Abstract
Poor regulation of emotions may involve impaired attention control. In the current paper, we report the results of two studies examining the interaction of anxiety, attention control, and cognitive load. In Study I, using a performance-based task to assess attention control, we examined whether anxiety is associated with impaired attention control, and whether these effects are influenced by working memory load. In Study II we examined these effects in patients with a diagnosis of Generalized Anxiety Disorder (GAD) compared to non-anxious control (NAC) participants. Results of Study I showed that high anxiety was associated with increased attention control, that is decreased interference from distractors, but only under high cognitive load. These results were replicated in Study II such that individuals with GAD showed increased attention control relative to NACs, but only under high cognitive load. These results help clarify previous predictions regarding the effect of anxiety on attention control.
Attention control is the ability to use cognitive resources selectively to inhibit the processing of certain stimuli. Posner and colleagues (Posner, 1980; Posner, Rothbart, Vizuetta, Levy, Thomas, & Clarkin, 2002; Posner & Rothbart, 2007) have posited that attention is not a unitary construct, but rather a system of components that carry out the functions of alerting, orienting, and executive control of attention. Alerting is involved in maintaining an appropriate sensitivity level to perceive and process stimuli; orienting involves the selection of information from among numerous sensory stimuli; and the executive control network specializes in conflict resolution and voluntary action control. In this article, we examine the interaction between the executive control of attention and anxiety.
Impaired attention control has been implicated in poor emotion regulation (e.g., Derryberry & Rothbart, 1988, 1997; Gross and Barret, 2011; Rothbart, Ellis, & Posner 2004). Extant research examining the association between attention control and emotion has focused on the notion of attention bias to threat cues. For example, anxious individuals tend to constrict their focus of attention on threatening stimuli -- when these stimuli compete for attentional resources with nonthreatening information -- by either attending preferentially to threatening information (Rothbart, Ellis, & Posner, 2007) or by exhibiting decreased attention control (Derakshan& Eysenck, 2009; Derryberry& Reed, 2002). Thus, greater attention control may allow an individual to inhibit involuntary attention to threat cues whereas lower attention control may enhance attention to threat cues (Derryberry& Reed, 2002; Reinholdt-Dunne, Mogg, & Bradley, 2009).
Fan, McClandliss, Sommer, Raz, and Posner (2002) developed the Attention Network Task (ANT) to measure the three attentional networks—alerting, orienting, and executive control of attention. The ANT is a computerized task in which participants see target stimuli (central arrow surrounded by other arrows or dashes) on the screen and are asked to identify the direction of the central arrow. Fan et al. indexed the efficiency of the alerting network by measuring changes in response latencies resulting from the presentation of a signal warning that a target will appear; efficiency of orienting is examined by changes in response latencies resulting from the presentation of cues indicating where the target will occur; efficiency of the executive attention control network is examined by differences in response latencies resulting from the presentation of the central arrow flanked by arrows pointing in a congruent versus incongruent direction. Pacheco-Unguetti, Acosta, Callejas, & Lupiáñez (2010) used the ANT to investigate the relationship between anxiety and components of attention. These researchers found that, when asked to focus on stimuli in the center of the visual field, individuals with high trait anxiety experienced more interference from distractors located in the peripheries of the visual field than did individuals with low anxiety. These results suggest that individuals with high anxiety exhibit low executive control of attention. In contrast, Finucane and Power (2010) found that healthy female participants demonstrated enhanced focus on the central target and were less distracted by peripheral information when performing a modified version of the ANT in which trials were interspersed with presentation of fear-eliciting images.
Most theories that explain the interaction between cognitive processing and emotion are based on the notion of a competition for resources required for cognitive processes such as executive (Eysenck & Calvo, 1992), perceptual (Lavie, 2000; 2010), or working memory (King & Schaefer, 2010) processes. Furthermore, some theories suggest that this interaction of cognitive processing and emotion is influenced differentially by the presence of different types of load. For example, Lavie (2005) suggested that distractors have a greater impact on low-load perceptual tasks relative to high-load perceptual tasks (e.g., Bishop, 2009); however, Lavie also found that distractors have a greater impact on high-load working memory tasks relative to low-load working memory tasks. This idea is consistent with Eysenck and Calvo’s (1992) processing efficiency theory, which states that high anxiety is most disruptive to cognition under conditions of high cognitive load because working memory resources compete with worry. Indeed, some researchers (e.g. Berggren et al, 2013; Judah et al., 2013) have shown that trait anxious individuals show larger effects of distraction under working memory load. As an alternative to Eysenck et al. (Eysenck & Calvo, 1992; Eysenck et al., 2007), Vytal et al. (2012) present King and Schaefer’s (2010) theory on top-down cognitive control of emotion and suggest that higher load reallocates resources towards task demands and results in a reduction in anxiety. Accordingly, in high anxiety, performance of perceptual and cognitive tasks is impaired under low load but not under high load where there is a competition of top-down cognitive resources. In their study, Vytal et al. (2012) used a threat induction procedure and a verbal n-back task to show that state anxiety led to impaired performance under low working memory load (1-back or 2-back) but not under high working memory load (3-back). However, as these researchers included threat stimuli in their study, it is not clear whether taxing working memory would affect attention control in the absence of threat.
