Abstract
Cryptosporidiosis, a gastrointestinal disease caused by protozoans of the genus Cryptosporidium, is a common cause of diarrheal diseases and often fatal in immunocompromised individuals. Cryptosporidium hominis (C. hominis)-specific bifunctional thymidylate synthase-dihydrofolate reductase (TS-DHFR) has been a molecular target for inhibitor design. C. hominis TS-DHFR inhibitors with nM potency at a biochemical level have been developed however drug delivery to achieve comparable antiparasitic activity in Cryptosporidium infected cell culture has been a major hurdle for designing effective therapies. Previous mechanistic and structural studies have identified compound 906 as a nM C. hominis TS-DHFR inhibitor in vitro, having μM antiparasitic activity in cell culture. In this work, proof of concept studies are presented using a nanotherapy approach to improve drug delivery and the antiparasitic activity of 906 in cell culture. We utilized PLGA nanoparticles that were loaded with 906 (NP-906) and conjugated with antibodies to the Cryptosporidium specific protein, CP2, on the nanoparticle surface in order to specifically target the parasite. Our results indicate that CP2 labeled NP-906 (CP2-NP-906) reduces the level of parasites by 200-fold in cell culture, while NP-906 resulted in 4.4-fold decrease. Moreover, the anticryptosporidial potency of 906 improved 15 to 78-fold confirming the utility of the antibody conjugated nanoparticles as an effective drug delivery strategy. 2009 Elsevier Ltd. All rights reserved.
Keywords: Thymidylate synthase, Dihydrofolate reductase, Cryptosporidium hominis, Nanoparticle, Drug delivery
Graphical Abstract
Cryptosporidiosis is a human gastrointestinal disease which is caused by the protozoans of the genus Cryptosporidium and was ranked fifth among the twenty-four most important global food-borne parasitic illnesses by a joint Food and Agriculture Organization/World Health Organization committee in 2012.1,2 Among the several Cryptosporidium species that can cause human disease, C. hominis and C. parvum are responsible for the majority of the human disease and share a high sequence identity (95–97%) at the genome level.3 Infection in humans is generally spread through contact with infected individuals or consumption of recreational water.4 This disease causes gastrointestinal distress, which can persist two weeks or more.4,5 However in immunocompromised individuals, such as those with malnutrition, HIV, cancer, or organ transplants, this disease can be debilitating and often fatal.4,5 Currently approved therapeutics, nitazoxanide and paromomycin have limited activity in immunocompromised individuals, creating an urgent need for the development of new anti-parasitic drugs.6–8
Attempts to develop new drugs to treat cryptosporidiosis have been hampered in part by the unique niche Cryptosporidium occupies in the host cell.4,9 The parasite is intracellular while remaining outside of the host cell cytoplasm.9 In essence Cryptosporidium surrounds its apical domain with cellular components of the host cell membrane forming the parasitophorous vacuole membrane (PVM), while fusing its basal membrane with the host cell membrane forming the feeder organelle.10 The PVM acts like a natural barrier to many therapeutics, whereas the feeder organelle is thought to modulate the transfer of many drugs, blocking uptake of drugs from the host cell cytoplasm.4,9,11,12 Besides PVM, the presence of ABC transporters or efflux pumps that transport the drugs out of the parasite is another obstacle for the effective treatment.13,14 Therefore, an effective drug delivery approach is needed to overcome these hurdles and facilitate drug transfer circumventing the issues raised above. Nanoparticles (NPs) have been shown to be a successful means of improving drug delivery.15,16 In the current study, a poly(lactic-co-glycolic acid) (PLGA) polymer was used to prepare nanoparticles conjugated with a Cryptosporidium specific antibody for the parasitic protein CP2. These NPs were used for delivering a model TS-DHFR inhibitor, 2-amino-4-oxo-4,7-dihydro-pyrrolo[2,3-d]pyrimidine-methyl-phenyl-L-glutamic acid (compound 906, Fig. 1).
Figure 1.
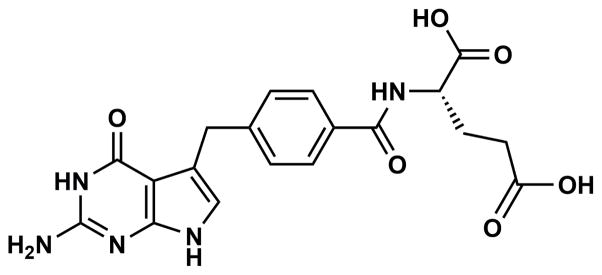
Chemical structure of compound 906.
