Abstract
Evolutionary pressure for protein function leads to unavoidable sampling of conformational states that are at risk of misfolding and aggregation. The resulting tension between functional requirements and the risk of misfolding and/or aggregation in the evolution of proteins is becoming more and more apparent. One outcome of this tension is sensitivity to mutation, in which only subtle changes in sequence that may be functionally advantageous can tip the delicate balance toward protein aggregation. Similarly, increasing the concentration of aggregation-prone species by reducing the ability to control protein levels or compromising protein folding capacity engenders increased risk of aggregation and disease. In this Perspective, we describe examples that epitomize the tension between protein functional energy landscapes and aggregation risk. Each case illustrates how the energy landscapes for the at-risk proteins are sculpted to enable them to perform their functions and how the risks of aggregation are minimized under cellular conditions using a variety of compensatory mechanisms.
Evolution selects protein sequences for the ability to function and not simply for stability or ability to fold, as long as folding is efficient enough for cell survival. This principle has many consequences. The sequence of a functional protein encodes: the ability of the protein to sample alternate conformations; the probability with which this sampling occurs, i.e., the conformational dynamics of the protein; the ease with which the protein folds and finds its native, functional state; and the tendency of the protein to interact with other proteins or itself. All of this is described by a protein’s energy landscape: the multidimensional surface that describes the choreography of protein folding. The energy landscapes for most proteins in the functional proteome will be evolutionarily selected to be rough, which means that a protein will have multiple energy minima available to it, depending on its environment and interactions. Rough energy landscapes correlate with frustration in folding1. They also explain the observation that kinetically stable states (deep minima with high barriers around them) may frequently be populated during folding2,3 and may form part of the ensemble of states populated under a given set of cellular conditions (pH, temperature or concentrations of partner ligands, for example). For folded proteins, cellular conditions and availability of binding partners may shift the energy landscape such that the bound conformation represents a deeper energy well and partially folded states are relatively disfavored. Only recently has an unavoidable dark side of functional protein energy landscapes—kinetic traps, frustration, metastability and sampling of alternative folds—been fully recognized.
Despite our ability to lock proteins into crystalline forms, it has long been known that they are not rocks. Quite the contrary: proteins are molecular machines composed of movable parts that work together to accomplish a wide range of physiological functions. From an energy landscape point of view, protein dynamics translate into excursions from one low-energy conformation to another. Hence, the dynamics of protein structures are crucial for their function by enabling the population of alternative conformations. The alternative conformations may enable functional interactions by exposing interactive surfaces, providing opportunities for new, favorable interactions (in functional terms). However, there is also a chance that the exposed interaction surfaces are aggregation prone, thus creating a risk of dysfunctional interactions, which are causative in an increasing family of pathologies4,5.
Even simple two-dimensional representations of energy landscapes can help to clarify these concepts. The depth of energy minima describes a protein’s thermodynamic stability; the heights of the barriers separating energy minima dictate the kinetic stability of a protein, that is, how readily it can leave one conformation and sample another; and the width of minima correlates with the breadth of the conformational ensemble within the energy well. The shape of a protein’s energy landscape is dictated by myriad weak and competing interactions that define the search for its native fold. These same interactions also enable proteins to undergo conformational changes and to use conformational malleability and dynamics for their roles, such as binding-induced folding6 and allosteric regulation7–9. As a consequence, the attributes of energy landscapes are sculpted by the requirements for proteins to perform their functions. In addition, the ability of proteins to evolve new functions places constraints on their energy landscapes that compete with the optimization of stability and function10. As illustrated by the examples discussed in this Perspective, the functionally required features of protein energy landscapes may put proteins at risk of misfolding, aggregation and/or polymerization.
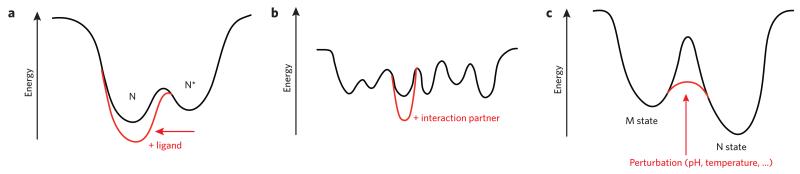
Schematic examples of possible energy landscapes for functional proteins are illustrated in Figure 1. Many proteins must change their conformations away from their native, or lowest, energy state for ligands to gain access to their binding site (or sites). An example of this behavior is the family of intracellular lipid-binding proteins (iLBPs) discussed in the first section below. iLBPs are characterized by an energy landscape that incorporates a near-native apo state (N*; Fig. 1a). In the absence of ligand, N* is dynamically visited from the native state N. Binding of ligand stabilizes and rigidifies the protein (Fig. 1a), leading to the native holoprotein (N). N and N* are generally separated by a relatively low energy barrier such that they readily interconvert. If the functionally required N* state exposes hydrophobic surface, it will also be susceptible to aggregation.
Figure 1. Schematic two-dimensional functional energy landscapes.
(a) Many proteins sample a near-native state (N*) to open a binding site and interact productively with a ligand. Formation of the complex with ligand stabilizes the protein in its native state (N). (b) IDPs have sequences that disfavor a unique folded state and instead lead to many conformational possibilities of nearly equivalent energy. Interaction(s) with partners leads to stabilization of the state(s) that have the capacity to bind the partner with the highest affinity, thus shifting the energy landscape in the presence of partner(s) to one or only a few more stable states. (c) The magnitude of barriers on energy landscapes has a profound impact on protein function. A state that is not the thermodynamically most stable state (here M) may be long lived because it is separated from the most thermodynamically stable state (N) by high-energy barriers. The barriers may be reduced in amplitude by various triggers, whether environmental or protein-protein interactions.
The energy landscapes of intrinsically disordered proteins (IDPs) are devoid of a single energy minimum and instead are characterized by many possible nearly isoenergetic conformational states separated by very low energy barriers, as schematically shown in Figure 1b. Interactions with partner proteins select from this heterogeneous collection of conformational ensembles and shift the landscape so that one conformation becomes preferentially populated. For these proteins as well as for natively folded or partially folded proteins, stability is enhanced by formation of a complex with ligands or protein partners. Thus, for IDPs, major changes in the energy landscape can result from binding, which can result in the formation of a clear energy minimum in the energy landscape (Fig. 1b), akin to that of their natively folded apo-protein counterparts.
Minima on protein conformational energy landscapes may be separated by high barriers, leading to the possibility of kinetically stable states. The example in Figure 1c shows a free energy landscape that includes a kinetically trapped metastable state (M) separated by a large energy barrier from the native state (N), such as that found in the serpin family or for proteins with a cis-proline, such as β2-microglobulin (β2m). The conformational population will shift to the more stable N state either through a slow, spontaneous process that may be catalyzed by environmental perturbations (serpin) or via proline isomerization (β2m). Here, as in the case of the N* state of the iLBP, the M state is vulnerable to intermolecular association to form pathological aggregates, amyloid fibrils or polymers.
