Abstract
Motor development and cognitive development in childhood have been found to be fundamentally interrelated, but less is known about the association extending over the life course. The aim of this study was to examine the association between early motor development and cognitive performance in early old age. From men and women belonging to the Helsinki Birth Cohort Study, who were born between 1934 and 1944 and resided in Finland in 1971, 1279 participated in cognitive performance tests (CogState®, version 3.0.5) between 2001 and 2006 at an average age of 64.2 years (SD 3.0). Of these, age at first walking extracted from child welfare clinic records was available for 398 participants. Longer reaction times in cognitive tasks measuring simple reaction time (SRT), choice reaction time (CRT), working memory (WM), divided attention (DA), and associated learning (AL) indicated poorer cognitive performance. Adjustment was made for sex, age at testing, father’s occupational status and own highest attained education, and occupation in adulthood. Average age of learning to walk was 12.2 months (SD 2.1). After adjusting for covariates, earlier attainment of learning to walk was associated with shorter reaction times in cognitive performance tasks (SRT 10.32 % per month, 95 % CI 0.48–21.12, p = 0.039; CRT 14.17 % per month, 95 % CI 3.75–25.63, p = 0.007; WM 15.14 % per month, 95 % CI 4.95–26.32, p = 0.003). People who learned to walk earlier had better cognitive performance in early old age. The earlier attainment of motor skills may track over to early old age and possibly reflect greater cognitive reserve in older age.
Keywords: Infant motor development, Age at first walking, Cognitive performance, Cognitive reserve, Older age
Introduction
It has been suggested that motor development and cognitive development in childhood are fundamentally interrelated (Diamond 2000). Motor development in infancy is a continuous process through which a child achieves new movement patterns and learns new motor skill (Gallahue et al. 2012). Maturation of neural circuits and rapid brain growth is associated with motor development in infancy (Malina 2004; Shenkin et al. 2009; Thelen 1995). Infancy has been suggested to be one of the critical periods of development for intellectual abilities in subsequent life (Gale et al. 2004; Räikkonen et al. 2009).
A life course approach to cognitive ageing provides a concept of cognitive reserve, which indicates that certain aspects of the brain structure such as brain size, neural density, and synaptic connectivity, and the function of brain such as efficiency of neural network, can buffer the effects of cognitive ageing (Richards and Deary 2005). Executive functioning, i.e., the cognitive abilities that control and guide goal-directed performance (Banich 2009), include processing speed, visual attention, and working memory. These cognitive domains are supported by prefrontal and frontal areas of brain and have been shown to be most vulnerable in the ageing process (Kramer et al. 1999, Ren et al. 2013). They are also significently and independently correlated with functional status in older ages (Royall et al. 2004).
So far, there are only a few studies that have investigated the association between infant motor development and cognitive functioning in adulthood. In the 1946 British Birth Cohort study, earlier age of learning to stand was found to be associated with better executive functioning in adulthood (Murray et al. 2006). In the Northern Finland, 1966 Birth Cohort study earlier infant motor development was found to be associated with better educational outcomes (Taanila et al. 2005) and level of intelligence (Murray et al. 2007) in adolescence and adulthood. Further, earlier motor development was found to be associated with increased gray and white matter densities and better executive function in adulthood (Ridler et al. 2006). However, little is still known about the relationship between early motor development and cognitive functioning in older age. Studying this relationship will further the understanding of early determinants of cognition in older age. The aim of this study was to investigate the association between age at first walking and cognitive performance in early old age at an average age of 64 years.
