exercise, and nutrition. Furthermore, the research team was available to provide one-to-one advice for participants as required.
2.10. Standard care arm and observational study
Patients randomized to the standard care arm or enrolled into the observational cohort study received a weekly telephone call from the research team to monitor general progress of recovery. Although in excess of routine standard care, this was mandated by the ethical review board. There was no specific advice on exercise rehabilitation provided during these telephone calls.
2.11. Core outcome measures
Outcome measures for both the RCT and observational study were assessed at baseline (hospital discharge) and at 3 months (Fig. 1) and included (1) exercise capacity—Incremental Shuttle Walk Test (ISWT) [31] and Six Minute Walking Test (6MWT) [32]; and (2) health-related quality of life—Short Form 36 v.2 questionnaire (SF-36, Acute Recall version) [33] physical (PCS) and mental (MCS) component scores and the Hospital Anxiety and Depression Scale (HADS) [34]. Feasibility outcomes, including screening, eligibility and recruitment rates, adherence to the EBRP, duration of exercise, information to guide education provision, results across a range of outcome measures, and clinical data associated with the ICU stay were collected and analyzed. Additional outcome measures are detailed in the online supplement (Section E3).
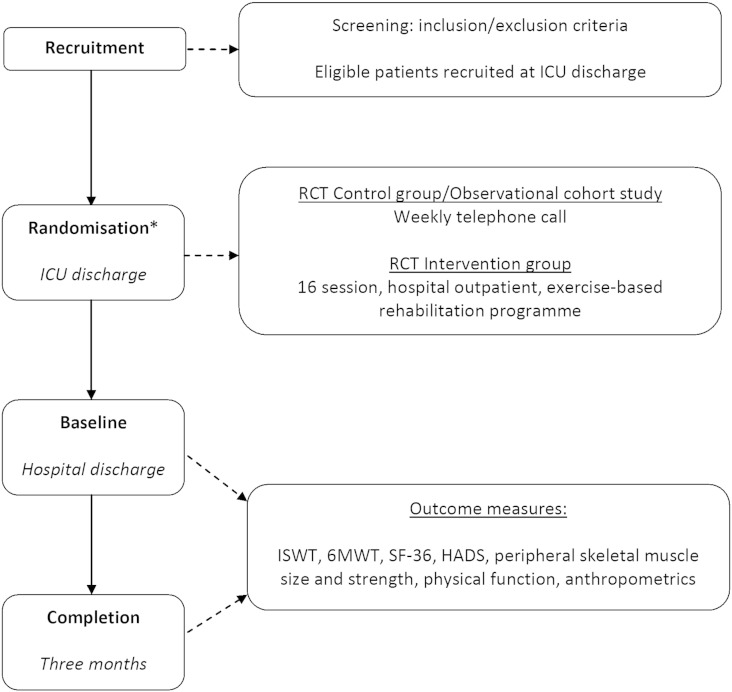
Fig. 1.
Schematic outline of pilot RCT and observational study pathway.
*Note that patients in the observational study did not undergo randomization but were managed in the same way as the control group of the RCT.
2.12. Statistical analysis
All data are expressed as mean ± SD or median (interquartile range [IQR]) as appropriate. Appropriate comparative tests were applied to determine within-group and between-group differences in both the pilot RCT and observational studies. For analyses between the randomized cohort with ICU-AW and the observational cohort without ICU-AW, these were limited to outcomes related to physical performance based on the rationale that these had the potential to be more influenced by degree of global peripheral skeletal muscle strength.
3. Results
3.1. Feasibility of the exercise-based rehabilitation intervention in patients with ICU-AW
3.1.1. Recruitment to a pilot feasibility RCT
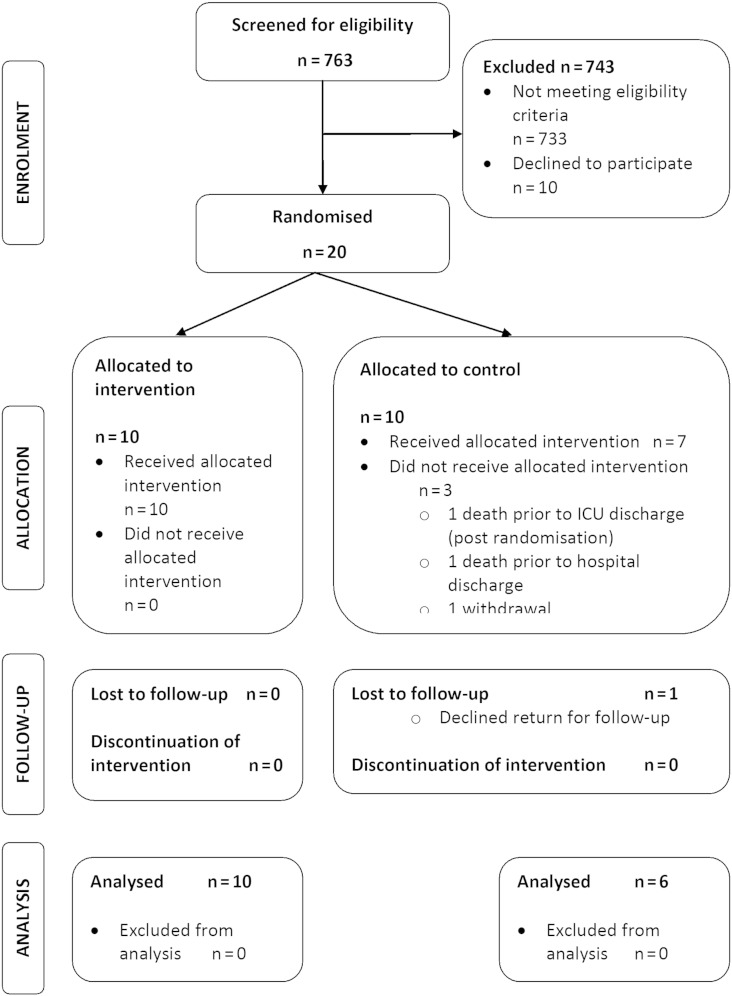
Patients were recruited between February 2010 and May 2012 with follow-up completed by August 2012. Participant flow-through is described in the Consolidated Standards of Reporting Trials [35] diagram (Fig. 2). A large proportion of all potentially eligible patients were excluded (n = 743), 10 of whom declined consent (Table 1). Further detail on the hospital discharge outcomes of excluded patients and interpretation of eligibility criteria are reported in the online supplement (Section E4). Twenty patients provided informed consent. These data indicate that 37 eligible patients were approached for 1 patient to be successfully recruited, albeit the logistics of screening across 2 study sites may have influenced this ratio. Consent rate in approached patients was 66.7% (20 of 30). Ten patients were randomized into each trial group. Baseline characteristics of the cohort are reported in Table 2. The groups were similar for all characteristics. MRC-SS for the cohort with ICU-AW was 43.0 (39.0-44.8).
