Abstract
Ornithine decarboxylase (ODC), the first enzyme of polyamine metabolism, is rapidly upregulated in response to agents that induce a pathological cardiac hypertrophy. Transgenic mice overexpressing ODC in the heart (MHC-ODC mice) experience a much more dramatic left ventricular hypertrophy in response to β-adrenergic stimulation with isoproterenol (ISO) compared to non-transgenic controls. ISO also induced arginase activity in transgenic hearts but not in controls. The current work studies the cooperation between the cardiac polyamines and L-arginine (L-Arg) availability in MHC-ODC mice. Although ISO-induced hypertrophy is well-compensated, MHC-ODC mice administered L-Arg along with ISO showed a rapid onset of systolic dysfunction and died within 48h. Myocytes isolated from transgenic mice administered L-Arg/ISO exhibited reduced contractility and altered calcium transients, suggesting an alteration in [Ca2+] homeostasis, and abbreviated action potential duration, which may contribute to arrhythmogenesis. The already elevated levels of spermidine and spermine were not further altered in MHC-ODC hearts by L-Arg/ISO treatment, suggesting alternative L-Arg utilization pathways lead to dysregulation of intracellular calcium. MHC-ODC mice administered an arginase inhibitor (Nor-NOHA) along with ISO died almost as rapidly as L-Arg/ISO-treated mice, while the iNOS inhibitor SMT was strongly protective against L-Arg/ISO. These results point to the induction of arginase as a protective response to β-adrenergic stimulation in the setting of high polyamines. Further, NO generated by exogenously supplied L-Arg may contribute to the lethal consequences of L-Arg/ISO treatment. Since considerable variations in human cardiac polyamine and L-Arg content are likely, it is possible that alterations in these factors may influence myocyte contractility.
Keywords: Arginase, Heart hypertrophy, Isoproterenol, Ornithine decarboxylase, Calcium homeostasis
Introduction
An increase in ventricular wall mass is a common beneficial adaptation of cardiac muscle in the face of stress. However, failure to limit myocardial hypertrophy can itself reduce cardiac output and lead to ventricular dysfunction. As such, left venticular hypertrophy (LVH) is considered an independent risk factor for heart failure (Levy et al. 1990).
Hypertrophic adaptations are believed to activate immediate early gene expression and protein synthesis. Ultimately these changes can modify the myocyte’s handling of Ca2+. Central to these protein alterations are fluctuations in polyamines (putrescine, spermidine and spermine), which are widely distributed in all cells and necessary for cell growth. Ornithine decarboxylase (ODC), the first and potentially rate-limiting enzyme of the polyamine pathway, is rapidly upregulated in response to various agents that induce a pathological cardiac hypertrophy, including thyroxine (Pegg et al., 1981), the β-adrenergic agonists isoproterenol (ISO) and clenbuterol, and the α1-adrenergic agonists phenylephrine and methoxamine (Cubria et al., 1998; Tipnis et al., 2000; Thompson et al.,1992). Administration of α-difluoromethylornithine (DFMO), a suicide inactivator of ODC (Wallace and Fraser 2004), reduced polyamine content and attenuated ISO- and clenbuterol-induced cardiac hypertrophy (Cubria et al., 1998; Tipnis et al., 2000), suggesting that high ODC activity is a factor in the development of hypertrophy.
ODC is part of a complex signaling cascade involving other compounds in addition to polyamines known to modulate cardiovascular homeostasis. Ornithine, the substrate for ODC, is produced by arginase, which catalyzes the conversion of L-arginine (L-Arg) to L-ornithine (L-Orn) and urea. L-Arg is also the substrate for nitric oxide synthases (NOS), which lead to the synthesis of NO, a molecule involved in several signal transduction pathways in cardiomyocytes (Pignatti et al. 1999). NO inhibits ODC (Bauer et al., 2001) and reduces polyamine content (Blachier et al., 1996). Likewise, polyamines can inhibit NOS (Hu et al., 1994). The activity of arginase is thought to play a regulatory role in the biosynthesis of both NO and polyamines (Li et al., 2001; Wu et al., 1998). These metabolic pathways may combine to regulate a variety of biochemical functions in the heart (reviewed in Giordano et al., 2003; Shantz et al., 2006). In contrast to ODC induction, NO deficiency has been linked to the development of cardiac hypertrophy using mouse models (Barouch et al., 2003; Cappola et al., 2003 Ozaki et al., 2002 Simko et al., 2000), and NO has been shown to attenuate the β-adrenergic response in cardiomyocytes (Balligand et al., 1999).
The central hypothesis of this work is that changes in polyamine and L-Arg metabolism that accompany upregulation of ODC, in cooperation with other signaling pathways, can mediate the development of myocardial hypertrophy and failure. Transgenic mice overexpressing ODC in the heart (MHC-ODC mice) experience a much more dramatic left ventricular hypertrophy in response to β-adrenergic stimulation compared to non-transgenic controls (Shantz et al., 2001). Here we show that although ISO-induced hypertrophy is well-compensated, MHC-ODC mice treated with L-Arg and ISO show a rapid onset of systolic dysfunction with a lethal outcome. The potential mechanism for this observation remains obscure. The purpose of these investigations was to establish the possible role of abnormal [Ca2+] in leading to ventricular dysfunction following elevation of polyamines. We found that the combination of L-Arg and ISO produces contractile dysfunction in myocytes from MHC-ODC mice, characterized by reduced contraction amplitude, as well as shortening and relengthening velocities at both low and high [Ca2+]o.
Material and Methods
2.1 Transgenic mice
Mice express a transgene containing a murine αMHC promoter upstream of a murine ODC cDNA with a stop codon at position 425 (Shantz et al., 2001). Heterozygous mice were bred from the original founder (Shantz et al., 2001) for at least 5 generations onto the B6D2 background for the current studies. Mice in these studies were treated at age 6-8 weeks. All transgenic animals used were heterozygous. All experiments described used transgenic mice compared to their littermate controls. The investigation conforms to the Recommendation from the Declaration of Helsinki and the Guiding Principles in the Care and Use of Animals. All methods were approved by the Penn State University Institutional Animal Care and Utilization Committee and conform to NIH guidelines.
2.2 Evaluation of hypertrophy
Mice received daily intraperitoneal (i.p.) injections of either saline or 20 mg/kg ISO in saline for 10 days, then were sacrificed by CO2-induced asphyxia. Upon sacrifice, each animal was weighed, the heart was excised, rinsed in phosphate buffered saline (PBS), dried and weighed. The ratio of heart weight in mg (H) to body weight in g (B) was used as an index of hypertrophy.
2.3 Arginine and ornithine supplementation
Initial observations used the following protocol: 5% L-Arg or L-Orn was supplied in the drinking water for three days before beginning ISO injections for 10 days. Mice were maintained on L-Arg or L-Orn for the entire experiment. To elucidate the mechanism of lethality, two ISO injections were given at 0 h and 24 h. Mice were sacrificed 6 h after the second ISO injection. When inhibitors were used, they were administered as described in the figures for either one or 3 days prior to the start of ISO injections, and continued until the end of the experiment.
2.4 Echocardiography
Echocardiography was performed using an Accuson Sequoia instrument paired with a 14 MHz transducer to study left ventricular hypertrophy and systolic function. After light anesthesia (100 mg/kg ketamine and 10 mg/kg xylazine) the animal was shaved, placed in a supine position and warmed using an isothermal heating pad. End-diastolic (LVEDD) and end-systolic LV dimension (LVESD) were measured from M-mode tracings, as well as interventricular septum (IVST) and posterior wall thickness (PWT). LV shortening fraction (SF) was calculated using the following equation: [LVEDD – LVESD/LVEDD] × 100. Ejection fraction (EF) was calculated as follows: [LV end-diastolic volume – LV end-systolic volume/ LV end-diastolic volume] × 100. Stroke volume (SV) was calculated as LV end-diastolic volume – LV end-systolic volume, while cardiac output (CO) equals SV × HR and is expressed per 10 g body weight. Hearts rates were similar in all animals under study.