Materials and methods
Study population
Of 8760 persons belonging to the Helsinki Birth Cohort Study (HBCS) who were born during 1934–1944 in Helsinki and who had attended child welfare clinics and resided in Finland in 1971 (Barker et al. 2005; Eriksson et al. 2001), random number tables were used to select a subset of 2902 persons for a clinical study. Of the 2003 persons who participated in the clinical study during 2001–2006, 1586 participants who were living in the greater Helsinki area were invited to participate in cognitive performance tests. Of them, 1279 persons participated (Paile-Hyvarinen et al. 2009). Among them, 398 individuals had valid data on age at first walking. These participants form the analytic sample in this study. Among the participants of cognitive tests, we compared those with and without data on age at first walking. There were no differences between them in birth weight (p = 0.847), but slightly more men (p = 0.040) had missing data on age at first walking. Participants with missing data on age at first walking were also younger at the time of cognitive testing (p = 0.002), and their fathers had more likely higher occupational status (p < 0.001) compared with those who had data available. The groups did not differ in adult occupational status (p = 0.641) or cognitive performance (p > 0.280). The study complies with the guidelines of the Declaration of Helsinki. The study was approved by the Ethics Committee of Epidemiology and Public Health of the Hospital District of Helsinki and Uusimaa and that of the National Public Health Institute, Helsinki. All participants gave a written informed consent.
Age at learning to walk
The age when the child learned to walk without support was asked from the mothers during their visits to the child welfare clinics and was recorded in months. The children visited the welfare clinics on average 11 times between birth and 2 years.
Cognitive performance
Cognitive performance was measured using the CogState (CogState®, version 3.0.5) computerized cognitive test battery. The tasks included in the battery are based on playing cards presented on a computer screen. The level of performance in the test battery does not depend on the language or socioeconomic background of the participant. CogState cognitive test battery has been validated (Maruff et al. 2009) and designed to be efficient and sensitive in identifying major cognitive domains such as psychomotor speed, visual attention, working memory, divided attention, and associated learning (Darby et al. 2002). CogState test battery has shown good reliability with minimal practice effects (Falleti et al. 2006). Each of the five tasks of the test battery consists of 30 to 50 repeated stimuli, and it takes approximately 15 min to complete the whole test battery. Reaction times were measured in milliseconds, and mean reaction times were calculated for each task. Accuracy of responses was recorded as the number of correct answers divided by the number of all answers given. The five tasks were as follows:
In simple reaction time task (SRT) measuring psychomotor speed, participants had to react by pressing the spacebar as quickly as possible whenever a card faced down on the screen was turned face up. This task was repeated 35 times with random time intervals. The SRT task was done twice, first, and last in the test, and the mean reaction time of the two performances was calculated.
In choice reaction time task (CRT), measuring visual attention and psychomotor speed participants had to react as quickly as possible by pressing K (“yes”) or D (“no”) if the turning card on the screen was red. This task was repeated 30 times.
In the working memory task (WM) that measured working memory, psychomotor speed and visual attention participants had to react as quickly as possible by pressing K (“yes”) or D (“no”) if the card showing on the screen was identical to the previous card. This task was repeated 30 times.
In divided attention task (DA) that measured divided attention, participants had to monitor five cards on the screen moving randomly between two horizontal lines above and below the cards. Participants were asked to press the spacebar quickly as possible if one of the cards touched the lines. This test was repeated 30 times.
In associated learning task (AL) that measured visual learning and memory, participants had to match pairs of cards on the screen. Five pairs of cards were shown on the top half of the screen and one random pair faced down on the bottom half of the screen. When the card pair below these five pairs turned face up, the participants had to press K (“yes”) or D (“no”) if the pair was identical with one of the pairs above. After matching the pair, the random pair on the bottom half of the screen turned face down, and after this, the participants had to use their memory to be able to produce the right matching. After every match, the pair of cards turned face up, allowing learning during the task. This task was repeated 50 times.
The tasks were performed by 409 participants with data on age at first walking. Of these, 11 participants had invalid result in one, two, or three tests, respectively. These test results were excluded from the analyses.