Fig. 2.
Consolidated standards of reporting trials diagram detailing patient flow-through within the RCT.
Table 1.
Factors accounting for ineligibility into the RCT
| Factor | Frequency of occurrence (%) | Classification |
|---|---|---|
| Ventilation ≥ 48 h | 294 (39.6) | Inclusion criterion not met |
| Extracontractual tertiary referral | 185 (24.9) | Exclusion criterion met |
| MRC-SS < 48/60 | 132 (17.8) | Inclusion criterion not met |
| Existing rehabilitation pathway | 131 (17.6) | Exclusion criterion met |
| Sufficient mobility | 97 (13.1) | Inclusion criterion not met |
| ICU admission ≥ 48 h | 95 (12.8) | Inclusion criterion not met |
| Expected survival to hospital discharge | 93 (12.5) | Inclusion criterion not met |
| Palliative/terminal prognosis | 89 (12.0) | Exclusion criterion met |
| Complex medical comorbidity | 82 (11.0) | Exclusion criterion met |
| Disabling condition precluding exercise | 80 (10.8) | Exclusion criterion met |
| Unstable cardiac diagnoses | 75 (10.1) | Exclusion criterion met |
| Neurological diagnoses | 64 (8.6) | Exclusion criterion met |
| Impaired GCS | 42 (5.7) | Exclusion criterion met |
| Psychiatric diagnoses | 42 (5.7) | Exclusion criterion met |
| Ongoing renal haemodialysis | 32 (4.3) | Exclusion criterion met |
| Acute limb amputation | 13 (1.7) | Exclusion criterion met |
| Acute peripheral vascular disease | 5 (0.7) | Exclusion criterion met |
| Age > 18 y | 4 (0.5) | Inclusion criterion not met |
Data are presented as number (percentage), and report frequency of reported occurrence of each criterion; therefore. total percentages exceed 100% (n = 733). Multiple factors could apply per patient. GCS indicates Glasgow Coma Scale.
Table 2.
Baseline characteristics for standard care and intervention arms of the pilot RCT and the observational cohort study
| Characteristic | Standard care group (n = 10) | Intervention group (n = 10) | Pooled randomized cohort (ICU-AW) (n = 20) | Observational cohort (MRC-SS ≥ 48/60) (n = 21) | P |
|---|---|---|---|---|---|
| Age (y) | 68.5 (64.3-78.0) | 63.0 (46.8-71.8) | 66.5 (54.5-73.3) | 63.0 (49.5-70.0) | .3 |
| Sex (male/female) | 3:7 | 3:7 | 6:14 | 16:5 | .005~ |
| ICU diagnosisa (%) | |||||
| Medical | 6 (60) | 7 (70) | 13 (65) | 15 (71.4) | .7~ |
| Surgical | 4 (40) | 3 (30) | 7 (35) | 6 (28.6) | n/a |
| Chronic diseasea (%) | |||||
| Respiratory | 4 (40) | 7 (70) | 11 (55) | 7 (33.3) | .07~ |
| Cardiac | 5 (50) | 4 (40) | 9 (45) | 7 (33.3) | .2~ |
| Otherb | 5 (50) | 3 (30) | 8 (40) | 10 (47.6) | .8~ |
| APACHE II | 23.5 (21.0-30.3) | 24.5 (18.8-29.5) | 23.5 (20.3-29.5) | 17.0 (12.5-19.5) | < .0001 |
| SOFA (ICU admission) | 12.0 (7.5-14.3) | 9.5 (8.0-12.5) | 11.0 (8.0-13.5) | 10.0 (7.5-12.0) | .4 |
| Duration MOF (days) | 10.5 (5.8-13.3) | 9.5 (6.8-15.3) | 10.0 (6.0-12.8) | 9.0 (3.5-14.5) | .6 |
| MV (d) | 11.2 (6.0-15.2) | 9.3 (6.0-13.9) | 10.2 (6.8-14.0) | 9.0 (4.3-20.4) | 1.0 |
| CPAP (d) | 2.0 (0.3-4.6) | 1.3 (0.04-6.9) | 1.3 (0.2-5.0) | 1.5 (0.4-2.6) | .6 |
| Tracheostomy (%) | 3 (30) | 5 (50) | 8 (40) | 8 (38.1) | – |
| ICU LOS (d) | 13.0 (9.8-20.5) | 14.5 (7.0-17.8) | 13.5 (8.5-19.3) | 10.0 (6.5-27.0) | .9 |
| CC LOS (d) | 18.0 (13.8-36.5) | 17.5 (9.0-27.3) | 18.0 (11.5-31.8) | 13.0 (10.0-37.0) | .8 |
| Ward LOS (d) | 27.5 (10.0-46.3) | 20.0 (10.0-43.0) | 23.5 (10.3-43.0) | 13.0 (6.5-19.5) | .03 |
| Hospital LOS (d) | 47.5 (26.5-68.5) | 39.0 (22.3-66.5) | 46.0 (25.0-61.8) | 30.0 (19.5-47.5) | .2 |
Data are presented as median (IQR) or number (percentage). Standard care and intervention groups were similar for all characteristics (all P = ns therefore not reported). P values derived from Mann-Whitney test or Fisher exact test~ and reflect difference between pooled data from groups within the randomized cohort with ICU-AW and the clinically strong observational cohort. Note: 1 P value applicable for contingency analysis of proportion of medical and surgical patients across both groups. APACHE indicates acute physiology and chronic health evaluation; SOFA, Sequential Organ Failure Assessment; MOF, multiorgan failure; CPAP, continuous positive airway pressure; LOS, length of stay; CC, critical care.
ICU diagnosis and chronic disease indicates frequency of occurrence. Patients could present with more than 1 comorbidity.