2.5 Biochemical assays
Polyamine content was analysed using reverse-phase HPLC on samples extracted using 10% trichloroacetic acid (Shantz et al., 1992). The concentrations of L-Arg and L-Orn were determined using HPLC analysis following o-pthalaldehyde derivatization of amino acids in heart extracts (Lynch et al., 2002). Arginase activity was tested by monitoring [14C]-urea formation from L-[guanido-14C]-arginine. Activity was assayed for 15 min at 37° C and expressed as the radioactivity of [14C]-urea per mg protein.
2.6 Isolation of adult murine cardiac myocytes
Cardiac myocytes were isolated from the septum and left ventricular free wall of wild-type and ODC overexpressed mice as described (Song et al., 2008; Tucker et al., 2006). Isolated myocytes were plated on laminin-coated glass cover slips and used within 2-8 h of isolation.
2.7 Myocyte shortening measurements
Myocytes adherent to cover slips were bathed in 0.6 ml of air- and temperature-equilibrated (37°C), HEPES-buffered (20 mM, pH 7.4) medium 199 containing 0.6, 1.8 or 5.0 mM [Ca2+]o. Measurements of myocyte contraction (1 Hz) were performed as described previously (Tucker et al., 2006; Zhang et al., 2001a).
2.8 [Ca2+]i transient measurements
Myocytes were exposed to 0.67 μM of fura-2 AM for 15 min. at 37°C. Fura-2 loaded myocytes were field-stimulated to contract (1 Hz, 37°C) in medium 100 containing 0.6, 1.8 or 5.0 mM [Ca2+]o. [Ca2+]i transient measurements, daily calibration of fura-2 fluorescent signals, and [Ca2+]i transient analyses were performed as described previously (Tucker et al., 2006; Zhang et al., 2001a).
2.9 Action potential measurements
Action potentials from wild-type and ODC overexpressing mice, both treated with L-Arg and ISO, were recorded using current-clamp configuration at 1.5× threshold stimulus and 4 ms duration (Tucker et al., 2006; Zhang et al., 2001b). Pipette solutions consisted of (in mM) 125 KCl, 4 MgCl2, 0.06 CaCl2, 10 HEPES, 5 K+-EGTA, 3 Na2ATP, and 5 Na2-creatine phosphate (pH 7.2). External solution consisted of (in mM) 132 NaCl, 5.4 KCl, 1.8 CaCl2, 1.8 MgCl2, 0.6 NaH2PO4, 7.5 HEPES, 7.5 Na+-HEPES, and 5 glucose, pH 7.4.
2.10 Statistics
All results are expressed as means ± SEM or S.D. as described in the figures. Comparison among groups was performed using two-tailed equal variance Student’s t-test. For analysis of a parameter (e.g., maximal contraction amplitude) as functions of group (e.g., wild-type vs. MHC-ODC + ISO) and [Ca2+]o, two-way ANOVA was used to determine statistical significance. For analysis of protein abundance, Cm and action potential parameters, Student’s unpaired t-test was used. A commercial software package (JMP version 4.05, SAS Institute, Cary, NC) was used. In all analyses, p<0.05 was taken to be statistically significant.
Results
3.1 Morphological and biochemical analysis of MHC-ODC mice and controls
MHC-ODC mice and littermate controls were injected with ISO daily for 10 days, and cardiac hypertrophy was evaluated using echocardiography. ISO-injected transgenic mice were characterized by significantly increased PWT and IVST compared to controls given ISO, and LV dimensions were smaller in ISO-treated versus saline-injected MHC-ODC mice, as shown by a decrease in LVESD (Table I). On the other hand, ISO injection produced no significant changes in wild-type mice (Table I). An increase in interstitial collagen in the hearts of MHC-ODC mice was also observed after treatment with ISO, suggesting that overexpression ODC leads to increased fibrosis in response to ISO (data not shown).
Table I.
Echocardiographic measurements in hearts of MHC-ODC (TG) non-transgenic (NTG) mice.
Mice were treated for 8 days with vehicle (S) or 20 mg/kg ISO, sacrificed and echocardiography was performed as described in the Material and Methods section.
| Mouse(n) | LV mass (mg) |
IVST (mm) |
LVEDD (mm) |
LVESD (mm) |
PWT (mm) |
EF (%) |
SF (%) |
|---|---|---|---|---|---|---|---|
| NTG-S(4) | 117.6 ± 11.0 | 0.88 ± 0.08 | 3.92 ± 0.29 | 1.95 ± 0.29 | 0.77 ± 0.08 | 85 ± 7 | 51 ±14 |
| TG-S(8) | 156.4 ± 14.9 | 0.88 ± 0.07 | 4.10 ± 0.18 | 2.36 ± 0.18 | 1.03 ± 0.07d | 79 ± 8 | 43 ± 7 |
| NTG-ISO(7) | 140.3 ± 14.2 | 0.86 ± 0.03 | 4.08 ± 0.18 | 2.15 ± 0.21 | 0.89 ± 0.04 | 85 ± 9 | 48 ±9 |
| TG-ISO (5) | 179.0 ± 22.5a | 1.22 ± 0.13a,b,c | 3.56 ± 0.18 | 1.62 ± 0.31b | 1.18 ± 0.05e,f | 88 ± 9 | 55 ±15 |
Values are mean ± S.E.M. n = number of mice; IVST, interventricular septum thickness; LVEDD, end-diastolic LV dimensions; LVESD, end-systolic LV dimensions; PWT, posterior wall thickness; EF, ejection fraction; SF, shortening fraction.
P<0.05 vs. NTG-S;
P<0.05 vs. TG-S;
P<0.05 vs. NTG-ISO;
P<0.01 vs. NTG-S;
P<0.005 vs. NTG-ISO;
P<0.005 vs. NTG-S
Because arginase has been implicated in modulation of polyamine levels through L-Arg availability (Li et al., 2001), we examined the activity of arginase in the hearts of MHC-ODC mice and their littermate controls. Arginase activity was below the limit of detection (0.1 nmol/15min/mg protein) in the hearts of both transgenic and control mice. However, when cardiac hypertrophy was induced using our standard protocol of daily ISO injections (20 mg/kg, i.p.) for 10 days, arginase activity in MHC-ODC mice was induced to 37.5 ± 7.2 (n=4) nmol/15min/mg protein. Activity in littermate controls was still below the limit of detection.
