Abstract
Background. A clinical trial of mass azithromycin distributions for trachoma created a convenient experiment to test the hypothesis that antibiotic use selects for clonal expansion of preexisting resistant bacterial strains.
Methods. Twelve communities in Ethiopia received mass azithromycin distributions every 3 months for 1 year. A random sample of 10 children aged 0–9 years from each community was monitored by means of nasopharyngeal swab sampling before mass azithromycin distribution and after 4 mass treatments. Swab specimens were tested for Streptococcus pneumoniae, and isolates underwent multilocus sequence typing.
Results. Of 82 pneumococcal isolates identified before treatment, 4 (5%) exhibited azithromycin resistance, representing 3 different sequence types (STs): 177, 6449, and 6494. The proportion of isolates that were classified as one of these 3 STs and were resistant to azithromycin increased after 4 mass azithromycin treatments (14 of 96 isolates [15%]; P = .04). Using a classification index, we found evidence for a relationship between ST and macrolide resistance after mass treatments (P < .0001). The diversity of STs—as calculated by the unbiased Simpson index—decreased significantly after mass azithromycin treatment (P = .045).
Conclusions. Resistant clones present before mass azithromycin treatments increased in frequency after treatment, consistent with the theory that antibiotic selection pressure results in clonal expansion of existing resistant strains.
Keywords: Streptococcus pneumoniae, Africa, MLST, multilocus sequence typing, clonal expansion
The use of large volumes of antibiotics in communities is thought to select for antibiotic resistance [1]. According to the prevailing theory, antibiotics clear susceptible strains of bacteria in the community but not the strains already resistant to the antibiotic. This in turn allows for clonal expansion of these resistant bacterial strains, leading to an increase in the prevalence of antibiotic resistance in the community. Although intuitive, the phenomenon of clonal expansion has been difficult to study, especially at the community level. Most studies claiming to show evidence of clonal expansion have been observational, hospital-based, retrospective studies of nonrandom samples that have demonstrated a high frequency of bacterial isolates that share identical genetic features [2–8]. There have been few if any prospective, population-based, interventional studies that have investigated the relationship between antibiotic selection pressure and clonal expansion.
In a recent cluster-randomized clinical trial, we found that communities exposed to mass azithromycin treatments for trachoma had higher levels of nasopharyngeal pneumococcal macrolide resistance than did untreated communities [9]. This study provided evidence that antibiotic selection pressure has a causative role in macrolide resistance but did not assess the mechanism by which the resistance is spread. Because this study monitored communities in a population-based manner both before and after several rounds of mass azithromycin treatments, it provides an ideal experiment to look for community-level evidence of clonal expansion. To this end, we performed multilocus sequence typing (MLST) on all pneumococcal isolates from the trial. MLST is a highly discriminative typing technique in which 7 unlinked housekeeping gene fragments are sequenced, allowing study of the population dynamics of bacterial clones [10]. In the current study, our goal was to better characterize how antibiotic resistance spreads within the nasopharyngeal pneumococcal reservoir in communities.
METHODS
Study Design
We obtained ethical approval for the study from the University of California–San Francisco Committee for Human Research, Emory University, and the Ethiopian Science and Technology Commission. This study is a nonprespecified analysis from a cluster-randomized clinical trial conducted in Goncha Siso Enese woreda, Amhara region, Ethiopia (clinical trials registration NCT00322972). In the trial, we randomly assigned 72 contiguous subkebeles (government-defined administrative units composed of approximately 350 households) that were easily accessible by vehicle to receive one of 6 different treatment strategies for trachoma [11]. We excluded the only large town in the woreda. The primary outcome of the trial was ocular chlamydial infection. In addition, we monitored 2 treatment arms for nasopharyngeal pneumococcus. Specifically, we compared macrolide resistance among nasopharyngeal pneumococci isolated from individuals in the 12 subkebeles randomized to receive quarterly mass azithromycin treatment with that for the 12 subkebeles randomized to receive no treatment [9]. Herein, we describe MLST results from the quarterly treatment arm from that report.