In all of these situations, non-native or unfolded protein conformations will inevitably be populated under some cellular conditions, for example, during protein biosynthesis or when a protein’s partner is absent or degraded. Because the evolutionary pressures for proteins to perform their functions drive the features of their energy landscapes, it is not surprising that we are increasingly recognizing new aggregation-based diseases and associating them with features encoded in protein sequences that correlate with their functions. The development of proteins as drugs is accompanied by similar risks, as aggregation poses one of the major bottlenecks in the exploitation of biologics for intervention in human health. It is also not surprising that a wide array of compensatory cellular strategies has evolved to minimize the accumulation of high-risk protein conformational states. Cellular factors that can offset the risk of aggregation include adjustments in expression levels11 and protein turnover12,13, both of which modulate cellular concentration; protection by molecular chaperones14; or the presence of intracellular ligands15. In addition, amino acid sequences themselves have evolved ways to minimize aggregation, including the appropriate positioning of proline or glycine residues; use of gatekeeper residues; minimization of sequence segments with high hydrophobicity, high β-sheet propensity and low net charge; insertion of protective elements at edge β-strands; and, indeed, folding into stable globular structures13. In a recent intriguing example of a protective mechanism to enable function and avoid aggregation, a chaperone in the type III bacterial secretion system exists as a dynamic, molten globule homodimer, poised to bind substrate at the dimer interface16. The aggregation-prone substrate-binding surface is protected by dimerization, and the dimer dynamics enable substrate binding. A similar mechanism has been reported by the HdeA family of bacterial chaperones17,18. Another recent study postulates that the mitochondrial protein frataxin experiences frustration owing to competition between folding and function; in this case, aggregation is avoided because aggregation-prone sequences are sequestered by misfolding events along the folding pathway19. Even though mechanisms have arisen to minimize the risks, vulnerability to aggregation is an inevitable cost of selective pressures for protein function, as seen for the examples discussed in the sections below: iLBPs, serpins, the immunoglobulin domain β2m and ataxin-3 (Atx3). These four very different proteins serve as exemplars of the delicate balance between the requirements for protein function (which frequently involve dynamics), rough energy landscapes and the threat of aggregation. Together, the examples portray how nature has used an array of strategies to enable function to evolve while avoiding the potential risks of aggregation.
iLBPs dynamically open to bind hydrophobic substrates
iLBPs comprise a family of β-barrel proteins that bind hydrophobic ligands in an interior cavity20. Their roles are varied, but all of them rely on their ability to solubilize and transport otherwise water-insoluble ligands. No aggregation or misfolding diseases have been reported for iLBPs, although in vitro studies have shown that the archetypal iLBP, cellular retinoic acid–binding protein 1 (CRABP1), is prone to aggregation as a purified protein or when expressed in Escherichia coli21. What is the origin of this aggregation propensity? How is aggregation avoided under physiological conditions? Formal possibilities include regulation of CRABP1 expression and clearance, so as to reduce its cellular concentration, or protection from aggregation by binding to ligand. Both may be protective in cells. Additionally, recent work has suggested that the folding mechanism of CRABP1 protects aggregation-prone sequences early in the formation of native structure, providing a neat mechanism of minimizing the risk of aggregation22.
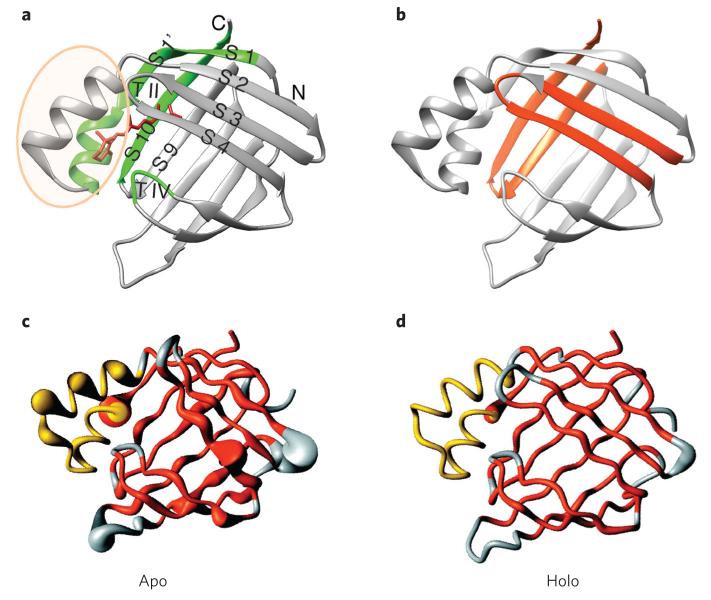
From early on, it was observed that iLBPs are dynamic in the absence of their ligand23. This dynamic character is intimately linked to the functional requirements and gives rise to rugged energy landscapes of iLBPs. The helical region of these proteins, which comprises a helix-loop-helix motif, has been dubbed ‘the helical portal’, as its movements allow the ligand access to the interior cavity within the ten-stranded β-barrel, where the ligand-binding surface is largely presented (Fig. 2). NMR studies of many iLBPs, including ileal lipid-binding protein24, intestinal fatty acid–binding protein (FABP)25, bile acid–binding protein26, liver FABP27 and CRABP1 (ref. 28), all show substantially higher fluctuations in the helical portal than in the β-barrel. The open form of iLBPs that allows ligand binding exemplifies an N* state, (Fig. 1a). In all cases, binding of ligand rigidifies the iLBP and, in particular, dampens movement of the helical portal, shifting the population to the N state.
Figure 2. CRABP1 exemplifies the structure and dynamics of the iLBP family.
(a) Structure of holo-CRABP1 (ref. 104) (Protein Data Bank (PDB) code 1CBR) showing the helical portal domain, proposed to dynamically open to allow ligand entry and exit30,105 (light orange ellipse), and several regions implicated in early folding events in CRABP1 (ref. 22) (green). (b) Regions of CRABP1 found to be sequestered in the cores of aggregates formed either in vitro or in E. coli cells21 are shown in orange. Note that the early folding events may provide a potential protective mechanism to offset the risk of aggregation during folding of apo-CRABP1. (c,d) Comparison showing increased dynamics of CRABP1 in the apo form (c) relative to holo-CRABP1 (d). The width of the polypeptide chain depicts the rate of exchange of backbone amides with solvent water, a clear indicator of chain dynamics28. Panel c is modified with permission from a figure in reference 28. Copyright 2000, American Chemical Society.
Several lines of evidence point to the importance of the dynamics of apo-iLBPs for ligand entry and egress from the binding site29. Some members of the subclass of iLBPs known as FABPs are postulated to bind their ligand directly from a lipid bilayer and then release the ligand into the bilayer. A portal-open form of the FABP has been postulated to dock onto the lipid bilayer, enabling the membrane-resident ligand to gain access to the binding cavity30. In other cases, such as the retinoic acid-binding iLBPs, direct interactions are postulated to occur with a protein partner, the nuclear retinoid receptor, and the ligand is proposed to be passed onto the partner via a mechanism that also requires opening of the helical portal31. The dynamics of the helical portal of the iLBP structure are therefore crucial to many of its functions. Major questions ask how much, or how often, the apo-protein is present inside cells and how cells protect themselves from the threat of apo-state aggregation.