Covariates
Based on earlier literature, we selected father’s occupational status, highest educational attainment, and occupational status in adulthood as covariates (Murray et al. 2007; Taanila et al. 2005). Father’s occupational status indicated by the highest occupational class was extracted from the child welfare and school healthcare records and was coded as upper middle class, lower middle class, manual workers, or unknown occupation (Central Statistics Office of Finland 1989). Own highest attained education in adulthood was recorded at 5-year intervals between 1970 and 2000 by Statistics of Finland and was categorized into basic or less or unknown, upper secondary, lower tertiary, and upper tertiary. Data on adult occupational status was extracted from the Finnish Population Register Center and was categorized into upper middle class, lower middle class, self-employed, and manual workers according to highest occupational class at 5-year intervals between 1970 and 2000.
Statistical analyses
Student’s t test was used for comparing means for normally distributed and Mann-Whitney U test for non-normally distributed continuous variables, and Pearson’s chi-squared test was used for comparing proportions for categorical variables. Linear regression analysis was used to investigate the association between age at first walking and cognitive performance in early older age. The reaction times of the cognitive performance tasks were log10 transformed in order to normalize the distribution, and standardized values were used in the regression models. The regression coefficients were expressed as percent changes per monthly change in the age of learning to walk with 95 % confidence intervals (CI). Multi-nominal regression analysis was used to investigate the associations between age at first walking and errors made in cognitive tasks.
We tested for the interaction between gender by age at first walking on cognitive performance tasks. A statistically significant association was observed for associated learning task (p = 0.042) but not for other cognitive tasks (p > 0.172). Based on these findings, we did gender-stratified analyses which revealed that associations between age at first walking and cognitive tasks were parallel except with associated learning task. Thus, we did analyses separately for men and women for associated learning task and pooled by gender for other cognitive tasks. Models were first adjusted for gender and age at cognitive testing, then for father’s occupational status and own highest attained education, and finally for adult occupational status. All tests were performed two-tailed, the level of significance was set at p < 0.05, and analyses were carried out with SPSS IBM version 22.0 (SPSS, Armonk, NY, IBM Corp).
Results
Characteristics of the study participants (172 men, 226 women) are presented in Table 1. Average age at learning to walk was 12.1 months (SD 2.1, range 6 to 24 months). Average age at the time of performing cognitive tests was 64.2 years (SD 3.0).
Table 1.
Characteristics of the participants (n = 398)
| Characteristics | Mean (SD) |
|---|---|
| Age at first walking (months) | 12.1 (2.1) |
| Age at cognitive tests (years) | 64.2 (3.0) |
| % | |
| Men | 43.2 |
| Father’s occupational status | |
| Upper middle | 8.8 |
| Lower middle | 23.5 |
| Manual worker | 67.7 |
| Attained education | |
| Upper tertiary | 11.8 |
| Lower tertiary | 23.4 |
| Upper secondary | 26.4 |
| Basic | 38.4 |
| Adult occupational status | |
| Higher official | 12.6 |
| Lower official | 45.5 |
| Self employed | 8.3 |
| Manual worker | 33.7 |
Median reaction times and accuracy of performance in cognitive tasks are shown in Table 2. We investigated the associations between age at first walking and errors made in cognitive tasks using multi-nominal regression analysis and found no statistically significant associations (data not shown).
Table 2.