Intervention
In the 12 subkebeles of the current study, all children aged 1–10 years were offered a single directly observed dose of azithromycin (20 mg/kg) administered by height-based dosing every 3 months (±1 month) for 1 year. No pneumococcal vaccination program existed at the time of the study.
Sample Collection
In a randomly selected community from each subkebele, a random sample of 10 children aged 0–9 years underwent nasopharyngeal swabbing at baseline (May 2006; before treatment) and at month 12 (May 2007; approximately 3 months after the fourth mass azithromycin treatment). A different random sample of children was chosen at each monitoring visit. Swabs were stored and transported in skim milk-tryptone-glucose-glycerin medium. Swabs were kept on ice for <8 hours while in the field, then at −20°C for several weeks, and then at −4°C for <96 hours during transport to San Francisco, where they were stored at −80°C until processed.
Laboratory Testing
Streptococcus pneumoniae was isolated with selective medium incubated at 35°C in 5% CO2 and confirmed with optochin disk testing (Remel; Lenexa, KS). Resistance to azithromycin, tetracycline, and penicillin was assessed using Etest strips (bioMérieux–AB Biodisk; Marcy l'Etoile, France) placed on Mueller-Hinton agar plates with 5% defibrinated sheep blood and incubated at 35°C in 5% CO2 for 20–24 hours, before determination of the minimum inhibitory concentration (MIC). We assigned MICs according to the Food and Drug Administration–approved package insert, using interpretation values for CO2 when provided. We defined azithromycin resistance as an MIC of ≥ 16 µg/mL and tetracycline resistance as an MIC of ≥ 8 µg/mL, each interpreted with CO2. We defined benzyl penicillin nonsusceptibility as an MIC of ≥ 0.12 µg/mL [12]. Polymerase chain reaction analysis was performed on all azithromycin-resistant isolates, using oligonucleotide primers to amplify a 348-bp segment containing the mefA/E gene or a 639-bp segment containing the ermB gene element [13]. Positive controls for each primer pair and a negative control strain (S. pneumoniae ATCC 49619) were included with all runs. MLST was performed on all pneumococcal isolates, based upon composite sequence types of 7 housekeeping gene fragments as previously described [10], with several modifications [14].
Statistical Analyses
We determined which STs were resistant to azithromycin before mass treatment and tested whether these same resistant STs accounted for a larger proportion of isolates after treatment, using mixed-effects logistic regression with community and individual as nested random effects. We set the significance level for the logistic regression at .05. We assessed for the relationship between ST and antibiotic resistance, using a classification index described by Jolley et al [15]. The classification index is interpreted as the probability of correctly classifying a ST as resistant or not, where classification is based on the difference in ST frequency between the resistant and susceptible populations. A value of 0 indicates that there are identical ST frequencies between the resistant and susceptible populations, whereas a value of 1 indicates no overlap between the populations. We calculated the classification index separately before and after treatment and separately for each antibiotic. We tested whether the classification index was significantly different from 0 by using permutation, stratified by subkebele (1000 repetitions). We set a significance level of .001 for this analysis because of the numerous P values that were calculated. Finally, we determined the diversity of STs in each community by calculating the unbiased Simpson index and compared this diversity before and after treatment by means of a Wilcoxon sign rank test, at a significance level of .05 [16]. We expressed diversity as 1 – Simpson index, with higher numbers indicating more diversity. We performed all analyses with Stata 12.1 (College Station, TX).
RESULTS
We performed nasopharyngeal swabbing on 110 children in 11 of the 12 communities before mass azithromycin treatment. The swabs from the remaining community were destroyed before they could be processed. We isolated 82 total pneumococcal isolates from 76 of these children (carriage frequency, 69.1%; 95% confidence interval [CI], 52.7%–81.8%). Of 82 isolates, we were able to determine the ST in 77, and we identified 61 distinct STs. Of these STs, 47 (77.0%) were identified in only 1 isolate.