Other chronic comorbidities included diabetes mellitus, osteoarthritis/gout, stable chronic renal disease.
3.1.2. Adherence to the EBRP
Of the 10 patients randomized to receive the EBRP, 8 successfully completed the intervention with 16.0 (9.3-16.0) sessions attended. Of the 2 patients who failed to complete the program, 1 attended only once due to recurrent chest infections with a subsequent reluctance to continue with the intervention. The second patient attended 7 sessions but was unable to continue participation due to a general deterioration in health related to a separate illness diagnosis (Addison's disease). For 2 of the 8 patients who, by definition, successfully completed the intervention, the reasons for not attending all 16 sessions included seasonal chest infections, family circumstances, and separate clinical appointments necessitating hospital attendance.
3.1.3. Adverse events
There were no adverse events during any of the EBRP sessions. One patient in the intervention arm was admitted to hospital for investigation of cardiac symptoms occurring outside of the program, which were unrelated to exercise participation. On discharge, this patient was medically approved to resume full participation in the intervention.
3.1.4. Patient exercise time per session
Within each session of the EBRP, patients could exercise for 25 minutes (total 40 minutes with warm-up and cool-down time). Patient exercise time per session was 21.9 (20.9-23.4) minutes, which remained consistent with the exclusion of noncompleters (21.9 (21.0-23.2) minutes.
3.1.5. Educational sessions
Patients attended an average of 5 education sessions. Individual advice was also provided by the research team where necessary. It was therefore not possible to quantify or analyze this input in a standardized manner.
3.1.6. Patient acceptability
Of the 8 patients successfully completing the EBRP, 6 completed a simple acceptability questionnaire requesting their opinion on the experience of participation with regard to the exercise component, and both the formal (classes) and informal (individual advice provided by research team) education sessions (Table 3). All 6 patients reported that, overall, they were “very satisfied” with the program and that it had assisted their recovery after their illness. Furthermore, all patients reported greater understanding and knowledge around exercise participation, and that increasing their fitness levels had assisted in performance of activities of daily living. Only 1 patient reported that attendance at the hospital for the EBRP was “too tiring.” Further details on patient acceptability of the EBRP are reported in the online supplement (Section E5).
Table 3.
Items and responses from patient acceptability questionnaire in the pilot RCT
| Item | Responses |
|---|---|
| 1. Attending the exercise program helped recovery from my illness | 66.7% strongly agree, 33.3% agree |
| 2. I have a clear picture of how exercise will help my fitness | 66.7% strongly agree, 33.3% agree |
| 3. I have a clear picture of how fitness will help in daily activities of my life | 66.7% strongly agree, 33.3% agree |
| 4. I feel confident doing exercise | 66.7% strongly agree, 33.3% agree |
| 5. I worry that exercise may be harmful to me | 33.3% strongly disagree, 66.7% disagree |
| 6. I felt very stressed doing the exercise | 33.3% strongly disagree, 66.7% disagree |
| 7. I found the visits to the hospital too tiring | 50% strongly disagree, 33.3% disagree, 16.7% agree |
| 8. Exercise has not helped me | 66.7% strongly disagree, 33.3% disagree |
| 9. The way the information was presented | 16.7% very satisfied, 66.7% satisfied, 16.7% unable to comment |
| 10. The information given | 16.7% very satisfied, 66.7% satisfied, 16.7% unable to comment |
| 11. The opportunities you had to discuss any concerns | 16.7% very satisfied, 66.7% satisfied, 16.7% unable to comment |
| 12. The way the staff answered your questions | 33.3% very satisfied, 50% satisfied, 16.7% unable to comment |
| 13. The range of education topics covered | 16.7% very satisfied, 50% satisfied, 16.7% neither satisfied or dissatisfied, 16.7% unable to comment |
Data from 6 patients successfully completing the EBRP. Items 1 to 8 refer to exercise component of program. Items 9 to 13 refer to both formal and informal education sessions. Where response indicates “Unable to comment,” this patient did not attend any education sessions.
3.1.7. Core outcomes
All patients in the intervention group and 6 patients in the standard care group completed follow-up at 3 months (Fig. 2). Results of outcome measures are reported in Table 4 and in the online supplement (Section E6). There were no between-group differences at baseline, change from baseline or at completion of the trial. Where minimum clinically important difference (MCID) data were available, both the intervention and the standard care group improved beyond these limits. This included 47.5 m for the ISWT [36] and 54 m for the 6MWT [37], albeit these data are established for chronic respiratory disease populations. Interestingly, but not unexpected, none of the patients achieved their predicted distance for 6MWT at completion [38]. Predicted distance for 6MWT for the cohort was 490.1 m (459.8-536.5 m), and percent predicted 6MWT achieved was 66.4% (46.2%-89.1%). By hospital discharge, commencement of the EBRP, the MRC-SS of the randomized cohort was 56.0 (52.0-58.0), increasing to 60.0 (56.0-60.0) at completion, indicating that these patients no longer exhibited ICU-AW, as measured by the MRC-SS threshold of < 48/60.
Table 4.
Results of outcome measures assessed in the pilot RCT
| Outcome measure | Standard care (n = 6) |
Intervention (n = 10) |
||||
|---|---|---|---|---|---|---|
| Baseline | Completion | Change | Baseline | Completion | Change | |
| ISWT (m) | 20.0 (10.0 to 60.0) | 190.0 (70.0 to 355.0) | 170.0 (40.0 to 315.0) | 55.0 (7.8 to 120.0) | 200.0 (132.5 to 340.0) | 115.0 (− 2.5 to 237.5) |
| 6MWT (m) | 150.0 (100.5 to 207.0) | 335.0 (177.5 to 455.0) | 185.0 (40.0 to 285.0) | 180.0 (125.0 to 221.5) | 328.5 (230.0 to 393.8) | 140.0 (35.8 to 210.3) |
| SF-36 v2 PCS (/100) | 20.6 (19.4 to 33.3) | 42.3 (27.9 to 47.6) | 11.0 (4.3 to 28.3) | 29.8 (24.1 to 33.2) | 33.2 (23.8 to 45.4) | 1.8 (− 6.8 to 15.9) |
| SF-36 v2 MCS (/100) | 50.9 (35.6 to 57.8) | 45.6 (34.3 to 54.7) | − 11.4 (− 19.0 to 19.1) | 31.6 (28.6 to 49.1) | 53.4 (39.5 to 58.8) | 14.3 (− 3.2 to 26.7) |
| HADS total (/42) | 14.0 (9.0 to 20.0) | 6.5 (5.5 to 10.3) | − 4.5 (− 13.3 to − 2.5) | 13.0(7.0 to 19.0) | 9.0 (3.5 to 10.3) | − 6 (− 9.3 to − 2.8) |
| HADS anxiety (/21) | 6.0 (1.5 to 11.5) | 4.0 (0.8 to 6.0) | 0.0 (− 7.0 to 0.0) | 7.0 (4.5 to 9.3) | 4.0 (1.8 to 5.5) | − 3.5 (− 5.0 to − 1.3) |
| HADS depression (/21) | 8.5 (7.3 to 10.0) | 2.5 (2.0 to 8.0) | − 4.5 (− 6.3 to − 1.8) | 5.5 (2.8 to 11.0) | 4.5 (1.0 to 7.3) | − 1.5 (− 3.3 to 2.0) |
Data are presented as median (IQR).