Extensive studies of the folding and aggregation of CRABP1 have been performed21,22,32–36. This has led to intriguing linkages: CRABP1 folds via multiple kinetic steps in which early hydrophobic collapse occurs in a few milliseconds along with formation of its helix-loop-helix motif. In a subsequent 100-ms step, the barrel topology organizes. Stable hydrogen bonding and van der Waals packing of side chains throughout the barrel occur only much later (time constant ~1 s), in a cooperative manner in the rate-determining step. Analysis of which topological interactions are organized earliest in CRABP1 folding showed that interactions between strands 10 and 1, and between helix 1, turn IV and strand 9 (Fig. 2), all favoring barrel closure, are organized first. These structural features sequester β-strand regions that are predicted to have the highest aggregation propensity. Indeed, assessment of the involvement of two different regions of the CRABP1 sequence in aggregates formed in E. coli and in vitro showed that they comprise β-strand 3–turn II–β-strand 4 and β-strands 9 and 10 (Fig. 2). Formation of structural contacts concomitant with early barrel closure sequester these very regions from intermolecular interactions22. It is thus striking that the functionally required exposure of hydrophobic surface in this archetypal iLBP gives rise to a high risk of aggregation. The residues evolutionarily conserved to mediate ligand binding are precisely those that create sequences of high predicted aggregation propensity, and a folding mechanism has evolved that enables protection of these regions during folding, not unlike the example of frataxin described above19. The hydrophobic collapse step in CRABP1 folding can be viewed as a kind of specific intramolecular aggregation. By the evolution of a folding mechanism that sequesters aggregation-prone sequences early through this collapse, intermolecular aggregation is effectively outcompeted. This iLBP exemplar epitomizes the ying of functionally required dynamics that leads to aggregation susceptibility and the yang of simultaneous offsetting coping mechanisms—in this case, the folding mechanism.
Serpins can get caught in their own ‘mouse trap’
Members of the ‘serine protease inhibitor’ or serpin superfamily are also paradigms of the delicate balance between function and folding-associated vulnerabilities. The inhibitory serpins inactivate their target proteases by dangling a scissile bond on a solvent-exposed reactive center loop (RCL). The RCL links the large central β-sheet A to the smaller β-sheet C in these α- and β-sheet proteins37 (Fig. 3a). Protease cleavage triggers RCL insertion into β-sheet A, whereupon it becomes the fourth strand in the now six-stranded sheet (Fig. 3a). This springing of the mouse trap translocates the covalently attached protease ~70 Å relative to the serpin, mechanically disrupting the protease active site by pulling on the single acyl-enzyme covalent bond between the protease active site serine (or cysteine) and the RCL38,39. Thus, serpins fold into a functionally active, yet metastable, kinetically trapped conformation (like the M state in Fig. 1c) that is poised to undergo a massive conformational change concomitant with the serpin’s inhibitory action on its target protease37,40. In the absence of cleavage by their target protease, serpins may slowly convert to an N-like state (Fig. 1c) via insertion of the RCL into β-sheet A; the resulting lower-energy, inactive conformation is termed ‘latent’41. The latency transition can be accelerated or decelerated by environmental perturbations including pH, temperature and the binding of protein partners40, all of which can alter the energy barrier between the active and latent states (Fig. 1c). When serpins perform the functional role of inhibiting proteases, proteolytic cleavage of the RCL initiates a similar conformational change, resulting in an even deeper energy minimum on the energy landscape and altered chain connectivity. These conformational changes support the notion that the serpin energy landscape has multiple energy minima, including the ‘cocked’ inhibitory state, with six strands in β-sheet A, and the more stable, strand-inserted state, with six strands in β-sheet A (Fig. 1c).
Figure 3. Functional and nonfunctional serpin conformational gymnastics.
(a) On the left is shown the active serpin conformation with the solvent-exposed RCL (red), sheet A (yellow) and the shutter, which must open to allow RCL insertion into sheet A. A target protease is shown as a blue space-filling structure as it interacts with the RCL in an initial encounter complex. On the right is shown the conformational transition required for mechanical inactivation of target proteases. (PDB codes 1OPH106 and 1EZX39 on the left and right, respectively). (b) Serpins can form multimers and polymers in vitro and in vivo. Possible interactions include, from left to right: addition of the RCL to the edge of a β sheet as observed in the antithrombin III dimer (PDB code 1E05 (ref. 107)), partial insertion of the RCL into sheet A of an adjacent monomer40 and domain swaps52.
A number of serpins, such as the hormone-binding globulins, do not inhibit proteases, but they still use RCL conformational changes to mediate function42–44. In addition, conformational changes in other regions, particularly the N-terminal helical region, often help regulate serpin function37,45–48. Conformational malleability is thus key to function throughout the serpin family.
This functionally essential conformational malleability comes at a cost: serpins form a variety of multimers and polymers (Fig. 3b), and formation of these species leads to loss of function and in some cases toxicity. This problem becomes more pronounced as RCL insertion into β-sheet A becomes more energetically favorable. Formation of serpin multimers is favored in vitro when the stability of the folded monomers is perturbed. The RCL with its β-strand character can add to the edge of β-sheets49,50. Serpin multimers can also be formed by domain swaps, either as a dimer (seen for ATIII)51 or a trimer (observed in α1-antitrypsin (A1AT)52) (Fig. 3b). In the A1AT trimer, the C-terminal 34 residues are swapped, and in vitro folding experiments53 as well as a number of disease- and polymerization-associated mutations at or near the serpin C terminus54 underscore the importance of the C terminus for proper folding and function. Hence, the folding energy landscape of serpins must have multiple wells, and destabilization of the metastable functional state can lead to competition with any of a number of inter-molecularly associated states.
The multimerization and polymerization reactions of serpins are a manifestation of the tug of war between the need to fold to a metastable functional state and the vulnerability of serpins to disease-associated polymerization events. Serpin polymers in cells are generally formed in the endoplasmic reticulum (ER), where secretory serpins fold and mature. In most cases, cellular quality control networks keep misfolding and polymerization in check. Nonetheless, the synthesis and accumulation of polymerization-prone serpin mutants in the ER can lead to cell death and diseases termed the serpinopathies55. The most prevalent serpinopathy arises from mutations in A1AT and leads to liver disease. Similarly, polymerization-prone neuroserpin mutants lead to epilepsy and dementia56. Disease severity correlates with the polymerization propensity of both A1AT and neuroserpin, as determined in vitro57,58. In the ER, the protein quality control network helps mutant serpins to fold and targets mutants for degradation through ER-associated degradation and/or autophagy59,60. Misfolded serpins may also be recognized by protein quality control in the Golgi61. Thus far, the most advanced therapy for A1AT serpinopathies is a small molecule that increases autophagy59. However, it remains unclear which specific members of the quality control network are most important to ameliorate serpinopathologies and how the network can best be tuned to minimize polymerization.
Mutations associated with serpin misfolding and polymerization in the ER often map to structural regions that are important for functionally required conformational changes37,40,54. Most of the neuroserpin mutations are in the shutter region (Fig. 3a), which must open to allow full insertion of the RCL into β-sheet A, and the A1AT Z mutation disrupts a conserved salt bridge that helps control the RCL conformation. This correlation suggests that function and folding rely on an overlapping set of key residues. The ease with which polymers may be formed in vitro even from wild-type serpins suggests that metastable serpins are precariously poised on their energy landscape and, given high enough concentrations and enough time in an on-pathway intermediate or misfolded state, serpins will polymerize. Although this may seem an error of evolution, the strong overlap between serpin folding and function suggests that some of the attributes that allow serpins to perform a host of functions and to change functions in a regulated manner are correlated with a susceptibility for polymerization and its consequent deleterious outcomes.
The dangerous plasticity of an immunoglobulin fold
The tug of war between folding and function is also demonstrated in the β-sandwich immunoglobulin (Ig) domains. Widely associated with antibodies, the all-β-sheet family of Ig domains are widespread in nature, with roles as scaffold proteins and binding partners and as tandem repeats within many multidomain proteins. The folding mechanisms of several Ig domains have been mapped by mutation and analysis of the stability and the folding and unfolding kinetics of the resulting sequences62. These studies have shown that a central tetrad of β-strands, conserved topologically in all Ig domains, needs to form first, acting as a key stepping stone in the search for the native state. Folding is challenged, however, by several complicating factors: most Ig domains are stabilized by a disulfide bond that links β-strands B and F, several have a cis X-Pro peptide bond in one or more of the loops, and the isomerization of this bond slows folding63,64. Additionally, the occurrence of Ig domains in long, tandem arrays increases the probability of protein misfolding by events such as domain swapping65. When isolated from their heavy-chain binding partners, aberrant folding of light chains can result in aggregation and disease (reviewed in ref. 66).