3.2 Development of a lethal phenotype
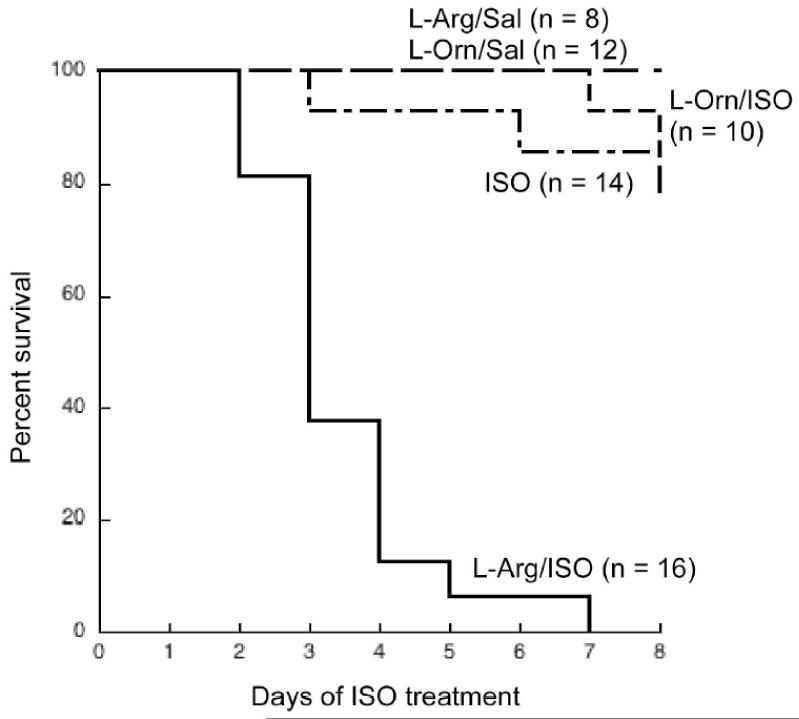
MHC-ODC hearts contain high levels of cadaverine, which is formed when ODC decarboxylates lysine (Shantz et al., 2001). L-Lys is a poor substrate for ODC, with a Km approximately 100-fold higher than that of L-Orn. This suggests that all of the available L-Orn is converted to putrescine, leaving L-Lys as an alternative substrate for ODC. Thus, the induction in arginase activity in transgenic hearts in response to ISO could reflect an attempt to increase L-Orn levels. To determine if this is the case, MHC-ODC mice and littermate controls were given 5% L-Arg in the drinking water, with or without ISO injections, and changes in polyamines as well as the development of hypertrophy were monitored. While the difference in LV mass between water-drinking, saline-injected MHC-ODC mice and their littermates did not reach statistical significance in echocardiographic experiments (Table I), measurements of H/B ratios showed MHC-ODC mice exhibit a slight hypertrophy compared to non-transgenic controls (Table II). Consistent with the echocardiographic data, injection of ISO for 10 days exaggerated the hypertrophy in transgenic mice (Table II). When L-Arg was added to the drinking water of saline-injected mice, H/B ratios were unaffected in both groups (Table II), and polyamine levels were also unaffected (data not shown). Surprisingly however, oral L-Arg administration to ODC-overexpressing mice at the time of ISO injection was lethal, while having no additional effect on control mice (Table II). This lethal effect appeared within a very short time period, beginning within 48h after the start of ISO injections. The survival curve shows that 90% of MHC-ODC mice were dead on Day 4 of ISO treatment, and all mice were dead by Day 6 (Fig. 1). Two lines of evidence suggest the effect was specific for L-Arg. First, MHC-ODC mice treated with L-Orn and ISO showed no increase in toxicity over mice receiving ISO alone (Fig. 1), suggesting that the L-Arg/ISO toxicity is not due to metabolism of L-Arg to L-Orn and the polyamines. Second, when L-glutamine was substituted for L-Arg, H/B ratios were not significantly different from water-drinking mice (data not shown).
Table II.
Effect of L-Arg/ISO treatment on nontransgenic and MHC-ODC mice.
Animals were given water with or without 5% L-Arg for 3 days, then given daily injections of saline or ISO in saline (20 mg/kg) for 10 days. Mice were sacrificed and H/B ratios determined as described in the Material and Methods section.
| Genotype (n) | Treatment | Heart weight (mg)/Body weight (g) after 10 days (mean ± SD) |
|---|---|---|
| NTG (3) | water/saline | 5.2 ± 0.4 |
| TG (5) | water/saline | 6.2 ± 0.2a |
| NTG (3) | L-Arg/saline | 5.4 ± 0.4 |
| TG (5) | L-Arg/saline | 6.4 ± 0.7 |
| NTG (3) | water/ISO | 6.0 ± 0.1 |
| TG (5) | water/ISO | 8.5 ± 0.2b |
| NTG (3) | L-Arg/ISO | 6.0 ± 0.3 |
| TG (5) | L-Arg/ISO | DEAD* |
All MHC-ODC mice treated with ISO/Arg died prior to day 10 of ISO treatment. n = number of mice;
P < 0.04 vs. NTG water/saline;
P < 0.0003 vs. NTG water/ISO.
Fig. 1.
L-Arg/ISO treatment is lethal in transgenic mice.
Survival curve of transgenic mice. Mice were given either L-Arg or L-Orn (5%) in the drinking water and injected i.p. with either ISO or saline as described in the Materials and Methods. N = number of mice in each group on Day 0. Statistical analysis of curves using log rank test showed that L-Arg/ISO survival curve is significantly different compared to all other curves (p < 0.0001).
3.3 Echocardiographic analysis of mice treated with L-Arg/ISO
We performed echocardiography on transgenic mice and control littermates, either untreated or treated for 3 days with L-Arg and 48 h with ISO. Nontransgenic and transgenic mice drinking either water or 5% L-Arg were given two injections of ISO 24 h apart and sacrificed 6h after the second injection. Both control and MHC-ODC mice treated with ISO alone for 48 h maintained a normal LV function, as measured by SF, EF and CO (Fig. 2). Unlike the 10 day ISO experiment, ISO produced no additional hypertrophy in any of the groups examined following this short-term exposure (data not shown). Administration of L-Arg alone also had no effect on control or transgenic mice (data not shown).
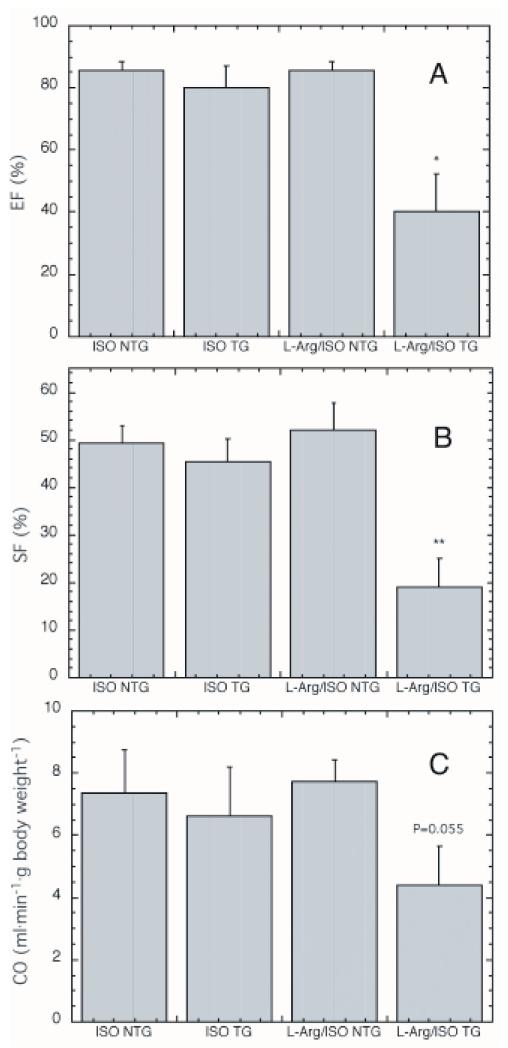
Fig. 2.
Measurement of cardiac function in mice treated with ISO alone or L-Arg/ISO.
Ejection fraction (A.), shortening fraction (B.) and cardiac output (C.) were measured in mice drinking either water or 5% L-Arg and treated for two days with ISO. ISO NTG (n = 5), ISO TG (n = 5) and L-Arg/ISO NTG (n = 7) all show normal heart function. L-Arg/ISO TG (n = 7) showed significantly impaired systolic function, as measured by EF and SF. * statistically significant compared to NTG + L-Arg/ISO, P < 0.01; ** statistically significant compared to NTG + L-Arg/ISO, P < 0.002.