Previous mechanistic and structural studies have shown that compound 906 inhibits the bifunctional thymidylate synthase-dihydrofolate reductase (TS-DHFR) of C. hominis (Ch TS-DHFR), which is an essential enzyme in folate biosynthesis.8,17 C. hominis encodes and expresses TS and DHFR as a bifunctional enzyme in contrast to the monofunctional forms of the enzyme found in humans.8
PLGA-based NPs are one of the most successfully used biodegradable nanotherapeutics utilized for medical purposes.18,19 PLGA degrades into lactic acid and glycolic acid, which are metabolized by the body via the Krebs cycle, creating minimal systemic toxicity.18,19 The unique size of nanoparticles makes them amenable to surface modifications, such as antibodies which can be used to directly target specific tissues.18 PLGA nanoparticles conjugated with antibodies specific to many cancer types have shown promise as a drug delivery strategy for cancer therapeutics, increasing target specificity and efficacy of the incorporated therapeutic compounds.20–22 Moreover, PLGA nanoparticles containing Indinavir, a protease inhibitor for the treatment of HIV-1 infection, which has been suggested to have anticryptosporidial activity, were modified with Cryptosporidium parvum antibodies to the COWP-190, a 190 kDa protein found in the Cryptosporidium oocyst cell wall.23 This study demonstrated a 1.5-fold decrease in the number of C. parvum infected cells in culture than with Indinavir alone.24
For our current study, an antibody (Ab) specific to the C. parvum CP2 protein was used for the modification of the NP surface. CP2, whose function has not been completely delineated, is a protein expressed in all stages of development in C. parvum and is localized in the parasites’ cytoplasm and amylopectin-like graduals as well as the host derived PVM.25 Moreover, CP2 has been shown to be essential for parasite viability.26 Using this drug delivery strategy as a proof of concept, we present the initial results of anticryptosporidial activity for compound 906 in C. parvum infected cells, which was incorporated into PLGA nanoparticles conjugated with CP2 Ab. We used C. parvum infected cells for our cell culture studies instead of C. hominis due to the ease of collection of oocysts from infected ruminants that are primary hosts for C. parvum.27 The sequence alignment of TS-DHFR from C. parvum and C. hominis showed a 100% sequence identity at the protein level, which allowed us to test the Ch TS-DHFR inhibitor in culture using C. parvum infected cells. Additionally, CP2 protein from C. parvum and C. hominis showed a high sequence identity (99%) as well.
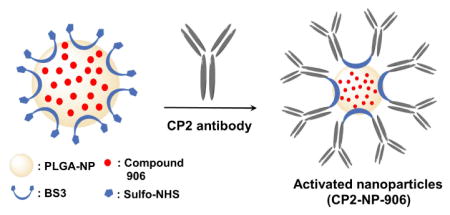
The compound 906 was synthesized according to the previously reported methods17 and the purified product was loaded into PLGA nanoparticles. In order to obtain high drug loading efficiencies, several different techniques were utilized for the preparation of several batches of compound 906 loaded nanoparticles (NP-906, Fig. 2).
Figure 2.
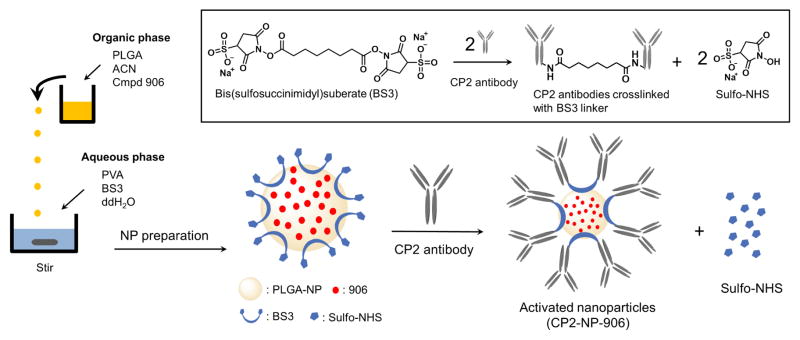
Schematic illustration of compound 906 loaded PLGA nanoparticle preparation and its surface functionalization reaction with CP2 antibody. The inlet shows the chemical reaction involved in covalent conjugation of CP2’s surface functionalized (activated) nanoparticles.
The production of NP-906 was carried out using nanoprecipitation as shown in Figure 2 (NP-00D; NP-01D; NP-02D; NP-04D; NP-06D; NP-08D; NP-10D), solvent evaporation (ESPV-02D; ESPV-05D; ESPF-02D; ESPF-05D) and double emulsion solvent evaporation techniques (W-PV-10W; W-PF-10W) (described in Supplemental methods).