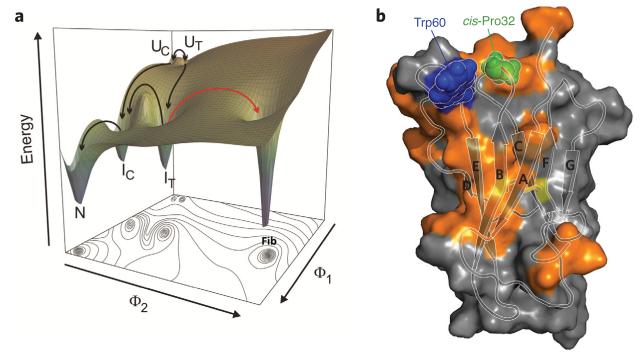
A striking example of the tension between function and folding is found in the Ig domain of β2m. This 99-residue protein has a canonical Ig fold comprising seven β-strands. The functional role of β2m is to chaperone the folding of its binding partner, the heavy chain of the major histocompatibility complex 1 (MHC-1), which is required for functional antigen presentation at the cell surface67. Like many Ig domains, β2m contains a disulfide bond linking residues Cys25 to Cys80 in β-strands B and F and a cis peptide bond linking residues His31 and Pro32. Studies of the folding of β2m have shown that the protein becomes trapped in a long-lived intermediate, known as IT, which has to wait for the slow (minute timescale) trans-cis isomerization of the His31-Pro32 bond to form its native structure63,64,68,69. The energy landscape of β2m, thus, is rough, with a near-native, long-lived and trapped intermediate blocking rapid folding to the native state (Fig. 4a). Structural studies of IT obtained by creation of a trapped metastable species that mimics IT by deletion of the N-terminal six amino acids (ΔN6)70 have shown that this species is able to fold to an Ig-like structure that differs only marginally from that of the native protein (r.m.s. deviation of backbone atoms of 1.5 Å)70. Most importantly, however, and by contrast with native β2m, IT and its structural analog, ΔN6, are highly aggregation prone64,71,72, assembling rapidly into amyloid fibrils that resemble those that form in the disease dialysis-related amyloidosis73,74. Moreover, this kinetically trapped non-native conformation of the protein can promote misfolding of the initially innocuous native state into an amyloidogenic conformer, analogous to conformational conversion associated with prion disease75. Thus, trapped partially folded proteins formed on rugged folding landscapes not only give rise to difficulties by creating folding bottlenecks and enhancing aggregate potential, but they can also wield a second blow by turning soluble proteins into aggregation precursors.
Figure 4. Folding landscape of β2m and its assembly with the MHC-1 heavy chain.
(a) Schematic free energy landscape of β2m monomer folding and aggregation. Φ1 and Φ2 indicate the increase of native intramolecular interactions during folding and non-native intermolecular interactions during aggregation, respectively. The unfolded protein with cis-Pro32 and trans-Pro32 are denoted UC and UT; the intermediate ensembles are denoted as IC, which is populated in vanishingly small amounts, and IT, which is ~5% populated; and the native state is N. The fibril is indicated as Fib. (b) Structure of β2m showing cis-Pro32 (green), Trp60 (blue) and the disulfide bond (C23–C80) (sticks) (PDB code 2XKS)70). The regions involved in binding to the MHC1 heavy chain108 are shown in orange on the surface. Panel a is reproduced from ref. 64.
Why, then, is Pro32 conserved across so many Ig domains in its cis isomer? And, why is wild-type β2m resilient to aggregation, when its close structural homolog ΔN6 aggregates spontaneously and rapidly in vitro? The answers, again, include the evolutionary pressure for function: cis-Pro32 is solvent exposed in wild-type β2m, forming a hydrophobic surface involved in binding to the MHC-1 heavy chain (Fig. 4b), thereby enabling it to chaperone MHC-1 complex assembly76. Formation of the MHC-1 complex involves interaction with Trp60, which is also highly conserved77 (Fig. 4). Despite being solvent exposed, mutation of Trp60 to Gly stabilizes native β2m and also reduces its propensity to aggregate, providing another example of function winning over folding and stability77,78. In a similar vein, the β2m variant with mutation D76N, which gives rise to a recently discovered form of hereditary systemic amyloid disease (in the absence of kidney dysfunction)79, is also more aggregation prone than its wild-type counterpart as this mutation reduces the stability of the native state relative to I 80T. In this case, however, the MHC-1 heavy chain stabilizes and thereby reduces the aggregation potential of the D76N mutant, protecting against aggregation and disease81. Other perturbations, for example, binding of Cu2+ ions during the dialysis procedure, combined with other truncations and chemical modifications, can enhance aggregation of β2m by increasing the population of the non-native trans-Pro32 forms (reviewed in ref. 82). Given that an intact adaptive immune response is vital for life in all higher eukaryotes, there is immense evolutionary pressure on the sequences of both the MHC-1 heavy chain and β2m to ensure that they fold and bind efficiently. This explains why the sequence of β2m is so highly conserved from cartilaginous fish to humans83 and why the smallest changes to its sequence that cause the isomerization of a single peptide bond can lead to aggregation and disease. In addition, the interactions between β2m and the MHC-1 heavy chain have coevolved in such a way as to minimize aggregation risks, although the susceptibility is ever present when the fragile fold of β2m is left on its own.
Aggregation-prone protein recognition sites in Atx3
Atx3 provides another example of the competition between function and aggregation and also illustrates a compensatory mechanism that again involves the protective role of functional protein-protein interactions. Atx3 belongs to the protein family associated with neurodegenerative diseases that are caused by the anomalous expansion of polymorphic polyglutamine (polyQ) tracts84. When above a threshold of approximately 37 repeats, these regions promote protein aggregation, misfolding and consequent cell death85. The polyQ-associated diseases include the well-known Huntington’s chorea as well as several spinocerebellar ataxias, among them spinocerebellar ataxia type 3, which is linked to Atx3. The proteins responsible share no similarities in terms of sequence, cellular localization or function. PolyQ expansion is a necessary requisite for disease development, but it is now established that other regions of the proteins are important contributors to aggregation84.
Atx3 is a soluble protein (~350 kDa) that can shuttle in and out of the nucleus (detailed reviews are in refs. 86–88). Although it is mainly cytoplasmic in unaffected brain and in normal neurons, Atx3 partitions to neuronal cell nuclei in diseased brains. Functionally, Atx3 is a deubiquitinating enzyme that preferentially cleaves ubiquitin chains longer than four repeats89. It is composed of an evolutionarily conserved N-terminal region, the Josephin domain, and an intrinsically unfolded C-terminal region that contains the polymorphic polyQ tract, a putative coiled-coil90,91 and two or three ubiquitin-interacting motifs, depending on the isoform. The Josephin domain accounts for enzymatic activity and forms a globular structure with a papain-like fold. The Atx3 Josephin domain contains a feature that is unusual among deubiquitinating enyzmes and more generally among cysteine proteases: a dynamic helical hairpin that protrudes out into solution from the main body of the globular domain.