Even after the very short exposure to L-Arg/ISO in this experiment, mean LV systolic function was significantly lower in MHC-ODC mice compared to control littermates, as measured by a depression in EF and SF (Fig. 2a, b). Mean CO was also lower in transgenic mice (Fig. 2c). All measurements in L-Arg/ISO-treated nontransgenic littermates were comparable to measurements in mice treated with ISO alone (Fig. 2).
3.4 Changes in cardiac arginine, ornithine and polyamines in response to L-Arg/ISO
When comparing water-drinking, saline-injected mice the cellular content of L-Arg and L-Orn are higher in nontransgenic mice than in their MHC-ODC counterparts. Surprisingly, despite the large amount of cadaverine present in transgenic hearts, L-Orn was still measurable (Table III). ISO administration for 48 h increased L-Arg and decreased L-Orn in both transgenic and control animals, consistent with increased arginine transport and induction of ODC activity by ISO. Administration of L-Arg, with or without ISO injection, increased the L-Arg content at least 15-fold in both transgenics and controls, confirming L-Arg transport, and L-Orn was also increased. Putrescine was increased by a greater fraction in transgenics than in controls (Table III). In contrast, spermidine and spermine were unchanged in mice of either genotype (data not shown), suggesting that the exogenously administered L-Arg is not metabolized to the higher polyamines. While L-Orn and putrescine were increased in all mice drinking L-Orn, L-Arg was increased only in transgenics (Table III). The reason for this increase in L-Arg is likely due to increased L-Arg transport. L-Orn administration increases the already very high putrescine content an additional 4-fold in MHC-ODC hearts. Arginine and ornithine share the same transport system, but arginine exhibits a lower Km and higher Vmax (White et al., 1982). Thus, it would not be surprising if both arginine and ornithine transport increased as ornithine is rapidly converted to putrescine in transgenic hearts. Again, even though putrescine was increased in transgenic mice in response to L-Orn, spermidine and spermine were unchanged (data not shown).
Table III.
Cardiac arginine, ornithine and putrescine levels.
Animals were treated with either 5% L-Arg or 5% L-Orn in the drinking water for 3 days, then given two injections of saline or ISO in saline (20 mg/kg) 24 h apart. Mice were sacrificed and arginine, ornithine and polyamine levels were measured as described in the Material and Methods section.
| Genotype | Treatment (n) | amino acid nmol/g wet weight ± SEM |
Putrescine nmol/mg protein ± SEM |
|
|---|---|---|---|---|
| arginine | ornithine | |||
| NTG | water/saline (9) | 250 ± 61 | 102 ± 20 | 0.23 ± 0.10 |
| water/ISO (6) | 677 ± 173a | 43 ± 4a | 0.30 ± 0.10 | |
| L-Arg/saline (5) | 4707 ± 306b | 237 ± 35b | 0.04 ± 0.003 | |
| L-Arg/ISO (5 | 6830 ± 324b | 315 ± 9b | 0.20 ± 0.05 | |
| L-Orn/saline (3) | 270 ± 46 | 252 ± 11b | 0.33 ± 0.07 | |
| TG | water/saline (8) | 91 ± 5.3c | 48 ± 15c | 2.17 ± 0.33d |
| water/ISO (4) | 217 ± 29c,e | 22 ± 7c | 3.36 ± 0.68d | |
| L-Arg/saline (5) | 1429 ± 180c,g | 277 ± 31g | 3.45 ± 0.54d,e | |
| L-Arg/ISO (8) | 1098 ± 174c,g | 74 ± 9g | 5.17 ± 1.26d,e | |
| L-Orn/saline (5) | 582 ± 127c,e | 74 ± 19c | 7.86 ± 1.24d,f | |
n = number of mice;
P < 0.05 vs. NTG water/saline;
P < 0.0005 vs. NTG water/saline;
P < 0.05 vs. identically-treated NTG;
P < 0.0001 vs. identically-treated NTG;
P < 0.05 vs. TG water/saline;
P < 0.01 vs. TG water/saline;
P < 0.001 vs. TG water/saline.
3.5 Inhibition of arginase or NOS
To investigate whether arginase inhibition would have a similar effect as L-Arg consumption in mice injected with ISO, N(omega)-nor-hydroxy-L-arginine (Nor-NOHA), an arginine analog that is an inhibitor of arginase activity but neither a substrate nor an inhibitor of NOS (Tenu et al., 1999), was used. Nor-NOHA treatment (50 mg/kg, i.p. daily for 8 days) in the absence of ISO had no effect on either control or transgenic mice (data not shown). When Nor-NOHA treatment was combined with ISO, MHC-ODC mice died almost as rapidly as mice treated with L-Arg/ISO (Fig. 3), while control mice were unaffected (data not shown). Amino acid analysis determined that the ratio of L-Arg to L-Orn increased from 3.0± 2.7 to 8.0 ± 2.8 in Nor-NOHA-treated mice, indicating that arginase was effectively inhibited.
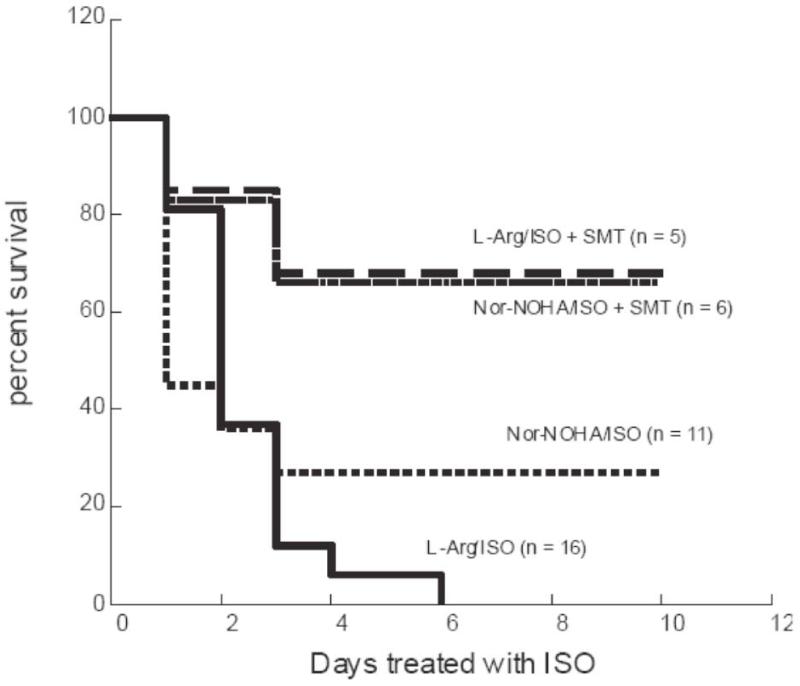
Fig. 3.
Treatment of MHC-ODC mice with inhibitors of arginase or NOS
MHC-ODC mice were treated with daily ISO injections (20 mg/kg i.p.) for 10 days. Mice also received one of the following: 5% L-Arg in the drinking water from three days prior to ISO injections until the end of the experiment; 50 mg/kg (i.p.) Nor-NOHA daily from 3 days prior to ISO treatment until the end of the experiment; L-Arg/ISO plus 10 mg/kg (i.p.) SMT daily from one day prior to ISO treatment until the end of the experiment; Nor-NOHA/ISO plus 10 mg/kg (i.p.) SMT daily from one day prior to ISO treatment until the end of the experiment. N = number of mice in each group on Day 0. Statistical analysis of curves using log rank test showed that L-Arg/ISO survival curve is significantly different compared to L-Arg/ISO + SMT and Nor-NOHA/ISO + SMT curves (p < 0.03). Nor-NOHA/ISO curve is not different from L-Arg/ISO survival curve.