When tested for percent drug loading, NP-02D, NP-04D, and W-PF-10W had percent drug loading of compound 906 greater than 90 %, and an average particle size of 135 nm, 155 nm and 300 nm respectively. Among these formulations, NP-04D was further selected for the CP2 antibody conjugation due to the highest drug loading efficiency and optimal particle size. Supplementary figure 1 illustrates the steps involved in the formulation of NP-04D via nanoprecipitation method. Following the NP loading, a homo-bifunctional chemical crosslinker, bis(sulfosuccinimidyl)suberate (BS3), was utilized for the conjugation of NP-906 with CP2 antibody (CP2-NP-906) via our previously established methodology (Fig. 2).28
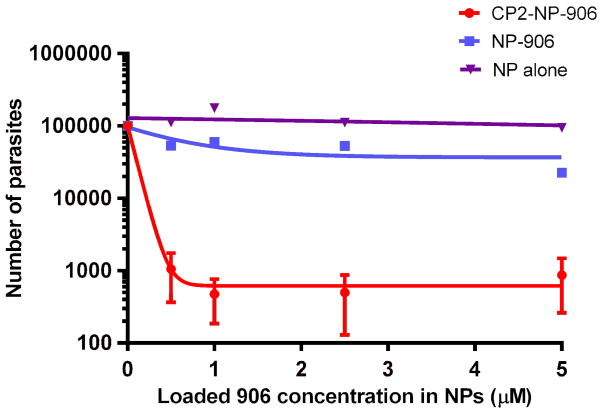
According to our earlier biochemical studies, compound 906 showed a half maximal inhibitory concentration (IC50) of 0.38 ± 0.04 μM against Ch-TS enzyme activity.17 However, when compound 906 was evaluated in cell culture, the half maximal effective concentration (EC50) required to significantly reduce C. parvum infection ranged between 1 and 5 μM.17 Therefore, compound 906 was a good model drug to test the CP2 Ab-conjugated NP (CP2-NP-906) delivery strategy to improve its therapeutic efficiency in cell culture. Indeed, as shown in Figure 3, the anti-cryptosporidial activity of CP2-NP-906 on C. parvum sporozoites in cell culture revealed an EC50 value in the 100–300 nM range, in agreement with results at the biochemical level.
Figure 3.
Antiparasitic effect of CP2-NP-906, NP-906 and NP alone on sporozoites and intracellular forms.
Additionally, the level of parasite infection in cell culture was decreased 200-fold when cells were preincubated with CP2-NP-906 compared with NP-906 lacking CP2 Ab conjugation, at the same concentration showed only a 4.4-fold decrease. We also tested unloaded NPs in cell culture as a negative control. As expected the level of parasites in cell culture remains relatively constant in the presence of NP alone, indicating that 906 was responsible for the reduction in infection in cell culture and not the components of the nanoparticles. Moreover, soluble CP2 Ab alone showed no change in infection similar to the unloaded NP at a concentration that was 10-fold higher (2.5 mg/ml) than the antibody conjugated NP-906.
These results indicate that CP2-NP-906 facilitates the efficient delivery of compound 906 to directly target C. parvum. The CP2 antibody conjugated to the NP-906 would be expected to aid in specificity for parasite while the PLGA-based NP-906 nanoparticles might assist entry of compound 906 through diffusion across the PVM of C. parvum.
In summary, these proof of concept studies demonstrate a new approach to deliver the Ch-TS inhibitor 906 for improved anticryptosporidial potency. The effective coupling of PLGA nanoparticles with CP2 antibody allowed a significant reduction in C. parvum infection in cell culture as compared to NP-906 and compound 906 alone. This nanodelivery approach provides very attractive and general strategy that could be readily adapted to improve efficacy of any type of drug in order to specifically target the Cryptosporidium parasite and maintain high antiparasitic activity. Future studies will investigate the important questions of nanoparticle stability as well as the most appropriate delivery route to target the unique environment of this intraluminal parasite with the host.