The full range of possible protein-protein interactions formed by Atx3 remains to be determined, but some of the partners have been identified92–94. Among the better-characterized partners are polyubiquitin chains and other components of the ubiquitin-proteasome pathway, including the transitional ER ATPase valocin-containing protein Vcp and the human homologs of Rad23, the HHR23 proteins. Atx3 can also bind monoubiquitin, and it does so through the multiple binding sites distributed along its length, albeit with low (20–400 μM) affinity. Two ubiquitin-binding sites have been described on Josephin, and they are located on either side of the helical hairpin95 (Fig. 5). Site 1 is essential for enzymatic activity96. Site 2, which is more exposed, confers K48 ubiquitin-chain linkage preference to Josephin and overlaps with the surface of interaction for the ubiquitin-like domain of the HHR23B protein89. Previous measurements suggest that the dynamics of the helical hairpin, which are distinct from that of the rest of the domain, may have a role in ubiquitin recognition and possibly determine substrate and ubiquitin linkage specificity95. The ubiquitin-interacting motifs are thought to be important for polyubiquitin binding and to enhance affinity.
Figure 5. Competition between ubiquitin binding and aggregation for the Josephin domain of Atx3.
A surface representation of Josephin (PDB code 2JRI) is shown in white with exposed hydrophobic surfaces in green. When in the presence of the natural partner ubiquitin (Ub; the two binding ubiquitins are shown as blue traces), the Josephin binding sites are saturated by the interaction and protected from aggregation. When interaction is impaired, for instance by polyQ expansion, which alters the affinity for the interactor, the two sites promote aggregation, as shown in the right panel. Adapted from ref. 15 with permission from Elsevier.
A tight relationship between function and aggregation is clear for Atx3. As in other polyQ-containing proteins, disease is determined by polyQ expansion, but the Josephin domain contributes to the process and is able per se to induce aggregation and misfold97–100. As shown in these references, the aggregates and fibrillar species of Josephin have morphologies and features very similar to those observed for full-length nonexpanded Atx3. Constructs lacking the polyQ-containing C-terminal region were shown to induce neurodegeneration in mice models101. Careful analysis of the regions of Josephin that promote aggregation showed that the two main aggregation hot spots coincide with the two ubiquitin-binding sites 1 and 2 and are formed by two exposed hydrophobic surfaces that have evolved to bind ubiquitin (Fig. 5). Accordingly, aggregation of Atx3 is strongly mitigated by mutations in Josephin that, at the same time, abolish ubiquitin binding102. Likewise, the presence of excess monoubiquitin has a similar effect and leads to amorphous aggregates102.
Consequently, ubiquitin recognition has been suggested to be an important factor that has a role in protecting Atx3 from protein aggregation in vivo102. A similar behavior in which the same regions of a protein are involved both in functional interactions and in aberrant aggregation is observed for ataxin-1, another member of the polyQ disease protein family103. This evidence teaches us an important lesson that, while confirming once again an intimate interconnection between a functional energy landscape and aggregation risk, also points to another cellular compensatory mechanism that might be harnessed pharmaceutically: the protective role of protein-protein interactions. Hence, a deeper understanding of protein function may inspire the generation of compounds that mimic cellular interactions and result in protection from aggregation and disease.
Concluding remarks
Life is full of compromises. It is important to remind ourselves that species, organisms and their component cells and molecular machines have been subjected to fitness pressures that require them to function well enough to survive and compete in their evolutionary niche. In the case of the evolutionary selection for molecular function, we are becoming increasingly aware of the compromises that are manifested in the vulnerability of proteins to altered conditions such as pH and temperature, stresses that challenge the cellular context such as proteostatic depletion and mutations that subtly alter the energetic landscape of the protein. The discovery of the role of misfolding in aggregation diseases highlights the fragility of proteins and the risk of the black pit of protein aggregation, which can readily outcompete folding once the scales are tipped toward that intermolecular process.
The four vignettes we have provided in this brief Perspective are intended to illustrate the tenuous balance between functional requirements on proteins and their susceptibility to competing misfolding and aggregation. These are not the only examples of functional constraints exacerbating the risk of protein aggregation—far from it. Nonetheless, these four examples show compellingly how the sculpting of an energy landscape for function can lead to dangerous characteristics, whether they be necessity for dynamics (the iLBPs), kinetically stable partially folded states (Ig folds), conformations that have exposed interactive (often hydrophobic) surfaces (Atx3) or metastability to enable a response required for function (serpins). We hope that the ideas described here will lead readers to look more deeply at the functional energy landscapes of proteins and in so doing gain insights that might help understanding of the cellular roles of proteins, the coping mechanisms employed in biology and possible intervention strategies one might invoke therapeutically.
Acknowledgments
We thank our many collaborators and co-authors whose experiments and ideas have contributed to our work. We also recognize that all relevant examples and references cannot be included in a short perspective such as this, and we apologize to those whose contributions we have omitted. We acknowledge, with thanks, funding from the National Institutes of Health (grants GM027616 and GM101644 to L.M.G., GM094848 to L.M.G. and A.G. and GM060418 to A.G.); the Medical Research Council (grant U117584256 to A.P.) and the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013; 322408) and the Wellcome Trust (WT092896MA to S.E.R.).
Footnotes
Competing financial interests The authors declare no competing financial interests.
References
- 1.Jenik M, et al. Protein frustratometer: a tool to localize energetic frustration in protein molecules. Nucleic Acids Res. 2012;40:W348–W351. doi: 10.1093/nar/gks447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutto L, Latzer J, Hegler JA, Ferreiro DU, Wolynes PG. Consequences of localized frustration for the folding mechanism of the IM7 protein. Proc. Natl. Acad. Sci. USA. 2007;104:19825–19830. doi: 10.1073/pnas.0709922104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng W, Schafer NP, Wolynes PG. Frustration in the energy landscapes of multidomain protein misfolding. Proc. Natl. Acad. Sci. USA. 2013;110:1680–1685. doi: 10.1073/pnas.1222130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014;15:384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 5.Uversky VN. Intrinsic disorder in proteins associated with neurodegenerative diseases. Front. Biosci. (Landmark Ed.) 2009;14:5188–5238. doi: 10.2741/3594. [DOI] [PubMed] [Google Scholar]