3.2. Natural recovery of post critical illness patients without ICU-AW
3.2.1. Recruitment to an observational cohort study
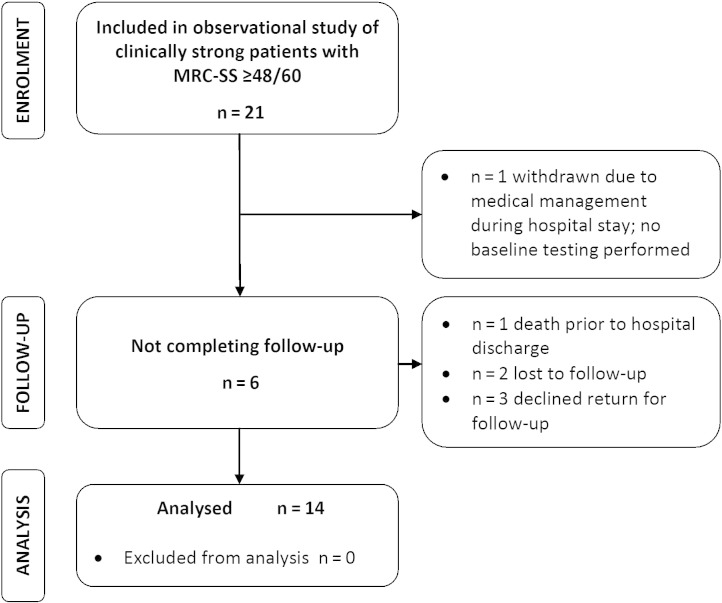
Failure to meet the inclusion criterion of demonstrating an MRC-SS < 48/60, that is, a diagnosis of ICU-AW, was evident in 132 of the 743 ineligible patients. Of these 132 patients, 21 met all other eligibility criteria and consented to participate in the observational follow-up study of patients without ICU-AW (Fig. 3). One patient was unable to complete any baseline testing during the hospital stay due to lack of availability for testing and was therefore withdrawn. A further 6 patients did not complete the study. Baseline characteristics for the observational cohort are reported in Table 2. MRC-SS for the group at enrolment at ICU discharge was 56.5 (53.3-59.8).
Fig. 3.
Flow diagram detailing participant flow through observational study of clinically strong patients.
3.2.2. Core outcomes
Results in outcome measures for the observational cohort are presented in Table 5 and the online supplement (Section E7). Improvements were observed in both ISWT and 6MWT that exceeded the reported MCID for each test [36,37]. Predicted 6MWT distance at completion for the cohort was 552.0 m (480.2-621.6 m), and percent predicted distance achieved was 74.4% (58.0%-93.5%). Improvements were also observed in health-related quality of life between baseline and completion (SF-36 PCS, 34.0 [28.2-41.4] to 42.7 [35.7-48.5], P = .01; SF-36 MCS, 44.4 [34.9-52.6] to 51.4 [43.1-57.2], P = .03). MRC-SS for the observational cohort at hospital discharge was 60.0 (57.0-60.0), and at completion, 60.0 (60.0-60.0).
Table 5.
Results of outcome measures assessed in the observational cohort study
| Outcome measure | Baseline | Completion | Change | P |
|---|---|---|---|---|
| ISWT (m) | 80.0 (30.0 to 212.5) | 365.0 (250.0 to 477.5) | 265.0 (207.5 to 300.0) | .0002 |
| 6MWT (m) | 167.0 (60.0 to 293.5) | 433.5 (318.3 to 481.0) | 157.5 (131.3 to 321.3) | .0002 |
| SF-36 v2 PCS (/100) | 34.0 (28.2 to 41.4) | 42.7 (35.7 to 48.5) | 6.4 (1.7 to 13.1) | .01 |
| SF-36 v2 MCS (/100) | 44.4 (34.9 to 52.6) | 51.4 (43.1 to 57.2) | 4.7 (− 1.8 to 11.4) | .03 |
| HADS | ||||
| Total (/42) | 10.0 (6.8 to 14.0) | 9.0 (6.5 to 13.0) | 0.5 (− 3.3 to 4.5) | .8 |
| Anxiety (/21) | 5.0 (3.0 to 7.5) | 4.0 (2.8 to 8.3) | 0.0 (− 2.0 to 3.3) | .7 |
| Depression (/21) | 5.5 (2.8 to 7.3) | 5.5 (1.8 to 7.5) | 1.0 (− 2.5 to 3.3) | .8 |
Data are presented as median (IQR) (n = 14). P values derived from Wilcoxon signed rank test and reflect change from baseline to completion.
3.3. Recovery of patients with ICU-AW and those without ICU-AW
Comparison of baseline characteristics between the randomized cohort with ICU-AW and the observational cohort without ICU-AW is presented in Table 2. The male-to-female ratio was greater in the observational cohort (P = .005). Patients with ICU-AW were sicker at ICU admission, with significantly higher illness severity (Acute Physiology and Chronic Health Evaluation II) scores than those without ICU-AW (23.5 [20.3-29.5] vs 17.0 [12.5-19.5], P < .0001). Furthermore, these patients experienced a longer post ICU in-hospital ward stay (23.5 [10.3-43.0] days vs 13.0 [6.5-19.5] days, P = .03), but only a trend to extended total hospital stay (46.0 (25.0-61.8] days vs 30.0 [19.5-47.5], P = 0.2).