In all communities, children aged 1–10 years received 4 quarterly mass azithromycin treatments. Azithromycin coverage exceeded 75% at each treatment, as published previously [11].
At the 12-month visit (3 months after the most recent mass azithromycin treatment), 119 children from 12 communities underwent nasopharyngeal swabbing. We identified 96 pneumococcal isolates from 93 swabbed children (carriage frequency, 78.2%; 95% CI, 65.2%–87.2%); STs were identified in all isolates, for a total of 61 unique STs. Of these STs, 43 (70.5%) were identified in a single isolate.
Before mass azithromycin treatments, 4 of 82 isolates (4.9%; 95% CI, 1.3%–17.1%) were resistant to azithromycin. We identified these 4 isolates as ST 177 (for 1 isolate), 6449 (for 1), and 6494 (for 2). We also determined the genetic resistance determinants: ST 177 was positive for ermB, and STs 6449 and 6494 were positive for mefA/E (Figure 1). After mass azithromycin, these 3 STs accounted for 14 of 96 isolates (14.6%; 95% CI, 8.3%–24.3%), all of which were resistant to azithromycin. Specifically, we identified ST 177 in 7 isolates, 6449 in 5 isolates, and 6494 in 2 isolates (Figure 1). After treatment, all isolates of ST 177 were positive for ermB, and all isolates of STs 6449 and 6494 were positive for mefA/E. Resistant isolates with one of these 3 STs were more common after mass azithromycin distributions, compared with before mass distributions (odds ratio [OR], 3.33; 95% CI, 1.04–10.6; P = .04).
Figure 1.
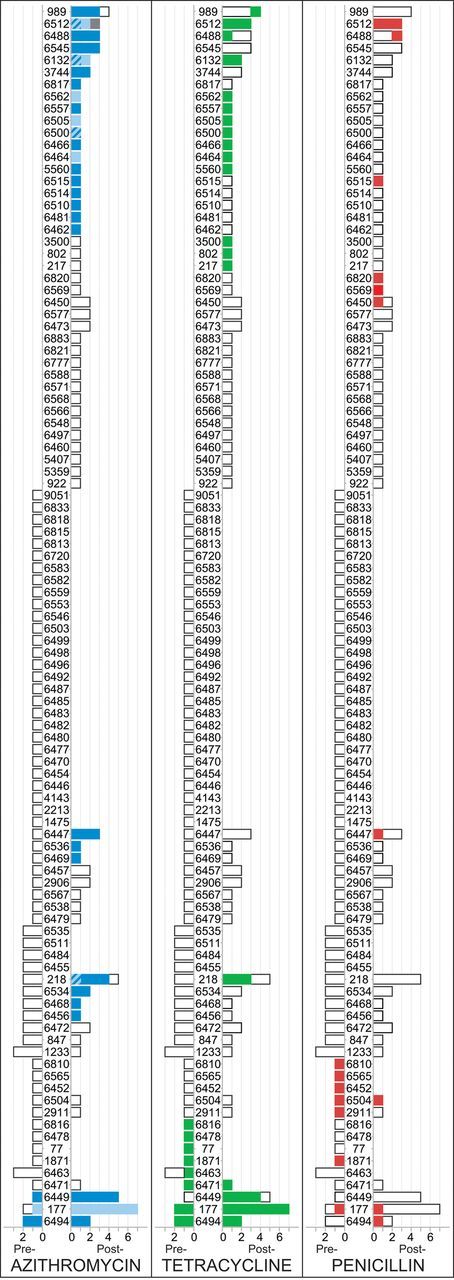
Multilocus sequence type (ST) and antibiotic resistance before and after repeated mass azithromycin distributions. For each class of antibiotic resistance, STs are listed in the center, and the frequency of each ST is displayed in a bar. Pretreatment frequencies are listed on the left of the ST, and posttreatment frequencies are listed on the right. An empty bar indicates a susceptible isolate, and a colored bar indicates a nonsusceptible isolate. In the case of azithromycin, the ermB genetic resistance determinant is represented by light blue, and mefA/E is represented by dark blue; isolates with both ermB and mefA/E are represented by light and dark blue stripes, and isolates with neither resistance determinant are represented by gray.