- 6.Wright PE, Dyson HJ. Linking folding and binding. Curr. Opin. Struct. Biol. 2009;19:31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma B, Tsai CJ, Haliloglu T, Nussinov R. Dynamic allostery: linkers are not merely flexible. Structure. 2011;19:907–917. doi: 10.1016/j.str.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalodimos CG. Protein function and allostery: a dynamic relationship. Ann. NY Acad. Sci. 2012;1260:81–86. doi: 10.1111/j.1749-6632.2011.06319.x. [DOI] [PubMed] [Google Scholar]
- 9.Motlagh HN, Wrabl JO, Li J, Hilser VJ. The ensemble nature of allostery. Nature. 2014;508:331–339. doi: 10.1038/nature13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tóth-Petróczy A, Tawfik DS. The robustness and innovability of protein folds. Curr. Opin. Struct. Biol. 2014;26:131–138. doi: 10.1016/j.sbi.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Tartaglia GG, Pechmann S, Dobson CM, Vendruscolo M. Life on the edge: a link between gene expression levels and aggregation rates of human proteins. Trends Biochem. Sci. 2007;32:204–206. doi: 10.1016/j.tibs.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 12.De Baets G, et al. An evolutionary trade-off between protein turnover rate and protein aggregation favors a higher aggregation propensity in fast degrading proteins. PLOS Comput. Biol. 2011;7:e1002090. doi: 10.1371/journal.pcbi.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monsellier E, Chiti F. Prevention of amyloid-like aggregation as a driving force of protein evolution. EMBO Rep. 2007;8:737–742. doi: 10.1038/sj.embor.7401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 15.Pastore A, Temussi PA. The two faces of Janus: functional interactions and protein aggregation. Curr. Opin. Struct. Biol. 2012;22:30–37. doi: 10.1016/j.sbi.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, et al. Structural instability tuning as a regulatory mechanism in protein-protein interactions. Mol. Cell. 2011;44:734–744. doi: 10.1016/j.molcel.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong W, Wu YE, Fu X, Chang Z. Periplasmic protein HdeA exhibits chaperone-like activity exclusively within stomach pH range by transforming into disordered conformation. J. Biol. Chem. 2005;280:27029–27034. doi: 10.1074/jbc.M503934200. [DOI] [PubMed] [Google Scholar]
- 18.Foit L, George JS, Zhang BW, Brooks CL, III, Bardwell JC. Chaperone activation by unfolding. Proc. Natl. Acad. Sci. USA. 2013;110:E1254–E1262. doi: 10.1073/pnas.1222458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianni S, Camilloni C, Giri R, Toto A, Bonetti D, Morrone A, Sormanni P, Brunori M, Vendruscolo M. Understanding the frustration arising from the competition between function, misfolding, and aggregation in a globular protein. Proc. Natl. Acad. Sci. USA. 2014 Sep 17; doi: 10.1073/pnas.1405233111. doi:10.1073/ pnas.1405233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernlohr DA, Simpson MA, Hertzel AV, Banaszak LJ. Intracellular lipid-binding proteins and their genes. Annu. Rev. Nutr. 1997;17:277–303. doi: 10.1146/annurev.nutr.17.1.277. [DOI] [PubMed] [Google Scholar]
- 21.Ferrolino MC, Zhuravleva A, Budyak IL, Krishnan B, Gierasch LM. Delicate balance between functionally required flexibility and aggregation risk in a β-rich protein. Biochemistry. 2013;52:8843–8854. doi: 10.1021/bi4013462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budyak IL, et al. Early folding events protect aggregation-prone regions of a β-rich protein. Structure. 2013;21:476–485. doi: 10.1016/j.str.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banaszak L, et al. Lipid-binding proteins: a family of fatty acid and retinoid transport proteins. Adv. Protein Chem. 1994;45:89–151. doi: 10.1016/s0065-3233(08)60639-7. [DOI] [PubMed] [Google Scholar]
- 24.Lücke C, Zhang F, Ruterjans H, Hamilton JA, Sacchettini JC. Flexibility is a likely determinant of binding specificity in the case of ileal lipid binding protein. Structure. 1996;4:785–800. doi: 10.1016/s0969-2126(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 25.Hodsdon ME, Cistola DP. Ligand binding alters the backbone mobility of intestinal fatty acid-binding protein as monitored by 15N NMR relaxation and 1H exchange. Biochemistry. 1997;36:2278–2290. doi: 10.1021/bi962018l. [DOI] [PubMed] [Google Scholar]
- 26.Eberini I, et al. Conformational and dynamics changes induced by bile acids binding to chicken liver bile acid binding protein. Proteins. 2008;71:1889–1898. doi: 10.1002/prot.21875. [DOI] [PubMed] [Google Scholar]
- 27.Cai J, et al. Solution structure and backbone dynamics of human liver fatty acid binding protein: fatty acid binding revisited. Biophys. J. 2012;102:2585–2594. doi: 10.1016/j.bpj.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan VV, Sukumar M, Gierasch LM, Cosman M. Dynamics of cellular retinoic acid binding protein I on multiple time scales with implications for ligand binding. Biochemistry. 2000;39:9119–9129. doi: 10.1021/bi000296l. [DOI] [PubMed] [Google Scholar]
- 29.Storch J, McDermott L. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 2009;50(Suppl):S126–S131. doi: 10.1194/jlr.R800084-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falomir-Lockhart LJ, Laborde L, Kahn PC, Storch J, Corsico B. Protein-membrane interaction and fatty acid transfer from intestinal fatty acid–binding protein to membranes. Support for a multistep process. J. Biol. Chem. 2006;281:13979–13989. doi: 10.1074/jbc.M511943200. [DOI] [PubMed] [Google Scholar]
- 31.Budhu A, Gillilan R, Noy N. Localization of the RAR interaction domain of cellular retinoic acid binding protein-II. J. Mol. Biol. 2001;305:939–949. doi: 10.1006/jmbi.2000.4340. [DOI] [PubMed] [Google Scholar]
- 32.Clark PL, Liu ZP, Rizo J, Gierasch LM. Cavity formation before stable hydrogen bonding in the folding of a β-clam protein. Nat. Struct. Biol. 1997;4:883–886. doi: 10.1038/nsb1197-883. [DOI] [PubMed] [Google Scholar]
- 33.Clark PL, Liu ZP, Zhang J, Gierasch LM. Intrinsic tryptophans of CRABPI as probes of structure and folding. Protein Sci. 1996;5:1108–1117. doi: 10.1002/pro.5560050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark PL, Weston BF, Gierasch LM. Probing the folding pathway of a β-clam protein with single-tryptophan constructs. Fold. Des. 1998;3:401–412. doi: 10.1016/s1359-0278(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 35.Eyles SJ, Gierasch LM. Multiple roles of prolyl residues in structure and folding. J. Mol. Biol. 2000;301:737–747. doi: 10.1006/jmbi.2000.4002. [DOI] [PubMed] [Google Scholar]
- 36.Gunasekaran K, Hagler AT, Gierasch LM. Sequence and structural analysis of cellular retinoic acid-binding proteins reveals a network of conserved hydrophobic interactions. Proteins. 2004;54:179–194. doi: 10.1002/prot.10520. [DOI] [PubMed] [Google Scholar]
- 37.Gettins PGW. Serpin structure, mechanism, and function. Chem. Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 38.Dementiev A, Dobo J, Gettins PGW. Active site distortion is sufficient for proteinase inhibition by serpins: structure of the covalent complex of α1-proteinase inhibitor with porcine pancreatic elastase. J. Biol. Chem. 2006;281:3452–3457. doi: 10.1074/jbc.M510564200. [DOI] [PubMed] [Google Scholar]
- 39.Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- 40.Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu. Rev. Biochem. 2009;78:147–176. doi: 10.1146/annurev.biochem.78.082107.133320. [DOI] [PubMed] [Google Scholar]
- 41.Mottonen J, et al. Structural basis of latency in plasminogen activator inhibitor-1. Nature. 1992;355:270–273. doi: 10.1038/355270a0. [DOI] [PubMed] [Google Scholar]
- 42.Klieber MA, Underhill C, Hammond GL, Muller YA. Corticosteroid-binding globulin, a structural basis for steroid transport and proteinase-triggered release. J. Biol. Chem. 2007;282:29594–29603. doi: 10.1074/jbc.M705014200. [DOI] [PubMed] [Google Scholar]