3.3.1. Core outcomes
Comparison of the outcomes between groups is summarized in Table 6. There were no consistent between-group differences at baseline and completion of the study. Furthermore, there was no difference in baseline to completion change. However, at a group level, patients without ICU-AW demonstrated greater ISWT distances at completion (365.0 m [250.0-477.5 m] vs 200.0 m [120.0-330.0 m], P = 0.03), with greater change (265.0 m (207.5-300.0 m] vs 120.0 m [10.0-230.0 m], P = .047). In addition, there was a higher SF-36 PCS (34.0 [28.2-41.4] vs 29.4 [19.7-32.9], P = .03) and SF-36 physical function domain (40.0 [15.0-66.3] vs 12.5 [5.0-27.5], P = .005) in those patients without ICU-AW at baseline, although this between-group difference was not present at completion. Patients without ICU-AW had enhanced physical performance as measured by the Timed Up And Go test at baseline (14.8 [13.1-18.5] vs 21.0 [16.0-36.0], P = .04), but there were no differences between the groups observed in the Barthel and Sit to Stand 5 tests.
Table 6.
Comparison of outcome measures between patients with ICU-AW and clinically strong patients
| Characteristic | Randomized cohort (ICU-AW) (n = 16) | Observational cohort (MRC-SS ≥ 48/60) (n = 14) | P |
|---|---|---|---|
| ISWT (m) | |||
| Baseline | 40 (10.0 to 80.0) | 80.0 (30.0 to 212.5) | 0.1 |
| Completion | 200.0 (120.0 to 330.0) | 365.0 (250.0 to 477.5) | 0.03 |
| Change | 120.0 (10.0 to 230.0) | 265.0 (207.5 to 300.0) | 0.047 |
| 6MWT (m) | |||
| Baseline | 160.0 (110.5 to 221.0) | 167.0 (60.0 to 293.5) | 0.8 |
| Completion | 330.0 (240.0 to 422.5) | 433.5 (318.3 to 481.0) | 0.1 |
| Change | 160.0 (36.5 to 208.5) | 157.5 (131.3 to 321.3) | 0.5 |
| SF-36 PCS (/100) | |||
| Baseline | 29.4 (19.7 to 32.9) | 34.0 (28.2 to 41.4) | 0.03 |
| Completion | 34.6 (46.5 to 55.1) | 42.7 (35.7 to 48.5) | 0.2 |
| Change | 6.3 (− 3.2 to 16.2) | 6.4 (1.7 to 13.1) | 0.9 |
| SF-36 PF (/100) | |||
| Baseline | 12.5 (5.0 to 27.5) | 40.0 (15.0 to 66.3) | 0.005 |
| Completion | 40.0 (20.0 to 73.8) | 70.0 (41.8 to 80.0) | 0.1 |
| Change | 20.0 (0.0 to 60.0) | 23.5 (3.8 to 31.3) | 0.8 |
| Barthel (/100) | |||
| Baseline | 87.5 (75.0 to 95.0) | 97.5 (85.0 to 100.0) | 0.08 |
| Completion | 100.0 (86.3 to 100.0) | 100.0 (98.8 to 100.0) | 0.5 |
| Change | 10.0 (1.3 to 23.8) | 0.0 (0.0 to 15.0) | 0.2 |
| TUAG (s) | |||
| Baseline | 21.0 (16.0 to 36.0) | 14.8 (13.1 to 18.5) | 0.04 |
| Completion | 10.0 (8.0 to 19.0) | 8.0 (6.8 to 10.0) | 0.06 |
| Change | − 7.0 (−24.0 to −2.0) | − 6.0 (− 9.3 to − 3.8) | 0.6 |
| STS-5 (s) | |||
| Baseline | 22.5 (16.8 to 29.5) | 19.0 (10.8 to 26.0) | 0.3 |
| Completion | 16.0 (11.5 to 20.0) | 11.4 (8.9 to 17.5) | 0.2 |
| Change | − 3.8 (− 15.0 to 1.9) | − 3.5 (− 11.0 to − 0.5) | 0.98 |
Data are presented as median (IQR). P values derived from Mann-Whitney test analysis. n = 14 for TUAG and STS-5 for the randomized cohort. SF-36 v2 PF indicates Short Form-36 v2 Physical Function domain; TUAG, Timed Up and Go; STS-5, Sit to Stand 5 times.
3.3.2. Relationship between ICU-AW and physical function
Extended analyses of the relationship between ICU-AW and physical function performance at study milestones is shown in the on line supplement (Sections E8).
3.3.3. Sample size
The calculated sample sizes for a future trial are reported in the online supplement (Section E9).
4. Discussion
This pilot RCT investigating exercise-based rehabilitation delivered after hospital discharge in survivors of critical illness with ICU-AW demonstrated that the intervention was feasible as evidenced by the high completion rate, an absence of adverse events, and strong patient-reported acceptability. However, the change in diagnostic status of ICU-AW between patient enrolment and commencement of the intervention indicates that using manual muscle testing for determining presence of weakness lacked robustness as an eligibility criterion for participation. Furthermore, the observation of the natural recovery of patients without ICU-AW revealed inconsistent differences in outcomes between this cohort and those patients with weakness, indicating that stratification for exercise rehabilitation requirements using this approach lacked satisfactory discrimination, which was demonstrated by the ceiling effect of the MRC-SS test.
4.1. Clinical significance of the findings
This study was not powered to detect differences between groups across any of the clinical, mechanistic, and patient-centered outcomes, and therefore cannot suggest effectiveness of the intervention limiting comparison with findings from previously reported trials [9,15,16]. Despite this, specification of a diagnosis of ICU-AW, measured by the MRC-SS at ICU discharge as a specific eligibility criterion was a novel aspect of this pilot trial, based on the extensive data reporting a causal association between an MRC-SS less than 48 and poor clinical outcome [17–23] and the rationale for greater rehabilitation requirements. Although since this trial was first designed, use of the MRC-SS threshold for diagnosing ICU-AW has been shown to lack both sensitivity and specificity to predict clinical outcome [39], in this study, the diagnosis ICU-AW failed to define the most appropriate target group for an exercise-based rehabilitation intervention. Indeed, at ICU discharge, patients with ICU-AW and those without had similar baseline characteristics. By hospital discharge, when the intervention commenced, those patients with ICU-AW at ICU discharge had improved clinically, demonstrating an MRC-SS greater than 48, such that they could be recategorized as without ICU-AW. This ceiling effect limits the clinical usefulness of MRC-SS testing.