The Nor-NOHA results suggest that induction of arginase in MHC-ODC hearts may be an effort to reduce NO synthesis by controlling L-Arg levels. Inhibition of NOS activity in MHC-ODC hearts may therefore provide a similar protective effect to L-Arg/ISO-treated mice. We examined the ability of S-methyl-isothiourea (SMT), which is reported to be a specific inhibitor of iNOS at the concentration used (Southan et al., 1996), to attenuate the pathological response of MHC-ODC mice to L-Arg/ISO. In mice drinking L-Arg, SMT (10 mg/kg) was injected simultaneously with ISO according to our standard protocol. The results indicate a clear protective effect of iNOS inhibition, with 70% of mice surviving for more than 8 days (Fig. 3). All mice receiving L-Arg/ISO alone were dead by 6 days. SMT also increased survival of Nor-NOHA/ISO treated mice (Fig. 3), and decreased H/B ratio in MHC-ODC mice treated with ISO alone for 8 days (data not shown). These experiments strongly support the concept that when polyamine levels are perturbed by ODC overexpression, L-Arg is shuttled away from polyamine synthesis and toward NO synthesis.
3.6 Effects of L-Arg/ISO treatment on contraction in ODC overexpressing myocytes
Our in vivo echocardiographic measurements indicate significantly reduced cardiac performance in transgenic hearts treated with L-Arg and ISO. To distinguish whether the decreased cardiac output is due to reduction in myocyte number or a defect in myocyte contractility, we examined contraction parameters in individual myocytes isolated from wild-type animals, with or without L-Arg/ISO treatment, and from MHC-ODC mice, after treatment with ISO alone or with L-Arg/ISO (3 days of L-Arg in the drinking water followed by a single i.p. injection of 20mg/kg ISO with sacrifice 2h later). In all groups, raising [Ca2+]o significantly increased maximal contraction amplitude (p<0.0001), as expected (Table IV). In wild-type myocytes, L-Arg/ISO treatment had no significant effects on maximal contraction amplitudes (p<0.75), maximal shortening (p<0.50) and relengthening (p<0.25) velocities (Table IV). Likewise, ISO treatment alone in MHC-ODC myocytes did not affect maximal contraction amplitudes (p<0.75), maximal shortening (p<0.40) and relengthening (p<0.10) velocities, when compared to wild-type myocytes. On the other hand, treatment of MHC-ODC myocytes with L-Arg/ISO resulted in significant depression in maximal contraction amplitudes (p<0.04), maximal shortening (p<0.003) and relengthening (p<0.001) velocities across the entire [Ca2+]o range, when compared to wild type myocytes receiving the same treatment (Table IV and Supplemental Fig. 1).
Table IV.
Effects of ODC overexpression and L-Arg (A)/ISO (I) treatment on myocyte shortening.
Mice were given 5% L-Arg in the drinking water for 3 days followed by a single i.p. injection of 20mg/kg ISO with sacrifice 2h later. Contraction parameters were measured as described in the Material and Methods section.
| [Ca2+]o | Wild-type | Wild-type+A/I | ODC+I | ODC+A/I |
|---|---|---|---|---|
| Maximal contraction amplitude, % resting cell length | ||||
| 0.6 | 2.93 ± 0.25 (25) | 2.71 ± 0.35 (13) | 2.55 ± 0.23 (12) | 2.02 ± 0.17* (13) |
| 1.8 | 6.39 ± 0.36 (26) | 6.24 ± 0.29 (13) | 6.27 ± 0.47 (15) | 5.54 ± 0.45* (22) |
| 5.0 | 10.09 ± 0.45 (32) | 10.07± 0.55 (15) | 10.04± 0.70 (20) | 8.46 ± 0.60* (25) |
| Maximal shortening velocity, cell length/s | ||||
| 0.6 | 0.56 ± 0.04 | 0.53 ± 0.08 | 0.52 ± 0.04 | 0.35 ± 0.04* |
| 1.8 | 1.12 ± 0.06 | 1.13 ± 0.05 | 1.16 ± 0.07 | 0.91 ± 0.08* |
| 5.0 | 1.57 ± 0.07 | 1.71 ± 0.11 | 1.71 ± 0.13 | 1.36 ± 0.10* |
| Maximal relengthening velocity, cell/length/s | ||||
| 0.6 | 0.41 ± 0.03 | 0.41 ± 0.07 | 0.41 ± 0.05 | 0.28 ± 0.03* |
| 1.8 | 0.96 ± 0.06 | 0.97 ± 0.04 | 1.04 ± 0.10 | 0.72 ± 0.08* |
| 5.0 | 1.23 ± 0.07 | 1.42 ± 0.11 | 1.44 ± 0.10 | 1.05 ± 0.09 |
Values are means ± SE. Nos. in parentheses are nos. of myocytes, without regard to the no. of cells contributed by each heart (n=3, 2, 3 and 4 hearts for wild-type, wild-type+A/I, ODC+I and ODC+A/I, respectively). [Ca2+]o, extracellular Ca2+ concentration; A/I, arginine + isoproterenol treatment; ODC, ornithine decarboxylase overexpression; I, isoproterenol treatment only.
p < 0.05, ODC+A/I vs. wild-type, or wild-type+A/I, or ODC+I.
3.7 Effects of L-Arg/ISO treatment on [Ca2+]i transients in MHC-ODC myocytes
The differences in contractility between wild-type and MHC-ODC myocytes may be due to changes in [Ca2+]i homeostasis. Indeed, compared with wild-type mice treated with L-Arg/ISO, myocytes from MHC-ODC mice had significantly decreased systolic [Ca2+]i values (Table V and Supplemental Fig. 2). This conclusion is supported by two-way ANOVA revealing highly significant group (p<0.0001) and group × [Ca2+]o interaction (p< 0.0085) effects, indicating that raising [Ca2+]o amplifies the differences in systolic [Ca2+]i between the two groups of myocytes. There were no significant differences in diastolic [Ca2+]i values between L-Arg/ISO treated wild-type and MHC-ODC myocytes (group effect, p<0.12; group × [Ca2+]o interaction effect, p<0.50). The percent increase in Fura-2 fluorescence intensity ratio is an accurate reflection of [Ca2+]i transient amplitude. Compared with wild-type myocytes post L-Arg/ISO treatment, ODC myocytes that had undergone the same treatment protocol had significantly (p<0.0001) decreased [Ca2+]i transient amplitudes at all three [Ca2+]o examined (Table V). In addition, the t1/2 of [Ca2+]i transient decline, an indicator of sarcoplasmic reticulum Ca2+ uptake activity (Zhang et al., 1999), was significantly (p<0.0065) prolonged in L-Arg/ISO treated MHC-ODC myocytes (Table V).
Table V.
Effects of ODC overexpression and L-Arg (A)/ISO (I) treatment on [Ca2+]i transients
Mice were treated as described in Table IV and changes in calcium homeostasis were examined as described in the Material and Methods section.
| [Ca2+]o | Wild-type+A/I |
ODC+A/I |
|---|---|---|
| Systolic [Ca2+]i, nM | ||
| 0.6 | 218 ± 18 (20) | 183 ± 15* (14) |
| 1.8 | 279 ± 13 (31) | 227 ± 15* (17) |
| 5.0 | 434 ± 18 (30) | 317 ± 13* (22) |
| Diastolic [Ca2+]i, nM | ||
| 0.6 | 116 ± 11 | 115 ± 9 |
| 1.8 | 137 ± 8 | 123 ± 12 |
| 5.0 | 146 ± 6 | 129 ± 8 |
| Increase in fluorescence intensity ratio, % | ||
| 0.6 | 17.6 ± 1.0 | 12.6 ± 1.2* |
| 1.8 | 22.1 ± 0.8 | 18.3 ± 1.0* |
| 5.0 | 38.1 ± 1.4 | 30.5 ± 1.3* |
| t1/2 of [Ca2+]i transient decline, ms | ||
| 0.6 | 168 ± 17 | 189 ± 14* |
| 1.8 | 146 ± 7 | 172 ± 10* |
| 5.0 | 133 ± 6 | 154 ± 10* |
Values are means ± SE. Nos. in parentheses are nos. of myocytes, without regard to the no. of cells contributed by each heart (n=5 and 4 hearts for wild-type+A/I and ODC+A/I, respectively). [Ca2+]o, extracellular Ca2+ concentration; [Ca2+]i, cytosolic Ca2+ concentration; A/I, arginine + isoproterenol treatment; ODC, ornithine decarboxylase overexpression.
p < 0.006, ODC+A/I vs. wild-type+A/I.