Median reaction times and accuracy of completing the cognitive performance tasks and their interquartile ranges
| Median (%) | 25th percentile | 75th percentile | |
|---|---|---|---|
| Simple reaction time (ms) | 332 | 295 | 373 |
| Hit rate (%) | 98.1 | ||
| Errors (%) | |||
| None | 47.7 | ||
| One | 25.4 | ||
| Two or more | 26.9 | ||
| Choice reaction time (ms) | 564 | 508 | 631 |
| Hit rate (%) | 96.8 | ||
| Errors (%) | |||
| None | 51.3 | ||
| One | 25.1 | ||
| Two or more | 23.6 | ||
| Working memory (ms) | 848 | 761 | 1031 |
| Hit rate (%) | 95.5 | ||
| Errors (%) | |||
| None | 36.4 | ||
| One | 27.1 | ||
| Two or more | 36.5 | ||
| Divided attention (ms) | 482 | 418 | 569 |
| Hit rate (%) | 91.7 | ||
| Errors (%) | |||
| None | 14.3 | ||
| One | 19.8 | ||
| Two or more | 65.9 | ||
| Associated learning (ms) | 1825 | 1552 | 2200 |
| Hit rate (%) | 72.0 | ||
| Errors (%) | |||
| None | 0 | ||
| One | 0 | ||
| Two or more | 100 | ||
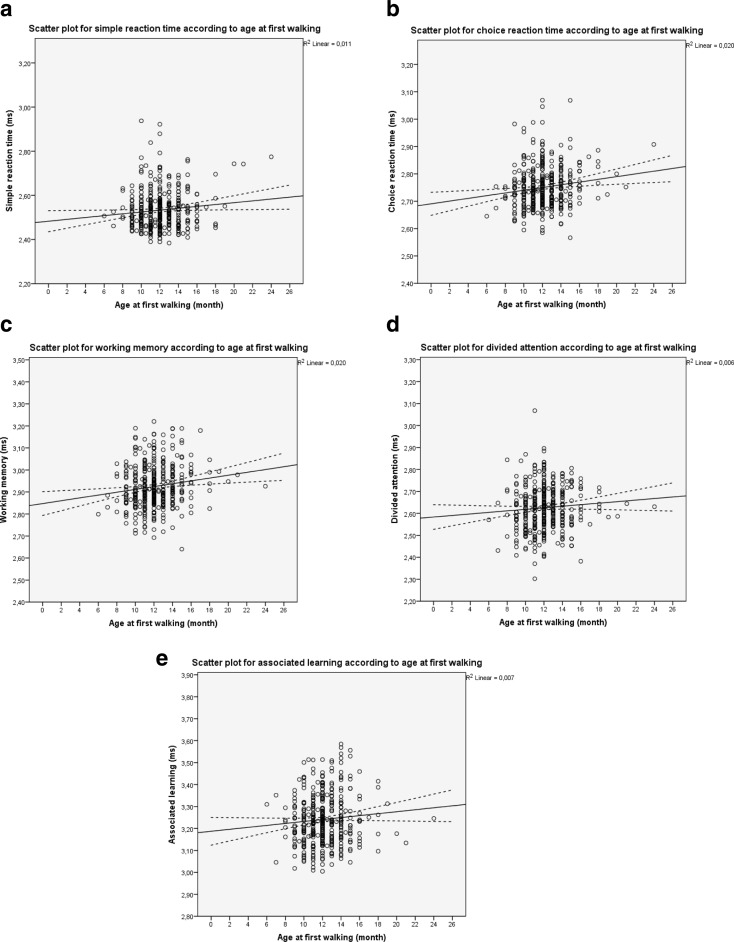
The association between age at first walking and each cognitive performance task and regression lines and their 95 % confidence intervals are displayed in Fig. 1. In the age- and gender-adjusted models, later attainment of learning to walk lengthened the reaction times in all cognitive performance tasks, associations being statistically significant in simple reaction time task (11.0 % per month, 95 % CI 1.14–21.89, p = 0.028), choice reaction task (14.8 % per month, 95 % CI 4.43–26.18, p = .004), and working memory task (14.8 % per month, 95 % CI 4.77–25.84, p = .003). Further adjustment for father’s occupational status and own maximum attained education (model 2), and for adult occupational status (model 3) did not attenuate the associations (Table 3). In the gender-stratified analyses, after adjustments, later attainment of learning to walk lengthened the reaction times in associated learning task for women (18.35 % per month, 95 % CI 3.94–34.77, p = 0.011). For men, the association between age at first walking and associated learning task was not statistically significant (data not shown). Further adjustment for gestational age did not change the results.
Fig. 1.
Scatterplots with regression lines and their 95 % confidence intervals for log10 transformed cognitive performances (ms) according to age at first walking (month)
Table 3.