We performed similar analyses to assess the relationship between ST and nonsusceptibility to penicillin. Seven isolates with 7 different STs were nonsusceptible to penicillin at baseline; these 7 STs accounted for 12 isolates at the 12-month time point, 2 of which were nonsusceptible to penicillin. We found no evidence that resistant isolates with these 7 STs were more common after mass azithromycin treatments (OR, 0.23; 95% CI, .05–1.13; P = .07). Of note, none of the penicillin-nonsusceptible isolates identified in this study had MICs of ≥ 2 µg/mL (range among 19 nonsusceptible isolates, 0.12–0.5 µg/mL).
We identified 11 tetracycline-resistant isolates before mass antibiotic treatments. The ST could be determined for 10 of these isolates; 8 different STs were identified (Figure 1). These same STs accounted for 10 isolates after mass treatments, all of which were resistant to tetracycline. There was no increase in the proportion of resistant isolates with these 8 STs after mass antibiotic treatments (OR, 0.81; 95% CI, .31–2.10; P = .67). Tetracycline resistance was detected in all 16 ermB-positive isolates from the posttreatment visit, compared with 18 of 80 ermB-negative isolates (22.5%; P < .001, by the Fisher exact test, ignoring clustering). In contrast, there was no association between tetracycline resistance and the mefA/E genetic resistance determinant: 16 of 43 mefA/E-positive isolates (37.2%) were resistant to tetracycline, compared with 18 of 53 mefA/E-negative isolates (34.0%; P = .83)
To determine whether ST was associated with antibiotic resistance, we calculated the classification index described by Jolley et al [15]. We performed this analysis separately for the pretreatment and posttreatment populations. We found no significant associations between ST and antibiotic resistance before mass azithromycin treatments, although this analysis may have been limited by a small number of resistant isolates (Table 1). In contrast, we found a significant association between ST and azithromycin resistance after mass azithromycin treatments, including strong associations with the ermB and mefA/E genetic resistant determinants responsible for the vast majority of pneumococcal macrolide resistance (P < .0001 for each). ST was also associated with tetracycline resistance after mass azithromycin treatments (P < .0001) but not with penicillin resistance (P = .14). We performed similar analyses to determine whether ST was associated with age or sex; these revealed no significant associations before or after mass azithromycin distributions (Table 1).
Table 1.
Relationship Between Multilocus Sequence Type (ST) and Resistance, According to the Classification Index Described by Jolley et al [15]
| Stratification Variable | Before Treatment (n = 77 STs) |
After Treatment (n = 96 STs) |
||||
|---|---|---|---|---|---|---|
| N* | Classification Index | P Values | N* | Classification Index | P Values | |
| Nonsusceptibility | ||||||
| To azithromycin | ||||||
| Overall | 4 | 0.974 | .28 | 56 | 0.929 | <.0001 |
| ermB | 1 | 0.974 | 1.0 | 16 | 0.922 | <.0001 |
| mefA/E | 3 | 1.0 | .04 | 43 | 0.889 | <.0001 |
| To tetracycline | 10 | 0.954 | .06 | 34 | 0.857 | <.0001 |
| To penicillin | 7 | 0.974 | .31 | 8 | 0.807 | .14 |
| Age <5 y | 30 | 0.786 | .99 | 46 | 0.619 | .64 |
| Female | 37 | 0.861 | .11 | 46 | 0.648 | .38 |
P values indicate whether the classification index estimate was significantly different from zero, assessed through permutation (1000 repetitions). The ST could not be determined for 5 isolates before treatment, which are not included in this analysis.
*Number of isolates fulfilling the stratification variable criterion.
