Abstract
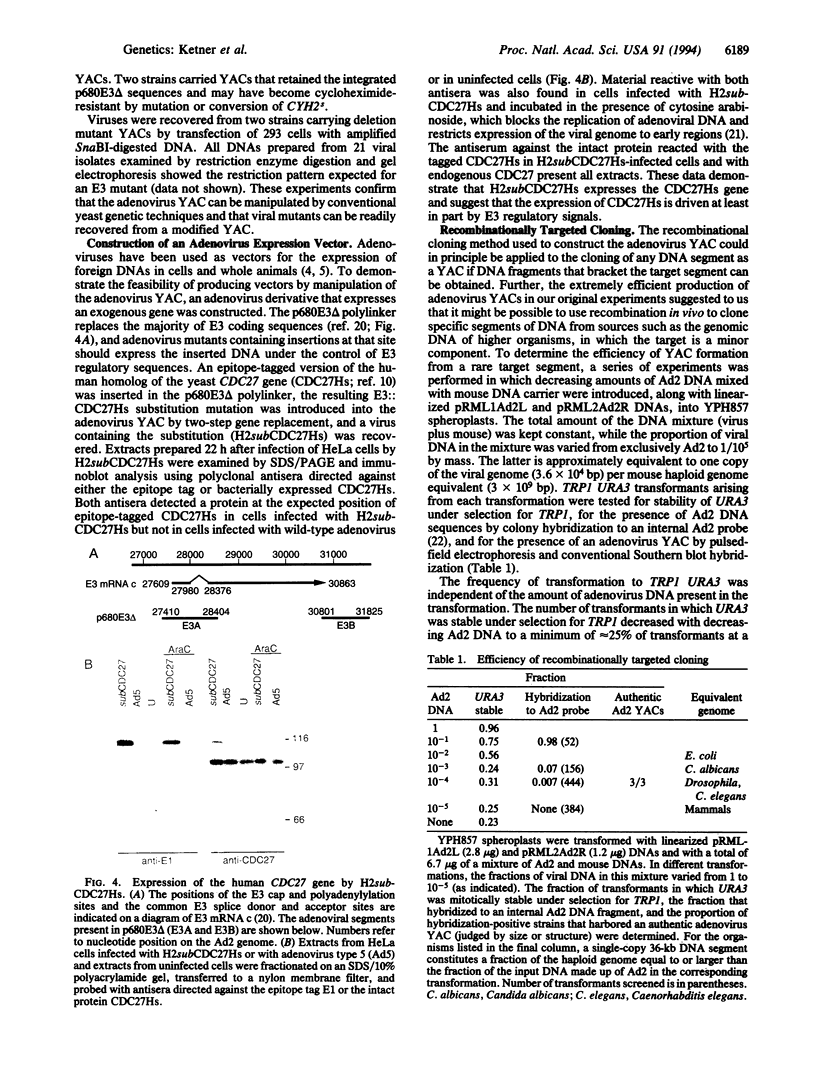
A yeast artificial chromosome (YAC) containing a complete human adenovirus type 2 genome was constructed, and viral DNA derived from the YAC was shown to be infectious upon introduction into mammalian cells. The adenovirus YAC could be manipulated efficiently using homologous recombination-based methods in the yeast host, and mutant viruses, including a variant that expresses the human analog of the Saccharomyces cerevisiae CDC27 gene, were readily recovered from modified derivatives of the YAC. The application of powerful yeast genetic techniques to an infectious adenovirus clone promises to significantly enhance the genetic analysis of adenovirus and to simplify the construction of adenovirus-based vectors for vaccines or for gene transfer to mammalian cells or whole animals. The adenovirus YAC was produced by homologous recombination in vivo between adenovirus 2 virion DNA and YAC vector plasmids carrying segments of the viral left and right genomic termini. This recombinational cloning strategy is generally applicable to the construction of YACs containing other DNA segments, such as the genomes of other viruses. Further, it is very efficient and may permit the targeted cloning of segments of the genomes of higher organisms directly from genomic DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Mentone S. A., Rose J. K., Farquhar M. G. The E1 glycoprotein of an avian coronavirus is targeted to the cis Golgi complex. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6944–6948. doi: 10.1073/pnas.87.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevec L., Campbell J. B., Christie B. S., Belbeck L., Graham F. L. A recombinant human adenovirus vaccine against rabies. J Infect Dis. 1990 Jan;161(1):27–30. doi: 10.1093/infdis/161.1.27. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Smyth A. P., Moir D. T. Amplification of large artificial chromosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8242–8246. doi: 10.1073/pnas.87.21.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Tugendreich S., Boguski M. S., Seldin M. S., Hieter P. Linking yeast genetics to mammalian genomes: identification and mapping of the human homolog of CDC27 via the expressed sequence tag (EST) data base. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10031–10035. doi: 10.1073/pnas.90.21.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. alpha-Aminoadipate as a primary nitrogen source for Saccharomyces cerevisiae mutants. J Bacteriol. 1985 May;162(2):579–583. doi: 10.1128/jb.162.2.579-583.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]