Increase (%) in reaction times on cognitive performance tasks (CogState) according to 1 month older age at first walking
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % | 95 % CI | p value | % | 95 % CI | p value | % | 95 % CI | p value | |
| SRT | 11.03 | 1.14, 21.89 | 0.028 | 10.68 | 0.88, 21.43 | 0.032 | 10.32 | 0.48, 21.12 | 0.039 |
| CRT | 14.79 | 4.43, 26.18 | 0.004 | 14.74 | 4.32, 26.17 | 0.005 | 14.17 | 3.75, 25.63 | 0.007 |
| WM | 14.82 | 4.77, 25.84 | 0.003 | 15.40 | 5.26, 26.53 | 0.002 | 15.14 | 4.95, 26.32 | 0.003 |
| DA | 7.51 | −1.87, 17.77 | 0.120 | 7.07 | −.2.28, 17.31 | 0.143 | 7.26 | −2.17, 17.59 | 0.135 |
Longer reaction times indicate poorer performance on the task
SRT simple reaction time task, CRT choice reaction time task, WM working memory, DA divided attention task, Model 1 adjusted for age and gender, Model 2 adjusted for model 1 and father’s occupational status and own highest attained education, Model 3 adjusted for model 2 and adult occupational status
Discussion
The main result of the current study was that people who had learned to walk at an earlier age performed better in cognitive tests in early old age. The associations were linear associations and not explained by potential socioeconomic or demographic confounders. Our study thus suggests that timing of motor development in infancy, which has been identified as a period of rapid brain growth, may track over to cognitive performance in early old age. We were, for the first time, able to study these associations from infancy to early old age. We were able to verify that the same trend in the associations between motor development and cognitive ability previous observed to extend to midlife can be found also in older age. Previously, based on data of the 1946 British Birth Cohort, it was observed that the earlier a child learned to stand, the better their subsequent intellectual function at ages 8, 26, and 53 years (Murray et al. 2007). In the Northern Finland 1966 birth cohort, those who reached key infant motor milestones earlier had better school performance at 16 years of age and educational attainment at 31 years of age (Taanila et al. 2005). Earlier motor development in infancy has been found to be linked with better executive functioning and greater gray matter density and white matter volume in the adult brain (Ridler et al. 2006). Another study from the Northern Finland 1966 birth cohort showed that earlier development in the cross motor domain, measured as the age of learning to stand, was associated with better executive functioning at ages 33–35 years. Furthermore, we have shown earlier in the HBCS data that lower weight, length, and head circumference at birth were associated with lower cognitive ability at age 68 years (Räikkönen et al. 2013). In all, infancy is a critical period of development of intellectual abilities in subsequent life (Gale et al. 2004; Räikkonen et al. 2009). Faster growth of the brain and central nervous system resulting in earlier motor development in infancy (Malina 2004) may have far-reaching effects on cognitive reserve capacity in older age (Murray et al. 2006), which might partly explain the finding of our study.
Although adjustment for educational attainment did not notably attenuate the associations in the present study, the results of the other studies might suggest that earlier motor development in infancy may increase opportunities to attain higher educational achievements in later life (Taanila et al. 2005) which are further associated with better cognitive performance (Deary et al. 2007). Earlier motor development together with faster maturation of basic neural circuits may lead to more favorable development of brain neural circuits which are involved in higher cognitive processes in later life (Murray et al. 2006).
As population ages, risks for decline in cognitive function threaten the independence and quality of life of older people and challenge the health care system (Dodge et al. 2005; Johansson et al. 2012). However, there is great variability among older persons in the rate of decline across various domains of cognitive performance (Wilson et al. 2002). This can be explained partly by neurobiological factor such as the size and structure of the brain (Haier et al. 2004). The concept of reserve in neuroscience indicates that structure and functioning of the brain can protect from the effects of neuropathology. The greater the reserve, the less severe the decline in cognitive performance (Richards and Deary 2005; Stern 2009). Cognitive performance outcomes in this study, i.e., psychomotor speed, visual attention, working memory, divided attention, and associated learning, all of which are dimensions of executive functioning, are needed for successful performance in everyday life (Bell-McGinty et al. 2002; Wang et al. 2002).