In the current paper, we report the results of two studies examining the interaction of anxiety, attention control, and working memory load in the absence of threat stimuli. In Study I we examined whether state anxiety increases interference from distractors. Moreover, we examined if these effects are influenced by working memory load. To this end, we administered the ANT (Fan et al., 2002) under both high and low cognitive load to anxious individuals and matched controls. We examined whether, under low load and high load, anxiety would be differentially associated with interference from distractors. In Study II we examined the same questions in patients with a diagnosis of Generalized Anxiety Disorder (GAD) compared to non-anxious control (NAC) participants.
Study I
Method
Participants
Participants were 108 individuals drawn from a pool of undergraduate students at a large university. They were unscreened volunteers who received course credit for their participation. On the basis of a median split of the STAI-state measure of anxiety, participants were included in the high anxiety group (HA, n = 57) or the low anxiety group (LA, n = 51). Mean STAI-state score for the HA group was 44.35 (SD = 8.26) and for the LA group was 28.02 (SD = 4.43).
Materials and Tasks
Self-Report Measures
The Spielberger State-Trait Anxiety Inventory (STAI-S/T: Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) is a 40-item questionnaire that assesses levels of anxiety. Participants completed the state and trait versions of the measure. This measure has adequate psychometric properties. In the present study, internal consistency for the sample was Cronbach’s alpha = .92 for STAI-State and .92 for STAI-Trait. Participants also completed the Beck Depression Inventory-II (BDI-II: Beck, Steer, & Brown, 1996), which is a reliable and well-validated 21-item self-report measure of symptoms of depression. The BDI-II has been shown to have good psychometric properties in college populations. In the present study, internal consistency for the sample was Cronbach’s alpha = .87.
Attention Network Task (ANT)
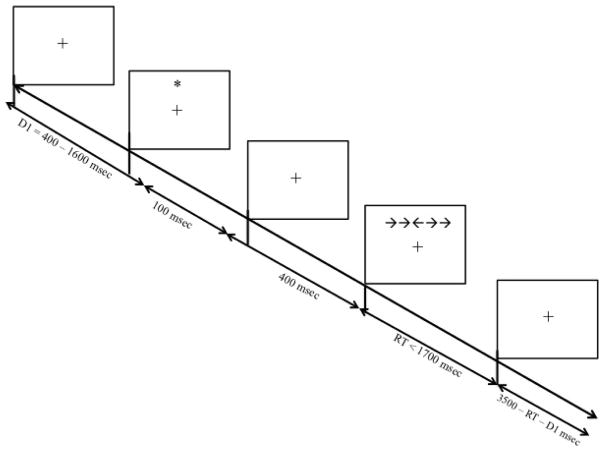
The ANT is a computerized task developed by Fan et al. (2002) to assess distinct components of attention. We used a shorter version of the original ANT task with 96 trials instead of the 288 trials in the original study. Based on the procedure outlined by Fan and colleagues (2002), each trial began with a fixation cross presented in the center of the computer screen for 400–1,600 msec. Next, the cross was replaced by either a blank screen or by asterisk cues that appeared either above, below, or both above and below the location of the cross for 100 msec. Next, a row of arrows appeared either above or below the location of the fixation cross. In the test trials, the center arrow was flanked by arrows that were either congruent (e.g., →→→→→) or incongruent (e.g., →→←→→). The arrows remained on the screen until the participant responded (see Figure 1.1). Participants were instructed to identify the direction (left or right) of the target (center) arrow by pressing the corresponding mouse button, and their response times (RT) were recorded by the software program.
Figure 1.
Attention Network Task adapted from Fan et al., (2002).
Participants first completed a set of 20 practice trials. Next, they completed 96 test trials, without a break, comprising five cueing conditions (no-cue, center-cue, double-cue, direct-cue top, direct-cue bottom) and three flanker conditions (control, congruent, incongruent). Control trials comprised flanking dashes (e.g., — —→ — —). Trials were presented in a new random order to each participant. All stimuli were presented in black against a grey background in a 12-point Arial font. Participants sat approximately 60 cm away from the screen. The distance between the beginning of the leftmost stimulus and the end of the rightmost stimulus was 4 cm. The computer program for this experiment was written in Delphi (Embarcadero Corp., Austin, TX, 2005).
Cognitive Load Task
Participants performed the ANT twice, once under a low load condition and once under a high load condition. The two load conditions comprised a concurrent counting task while completing the ANT. In the low load condition participants were instructed to count backward from 100 by 1’s while completing the ANT whereas in the high load condition participants were instructed to count backward from 100 by 3’s while completing the ANT (e.g., van den Hout et al., 2010). The order in which the load conditions were presented was counterbalanced across participants.
Procedure
All procedures were approved by the institutional review board. Participants completed an informed consent form, a demographics questionnaire, STAI-S/T, and BDI-II, followed by the Attention Network Task.
Results
Data Exclusions
Trials with incorrect responses were removed (3.31%). Outliers were removed in keeping with recommendations from Ratcliff (1993). Response latencies ±3 SD from each participant’s mean response latency were also excluded from analysis (2.13% of remaining trials). Participants with mean accuracy less than 75% were excluded from analysis, which resulted in the exclusion of three participants from the LA group and three participants from the HA group.
Demographics and Self-Report Data
Table 1 summarizes the demographic and self-report data for the resulting HA and LA groups. The two groups did not differ in age, education, or sex. As expected, participants in the HA group had significantly greater STAI-state, STAI-trait, and BDI scores compared to those in the LA group.