Presence of ICU-AW at ICU discharge did, however, alter the ward-based therapy provision for these patients and influence their trajectory of recovery during this period. These patients were the sickest patients at ICU admission, and based on our previous data were those likely to have undergone greater muscle wasting during the first week of critical illness [1]. It is postulated that the ward stay of such patients was protracted while they received more intense rehabilitation interventions than those without ICU-AW to achieve a level of function sufficient for hospital discharge, at which point the MRC-SS could not distinguish between the groups. Unfortunately, these data were not captured in this study, nor indeed were data regarding the dose of rehabilitation or other ICU exposures received by patients during their preceding ICU stay. Future trials must carefully characterize the pretrial period rehabilitation and other factors that could influence outcome and recovery in patients, in detail [40].
Both trial and observational study cohorts demonstrated improvements in exercise capacity and subjective physical function scores beyond currently available MCID data, albeit specific values for the post–critical illness population have yet to be established, and we acknowledge these were pilot patient numbers. However, natural recovery exceeding the current MCID has clinical relevance, and it is important to note as any future rehabilitation intervention would need to demonstrate effectiveness beyond this level. In addition, the rate of recovery trajectory also requires consideration to deliver the intervention at the optimal time point during recovery. MCID data for outcomes should be applicable to the relative stage of recovery, as this will ultimately influence the effect of the intervention. Satisfactory recovery with standard management in the early recovery period could result in resource allocation to the later stages of the patient pathway when there is a greater clinical need.
4.2. Critique of the method
Process evaluation can distinguish failure of an intervention to cause an effect from failure of effective delivery of the intervention [41,42] and is integral to the development, evaluation, and reporting of complex interventional trials [43], such as exercise rehabilitation in the post–critical illness population. The pilot nature of this trial precluded detecting effectiveness of the intervention, and so, we focused on retrospective evaluation of methodological considerations around trial design, an important feature and outcome associated with pilot trials [44]. Undertaking this process is a particular strength of this feasibility study, informing our understanding of aspects that would require consideration in a future, larger scale study. We adopted the population, intervention, control, and outcome construct, so called “PICO” principle, to assess the present trial and provide a framework for discussion [45,46].
4.2.1. Population
Heterogeneity of post–critical illness patients challenges the delivery and evaluation of rehabilitation throughout the recovery pathway, with individual patterns of response to interventions evident. Trial eligibility criteria aim to enrol more homogenous cohorts, and those adopted in this study were consistent with previous trials. This resulted, however, in an extremely low ratio of included to excluded patients and indeed has attributed to failure to reach a priori sample sizes in prior larger scale studies [9,16]. Further definition of the patients most likely to benefit from post–hospital discharge rehabilitation is required [47] to maximize recruitment. Experience from this study challenges the usefulness of measuring peripheral skeletal muscle strength using manual muscle testing. Measures of physical functional performance that reflect residual impairment are more likely to be useful, but require validation. The severity of the acute critical illness determines the degree of skeletal muscle wasting, and the presence of preexisting chronic disease states determines the trajectory of the recovery pathway, and both need to be considered in any future trials [48]. Assessment for eligibility, randomization, and commencement of the intervention should be synchronous to ensure that natural recovery is accommodated into the trial design in both the control and intervention group, and the intervention is being tested effectively. Furthermore, in light of greater recognition of the cognitive affects associated with critical illness, in any future trial of exercise-based rehabilitation, the cognitive ability of patients will need to be assessed and delirium excluded along with the usual assesment for mental capacity status'.
4.2.2. Intervention
Post–intensive care syndrome encompasses a range of physical, psychological, cognitive, and physical impairments for survivors of critical illness [2]. The current trial focused on an exercise-based intervention primarily targeted toward physical recovery. The failure to address other morbidities of critical illness [47] may have contributed to the outcome, although this would only be confirmed by a fully powered trial. In the future, multidisciplinary, multimodal rehabilitation programs may elicit greater response through the synergistic effect of optimizing 1 element on another, for example, physical performance and mental health.
The optimum exercise prescription to enhance physical recovery in post–critical illness patients has yet to be determined. This pilot study conformed with recommendations for nonpharmacological trials by closely detailing the intervention [49]. Nonetheless, the chosen content, format, and route of delivery may not have been preferable for all participants. That said, high completion rates were evident, but we acknowledge that this may have, in part, been influenced by the provision of transport to and from the hospital, which may not always be available in routine clinical practice. Clinical factors accounted for the majority of nonattendances, which may also affect adherence to rehabilitation delivered via other formats.
Because this is a pilot feasibility trial, future studies are required to test if the current exercise prescription would be sufficient to cause physiological improvement in a larger sample, even with the employment of target intensity levels and measures for monitoring this. We delivered the intervention in tandem with existing pulmonary rehabilitation programs for patients with chronic respiratory disease for pragmatic and logistical reasons, and we acknowledge the influence of this in the design of our intervention. Close attention is required to patient education in self-monitoring of exercise and to individual exercise prescription and progression. Cardiopulmonary exercise testing could have a role in determining optimum exercise intensity and guiding exercise delivery, albeit with pragmatic limitations for its frequent widespread clinical availability and implementation.
4.2.3. Standard care (control) group
Accurate description of the standard care or control arm in a complex intervention trial is important for enhanced interpretation and generalizability of the results [50,51]. Methods such as benchmarking or point prevalence studies to detail and evaluate existing practice have recently reported to the timing and intensity of exercise rehabilitation in the ICU [52–54]. Standardized reporting of usual practice can improve safety monitoring, facilitate and accelerate the translation of research findings into local clinical practice, and enhance the understanding of differences between groups with regard to intensity of interventions received [55,56].
In this study, the standard care arm received a weekly telephone call from the research team, mandated by the ethical review board. While no specific advice regarding exercise rehabilitation was provided during these phone calls, this represents additional input than these patients would normally receive. Furthermore, standard care patients could also be discharged from hospital in receipt of generic rehabilitation services such as intermediate care or community therapy at the discretion of the clinical team. Lack of formal evaluation of additional services received by these patients represents a methodological weakness of the current protocol. These interventions could have contributed to expediting recovery, albeit given the small sample size this would not have been possible to determine in the current trial.