We calculated the unbiased form of Simpson index for each community to assess ST diversity (Table 2). Community-level ST diversity decreased after mass azithromycin treatments, from a median of 0.978 before treatment to 0.952 after treatment (P = .045, by the Wilcoxon signed rank test).
Table 2.
Diversity of Pneumococcal Multilocus Sequence Type (ST) in Each of 11 Communities Monitored Before and After 4 Rounds of Mass Azithromycin Treatments
| Subkebele | Before Treatment |
After Treatment |
||||
|---|---|---|---|---|---|---|
| Isolates, No. | Distinct STs, No. | 1 – Simpson Indexa | Isolates, No. | Distinct STs, No. | 1 – Simpson Indexa | |
| 1 | 9 | 8 | 0.972 | 8 | 7 | 0.964 |
| 2 | 5 | 4 | 0.900 | 8 | 6 | 0.929 |
| 4 | 8 | 6 | 0.893 | 7 | 5 | 0.857 |
| 5 | 10 | 9 | 0.978 | 11 | 10 | 0.982 |
| 6 | 6 | 6 | 1.000 | 8 | 7 | 0.964 |
| 7 | 3 | 3 | 1.000 | 4 | 3 | 0.667 |
| 8 | 10 | 8 | 0.933 | 10 | 7 | 0.911 |
| 9 | 4 | 4 | 1.000 | 7 | 6 | 0.952 |
| 10 | 9 | 9 | 1.000 | 8 | 6 | 0.929 |
| 11 | 5 | 5 | 1.000 | 10 | 9 | 0.978 |
| 12 | 8 | 7 | 0.964 | 9 | 8 | 0.972 |
a Unbiased Simpson index. Higher numbers indicate more diversity.
DISCUSSION
This study provides evidence that antibiotic selection pressure increases antibiotic resistance through clonal expansion. The 3 analyses performed were each consistent with the hypothesis of clonal expansion. First, we found that the group of pneumococcal STs that were resistant before mass treatments became significantly more common after mass treatments. Second, we found that ST was significantly associated with macrolide resistance when assessed by the classification index published by Jolley et al. Finally, we found that the community-level diversity of STs decreased after mass azithromycin treatments. These results suggest that pneumococcal clones resistant to macrolides spread horizontally throughout the community after extreme macrolide selection pressure, resulting in a higher proportion of these resistant clones and a less diverse pneumococcal population.
Twelve Ethiopian communities received mass azithromycin distribution for trachoma every 3 months for 1 year. Before treatment, macrolide resistance was identified in 3 distinct STs from approximately 5% of pneumococcal isolates. After 4 rounds of mass azithromycin, resistant isolates carrying one of these 3 STs accounted for almost 15% of pneumococcal isolates, although the increase in prevalence was due to 2 of the 3 STs (ie, STs 177 and 6449). Each of the STs was associated with the same genetic resistance determinant before and after mass azithromycin treatment (eg, ermB for ST 177 and mefA/E for STs 6449 and 6494). The most likely explanation for these findings is clonal expansion. Mass azithromycin is known to greatly reduce the prevalence of nasopharyngeal pneumococcal carriage [17]; clearance of susceptible strains would shift the competitive balance in favor of the existing resistant strains and allow horizontal spread of these resistant clones.
As expected, ST and macrolide resistance were associated after mass azithromycin treatments (Table 1). Specifically, the posttreatment classification index for ST and azithromycin resistance was 0.929, which can be interpreted as a 92.9% chance of classifying whether a pneumococcal isolate is or is not resistant to macrolides solely on the basis of knowledge of its ST classification. A similar effect was found for both the ermB and mefA/E genetic resistance determinants. This result is what would be expected in the case of clonal expansion: certain clones are resistant at the time of antibiotic selection pressure, and these clones expand within the population, maintaining their association with resistance. If ST was not associated with resistance, this would suggest that antibiotic selection pressure might function through de novo mutation of random clones. We were unable to demonstrate an association between ST and azithromycin resistance before mass azithromycin treatments, only afterwards. There are several potential explanations for this. It is possible that the finding is merely a power issue, since the coefficients are very similar before and after treatments, but the number of resistant isolates is much smaller before mass treatments. However, it is also possible that random recombination events resulted in a certain number of isolates that were resistant to macrolides before mass azithromycin distribution. In this scenario, an association between ST and resistance would not be expected before mass treatments. The azithromycin selection pressure would then allow clonal expansion of these randomly generated resistant strains, resulting in a significant association between ST and resistance after treatment. A larger study may be needed to fully elucidate these 2 possibilities.