Table 1.
Demographics and questionnaire data for Study I and Study II
| Study I | |||
|---|---|---|---|
| Anxiety Group | |||
| LA (n = 54) | HA (n = 48) | ||
| % female* | 67.93 | 60.42 | χ2(1) = 0.62, p = .43 |
| Age | 19.63 (3.42) | 19.31 (2.16) | t(100) = 0.55, p = .58 |
| Education | 13.48 (1.17) | 12.13 (5.61) | t(100) = 1.74, p = .09 |
| STAI state | 28.33 (4.33) | 44.67 (8.39) | t(100) = −12.56, p< .001 |
| STAI trait* | 33.30 (9.04) | 46.92 (10.49) | t(99) = −7.01, p< .001 |
| BDI-II | 6.41 (4.96) | 11.79 (8.17) | t(100) = −4.07, p< .001 |
| Study II | |||
|---|---|---|---|
| Group | |||
| NAC (n = 26) | GAD (n = 24) | ||
| % female* | 60 | 75 | χ2(1) = 1.25, p = .26 |
| Age | 35.50 (14.62) | 38.46 (12.51) | t(48) = 0.77, p = .45 |
| Education* | 15.00 (1.87) | 15.61 (2.13) | t(45) = 1.05, p = .30 |
| STAI state | 26.46 (6.66) | 54.13 (12.73) | t(48) = 9.74, p< .001 |
| STAI trait | 26.38 (5.73) | 63.79 (7.81) | t(48) = 19.41, p< .001 |
| BDI-II* | 1.20 (1.38) | 29.42 (8.86) | t(47) = 15.73, p< .001 |
Note. STAI-S/T: Spielberger State/Trait Anxiety Inventory; BDI-II: Beck Depression Inventory II
In Study I, one participant declined to report sex; STAI-trait was missing for one participant.
In Study II, one participant in the GAD group and two participants in the NAC group declined to report Education; One participant in the NAC group declined to report sex; BDI-II was missing for one participant in the NAC group.
Cognitive Load Manipulation Check
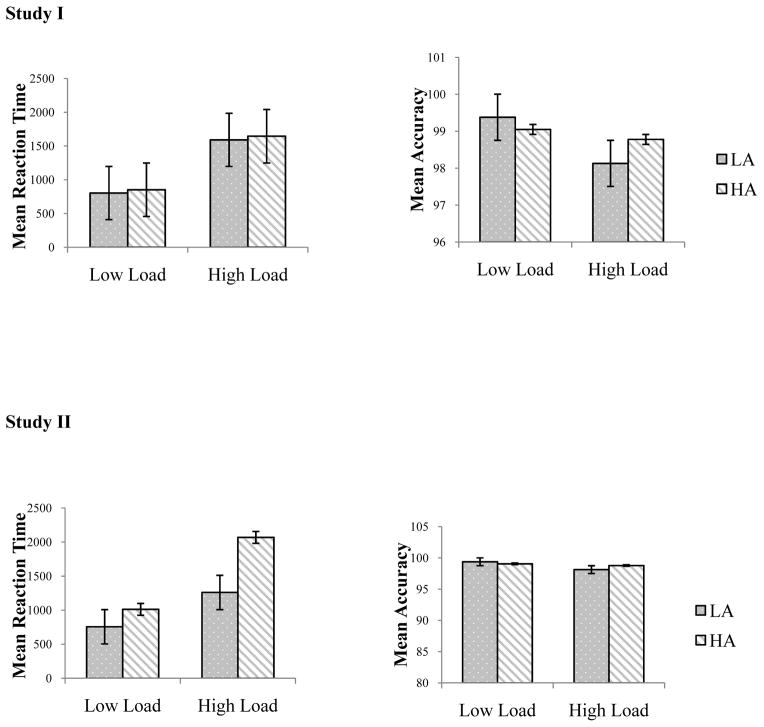
To check that high cognitive load was characterized by slower speed and lower accuracy relative to the low load condition, we submitted mean RTs to a 2 (Group: LA, HA) × 2 (Load: low, high) repeated measures ANOVA with Group as the between subjects variable and Load as the within subjects variable. As predicted, we found a significant main effect of load [F(1,100) = 141.44, p < .001, η2 = 0.59] such that mean RTs for the high load condition were significantly larger than for the low load condition. The main effect of anxiety was not significant [F(1,100) = .03, p = .60, η2 = 0.003]. The interaction of load and anxiety group was not significant [F(1,100) = .001, p =.98, η2 = 0.00] (See Figure 2; Table 2).
Figure 2.
Mean RT and Mean Accuracy for anxiety groups under low and high cognitive load in Study I and Study II (standard error bars)
Table 2.