4.2.4. Outcome measures
Various outcome measures are used in survivors of critical illness to assess physical functional and performance, although the psychometric properties of these tests have yet to be ascertained [57]. Indeed, appropriate outcome measures for evaluation of rehabilitation interventions remain undetermined [47]. Outcome measures must be responsive to change with close consideration for the relative floor and ceiling effects. For complex intervention trials, a core outcome set may be required to best reflect the response to an intervention, which would be applicable to the specific stage of recovery [9]. In contrast, implementing such a battery of assessment measures may be difficult to achieve due to excessive testing burden, and a clear balance between testing burden and robust outcome data must be found. In the current trial, we observed “testing burden and measurement fatigue” potentially resulting in poor test performance and missing data, which in a larger trial would result in increased trial attrition. A consensus-driven core set of outcomes to evaluate effectiveness of rehabilitation interventions would guide future trial protocols [58,59]. As this outcome set, there would also be a requirement to develop a specific bespoke post–critical illness tool to monitor recovery based on the qualitative assessment of patient experiences.
5. Conclusion
In this pilot trial, an EBRP after hospital discharge for survivors of critical illness with ICU-AW was feasible in delivery and patient acceptance, albeit the study was underpowered to demonstrate intervention effectiveness. Diagnosis of ICU-AW, based on results of manual muscle testing, conferred limited clinical use as an eligibility criterion for participation in such a rehabilitation program, and in identifying patients most likely to benefit from rehabilitation after hospital discharge. Process evaluation identified methodological factors for consideration in the design, conduct, and implementation of a future trial, notably focused on eligibility criteria, intensity, and timing of intervention delivery and appropriate outcomes for evaluating effectiveness.
Author Contributions
BC contributed to study design and conception, was responsible for data acquisition, analysis and interpretation, and drafted, revised, and agreed on the final manuscript version for submission. AT contributed to data acquisition. AD assisted with statistical analysis. JM contributed to study design and conception. NH contributed to study design and conception, data interpretation, manuscript revision and agreed on the final version for submission. Furthermore, BC and NH act as the guarantors for the intellectual integrity of the work.
Acknowledgments
The authors would like to acknowledge the support of the Pulmonary Rehabilitation teams at Guy's & St. Thomas' NHS Foundation Trust and King's College Hospital NHS Foundation Trust, London, UK, in particular Mrs Lauren Hogg and Miss Helene Bellas.
Footnotes
Sources of funding: This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health. Additional support was received from the Lane Fox Respiratory Unit Patient Association.
Conflict of interest: The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary material
References
- 1.Puthucheary Z.A., Rawal J., McPhail M., Connolly B., Ratnayake G., Chan P. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 2.Needham D.M., Davidson J., Cohen H., Hopkins R.O., Weinert C., Wunsch H. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 3.Bailey P., Thomsen G.E., Spuhler V.J., Blair R., Jewkes J., Bezdjian L. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 4.Burtin C., Clerckx B., Robbeets C., Ferdinande P., Langer D., Troosters T. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 5.Morris P.E., Goad A., Thompson C., Taylor K., Harry B., Passmore L. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 6.Needham D.M. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert W.D., Pohlman M.C., Pohlman A.S., Nigos C., Pawlik A.J., Esbrook C.L. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayambu G., Boots R., Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 9.Denehy L., Skinner E., Edbrooke L., Haines K., Warrillow S., Hawthorne G. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17:R156. doi: 10.1186/cc12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh T. 2010. The RECOVER study: rehabilitation after intensive care. [ISRCTN09412438Available at www.controlled-trials.com] [Google Scholar]
- 11.Hopkins R., Miller R., Rodriguez L., Spuhler V., Thomsen G. Physical therapy on the wards after early physical activity and mobility in the intensive care unit. Phys Ther. 2012;92:1518–1523. doi: 10.2522/ptj.20110446. [DOI] [PubMed] [Google Scholar]
- 12.NICE . NICE Clinical Guideline 83. National Institute for Health and Care Excellence; London, UK: 2009. Rehabilitation after critical illness. [available at http://www.nice.org.uk/guidance/cg83] [Google Scholar]
- 13.Connolly B., Douiri A., Steier J., Moxham J., Denehy L., Hart N. A UK survey of rehabilitation following critical illness: implementation of NICE Clinical Guidance 83 (CG83) following hospital discharge. BMJ Open. 2014;4:e004963. doi: 10.1136/bmjopen-2014-004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly B., Denehy L., Brett S., Elliott D., Hart N. Exercise rehabilitation following hospital discharge in survivors of critical illness: an integrative review. Crit Care. 2012;16:R226. doi: 10.1186/CC11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batterham A.M., Bonner S., Wright J., Howell S.J., Hugill K., Danjoux G. Effect of supervised aerobic exercise rehabilitation on physical fitness and quality-of-life in survivors of critical illness: an exploratory minimized controlled trial (PIX study) Br J Anaesth. 2014;113:130–137. doi: 10.1093/bja/aeu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott D., McKinley S., Alison J., Aitken L., King M., Leslie G. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15:R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali N.A., O'Brien J.M., Jr., Hoffmann S.P., Phillips G., Garland A., Finley J.C.W. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 18.De Jonghe B., Sharshar T., Lefaucheur J.-P., Authier F.-J., Durand-Zaleski I., Boussarsar M. Paresis acquired in the intensive care unit. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 19.Nanas S., Kritikos K., Angelopoulos E., Siafaka A., Tsikriki S., Poriazi M. Predisposing factors for critical illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol Scand. 2008;118:175–181. doi: 10.1111/j.1600-0404.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharshar T., Bastuji-Garin S., Stevens R.D., Durand M.C., Malissin I., Rodriguez P. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37:3047–3053. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 21.Needham D., Colantuoni E., Mendez-Tellez Dinglas V., Sevransky J., Himmelfarb C., Desai S. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Needham D., Dinglas V., Bienvenu O.J., Colantuoni E., Wozniak A.W., Rice T.W. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herridge M.S., Tansey C.M., Matté A., Tomlinson G., Diaz-Granados N., Cooper A. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 24.Fan E., Cheek F., Chlan L., Gosselink R., Hart N., Herridge M.S. An official American Thoracic Society Clinical Practice Guideline: the diagnosis of intensive care unit–acquired weakness in adults. Am J Respir Crit Care Med. 2014;190:1437–1446. doi: 10.1164/rccm.201411-2011ST. [DOI] [PubMed] [Google Scholar]