The results of the diversity analysis (0.978 before treatment vs 0.952 after treatment) can be interpreted as meaning that the chance of any 2 randomly chosen isolates being the same was 2.2% before treatment and increased to 4.8% after mass azithromycin distribution. The finding of reduced ST diversity after antibiotic selection pressure is consistent with clonal expansion. Clonal expansion would allow a relatively small group of azithromycin-resistant strains to outcompete the diverse array of susceptible strains. A reduction of the susceptible strains, accompanied by an increase in a relatively small group of resistant strains, would result in reduced ST diversity after mass azithromycin treatments. We acknowledge that the diversity analysis in this study should be interpreted with caution, since the absolute diversity measurements are dependent on a small number of observations per community. However, we used the unbiased Simpson index, which takes into account differing numbers of isolates per community and should thus protect the analysis from bias due to small community size.
In this study, we performed similar analyses for resistance to penicillin and to tetracycline, which served as negative controls. The STs accounting for tetracycline and penicillin resistance were not found to increase after mass azithromycin treatments, suggesting that clonal expansion did not occur for these organisms. This is to be expected, since the antibiotic selection pressure for each of these antibiotics was minimal to nonexistent. In contrast, the classification index did find an association between ST and tetracycline resistance but not penicillin resistance. It should be noted that the tetM genetic determinant of tetracycline resistance is associated with the ermB determinant for azithromycin resistance, so this finding is not surprising [18]. It is also important to emphasize that we detected no pneumococcal isolates with penicillin resistance (MIC ≥ 2 µg/mL) but instead found only isolates with intermediate penicillin resistance (MIC ≥ 0.12 to ≤1 µg/mL). It is possible that these intermediately resistant isolates would have enjoyed little competitive advantage over isolates with lower MICs even if selection pressure had been present, which would have made clonal behavior difficult to appreciate.
This study provides a prospective population-based assessment of nasopharyngeal pneumococcal population dynamics after mass azithromycin treatment. Because it was conducted as part of a clinical trial, the intervention was performed in a similar way in all communities, and the data collected in a standardized manner. The study has several limitations. MLST, although recognized as an efficient way to perform genetic epidemiological studies, may not completely discriminate between closely related clones, since it relies only on 7 housekeeping genes. While these housekeeping genes allow for a high degree of discrimination between clones, newer techniques such as whole-genome sequencing could provide a more complete strain classification [19]. Nonetheless, most of the STs identified in this study belonged to a single isolate, suggesting that MLST was sufficient for the purposes of the current study. We did not use macrolide-containing medium when isolating S. pneumoniae, which may have reduced the number of azithromycin-resistant isolates identified, especially before mass antibiotic treatments. The sample size and sampling schedule were not sufficient to detect all resistant isolates before or after mass azithromycin treatments. However, such a study would be time and cost prohibitive. The current study selected a random sample of children, allowing equally unbiased conclusions, although with some compromised precision.
In summary, a cluster-randomized trial of mass azithromycin treatments for trachoma allowed us to monitor nasopharyngeal pneumococcal populations before and after a defined, massive amount of antibiotic selection pressure. The results of 3 separate analyses were each consistent with clonal expansion being the underlying mechanism for the increase in antibiotic resistance following antibiotic selection pressure.
Notes
Acknowledgments. We thank Imperial College London for use of the pneumococcal MLST database, which is funded by the Wellcome Trust.