- 43.Qi X, Chan WL, Read RJ, Zhou A, Carrell RW. Temperature-responsive release of thyroxine and its environmental adaptation in Australians. Proc. Biol. Sci. 2014;281:20132747. doi: 10.1098/rspb.2013.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou A, Wei Z, Read RJ, Carrell RW. Structural mechanism for the carriage and release of thyroxine in the blood. Proc. Natl. Acad. Sci. USA. 2006;103:13321–13326. doi: 10.1073/pnas.0604080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baek J-H, Yang WS, Lee C, Yu M-H. Functional unfolding of α1-antitrypsin probed by hydrogen-deuterium exchange coupled with mass spectrometry. Mol. Cell. Proteomics. 2009;8:1072–1081. doi: 10.1074/mcp.M800365-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrell RW, Stein PE, Fermi G, Wardell MR. Biological implications of a 3 Å structure of dimeric antithrombin. Structure. 1994;2:257–270. doi: 10.1016/s0969-2126(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 47.Trelle MB, Madsen JB, Andreasen PA, Jørgensen TJD. Local transient unfolding of native state PAI-1 associated with serpin metastability. Angew. Chem. Int. Ed. Engl. 2014;53:9751–9754. doi: 10.1002/anie.201402796. [DOI] [PubMed] [Google Scholar]
- 48.Zhou A, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marszal E, Shrake A. Serpin crystal structure and serpin polymer structure. Arch. Biochem. Biophys. 2006;453:123–129. doi: 10.1016/j.abb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Law RHP, Bottomley SP, Whisstock JC, Buckle AM. A structural basis for loop C-sheet polymerization in serpins. J. Mol. Biol. 2008;376:1348–1359. doi: 10.1016/j.jmb.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 51.Yamasaki M, Li W, Johnson DJD, Huntington JA. Crystal structure of a stable dimer reveals the molecular basis of serpin polymerization. Nature. 2008;455:1255–1258. doi: 10.1038/nature07394. [DOI] [PubMed] [Google Scholar]
- 52.Yamasaki M, Sendall TJ, Pearce MC, Whisstock JC, Huntington JA. Molecular basis of α1-antitrypsin deficiency revealed by the structure of a domain-swapped trimer. EMBO Rep. 2011;12:1011–1017. doi: 10.1038/embor.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolmer K, Gettins PG. How the serpin α1-proteinase inhibitor folds. J. Biol. Chem. 2012;287:12425–12432. doi: 10.1074/jbc.M111.315465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein PE, Carrell RW. What do dysfunctional serpins tell us about molecular mobility and disease? Nat. Struct. Biol. 1995;2:96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- 55.Lomas DA, Carrell RW. Serpinopathies and the conformational dementias. Nat. Rev. Genet. 2002;3:759–768. doi: 10.1038/nrg907. [DOI] [PubMed] [Google Scholar]
- 56.Davis RL, et al. Familial dementia caused by polymerization of mutant neuroserpin. Nature. 1999;401:376–379. doi: 10.1038/43894. [DOI] [PubMed] [Google Scholar]
- 57.Hagen MC, et al. Encephalopathy with neuroserpin inclusion bodies presenting as progressive myoclonus epilepsy and associated with a novel mutation in the Proteinase Inhibitor 12 gene. Brain Pathol. 2011;21:575–582. doi: 10.1111/j.1750-3639.2011.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miranda E, et al. The intracellular accumulation of polymeric neuroserpin explains the severity of the dementia FENIB. Hum. Mol. Genet. 2008;17:1527–1539. doi: 10.1093/hmg/ddn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perlmutter DH. α-1-antitrypsin deficiency: importance of proteasomal and autophagic degradative pathways in disposal of liver disease–associated protein aggregates. Annu. Rev. Med. 2011;62:333–345. doi: 10.1146/annurev-med-042409-151920. [DOI] [PubMed] [Google Scholar]
- 60.Schipanski A, et al. The lectin OS-9 delivers mutant neuroserpin to endoplasmic reticulum associated degradation in familial encephalopathy with neuroserpin inclusion bodies. Neurobiol. Aging. 2014;35:2394–2403. doi: 10.1016/j.neurobiolaging.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Gelling CL, Dawes IW, Perlmutter DH, Fisher EA, Brodsky JL. The endosomal protein-sorting receptor sortilin has a role in trafficking α-1 antitrypsin. Genetics. 2012;192:889–903. doi: 10.1534/genetics.112.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nickson AA, Wensley BG, Clarke J. Take home lessons from studies of related proteins. Curr. Opin. Struct. Biol. 2013;23:66–74. doi: 10.1016/j.sbi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiti F, et al. Detection of two partially structured species in the folding process of the amyloidogenic protein β2-microglobulin. J. Mol. Biol. 2001;307:379–391. doi: 10.1006/jmbi.2000.4478. [DOI] [PubMed] [Google Scholar]
- 64.Jahn TR, Parker MJ, Homans SW, Radford SE. Amyloid formation under physiological conditions proceeds via a native-like folding intermediate. Nat. Struct. Mol. Biol. 2006;13:195–201. doi: 10.1038/nsmb1058. [DOI] [PubMed] [Google Scholar]
- 65.Wright CF, Teichmann SA, Clarke J, Dobson CM. The importance of sequence diversity in the aggregation and evolution of proteins. Nature. 2005;438:878–881. doi: 10.1038/nature04195. [DOI] [PubMed] [Google Scholar]
- 66.Feige Y, Buchner J. Principles and engineering of antibody folding and assembly. Biochim. Biophys. Acta. 2014 Jun 13; doi: 10.1016/j.bbapap.2014.06.004. doi:10.1016/j.bbapap.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Eichner T, Radford SE. Understanding the complex mechanisms of β2-microglobulin amyloid assembly. FEBS J. 2011;278:3868–3883. doi: 10.1111/j.1742-4658.2011.08186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiti F, et al. A partially structured species of β2-microglobulin is significantly populated under physiological conditions and involved in fibrillogenesis. J. Biol. Chem. 2001;276:46714–46721. doi: 10.1074/jbc.M107040200. [DOI] [PubMed] [Google Scholar]
- 69.Kameda A, et al. Nuclear magnetic resonance characterization of the refolding intermediate of β2-microglobulin trapped by non-native prolyl peptide bond. J. Mol. Biol. 2005;348:383–397. doi: 10.1016/j.jmb.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 70.Eichner T, Kalverda AP, Thompson GS, Homans SW, Radford SE. Conformational conversion during amyloid formation at atomic resolution. Mol. Cell. 2011;41:161–172. doi: 10.1016/j.molcel.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eichner T, Radford SE. A generic mechanism of β2-microglobulin amyloid assembly at neutral pH involving a specific proline switch. J. Mol. Biol. 2009;386:1312–1326. doi: 10.1016/j.jmb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 72.Esposito G, et al. Removal of the N-terminal hexapeptide from human β2-microglobulin facilitates protein aggregation and fibril formation. Protein Sci. 2000;9:831–845. doi: 10.1110/ps.9.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White HE, et al. Globular tetramers of β2-microglobulin assemble into elaborate amyloid fibrils. J. Mol. Biol. 2009;389:48–57. doi: 10.1016/j.jmb.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Su Y, et al. Secondary structure in the core of amyloid fibrils formed from human β2m and its truncated variant ΔN6. J. Am. Chem. Soc. 2014;136:6313–6325. doi: 10.1021/ja4126092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karamanos TK, Kalverda AP, Thompson GS, Radford SE. Visualization of transient protein-protein interactions that promote or inhibit amyloid assembly. Mol. Cell. 2014;55:214–226. doi: 10.1016/j.molcel.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zijlstra M, et al. β2-microglobulin deficient mice lack CD4–8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 77.Ricagno S, Raimondi S, Giorgetti S, Bellotti V, Bolognesi M. Human β2 microglobulin W60V mutant structure: implications for stability and amyloid aggregation. Biochem. Biophys. Res. Commun. 2009;380:543–547. doi: 10.1016/j.bbrc.2009.01.116. [DOI] [PubMed] [Google Scholar]