- 25.Sessler C., Gosnell M., Grap M., Brophy G., O'Neal P., Keane K. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 26.Kleyweg R.P., van der Meche F.G., Schmitz P.I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. 1991;14:1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 27.De Jonghe B., Bastuji-Garin S., Durand M.-C., Malissin I., Rodrigues P., Cerf C. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 28.De Jonghe B., Bastuji-Garin S., Sharshar T., Outin H., Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30:1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 29.Guarneri B., Bertolini G., Latronico N. Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry. 2008;79:838–841. doi: 10.1136/jnnp.2007.142430. [DOI] [PubMed] [Google Scholar]
- 30.Sewell L., Singh S., Williams J., Collier R., Morgan M. How long should outpatient pulmonary rehabilitation be? A randomised controlled trial of 4 weeks versus 7 weeks. Thorax. 2006;61:767–771. doi: 10.1136/thx.2005.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S.J., Morgan M.D., Scott S., Walters D., Hardman A.E. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47:1019–1024. doi: 10.1136/thx.47.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Thoracic Society Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 33.Ware J., Kosinski M., Dewey J. 2001. How to score version 2 of the SF-36 health survey. [Google Scholar]
- 34.Zigmond A., Snaith R. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 35.Moher D., Hopewell S., Schulz K.F., Montori V., Gøtzsche P.C., Devereaux P.J. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh S.J., Jones P.W., Evans R., Morgan M.D.L. Minimum clinically important improvement for the incremental shuttle walking test. Thorax. 2008;63:775–777. doi: 10.1136/thx.2007.081208. [DOI] [PubMed] [Google Scholar]
- 37.Redelmeier D., Bayoumi A., Goldstein R., Guyatt G. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 38.Enright P., Sherrill D. Reference equations for the Six-Minute Walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 39.Connolly B., Jones G., Curtis A., Murphy P., Douiri A., Hopkinson N. Clinical predictive value of manual muscle strength testing during critical illness: an observational cohort study. Crit Care. 2013;17:R229. doi: 10.1186/cc13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh T.S., Salisbury L.G., Boyd J., Ramsay P., Merriweather J., Huby G. A randomised controlled trial evaluating a rehabilitation complex intervention for patients following intensive care discharge: the RECOVER study. BMJ Open. 2012;2:e001475. doi: 10.1136/bmjopen-2012-001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakley A., Strange V., Bonell C., Allen E., Stephenson J. Process evaluation in randomised controlled trials of complex interventions. BMJ. 2006;332:413–416. doi: 10.1136/bmj.332.7538.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saunders R., Evans M., Joshi P. Developing a process-evaluation plan for assessing health promotion program implementation: a how-to guide. Health Promot Pract. 2005;6:134–147. doi: 10.1177/1524839904273387. [DOI] [PubMed] [Google Scholar]
- 43.Council M.R. 2008. Medical Research Council: developing and evaluating complex interventions: new guidance. [Available at http://wwwmrcacuk/Utilities/Documentrecord/indexhtm?d=MRC004871 Accessed 26th October 2013] [Google Scholar]
- 44.Arnold D., Burns K., Adhikari N., Kho M., Meade M., Cook D. Vol. 37. 2008. The design and interpretation of pilot trials in clinical research in critical care; pp. S69–S74. (Critical care medicine improving clinical trials in the critically ill: proceedings of a Roundtable Conference in Brussels, Belgium). [DOI] [PubMed] [Google Scholar]
- 45.Brown P., Brunnhuber K., Chalkidou K., Chalmers I., Clarke M., Fenton M. How to formulate research recommendations. BMJ. 2006;333:804–806. doi: 10.1136/bmj.38987.492014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson W., Wilson M., Nishikawa J., Hayward R. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–A13. [PubMed] [Google Scholar]
- 47.Herridge M. The challenge of designing a post-critical illness rehabilitation intervention. Crit Care. 2011;15:1002. doi: 10.1186/cc10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwashyna T.J. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186:302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boutron I., Moher D., Altman D., Schulz K., Ravaud P., Group ftC Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 50.Parker A., Tehranchi K., Needham D. Critical care rehabilitation trials: the importance of 'usual care'. Crit Care. 2013;17:183. doi: 10.1186/cc12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson B.T., Schoenfeld D. Usual care as the control group in clinical trials of nonpharmacologic interventions. Proc Am Thorac Soc. 2007;4:577–582. doi: 10.1513/pats.200706-072JK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berney S., Harrold M., Webb S., Seppelt I., Patman S., Thomas P. ICU mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15:260–265. [PubMed] [Google Scholar]
- 53.Nydahl P., Ruhl A.P., Bartoszek G., Dubb R., Filipovic S., Flohr H.J. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42:1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 54.Stevenson L., Salisbury L. A service evaluation of physiotherapy practice within one Scottish intensive care unit. J Assoc Chartered Physiotherapists Respir Care. 2011;43:17–25. [Google Scholar]
- 55.Hart T., Bagiella E. Design and implementation of clinical trials in rehabilitation research. Arch Phys Med Rehabil. 2012;93:S117–S126. doi: 10.1016/j.apmr.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 56.Minneci P., Eichacker P., Danner R., Banks S., Natanson C., Deans K. The importance of usual care control groups for safety monitoring and validity during critical care research. Intensive Care Med. 2007;34:942–947. doi: 10.1007/s00134-008-0999-6. [DOI] [PubMed] [Google Scholar]
- 57.Elliott D., Denehy L., Berney S., Alison J.A. Assessing physical function and activity for survivors of a critical illness: a review of instruments. Aust Crit Care. 2011;24:155–166. doi: 10.1016/j.aucc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Williamson P., Altman D., Blazeby J., Clarke M., Devane D., Gargon E. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Connolly B., Hart N. Development of a core outcomes set for trials of rehabilitation following critical illness Core Outcome Measures in Effectiveness Trials Initiative. 2013. http://www.comet-initiative.org/studies/details/288?result=true Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material