Mean RT and Accuracy Data for Study I and Study II
| Study I | |||
|---|---|---|---|
| LA | HA | ||
| Incongruent Trials – Low Load | 861(221) | 935(305) | t(100) = −1.41, p = .16 |
| Incongruent Trials – High Load | 1645(713) | 1671(875) | t(100) = −0.16, p = .87 |
| Congruent Trials – Low Load | 781(215) | 826(325) | t(100) = −0.83, p = .41 |
| Congruent Trials – High Load | 1592(679) | 1645(909) | t(100) = −0.74, p = .46 |
| % Accuracy – Low Load | 99.38 | 99.05 | t(100) = 1.44, p = .15 |
| % Accuracy – High Load | 98.13 | 98.78 | t(100) = −1.98, p = .051 |
| Study II | |||
|---|---|---|---|
| NAC | GAD | ||
| Incongruent Trials – Low Load | 814(209) | 1034(615) | t(48) = 1.072, p = .09 |
| Incongruent Trials – High Load | 1358(682) | 2012(981) | t(48) = 2.76, p = .01 |
| Congruent Trials – Low Load | 728(165) | 915(428) | t(48) = 2.07, p = .04 |
| Congruent Trials – High Load | 1193(432) | 2160(1345) | t(48) = 3.48, p = .001 |
| % Accuracy – Low Load | 99.24 | 99.26 | t(48) = 0.09, p = .93 |
| % Accuracy – High Load | 97.40 | 98.26 | t(48) = 0.98, p = .33 |
Next, we submitted mean accuracy to a 2 (Group: LA, HA) × 2 (Load: low, high) repeated measures ANOVA with Group as the between subjects variable and Load as the within subjects variable. Once again, we found a significant main effect of load [F(1,100) = 18.41, p< .001, η2 = 0.16] such that mean accuracies for the high load condition were significantly lower than for the low load condition. There was no significant main effect of anxiety [F(1,100) = .49, p = .48, η2 = 0.005]. The interaction between load and anxiety group was significant [F(1,100) = 7.92, p = .01, η2 = 0.07] (See Figure 2). However, follow-up t-tests did not reveal significant differences in mean accuracies between the LA and HA groups for the low or high load conditions (ps> .05).
Based on these findings of slower speed and lower accuracy in the high load condition, we concluded that the high load condition did indeed tax working memory more so than did the low load condition (van den Hout et al., 2010).
Attention Network Task Measures
We computed the three components of attention – alerting, orienting, and executive attention control – as described by Fan et al. (2002). The alerting index was calculated by subtracting mean RT for double-cue trials from mean RT for no-cue trials. The orienting index was calculated by subtracting mean RT for direct-cue trials from mean RT for center-cue trials. The attention control index was calculated by subtracting mean RT for congruent trials from mean RT for incongruent trials. A higher score for the conflict index indicates lower attention control.
Effect of anxiety on the components of attention under low cognitive load
We used independent samples t-tests to examine the effect of anxiety on the three components of attention under low cognitive load. There was no significant difference between LA and HA groups in their alert scores [t(100) = 0.59, p = .56, d = 0.12], orient scores [t(100) = 0.28, p = .78, d = 0.06], or conflict scores [t(100) = −1.25, p = .22, d = −0.25].
Effect of anxiety on the components of attention under high cognitive load
We used independent t-tests to examine the effect of anxiety on the three components of attention under high cognitive load. There was no significant difference between LA and HA groups in their alert scores [t(100) = −0.35, p = .73, d = 0.07], orient scores [t(100) = −1.43, p = .16, d = −0.29], or conflict scores [t(100) = 1.64, p = .10, d = 0.33].
Effect of cognitive load and anxiety on attention control
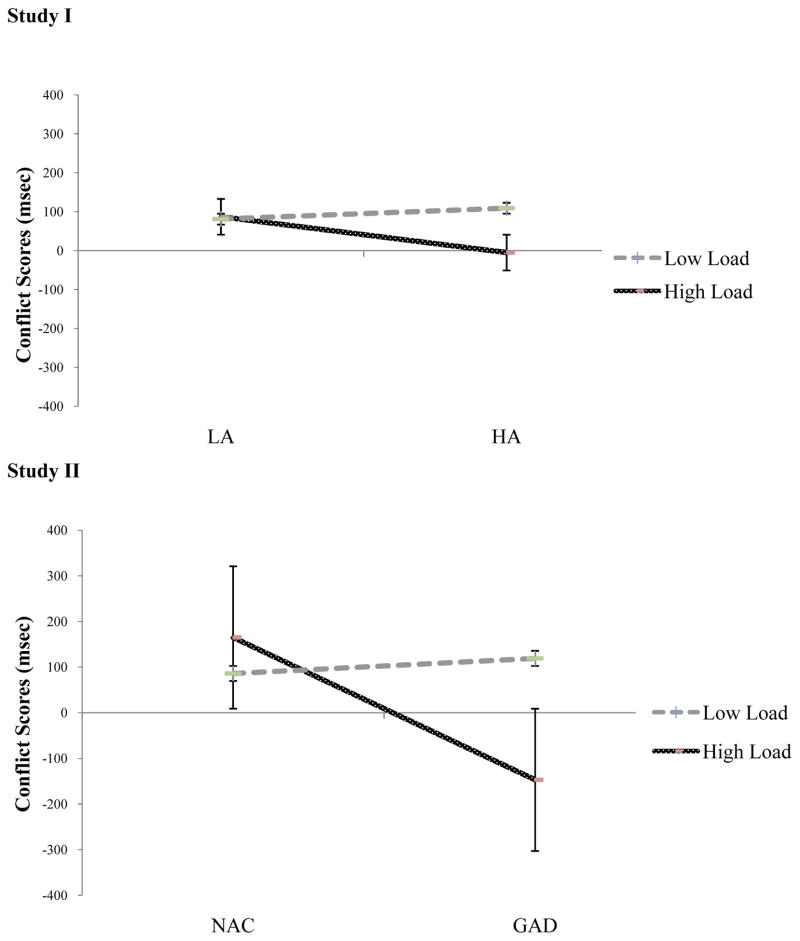
To examine the effect of cognitive load and anxiety on interference from distractors, we submitted the scores from the conflict component of attention from the high and low cognitive load condition to a 2 (Group: HA, LA) × 2 (Load: High, Low) repeated measures ANOVA with repeated measures on the second factor. These results revealed a significant interaction of Group x Cognitive Load [F(1,100) = 4.18, p = .04, η2 = 0.04]. The main effect of Group was not significant [F(1,100) = 1.04, p = .31, η2 = .01], and nor was the main effect of Load [F(1,100) = 3.38, p = .07, η2 = .03] (See Figure 3). To follow up the 2 × 2 interaction, we conducted independent samples t-tests to assess the effect of anxiety group on conflict scores for each load condition. As noted above, there was no significant difference in conflict scores between the LA and HA groups for the low load condition [t(100) = −1.25, p = .22, d = −0.25] or the high load condition [t(100) = 1.64, p = .10, d = 0.33]. We also conducted paired sample t-tests to assess the effect of load within each anxiety group. For the LA group, there was no significant difference in conflict scores between the high and low load conditions [t(53)= −0.14, p = .89]. However, results for HA group revealed a significantly lower conflict score in the high load condition (M = −5.29, SD = 271.05) compared to the low load condition (M = 109.48, SD = 149.96), [t(47) = 2.83, p = .01].