One of the strengths of this study is the longitudinal study design. We were able to use register-based data on age at first walking gathered from child welfare clinic records which had been filled in by nurses at the close proximity to the age the child learned to walk. Cognitive performance was measured using a sensitive validated computerized testing battery. A computerized test is more instructor-independent resulting in fewer data entry errors compared to traditional paper-and-pencil neuropsychological tests (Collie and Maruff 2003). In addition, we were able to extend the study to a later age than ever before. There are also some limitations in our study. First, we cannot be completely sure that our results can be generalized to other cohorts. Even though the achievement of motor milestones in infancy is fairly universal, the age of attainment may be culturally influenced (WHO 2006). Second, our study population comprised people who were born in Helsinki and had attended child welfare clinics. It is likely that this does not bias the sample extensively, because the clinics were free. We cannot entirely rule out the people who were, deceased, declined to participate, or not willing to participate in the clinical examinations between the years 2004 and 2006 that were somehow different from those not interested. However, the participants consist of men and women from all social strata, so this is not likely to bias the results materially either. Unfortunately, we were not able to control for some potentially important early exposures such as parental characteristics because such data were not recorded in the clinics at the time the participants were growing up. Finally, there might have been other possible factors during the life course that may have affected the association between age at first walking and cognitive performance in early old age which we have not been able to control for in this study.
Conclusion
To conclude, our research has shown that persons who learned to walk earlier had better cognitive performance in early old age. Hence, the effects of earlier attainment of motor skills seem to track over to better cognitive functioning in early old age. The contribution of infant motor development should be further explored as an early predictor of cognitive decline.
Acknowledgments
Funding
HBCS was supported by Emil Aaltonen Foundation, Finnish Foundation for Diabetes Research, Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, Samfundet Folkhälsan, Finska Läkaresällskapet, Liv och Hälsa, and Finnish Foundation for Cardiovascular Research. TP-C was supported by Yrjö Jahnsson Foundation and Folkhälsan. The Academy of Finland supported MBvB (grant no. 257239), TR (grant no. 255403), EK (grant no. 127437, 129306, 130326, 134791, and 2639249), and JGE (grant no. 129369, 129907, 135072, 129255, and 126775). The research leading to these results has received funding from the European Commission within the 7th Framework Programme (DORIAN, grant agreement no 278603).
Conflict of interest
No declared.
References
- Banich MT. Executive function the search for an integrated account. Curr Dir Psychol Sci. 2009;18:89–94. doi: 10.1111/j.1467-8721.2009.01615.x. [DOI] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children Who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P. Computerised neuropsychological testing. Br J Sports Med. 2003;37:2–3. doi: 10.1136/bjsm.37.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby D, Maruff P, Collie A, McStephen M. Mild cognitive impairment can be detected by multiple assessments in a single Day. Neurology. 2002;59:1042–1046. doi: 10.1212/WNL.59.7.1042. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13–21. doi: 10.1016/j.intell.2006.02.001. [DOI] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Dodge HH, Kadowaki T, Hayakawa T, Yamakawa M, Sekikawa A, Ueshima H. Cognitive impairment as a strong predictor of incident disability in specific ADL–IADL tasks among community-dwelling elders: the Azuchi study. Gerontologist. 2005;45:222–230. doi: 10.1093/geront/45.2.222. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falleti MG, Maruff P, Collie A, Darby DG. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, One week and One month test-retest intervals. J Clin Exp Neuropsychol. 2006;28:1095–1112. doi: 10.1080/13803390500205718. [DOI] [PubMed] [Google Scholar]