3.8 Effects ofL-Arg/ISO treatment on action potential in MHC-ODC myocytes
Another mechanism to account for excessive mortality in MHC-ODC mice after L-Arg/ISO treatment is enhanced arrhythmogenesis. We therefore measured action potential parameters in wild-type and MHC-ODC myocytes after in vivo treatment with L-Arg/ISO as described above. Resting membrane potential (p<0.81), action potential amplitude (p<0.10), APD50 (p<0.58) and Cm (p<0.13) were similar between the 2 groups of myocytes (Table VI). In L-Arg/ISO treated ODC myocytes, however, action potential duration was significantly (p<0.02) abbreviated when compared with wild-type myocytes that had undergone the same treatment, as indicated by the shortened APD90 values (Table VI and Supplemental Fig. 3). These results are consistent with an increased possibility of arrhythmias in MHC-ODC mice.
Table VI.
Effects of ODC overexpression and L-Arg (A)/ISO (I) treatment on action potential parameters
Mice were treated as described in Table IV and action potential parameters were measured as described in the Material and Methods section.
| Wild-type+A/I |
ODC+A/I | |
|---|---|---|
| Resting membrane potential, mV | −69.2 ± 1.9 (11) | −68.4 ± 2.5 |
| Action potential amplitude, mV | 100.5 ± 3.9 | 111.4 ± 4.8 |
| APD50, ms | 3.85 ± 0.21 | 3.55 ± 0.49 |
| APD90, ms | 35.19 ± 4.09 | 22.13 ± 3.17* |
| Cm, pF | 151.3 ± 2.9 (6) | 159.7 ± 4.1 (6) |
Values are means ± SE. APD50 and APD90, action potential duration at 50 and 90% repolarization, respectively. Cells were paced at 1 Hz.
p < 0.02, ODC+A/I vs. wild-type+A/I.
Discussion
The increase in cardiac mass in transgenic mice in response to ISO was characterized by increases in IVST and PWT, and the LVESD was decreased in ISO-treated MHC-ODC mice. These changes are consistent with concentric hypertrophy (Yang et al., 1999) and our previous report (Shantz et al., 2001). Despite LV hypertrophy, significant changes in SF and EF were not detected in transgenic mice. This observation suggests that LV function was well compensated in MHC-ODC mice in the absence of additional stress. Chronic (10 day) ISO administration induced a striking elevation in the activity of transgenic cardiac arginase. No measurable increase in arginase activity was observed in control animals given ISO. This suggests that the high levels of ODC in the transgenic hearts influence the induction of arginase by β-adrenergic stimulation.
The large increase in cardiac ODC activity in the MHC-ODC mice might be expected to lead to a reduction in the L-Orn content of the heart. Although arginase was dramatically increased in the hearts of MHC-ODC mice treated with ISO, surprisingly there was no further increase in the synthesis of polyamines and cadaverine was not reduced. These results suggest the intracellular compartmentation of the L-Orn pools. Our data indicating that L-Orn can be detected at reduced but measurable levels in the hearts of MHC-ODC mice also support this concept. ODC is extra-mitochondrial (Schipper et al., 2004), whereas L-Orn may be located in the mitochondria. Arginase exists as two isoforms, with arginase I targeted to the cytoplasm and arginase II to the mitochondria, and the arginase assay does not differentiate between these forms (reviewed in Wu et al., 1998). Further study is needed to investigate whether L-Orn generated at a mitochondrial site can become available to ODC.
The left ventricular hypertrophy in MHC-ODC mice treated with ISO progresses to dysfunction upon the addition of L-Arg to the drinking water. Echocardiography measured cardiac decompensation, indicated by decreased SF and CO, as little as 48 h after beginning L-Arg/ISO treatment. The obvious explanation for the mechanism of L-Arg toxicity in these studies is that it is converted into L-Orn and that this provides the substrate for additional polyamine synthesis by the high level of transgenic ODC. However, our studies do not support this hypothesis. There was not a significant increase in cardiac spermidine or spermine in response to L-Arg/ISO, and administration of L-Orn instead of L-Arg does not have the same toxic effect in MHC-ODC mice. In addition, L-Arg accumulated to 4-fold higher levels in nontransgenic mice drinking L-Arg, with or without ISO treatment, suggesting an alternate utilization of L-Arg in transgenic mice. These results suggest that the effect of supplying exogenous L-Arg in combination with ISO includes the activation of other pathways, such as those leading to NO synthesis.
It is possible that the induction of arginase seen with long-term treatment of MHC-ODC mice with ISO alone may be a protective response attempting to limit the availability of L-Arg for NO synthesis. Our results with the arginase inhibitor Nor-NOHA, which in combination with ISO was almost as effective as L-Arg in inducing death, support this hypothesis. The likelihood that NO may contribute to the lethal phenotype of L-Arg/ISO-treated mice is also supported by the finding that inhibition of iNOS with SMT protects MHC-ODC mice from the toxic effects of both L-Arg/ISO and Nor-NOHA/ISO. SMT administration was also shown to prevent cardiac dysfunction in a rat myocardial infarction model (Saito et al., 2002).
At the cellular level, myocytes isolated from ODC overexpressing mice treated with L-Arg/ISO exhibited reduced contractility at both low and high [Ca2+]o, when compared to wild-type myocytes. The acute cellular dysfunction was not due to L-Arg/ISO treatment in itself because myocytes from wild-type mice receiving this treatment showed no contractile abnormality. In addition, treatment of MHC-ODC mice with ISO alone did not result in myocyte contractile dysfunction. Thus our results indicate that treatment with both L-Arg and ISO in the setting of ODC overexpression is required to induce rapid-onset cellular contractile failure.
The major feature of contractile dysfunction in myocytes from MHC-ODC mice treated with L-Arg and ISO is a reduction in the contraction amplitude, as well as in the shortening and relengthening velocities, at both low and high [Ca2+]o. Although there are many potential aberrations in the excitation-contraction pathway that can result in this phenotype, alterations in Na+/Ca2+ exchanger activities are unlikely to account for the observed contractile failure. This is because reduction in Na+/Ca2+ exchange activity will result in higher contractile amplitude at 0.6 mM [Ca2+]o and lower contractile amplitude at 5.0 mM [Ca2+]o (Tadros et al., 2002), while increased Na+/Ca2+ exchange activity results in lower contractile amplitude at 0.6 mM [Ca2+]o and higher contractile amplitude at 5.0 mM [Ca2+]o (Zhang et al., 2001a). This phenotype was not observed in MHC-ODC myocytes treated with L-Arg/ISO. Rather, the pattern of contractile dysfunction is consistent with decreased Ca2+ entry via L-type Ca2+ channels, reduced Ca2+ influx due to abbreviated action potential duration, increased Ca2+ efflux secondary to enhanced Na+-K+-ATPase activity, decreased SR Ca2+ content as a consequence of reduced SERCA2 activity and/or increased SR Ca2+ leak (hyperphosphorylation of ryanodine receptor), and changes in Ca2+ sensitivity of the myofilaments.