Supplementary Material
Acknowledgments
This work is supported by NIAID Grant (AI083146) to K.S.A., Training Grant (5T32AI007404-23) to D.C., the Paul R. Stalnaker MD Distinguished Professorship to A.C.W. and NIH Grant (AI44616) to W.L.J. We would like to thank InBios International Inc. for their generosity in providing us the CP2 antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ryan U, Fayer R, Xiao L. Parasitology. 2014;141:1667. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Lancet. 2013;382:209. [Google Scholar]
- 3.Mazurie AJ, Alves JM, Ozaki LS, Zhou S, Schwartz DC, Buck GA. Int J Genomics. 2013;2013:832756. doi: 10.1155/2013/832756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Checkley W, White AC, Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen X, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA, Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. Lancet Infect Dis. 2015;15:85. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hara SP, Chen XM. Microbes Infect. 2011;13:721. doi: 10.1016/j.micinf.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. Br J Clin Pharmacol. 2007;63:387. doi: 10.1111/j.1365-2125.2007.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gargala G, Delaunay A, Li X, Brasseur P, Favennec L, Ballet JJ. J Antimicrob Chemother. 2000;46:57. doi: 10.1093/jac/46.1.57. [DOI] [PubMed] [Google Scholar]
- 8.Kumar VP, Frey KM, Wang Y, Jain HK, Gangjee A, Anderson KS. Bioorg Med Chem Lett. 2013;23:5426. doi: 10.1016/j.bmcl.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenman K, Wanyiri JW, Bhat N, Ward HD, Coppens I. Cell Microbiol. 2013;15:1182. doi: 10.1111/cmi.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths JK, Balakrishnan R, Widmer G, Tzipori S. Infect Immun. 1998;66:3874. doi: 10.1128/iai.66.8.3874-3883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead JR. Drug Resist Updat. 2002;5:47. doi: 10.1016/s1368-7646(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 12.Tzipori S, Ward H. Microbes Infect. 2002;4:1047. doi: 10.1016/s1286-4579(02)01629-5. [DOI] [PubMed] [Google Scholar]
- 13.Xu P, Widmer G, Wang Y, Ozaki LS, Alves JM, Serrano MG, Puiu D, Manque P, Akiyoshi D, Mackey AJ, Pearson WR, Dear PH, Bankier AT, Peterson DL, Abrahamsen MS, Kapur V, Tzipori S, Buck GA. Nature. 2004;431:1107. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- 14.Benitez AJ, McNair N, Mead J. Parasitol Res. 2007;101:1611. doi: 10.1007/s00436-007-0701-x. [DOI] [PubMed] [Google Scholar]
- 15.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. J Control Release. 2001;70:1. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 16.De Jong WH, Borm PJ. Int J Nanomedicine. 2008;3:133. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar VP, Cisneros JA, Frey KM, Castellanos-Gonzalez A, Wang Y, Gangjee A, White AC, Jr, Jorgensen WL, Anderson KS. Bioorg Med Chem Lett. 2014;24:4158. doi: 10.1016/j.bmcl.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. J Control Release. 2012;161:505. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Kumari A, Yadav SK, Yadav SC. Colloids Surf B Biointerfaces. 2010;75:1. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Yallapu MM, Khan S, Maher DM, Ebeling MC, Sundram V, Chauhan N, Ganju A, Balakrishna S, Gupta BK, Zafar N, Jaggi M, Chauhan SC. Biomaterials. 2014;35:8635. doi: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukerjee A, Ranjan AP, Helson L, Vishwanatha JK. International Journal of Nanotechnology. 2014;11:676. [Google Scholar]
- 22.Byrne JD, Betancourt T, Brannon-Peppas L. Adv Drug Deliv Rev. 2008;60:1615. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Ranucci L, Muller HM, La Rosa G, Reckmann I, Morales MA, Spano F, Pozio E, Crisanti A. Infect Immun. 1993;61:2347. doi: 10.1128/iai.61.6.2347-2356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bondioli L, Ludovisi A, Tosi G, Ruozi B, Forni F, Pozio E, Vandelli MA, Gomez-Morales MA. Parasitology. 2011;138:1384. doi: 10.1017/S0031182011001119. [DOI] [PubMed] [Google Scholar]
- 25.O’Hara SP, Yu JR, Lin JJ. Parasitol Res. 2004;92:317. doi: 10.1007/s00436-003-1057-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee SU, Joung M, Ahn MH, Huh S, Song H, Park WY, Yu JR. Parasitol Res. 2008;102:381. doi: 10.1007/s00436-007-0772-8. [DOI] [PubMed] [Google Scholar]
- 27.Leoni F, Amar C, Nichols G, Pedraza-Diaz S, McLauchlin J. J Med Microbiol. 2006;55:703. doi: 10.1099/jmm.0.46251-0. [DOI] [PubMed] [Google Scholar]
- 28.Thamake SI, Raut SL, Ranjan AP, Gryczynski Z, Vishwanatha JK. Nanotechnology. 2011;22:035101. doi: 10.1088/0957-4484/22/3/035101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.