- Gale CR, O’Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain. 2004;127:321–329. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- Gallahue D, Ozmun J, Goodway J (2012) Understanding motor development: infants, children, adolescents, adults. McGraw-Hill International edition. 14 p
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Johansson MM, Marcusson J, Wressle E. Cognition, daily living, and health-related quality of life in 85-year-olds in Sweden. Aging Neuropsychol Cognit. 2012;19:421–432. doi: 10.1080/13825585.2011.629290. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Malina RM. Motor development during infancy and early childhood: overview and suggested directions for research. Int J Sport Health Sci. 2004;2:50–66. doi: 10.5432/ijshs.2.50. [DOI] [Google Scholar]
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, Pietrzak RH. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- Murray GK, Veijola J, Moilanen K, Miettunen J, Glahn DC, Cannon TD, Jones PB, Isohanni M. Infant motor development is associated with adult cognitive categorisation in a longitudinal birth cohort study. J Child Psychol Psychiatry. 2006;47:25–29. doi: 10.1111/j.1469-7610.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- Murray GK, Jones PB, Kuh D, Richards M. Infant developmental milestones and subsequent cognitive function. Ann Neurol. 2007;62:128–136. doi: 10.1002/ana.21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paile-Hyvarinen M, Raikkonen K, Kajantie E, Darby D, Yliharsila H, Salonen MK, Osmond C, Eriksson JG. Impact of glucose metabolism and birth size on cognitive performance in elderly subjects. Diabetes Res Clin Pract. 2009;83:379–386. doi: 10.1016/j.diabres.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Räikkonen K, Forsen T, Henriksson M, Kajantie E, Heinonen K, Pesonen AK, Leskinen JT, Laaksonen I, Osmond C, Barker DJ, Eriksson JG. Growth trajectories and intellectual abilities in young adulthood: the Helsinki birth cohort study. Am J Epidemiol. 2009;170:447–455. doi: 10.1093/aje/kwp132. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Kajantie E, Pesonen AK, Heinonen K, Alastalo H, Leskinen JT, Nyman K, Henriksson M, Lahti J, Lahti M, Pyhala R, Tuovinen S, Osmond C, Barker DJ, Eriksson JG. Early life origins cognitive decline: findings in elderly Men in the Helsinki birth cohort study. PLoS One. 2013;8:e54707. doi: 10.1371/journal.pone.0054707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Wu YD, Chan JS, Yan JH. Cognitive aging affects motor performance and learning. Geriatr Gerontol Int. 2013;13:19–27. doi: 10.1111/j.1447-0594.2012.00914.x. [DOI] [PubMed] [Google Scholar]
- Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Ridler K, Veijola JM, Tanskanen P, Miettunen J, Chitnis X, Suckling J, Murray GK, Haapea M, Jones PB, Isohanni MK, Bullmore ET. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but Not in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:15651–15656. doi: 10.1073/pnas.0602639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the freedom house study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Deary IJ, Starr JM. Birth parameters and cognitive ability in older Age: a follow-Up study of people born 1921-1926. Gerontology. 2009;55:92–98. doi: 10.1159/000163444. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taanila A, Murray GK, Jokelainen J, Isohanni M, Rantakallio P. Infant developmental milestones: a 31-year follow-Up. Dev Med Child Neurol. 2005;47:581–586. doi: 10.1111/j.1469-8749.2005.tb01207.x. [DOI] [PubMed] [Google Scholar]
- Thelen E. Motor development. A New synthesis. Am Psychol. 1995;50:79–95. doi: 10.1037/0003-066X.50.2.79. [DOI] [PubMed] [Google Scholar]
- Wang L, van Belle G, Kukull WB, Larson EB. Predictors of functional change: a longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002;50:1525–1534. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- WHO Motor Development Study Windows of achievement for Six gross motor development milestones. Acta Paediatr Suppl. 2006;450:86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. doi: 10.1037/0882-7974.17.2.179. [DOI] [PubMed] [Google Scholar]