Figure 3.
Effects of cognitive load and anxiety group on attention control in Study I and Study II (standard error bars)
Study I Discussion
In Study I we found that high anxiety was associated with increased interference from distractors under high load relative to low load, implying decreased attention control under high load for the high anxious group. These results are consistent with findings from King and Schaefer (2010) and Vytal et al. (2012). Although these results suggest that cognitive load may interact with the effect of anxiety on attention control, it is not clear whether this effect has clinical relevance as participants in the current study were an analogue sample. We addressed this question by replicating Study I using a sample of individuals with a clinical diagnosis of GAD in Study II.
Study II
Method
Participants
All participants were recruited through posted announcements in community settings and local newspapers that described the program and provided a telephone contact number. Diagnostic assessment was based on an initial telephone screening followed by an in-person diagnostic interview. Participant in the GAD group were 30 individuals (8 men, 22 women) who met the diagnostic criteria for GAD based on a diagnostic interview using the Structured Clinical Interview for the DSM-IV (SCID: First & Williams, 1997). Participants were included in the GAD group if they had a principal DSM-IV (American Psychiatric Association, 2000) Axis I diagnosis of GAD. Of the participants in the GAD group, 5 had a comorbid diagnosis of Major Depressive Disorder, 3 had a comorbid diagnosis of Posttraumatic Stress Disorder, 1 had a comorbid diagnosis of Social Phobia, 1 had a comorbid diagnosis of Obsessive-Compulsive Disorder, 1 had a comorbid diagnosis of Panic Disorder with Agoraphobia, and 1 had a comorbid diagnosis of Specific Phobia. Participant in the NAC group were 28 individuals (10 men, 17 women, 1 participant declined to report sex) who were included in the study if they showed no evidence for any current or past DSM-IV Axis I diagnoses on the diagnostic interview using the SCID. Participants were excluded from both groups if they showed evidence of suicidal intent, evidence of substance abuse or dependence in the past 3 months, or evidence of current or past schizophrenia, bipolar disorder, or organic mental disorder.
Materials and Tasks
Self-Report Measures
The self-report measures in the current study were the same as the ones administered in Study I. In the present study, internal consistency for the GAD sample was Cronbach’s alpha = .86 for STAI-State, .80 for STAI-Trait, and .87 for BDI-II; internal consistency for the NAC sample was Cronbach’s alpha = .90 for STAI-State, .80 for STAI-Trait, and .47 for BDI-II.
Attention Network Task (ANT)
The ANT in the current study was the same as the one administered in Study I.
Cognitive Load Task
The cognitive load tasks in the current study were the same as the ones administered in Study I.
Procedure
All procedures were approved by the institutional review board. After providing informed consent, participants completed an initial assessment for study eligibility that comprised the diagnostic interview (SCID: First & Williams, 1997) and administration of the demographics and self-report questionnaires. Next, they completed the Attention Network Task.
Results
Data Exclusions
Trials with incorrect responses were removed (7.47%). Response latencies ±3 SD from each participant’s mean response latency were also eliminated from analysis (2.07% of remaining trials). Participants with mean accuracy less than 75% were excluded from analysis, which resulted in the exclusion of one participants from the NAC group and six participants from the GAD group. Finally, one participants from the NAC group was removed for not being able to follow task instructions.
Demographics and Self-Report Data
Table 1 summarizes the demographic and self-report data for the resulting sample.
Cognitive Load Manipulation Check
To check that high cognitive load was characterized by slower speed and lower accuracy relative to the low load condition, we submitted mean RTs to a 2 (Group: GAD, NAC) x 2 (Load: low, high) repeated measures ANOVA with Group as the between subjects variable and Load as the within subjects variable. As predicted, we found a significant main effect of load [F(1,48) = 89.38, p < .001, η2 = 0.65] such that mean RTs for the high load condition were significantly larger than for the low load condition. The the main effect of group [F (1,48) = 8.30, p = .006, η2 = 0.15] was significant, as was the interaction of load and group [F(1,48) = 11.20, p =.002, η2 = 0.19], such that mean RTs were larger for the GAD group than for the NAC group in the high load condition.