- 78.Esposito G, et al. The controlling roles of Trp60 and Trp95 in β2-microglobulin function, folding and amyloid aggregation properties. J. Mol. Biol. 2008;378:887–897. doi: 10.1016/j.jmb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Valleix S, et al. Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N. Engl. J. Med. 2012;366:2276–2283. doi: 10.1056/NEJMoa1201356. [DOI] [PubMed] [Google Scholar]
- 80.Mangione PP, et al. Structure, folding dynamics, and amyloidogenesis of D76N β2-microglobulin: roles of shear flow, hydrophobic surfaces, and α-crystallin. J. Biol. Chem. 2013;288:30917–30930. doi: 10.1074/jbc.M113.498857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halabelian L, et al. Class I major histocompatibility complex, the trojan horse for secretion of amyloidogenic β2-microglobulin. J. Biol. Chem. 2014;289:3318–3327. doi: 10.1074/jbc.M113.524157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corlin DB, Heegaard NH. β2-microglobulin amyloidosis. Subcell. Biochem. 2012;65:517–540. doi: 10.1007/978-94-007-5416-4_19. [DOI] [PubMed] [Google Scholar]
- 83.Raimondi S, et al. The two tryptophans of β2-microglobulin have distinct roles in function and folding and might represent two independent responses to evolutionary pressure. BMC Evol. Biol. 2011;11:159. doi: 10.1186/1471-2148-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zoghbi HY, Orr HT. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1. J. Biol. Chem. 2009;284:7425–7429. doi: 10.1074/jbc.R800041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Orr HT. Cell biology of spinocerebellar ataxia. J. Cell Biol. 2012;197:167–177. doi: 10.1083/jcb.201105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matos CA, de Macedo-Ribeiro S, Carvalho AL. Polyglutamine diseases: the special case of ataxin-3 and Machado-Joseph disease. Prog. Neurobiol. 2011;95:26–48. doi: 10.1016/j.pneurobio.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 87.Paulson H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb. Clin. Neurol. 2012;103:437–449. doi: 10.1016/B978-0-444-51892-7.00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Almeida B, Fernandes S, Abreu IA, Macedo-Ribeiro S. Trinucleotide repeats: a structural perspective. Front Neurol. 2013;4:76. doi: 10.3389/fneur.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nicastro G, et al. The solution structure of the Josephin domain of ataxin-3: structural determinants for molecular recognition. Proc. Natl. Acad. Sci. USA. 2005;102:10493–10498. doi: 10.1073/pnas.0501732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fiumara F, Fioriti L, Kandel ER, Hendrickson WA. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and PolyQ proteins. Cell. 2010;143:1121–1135. doi: 10.1016/j.cell.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pelassa I, et al. Association of polyalanine and polyglutamine coiled coils mediates expansion disease-related protein aggregation and dysfunction. Hum. Mol. Genet. 2014;23:3402–3420. doi: 10.1093/hmg/ddu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doss-Pepe EW, Stenroos ES, Johnson WG, Madura K. Ataxin-3 interactions with rad23 and valosin-containing protein and its associations with ubiquitin chains and the proteasome are consistent with a role in ubiquitin-mediated proteolysis. Mol. Cell. Biol. 2003;23:6469–6483. doi: 10.1128/MCB.23.18.6469-6483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang G, Sawai N, Kotliarova S, Kanazawa I, Nukina N. Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B. Hum. Mol. Genet. 2000;9:1795–1803. doi: 10.1093/hmg/9.12.1795. [DOI] [PubMed] [Google Scholar]
- 94.Zhong X, Pittman RN. Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum. Mol. Genet. 2006;15:2409–2420. doi: 10.1093/hmg/ddl164. [DOI] [PubMed] [Google Scholar]
- 95.Nicastro G, et al. Josephin domain of ataxin-3 contains two distinct ubiquitin-binding sites. Biopolymers. 2009;91:1203–1214. doi: 10.1002/bip.21210. [DOI] [PubMed] [Google Scholar]
- 96.Nicastro G, et al. Understanding the role of the Josephin domain in the PolyUb binding and cleavage properties of ataxin-3. PLoS ONE. 2010;5:e12430. doi: 10.1371/journal.pone.0012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellisdon AM, Pearce MC, Bottomley SP. Mechanisms of ataxin-3 misfolding and fibril formation: kinetic analysis of a disease-associated polyglutamine protein. J. Mol. Biol. 2007;368:595–605. doi: 10.1016/j.jmb.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 98.Ellisdon AM, Thomas B, Bottomley SP. The two-stage pathway of ataxin-3 fibrillogenesis involves a polyglutamine-independent step. J. Biol. Chem. 2006;281:16888–16896. doi: 10.1074/jbc.M601470200. [DOI] [PubMed] [Google Scholar]
- 99.Gales L, et al. Towards a structural understanding of the fibrillization pathway in Machado-Joseph’s disease: trapping early oligomers of non-expanded ataxin-3. J. Mol. Biol. 2005;353:642–654. doi: 10.1016/j.jmb.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 100.Masino L, et al. Characterization of the structure and the amyloidogenic properties of the Josephin domain of the polyglutamine-containing protein ataxin-3. J. Mol. Biol. 2004;344:1021–1035. doi: 10.1016/j.jmb.2004.09.065. [DOI] [PubMed] [Google Scholar]
- 101.Hübener J, et al. N-terminal ataxin-3 causes neurological symptoms with inclusions, endoplasmic reticulum stress and ribosomal dislocation. Brain. 2011;134:1925–1942. doi: 10.1093/brain/awr118. [DOI] [PubMed] [Google Scholar]
- 102.Masino L, et al. The Josephin domain determines the morphological and mechanical properties of ataxin-3 fibrils. Biophys. J. 2011;100:2033–2042. doi: 10.1016/j.bpj.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Chiara C, Menon RP, Kelly G, Pastore A. Protein-protein interactions as a strategy towards protein-specific drug design: the example of ataxin-1. PLoS ONE. 2013;8:e76456. doi: 10.1371/journal.pone.0076456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kleywegt GJ, et al. Crystal structures of cellular retinoic acid binding proteins I and II in complex with all-trans-retinoic acid and a synthetic retinoid. Structure. 1994;2:1241–1258. doi: 10.1016/s0969-2126(94)00125-1. [DOI] [PubMed] [Google Scholar]
- 105.Corsico B, Cistola DP, Frieden C, Storch J. The helical domain of intestinal fatty acid binding protein is critical for collisional transfer of fatty acids to phospholipid membranes. Proc. Natl. Acad. Sci. USA. 1998;95:12174–12178. doi: 10.1073/pnas.95.21.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dementiev A, Simonovic M, Volz K, Gettins P. Canonical inhibitor-like interactions explain reactivity of α1-proteinase inhibitor Pittsburgh and antithrombin with proteinases. J. Biol. Chem. 2003;278:37881–37887. doi: 10.1074/jbc.M305195200. [DOI] [PubMed] [Google Scholar]
- 107.McCoy AJ, Pei XY, Skinner R, Abrahams JP, Carrell RW. Structure of β-antithrombin and the effect of glycosylation on antithrombin’s heparin affinity and activity. J. Mol. Biol. 2003;326:823–833. doi: 10.1016/s0022-2836(02)01382-7. [DOI] [PubMed] [Google Scholar]
- 108.Tysoe-Calnon VA, Grundy JE, Perkins SJ. Molecular comparisons of the β2-microglobulin-binding site in class I major-histocompatibility-complex α-chains and proteins of related sequences. Biochem. J. 1991;277:359–369. doi: 10.1042/bj2770359. [DOI] [PMC free article] [PubMed] [Google Scholar]