Financial support. This work was supported by the National Institutes of Health (NEI U10 EY016214, NIH/NCRR/OD UCSF-CTSI KL2RR024130, and NIH/NEI K23EY019071), the Bernard Osher Foundation, That Man May See, the Harper Inglis Trust, the Bodri Foundation, the South Asia Research Fund, Research to Prevent Blindness, and the International Trachoma Initiative (for the azithromycin used for this study).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg Infect Dis. 2002;8:347–54. doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Den Braak N, van Belkum A, Kreft D, te Witt R, Verbrugh HA, Endtz HP. The prevalence and clonal expansion of high-level gentamicin-resistant enterococci isolated from blood cultures in a Dutch university hospital. J Antimicrob Chemother. 1999;44:795–8. doi: 10.1093/jac/44.6.795. [DOI] [PubMed] [Google Scholar]
- 3.Le TA, Fabre L, Roumagnac P, Grimont PA, Scavizzi MR, Weill FX. Clonal expansion and microevolution of quinolone-resistant Salmonella enterica serotype typhi in Vietnam from 1996 to 2004. J Clin Microbiol. 2007;45:3485–92. doi: 10.1128/JCM.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solnik-Isaac H, Weinberger M, Tabak M, Ben-David A, Shachar D, Yaron S. Quinolone resistance of Salmonella enterica serovar Virchow isolates from humans and poultry in Israel: evidence for clonal expansion. J Clin Microbiol. 2007;45:2575–9. doi: 10.1128/JCM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy AD, Otto M, Braughton KR, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A. 2008;105:1327–32. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsen O, Toleman MA, Sundsfjord A, et al. Molecular epidemiology of metallo-beta-lactamase-producing Pseudomonas aeruginosa isolates from Norway and Sweden shows import of international clones and local clonal expansion. Antimicrob Agents Chemother. 2010;54:346–52. doi: 10.1128/AAC.00824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rensburg MJ, Whitelaw AC, Elisha BG. Genetic basis of rifampicin resistance in methicillin-resistant Staphylococcus aureus suggests clonal expansion in hospitals in Cape Town, South Africa. BMC Microbiol. 2012;12:46. doi: 10.1186/1471-2180-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loman NJ, Gladstone RA, Constantinidou C, et al. Clonal Expansion within Pneumococcal Serotype 6C after Use of Seven-Valent Vaccine. PLoS One. 2013;8:e64731. doi: 10.1371/journal.pone.0064731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377. doi: 10.1371/journal.pmed.1000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–60. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 11.House JI, Ayele B, Porco TC, et al. Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. Lancet. 2009;373:1111–8. doi: 10.1016/S0140-6736(09)60323-8. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein MP, Klugman KP, Jones RN. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin Infect Dis. 2009;48:1596–600. doi: 10.1086/598975. [DOI] [PubMed] [Google Scholar]
- 13.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–6. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore MR, Gertz RE, Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–27. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 15.Jolley KA, Wilson DJ, Kriz P, McVean G, Maiden MC. The influence of mutation, recombination, population history, and selection on patterns of genetic diversity in Neisseria meningitidis. Mol Biol Evol. 2005;22:562–9. doi: 10.1093/molbev/msi041. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig ML. Species diversity in space and time. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- 17.Leach AJ, Shelby-James TM, Mayo M, et al. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis. 1997;24:356–62. doi: 10.1093/clinids/24.3.356. [DOI] [PubMed] [Google Scholar]
- 18.Seral C, Castillo FJ, Rubio-Calvo MC, Fenoll A, Garcia C, Gomez-Lus R. Distribution of resistance genes tet(M), aph3′-III, catpC194 and the integrase gene of Tn1545 in clinical Streptococcus pneumoniae harbouring erm(B) and mef(A) genes in Spain. J Antimicrob Chemother. 2001;47:863–6. doi: 10.1093/jac/47.6.863. [DOI] [PubMed] [Google Scholar]
- 19.Croucher NJ, Finkelstein JA, Pelton SI, et al. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet. 2013;45:656–63. doi: 10.1038/ng.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]