Next, we submitted mean accuracy to a 2 (Group: GAD, NAC) × 2 (Load: low, high) repeated measures ANOVA with Group as the between subjects variable and Load as the within subjects variable. Once again, we found a significant main effect of load [F(1,48) = 12.39, p = .001, η2 = 0.21] such that mean accuracies for the high load condition were significantly lower than for the low load condition. There was no significant main effect of group [F(1,48) = 0.76, p = .39, η2 = 0.02]. The interaction between load and group was also not significant [F(1,48) = 1.10, p = .30, η2 = 0.02].
Based on these findings of slower speed and lower accuracy in the high load condition, we concluded that the high load condition did indeed tax working memory more so than did the low load condition, and that it did not do so differently in the GAD and NAC groups (van den Hout et al., 2010).
Attention Network Task Measures
Effect of clinical group on the components of attention under low cognitive load
We used independent samples t-tests to examine the effect of clinical group on the three components of attention under low cognitive load. There was no significant difference between the GAD and NAC groups in their alert scores [t(48) = 0.69, p = .49, d = 0.20], orient scores [t(48) = −1.75, p = .09, d = 0.51], or conflict scores [t(48) = 0.57, p = .57, d = 0.16].
Effect of clinical group on the components of attention under high cognitive load
We used independent t-tests to examine the effect of anxiety on the three components of attention under high cognitive load. There was no significant difference between the GAD and NAC groups in their alert scores [t(48) = −0.57, p = .57, d = 0.16] and orient scores [t(48) = 1.39, p = .17, d = 0.40]. However, conflict scores for the GAD group were significantly smaller than for the NAC group, [t(48) = −2.45, p = .02, d = 0.71].
Effect of cognitive load and clinical group on attention control
To examine the effect of cognitive load and clinical on interference from distractors, we submitted conflict scores from the high and low cognitive load conditions to a 2 (Group: GAD, NAC) × 2 (Load: low, high) repeated measures ANOVA with Group as the between subjects variable and Load as the within subjects variable. The interaction between load and group was significant [F(1,48) = 4.27, p = .04, η2 = 0.08] (See Figure 3), as was the main effect of group [F(1,48) = 6.82, p = .01, η2 = 0.12]. The main effect of load was not significant [F(1,48) = 1.26, p = .27, η2 = 0.03]. To follow up the 2 × 2 interaction, we conducted independent samples t-tests to assess the effect of group on conflict scores for each load condition. As noted above, there was no significant difference in conflict scores between the GAD and NAC groups for the low load condition [t(48)= 0.57, p = .57, d = 0.16]. However, for the high load condition, the conflict score for the GAD group was significantly smaller than that of the NAC group [t(48)= −2.45, p = .02, d = −0.71]. We also conducted paired sample t-tests to assess the effect of load within each group. There was no significant difference in conflict scores between the low and high load conditions for the GAD group [t(23) = 1.65, p = .11] or the NAC group [t(25) = −1.31, p = .20].
Study II Discussion
In Study II we tested whether our findings from Study I had implications for clinical levels of anxiety. We found that individuals with GAD, relative to NACs, experienced decreased interference from distractors, but only in the high cognitive load condition. This finding is consistent with findings from King and Schaefer (2010) and Vytal et al. (2012).
General Discussion
In the current paper, we reported the results of two studies examining the interaction of anxiety, attention control, and working memory load. In Study I we found that, consistent with findings from King and Schaefer (2010) and Vytal et al. (2012), under high load anxiety was characterized by decreased interference from distractors, that is, increased attention control. In Study II, we considered the clinical implications of the construct of attention control -- as measured by the ANT -- by examining the same questions with individuals with a clinical diagnosis of GAD relative to non-anxious individuals. We found that, individuals with GAD, relative to NACs, experienced decreased interference from distractors, but only in the high cognitive load condition.
Our findings help to clarify apparently contradictory predictions regarding the influence of anxiety on attention. Our findings suggest that these effects are dependent on the presence of low or high cognitive load. On the one hand, several researchers (e.g. Berggren, Derryberry, Derakshan, Eysenck, and colleagues) have shown that anxious individuals show larger effects of distraction under working memory load, indicative of competition for cognitive resources, as stated in the processing efficiency theory (Eysenck & Calvo, 1992). Our findings similarly suggest that the competition for attentional resources causes anxiety-related impairment in task performance; however, in contrast to the processing efficiency theory, we find that a low cognitive load task leaves attentional resources free for anxiety-related impairment in task performance, whereas a high cognitive load task competes for attentional resources with worry and diminishes anxiety-related impairment in task performance, similar to what is described by Vytal et al. (2012).
Our results appear at odds with some findings in the extant research on this topic. For instance, Pacheco-Unguetti, Acosta, Lupiáñez, Román, & Derakshan (2012) used a go/no-go task to examine the effect of load and state anxiety on response inhibition and concluded that state anxiety results in lower attention control and increased interference from peripheral distractors. However, the focus of their study was a perceptual load rather than a working memory load and their task included threatening stimuli. Similarly, findings such as those of Judah et al. (2012) that suggest that high working memory load causes impairment in attentional bias tasks may appear at odds with ours. However, the focus of their study was the effect of working memory load on processing of threat-related stimuli, whereas in our study we focused on neutral stimuli.