Reduced contractility in ODC overexpressing myocytes was associated with altered [Ca2+]i transients. Specifically, both systolic [Ca2+]i and amplitude of [Ca2+i transient were lower in L-Arg/ISO treated MHC-ODC myocytes, when compared to wild-type myocytes from mice receiving an identical treatment. This observation suggests that alteration in [Ca2+]i homeostasis, rather than changes in Ca2+ sensitivity of the contractile apparatus, is a major factor accounting for the observed decreased contractility. In addition, SR Ca2+ uptake activity, as reflected by the t1/2 of [Ca2+]i transient decline (Zhang et al., 1999), was significantly slower in myocytes from ODC overexpressing mice treated with L-Arg/ISO. Thus, reductions in SERCA2 amounts/activity likely contribute to the suppression of contractility in ODC overexpressed myocytes treated with L-Arg and ISO. This conclusion is supported by our previous observation that SERCA2 overexpression in rat myocytes resulted in enhanced contractility and [Ca2+]i transient amplitudes at both low and high [Ca2+]o (Ahlers et al, 2005). It is important to note that SERCA2 protein levels were similar between wild-type and ODC overexpressed myocytes treated with L-Arg/ISO (data not shown), suggesting that regulation of SERCA2 activity, rather than its expression/degradation, is affected.
In addition to reductions in contractility and altered [Ca2+]i transients in MHC-ODC myocytes, another major finding was the reduction of action potential duration. Shortened action potential duration not only limits the duration of Ca2+ influx, thereby affecting myocyte [Ca2+]i homeostasis and contractility, but also contributes to arrhythmogenesis and sudden death.
The experiments described here show the rapid onset of severe cardiac dysfunction triggered by a defined procedure within a few days, unlike other published models of heart failure (Du et al., 2000; Engelhardt et al., 1999; Molkentin, 1998). Therefore, our model is more useful for studying possible procedures for acute intervention. Furthermore, although the ODC levels in the hearts of MHC-ODC mice are substantially increased when assayed in vitro, it is likely that the increase in cardiac polyamines is limited by the availability of L-Orn and decarboxylated S-adenosylmethionine, because S-adenosylmethionine decarboxylase may become rate limiting in these mice (Ikeguchi et al., 2004; Nisenberg et al., 2006). This suggests that much smaller alterations in the activities of these enzymes, particularly in response to inducing stimuli, may influence cardiac polyamines and myocyte contractility.
In summary, we describe a lethal dysfunction in mice with cardiac-specific expression of ODC. This lethal phenotype is induced only by L-Arg/ISO administration and is not accompanied by further alterations in the levels of cardiac polyamines, suggesting alternative L-Arg utilization pathways are involved, leading to dysregulation of intracellular calcium. It is likely that there is considerable variation in human cardiac polyamine and L-Arg levels, given that known polymorphisms in the ODC gene can affect its transcription (Martinez et al., 2003). Consequently, the possibility that high levels of these factors contribute to cardiac hypertrophy and dysfunction should be considered.
Supplementary Material
Supplemental Fig. 1. Contractile abnormalities in myocytes isolated from MHC-ODC mice ertated with L-Arg/ISO. Myocytes were paced to contract at 37°C and [Ca2+]o of 0.6, 1.8 and 5.0 mM. Results are summarized in Table 4.
Supplemental Fig. 2. L-Arg/ISO alters cytosolic Ca2+ concentration ([Ca2+]i) transients in MHC-ODC myocytes. Myocytes isolated from either wild-type or MHC-ODC mice treated with L-Arg/ISO were loaded with the Ca2+ indicator fura-2 and then paced (1 Hz) to contact at 37°C and [Ca2+]o of 0.6, 1.8 or 5.0 mM. Results are summarized in Table 5.
Supplemental Fig. 3. Action potential duration is abbreviated in MHC-ODC myocytes from L-Arg/ISO-treated mice. Action potentials were measured at 30°C and 1.8 mM [Ca2+]o in wild-type (A) and MHC-ODC (B) myocytes isolated from mice treated with L-Arg/ISO. Results are summarized in Table 6.
Acknowledgements
We want to remember here the late Dr. Thomas C. Vary. His unexpected passing deprives us of friend. EG acknowledges the Italian National Institute for Cardiovascular Research (INRC) and the Pennsylvania State University, each providing partial resources for his visit to the Hershey Medical Center, and is recipient of funding from the University of Bologna (RFO) and MIUR (PRIN).
Supported by grant 004014N from the American Heart Association (LMS), and NIH grants CA18138 (AEP), HL58672 (JYC), HL74854 (JYC) and AA12814 (TCV). The echocardiographic studies were supported by a Commonwealth of Pennsylvania Tobacco Settlement Award (TCV) and a Penn State University Dean’s Feasibility grant (AEP, LMS).
We are grateful to Suzanne Sass-Kuhn Patricia Welsh and Gina Deiter for technical assistance.
Abbreviations
- DFMO
α-difluoromethylornithine
- ISO
isoproterenol
- MHC
myosin heavy chain
- ODC
Ornithine decarboxylase
References
- Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song J, Wang J, Geddis LM, Tucker AL, Mounsey JP, Cheung JY. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem. 2005;280:19875–19882. doi: 10.1074/jbc.M414703200. [DOI] [PubMed] [Google Scholar]
- Balligand J-L. Regulation of cardiac -adrenergic response by nitric oxide. Cardiovascular Res. 1999;43:607–620. doi: 10.1016/s0008-6363(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez EG, Huang PL, Lima JAC, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–340. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- Bauer PM, Buga GM, Fukuto JM, Pegg AE, Ignarro LJ. Nitric oxide inhibits ornithine decarboxylase via S-nitrosylation of cysteine 360 in the active site of the enzyme. J Biol Chem. 2001;276:34458–34464. doi: 10.1074/jbc.M105219200. [DOI] [PubMed] [Google Scholar]
- Blachier F, Robert V, Selamnia M, Mayeur C, Duee PH. Sodium nitroprusside inhibits proliferation and putrescine synthesis in human colon carcinoma cells. FEBS Lett. 1996;396:315–318. doi: 10.1016/0014-5793(96)01122-2. [DOI] [PubMed] [Google Scholar]
- Cubria JC, Reguera R, Balana-Fouce R, Ordonez C, Ordonez D. Polyamine-mediated heart hypertrophy induced by clenbuterol in the mouse. J Pharm Pharmacol. 1998;50:91–96. doi: 10.1111/j.2042-7158.1998.tb03310.x. [DOI] [PubMed] [Google Scholar]
- Du X-J, Gao X-M, Wang B, Jennings GL, Woodcock EA, Dart AM. Age dependent cardiomyopathy and heart failure phenotype in mice overexpressing 2-adrenergic receptors in the heart. Cardiovasc Res. 2000;48:448–454. doi: 10.1016/s0008-6363(00)00187-5. [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in 1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano E, Shantz LM, Caldarera CM, Pegg AE. L-Arginine at the crossroads of biochemical pathways involved in myocardial hypertrophy. In: Dhalla NS, Takeda N, Kardami E, Singal PK, editors. Signal Transduction and Cardiac Hypertrophy. Kluwer Academic Publishers; Boston: 2003. [Google Scholar]
- Hu J, Mahmoud MI, El-Fakahany EE. Polyamines inhibit nitric oxide synthase in rat cerebellum. Neurosci Lett. 1994;175:41–45. doi: 10.1016/0304-3940(94)91073-1. [DOI] [PubMed] [Google Scholar]