Indeed, most studies examining interference from distractors have focused on threat-related distractors. For instance, electroencephalography (EEG) studies have identified the late positive potential (LPP) as an index of attention towards task-irrelevant stimuli and have shown that high working memory load decreases LPP to threatening picture distractors. In other words, high load hinders the processing of task-irrelavent stimuli, as evidenced by LPP. Contrary to our findings, these studies also show that the effect of working memory load on attention to task-irrelevant stimuli is attenuated in high anxiety, both in subclinical anxiety (MacNamara, Ferri, & Hajcak, 2011) and in GAD (MacNamara & Proudfit, 2014). It is important to note that this effect was not significant for neutral pictures. Taken together, it appears that high cognitive load reveals attention control deficits in high anxiety but only in the presence of threat distractors.
The finding that anxiety is associated with lower attention control both in subclinical and clinical samples has been documented previously (e.g., Derryberry & Rothbart, 1988, 1997; Rothbart et al., 2004). We did not find evidence consistent with this hypothesis under conditions of low load in either the subclinical group (Study I) or the clinical group (Study II). One possibility is that many previous studies have examined attention control using self-report measures; the attention control component assessed by ANT accesses cognitive processing using a performance-based measure and may indeed have better predictive validity than do symptom measures. At the very least, these findings challenge an unqualified reliance on self-report and are consistent with earlier studies that have reported non-significant associations between the self-report and performance-based measures of attention control (e.g., Reinholdt-Dunne et al., 2009).
Do our findings suggest that high cognitive load is beneficial for individuals with elevated anxiety? We suppose that the answer likely depends on the contents of the target stimulus relative to the content of the distractors. For example, if our target is a neutral or positive stimulus, then distractors are likely to be undesirable (e.g., threat cues in the environment; worries). Hence, greater attention control would be preferable, which would be facilitated by conditions of high cognitive load. In this example greater attention control may allow an individual to inhibit involuntary attention to threat cues or worries whereas lower attention control may enhance attention to threat cues (e.g., Reinholdt-Dunne et al., 2009). Consistent with this notion, research by Forster and Lavie (2009) has shown that mind-wandering rates are reduced significantly under high load. Indeed, mind-wandering towards worries is a central feature of GAD. If our central focus is an internally generated target such as worries in GAD, then distractors are likely to be desirable. In this example, lower attention control would be preferable and would be hindered by conditions of high load.
The current studies have limitations. First, we did not include a measure of participant error in performing the counting task and hence cannot verify that participants were performing the cognitive load tasks as intended. We did, however, conduct a spot check for a random sample of 10% of the participants in Study I and found 100% accuracy in counting backward by 1’s in the low load task for both low and high anxious groups, and 90.8% and 91.4% accuracy in counting backward by 3’s in the high load task for the low and high anxious groups respectively. Nonetheless, that we did not assess counting accuracy for the entire sample is a limitation of the current studies. A second limitation is that, both the high anxious sample from Study I and the GAD sample in Study II comprised participants with elevated BDI scores; therefore it remains untested whether our findings are specific to anxiety, depression, or comorbid anxiety and depression. Indeed, in the MacNamara and Proudfit (2014) study mentioned above, the authors found that anhedonic depression within the GAD group showed a reduced effect of working memory load on LPP for neutral distractors. Follow-up studies conducted to compare individuals diagnosed with an anxiety disorder with differing levels of depressive symptoms would greatly improve the conclusions that can be drawn regarding the effect of anxiety and cognitive load on attention control. Finally, we chose to use state anxiety to classify our groups in Study I because we were interested in examining the effect of anxiety at the time of administering the ANT. However, we (and others, e.g., Bishop (2009)) acknowledge that the state and trait anxiety measures are highly correlated and hence we must be cautious in making differential inferences.
These limitations notwithstanding, our study demonstrates the first attempt to elucidate the precise relationship between anxiety, cognitive load, and attention control for neutral information. Recent successes in cognitive bias modification techniques suggest the possibility of altering cognitive processes in anxiety. Given the potentially harmful correlates of low and high attention control under different load conditions, a promising direction for future research might be to develop programs to train individuals to enhance attention control and to use it selectively in conditions of low and high load.
Acknowledgments
This work was funded by NIMH Grant # R01MH087623-01A1 awarded to Dr. Amir.