- Ikeguchi Y, Wang X, McCloskey DE, Coleman CS, Nelson P, Hu G, Shantz LM, Pegg AE. Characterization of transgenic mice with widespread overexpression of spermine synthase. Biochem J. 2004;381:701–707. doi: 10.1042/BJ20040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricle mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Li H, Meininger CJ, Hawker JR, Jr., Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr., Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab. 2002;283:E824–E835. doi: 10.1152/ajpendo.00085.2002. [DOI] [PubMed] [Google Scholar]
- Martinez ME, O’Brien TG, Fultz KE, Babbar N, Yerushalmi H, Qu N, Guo Y, Boorman D, Einspahr J, Alberts DS, Gerner EW. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci USA. 2003;100:7859–7864. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenberg O, Pegg AE, Welsh PA, Keefer K, Shantz LM. Overproduction of cardiac S-adenosylmethionine decarboxylase in transgenic mice. Biochem J. 2006;393:295–302. doi: 10.1042/BJ20051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M, Kawashima S, Yamashita T, Hirase T, Ohashi Y, Inoue N, Hirata K, Yokoyama M. Overexpression of endothelial nitric oxide synthase attenuates cardiac hypertrophy induced by chronic isoproterenol infusion. Circ J. 2002;66:851–856. doi: 10.1253/circj.66.851. [DOI] [PubMed] [Google Scholar]
- Pegg AE. Effect of -difluoromethylornithine on cardiac polyamine content and hypertrophy. J Mol Cell Cardiol. 1981;13:881–887. doi: 10.1016/0022-2828(81)90287-x. [DOI] [PubMed] [Google Scholar]
- Pignatti C, Tantini B, Stefanelli C, Giordano E, Bonavita F, Clo’ C, Caldarera CM. Nitric oxide mediates either proliferation or cell death in cardiomyocytes. Involvement of polyamines. Amino Acids. 1999;16:181–190. doi: 10.1007/BF01321535. [DOI] [PubMed] [Google Scholar]
- Saito T, Hu F, Tayara L, Fahas L, Shennib H, Giaid A. Inhibition of NOS II prrevents cardiac dysfunction in myocardial infarction and congestive heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H339–H345. doi: 10.1152/ajpheart.00596.2001. [DOI] [PubMed] [Google Scholar]
- Schipper RG, Cuijpers VM, De Groot LH, Thio M, Verhofstad AA. Intracellular localization of ornithine decarboxylase and its regulatory protein, antizyme-1. J Histochem Cytochem. 2004;52:1259–1266. doi: 10.1177/002215540405201002. [DOI] [PubMed] [Google Scholar]
- Shantz LM, Holm I, Jänne OA, Pegg AE. Regulation of S-adenosylmethionine decarboxylase activity by alterations in the intracellular polyamine content. Biochem J. 1992;288:511–518. doi: 10.1042/bj2880511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantz LM, Feith DJ, Pegg AE. Targeted overexpression of ornithine decarboxylase enhances - adrenergic agonist-induced cardiac hypertrophy. Biochem J. 2001;358:25–32. doi: 10.1042/0264-6021:3580025. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantz LM, Giordano E. Polyamine metabolism and the hypertrophic heart. In: Wang J-Y, Casero RA Jr., editors. Polyamine cell signaling. Humana Press; Totowa: 2006. pp. 123–137. [Google Scholar]
- Shantz LM, Levin VA. Regulation of ornithine decarboxylase during oncogenic transformation: mechanisms and therapeutic potential. Amino Acids. 2007;33:213–223. doi: 10.1007/s00726-007-0531-2. [DOI] [PubMed] [Google Scholar]
- Simko F, Simko J. The potential role of nitric oxide in the hypertrophic growth of the left ventricle. Physiol Res. 2000;49:37–46. [PubMed] [Google Scholar]
- Song J, Zhang XQ, Wang J, Cheskis E, Chan TO, Feldman AM, Tucker AL, Cheung JY. Regulation of cardiac myocyte contractility by phospholemman:Na+/Ca2+ exchange vs. Na+-K+-ATPase. Am J Physiol Heart Circ Physiol. 2008;295:H1615–1625. doi: 10.1152/ajpheart.00287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Tadros GM, Zhang XQ, Song J, Carl LL, Rothblum LI, Tian Q, Dunn J, Lytton J, Cheung JY. Effects of Na(+)/Ca(2+) exchanger downregulation on contractility and [Ca(2+)](i) transients in adult rat myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H1616–H1626. doi: 10.1152/ajpheart.00186.2002. [DOI] [PubMed] [Google Scholar]
- Tenu JP, Lepoivre M, Moali C, Brollo M, Mansuy D, Boucher JL. Effects of the new arginase inhibitor N(omega)-hydroxy-nor-L-arginine on NO synthase activity in murine macrophages. Nitric Oxide. 1999;3:427–438. doi: 10.1006/niox.1999.0255. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Friberg P, Adams MA. Vasodilators inhibit acute alpha 1-adrenergic receptor-induced trophic responses in the vasculature. Hypertension. 1992;20:809–815. doi: 10.1161/01.hyp.20.6.809. [DOI] [PubMed] [Google Scholar]
- Tipnis UR, He GY, Campbell G, Boor PJ. Attenuation of isoproterenol mediated myocardial injury in rat by an inhibitor of polyamine synthesis. Cardiovasc Pathol. 2000;9:273–280. doi: 10.1016/s1054-8807(00)00038-7. [DOI] [PubMed] [Google Scholar]
- Tucker AL, Song J, Zhang XQ, Wang J, Ahlers BA, Carl LL, Mounsey JP, Moorman JR, Rothblum LI, Cheung JY. Altered contractility and [Ca2+]I homeostasis in phospholemman-deficient murine myocytes: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol. 2006;291:H2199–H2209. doi: 10.1152/ajpheart.01181.2005. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF, Christensen HN. The two-way flux of cationic amino acids across the plasma membrane of mammalian cells is largely explained by a single transport system. J Biol Chem. 1982;257:10069–10080. [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-P, Liu Y-H, Rhaleb N-E, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Ng YC, Moore RL, Musch TI, Cheung JY. In situ SR function in postinfarction myocytes. J Appl Physiol. 1999;87:2143–2150. doi: 10.1152/jappl.1999.87.6.2143. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Song J, Rothblum LI, Lun M, Wang X, Ding F, Dunn J, Lytton J, McDermott PJ, Cheung JY. Overexpression of Na+/Ca2+ exchanger alters contractility and SR Ca2+ content in adult rat myocytes. Am J Physiol Heart Circ Physiol. 2001;281:H2079–2088. doi: 10.1152/ajpheart.2001.281.5.H2079. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Zhang LQ, Palmer BM, Ng YC, Musch TI, Moore RL, Cheung JY. Sprint training shortens prolonged action potential duration in postinfarction rat myocyte: mechanisms. J Appl Physiol. 2001;90:1720–1728. doi: 10.1152/jappl.2001.90.5.1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Contractile abnormalities in myocytes isolated from MHC-ODC mice ertated with L-Arg/ISO. Myocytes were paced to contract at 37°C and [Ca2+]o of 0.6, 1.8 and 5.0 mM. Results are summarized in Table 4.
Supplemental Fig. 2. L-Arg/ISO alters cytosolic Ca2+ concentration ([Ca2+]i) transients in MHC-ODC myocytes. Myocytes isolated from either wild-type or MHC-ODC mice treated with L-Arg/ISO were loaded with the Ca2+ indicator fura-2 and then paced (1 Hz) to contact at 37°C and [Ca2+]o of 0.6, 1.8 or 5.0 mM. Results are summarized in Table 5.
Supplemental Fig. 3. Action potential duration is abbreviated in MHC-ODC myocytes from L-Arg/ISO-treated mice. Action potentials were measured at 30°C and 1.8 mM [Ca2+]o in wild-type (A) and MHC-ODC (B) myocytes isolated from mice treated with L-Arg/ISO. Results are summarized in Table 6.