Footnotes
Disclosure of Interest: Dr. Amir has a financial interest in Cognitive Retraining Technologies Incorporated, a company that markets anxiety relief products.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorder. 4. Washington DC: Author; 2000. text rev. [Google Scholar]
- Bar-Haim Y, Bakermans-Kraneburg MJ, Pergamin L, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bishop S. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Berggren N, Richards A, Taylor J, Derakshan N. Affective attention under cognitive load: reduced emotional biases but emergent anxiety-related costs to inhibitory control. Frontiers in Human Neuroscience. 2013;7:1–7. doi: 10.3389/fnhum.2013.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Costello E. Efficacy of applied relaxation and cognitive-behavioral therapy in the treatment of generalized anxiety disorder. Journal of Consulting and Clinical Psychology. 1983;61(4):611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: Some characteristics and processes. Behaviour research and therapy. 1983;21(1):9–16. doi: 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Eysenck MW. Anxiety, processing efficiency, and cognitive performance. European Psychologist. 2009;14(2):168–176. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111(2):225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Arousal, Affect, and attention as components of temperament. Journal of Personality and Social Psychology. 1988;55(6):958–966. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Developmental and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Attentional control, trait anxiety, and the regulation of irrelevant response information. 2001. Manuscript in review. [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fajkowska M, Derryberry D. Psychometric properties of Attentional Control Scale: The preliminary study on a Polish sample. Polish Psychological Bulletin. 2010;41(1):1–7. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Finucane AM, Power MJ. The effect of fear on attentional processing in a sample of healthy females. Journal of Anxiety Disorders. 2010;24(1):745–761. doi: 10.1016/j.janxdis.2009.08.005. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D. C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- First MB, Gibbon M. User’s guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. Amer Psychiatric Pub Incorporated; 1997. [Google Scholar]
- Forster S, Lavie N. Harnessing the wandering mind: the role of perceptual load. Cognition. 2009;111(3):345–355. doi: 10.1016/j.cognition.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Barrett LF. Emotion generation and emotion regulation: One or two depends on your point of view. Emotion Review. 2011;3:8–16. doi: 10.1177/1754073910380974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23(1):56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judah MR, DeMond MG, Lechner WV, Mills AC. Working memory load moderates late attentional bias in social anxiety. Cognition and Emotion. 2012:1–10. doi: 10.1080/02699931.2012.719490. [DOI] [PubMed] [Google Scholar]
- King R, Schaefer A. The emotional startle effect is disrupted by a concurrent working memory task. Psychophysiology. 2010;48:269–272. doi: 10.1111/j.1469-8986.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- Lavie N. Selective attention and cognitive control: Dissociating attentional functions through different types of load. In: Monsell S, Driver J, editors. Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 175–194. [Google Scholar]
- Lavie N. Distracted and confused? Selective attention under load. Trends in Cognitive Sciences. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N. Attention, distraction, and cognitive control under load. Current Directions in Psychological Science. 2010;19(3):143–148. [Google Scholar]
- Macleod C. Cognition in clinical psychology: measures, methods or models? Behaviour Change. 1993;10(3):169–195. [Google Scholar]
- MacLeod C, Hagan R. Individual differences in the selective processing of threatening information, and emotional responses to a stressful life event. Behaviour research and therapy. 1992;30(2):151–161. doi: 10.1016/0005-7967(92)90138-7. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Ferri J, Hajcak G. Working memory reduces the LPP and this effect is attenuated with increasing anxiety. Cognitive, Affective & Behavioral Neuroscience. 2011;11:321–331. doi: 10.3758/s13415-011-0036-z. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Proudfit GH. Cognitive Load and Emotional Processing in Generalized Anxiety Disorder: Electrocortical Evidence for Increased Distractibility. Journal of Abnormal Psychology. 2014 Jun 16; doi: 10.1037/a0036997. Advance online publication. http://dx.doi.org/10.1037/a0036997. [DOI] [PMC free article] [PubMed]
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders. 1988;14(1):61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State worry questionnaire. Behaviour research and therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. 1994. [Google Scholar]
- Najmi S, Kuckertz JM, Amir N. Attentional impairment in the anxiety: inefficiency in expanding the scope of attention. Depression and Anxiety. 2012;29(3):243–249. doi: 10.1002/da.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett RE, Wilson TD. Telling more than we can know: Verbal reports on mental processes. Psychological Review; Psychological Review. 1977;84(3):231. [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupiáñez J. Attention and anxiety: different attentional functioning under state and trait anxiety. Psychological Science. 2010;21(2):298–304. doi: 10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly journal of experimental psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Vizuetta N, Levy K, Thomas KM, Clarkin JF. Attentional mechanisms of borderline personality disorder. Proceedings of the National Academy of Science, USA. 2002;99(25):16366–16370. doi: 10.1073/pnas.252644699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychological Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Reinholdt-Dunne ML, Mogg K, Bradley BP. Effects of anxiety and attention control on processing pictorial and linguistic emotional information. Behaviour research and therapy. 2009;47(5):410–417. doi: 10.1016/j.brat.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Reinholdt-Dunne ML, Mogg K, Bradley BP. Attention control: relationships between self-report and behavioural measures, and symptoms of anxiety and depression. Cognition and Emotion. 2012;1:1–11. doi: 10.1080/02699931.2012.715081. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71(6):1115–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Posner MI. Temperament and self-regulation. Handbook of self-regulation: Research, theory, and applications. 2004:357–370. [Google Scholar]
- Ruscio AM, Borkovec TD. Experience and appraisal of worry among high worriers with and without generalized anxiety disorder. Behaviour research and therapy. 2004;42(12):1469–1482. doi: 10.1016/j.brat.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- van den Hout MA, Engelhard IM, Smeets MAM, Hornsveld H, Hoogeveen E, de Heer E, Rijkeboer M. Counting during recall: taxing working memory and reduced vividness and emotionality of negative memories. Applied Cognitive Psychology. 2010;24(3):303–311. [Google Scholar]
- Vytal K, Cornwell C, Arkin N, Grillon C. Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]