Abstract
Angiogenic remodeling during embryonic development and in adult tissue homeostasis is orchestrated by cooperative signaling between several distinct molecular pathways, which are often exploited by tumors. Indeed, tumors upregulate pro-angiogenic molecules while simultaneously suppressing angiostatic pathways in order to recruit blood vessels for growth, survival, and metastatic spread. Understanding how cancers exploit pro- and anti-angiogenic signals is a key step in developing new, molecularly targeted anti-angiogenic therapies. While EphA2, a receptor tyrosine kinase (RTK), is required for vascular endothelial growth factor (VEGF)-induced angiogenesis, the mechanism through which these pathways intersect remains unclear. Slit2 expression is elevated in EphA2-deficient endothelium, and here it is reported that inhibiting Slit activity rescues VEGF-induced angiogenesis in cell culture and in vivo, as well as VEGF-dependent tumor angiogenesis, in EphA2-deficient endothelial cells and animals. Moreover, blocking Slit activity or Slit2 expression in EphA2-deficient endothelial cells restores VEGF-induced activation of Src and Rac, both of which are required for VEGF-mediated angiogenesis. These data suggest that EphA2 suppression of Slit2 expression and Slit angiostatic activity enables VEGF-induced angiogenesis in vitro and in vivo, providing a plausible mechanism for impaired endothelial responses to VEGF in the absence of EphA2 function.
Keywords: EphA2, Slit2, VEGF, endothelium, tumor angiogenesis
Introduction
Angiogenic remodeling, which generates new vessel sprouts from pre-existing vessels, is essential for proper embryonic development, normal tissue homeostasis, and contributes to the pathogenesis and progression of cancer. Proper vessel formation requires a balance between angiogenic stimuli, which regulate endothelial cell invasion and migration, proliferation, and tubulogenesis, and angiostatic factors that terminate or inhibit these processes upon vessel maturation to promote vascular stability (Reviewed in [1]). Vascular endothelial growth factor (VEGF), the best-characterized pro-angiogenic factor, is a key regulator of physiologic angiogenesis and tumor neovascularization (Reviewed in [2, 3]). In addition to VEGF, the Eph family of receptor tyrosine kinases (RTKs) and their cell surface membrane-bound ephrin ligands also regulate physiologic and pathologic angiogenesis. Specifically, EphA2 and its primary ligand, ephrin-A1, have become the targets of intensive investigation due to their functions in tumorigenesis and neovascularization (Reviewed in [4–7]).
Though VEGF regulates endothelial cell activation, proliferation, migration, and morphogenesis, this factor does not act in isolation. Indeed, coordinated signaling between VEGF and a plethora of other factors, such as Notch, transforming growth factor β (TGF-β), angiopoietins, platelet derived growth factors (PDGF), and ephrins/Eph RTKs, is essential for normal physiologic angiogenesis (Reviewed in [8, 9]). Previous studies from our laboratory and others demonstrated that the VEGF pathway also cooperates with ephrin/Eph signaling to regulate angiogenesis. Specifically, soluble EphA receptors, EphA2-deficiency, or antibodies targeting EphA2 impair VEGF-induced angiogenesis, as well as angiogenic responses induced by ephrins [10–15]. The mechanism through which blocking EphA2 function interferes with VEGF-mediated angiogenesis remains unclear.
Members of the Slit/roundabout (Robo) gene family also regulate vascular remodeling and homeostasis (Reviewed in [16]). The three Slit proteins (Slit1-3) identified in vertebrates interact with receptors of the Robo family (Robo1-4), Robo1 and Robo4 being most highly expressed in endothelial cells [17]. The role of Slit proteins in regulation of angiogenesis remains controversial, however, with reported pro- [18–22] and anti-angiogenic activities [23–27]. Recent investigations clearly demonstrated that Slit2 inhibits VEGF-induced vascular remodeling [23–26, 28].
In a previous study, we reported elevated slit2 mRNA expression in EphA2 -deficient endothelial cells relative to wild-type controls, and determined that Slit functioned as an inhibitory angiocrine factor. Inhibition of Slit function in conditioned media harvested from EphA2-deficient endothelium alleviated repression of mammary tumor cell growth and motility in culture and in vivo [29], consistent with the chemorepulsive, growth inhibitory, and tumor suppressive function of Slit2 in mammary epithelium and breast cancer [30–37]. These data suggest that elevated Slit2 expression in EphA2-deficient endothelium contributes to reduced tumor growth in EphA2-deficient mice.
We previously reported that the pro-angiogenic effects of ephrin-A1 were suppressed in the presence of Slit2 [38], suggesting cross-talk between EphA receptor signaling and the Slit-Robo pathway may also regulate angiogenesis. Because Slit2 expression is significantly elevated in EphA2-deficient endothelium, we hypothesized that overexpression of this angiostatic factor could account for impaired VEGF-induced angiogenesis in the absence of EphA2. To test this hypothesis, we blocked Slit activity in EphA2-deficient endothelium using soluble Robo1-Fc receptor as a ligand trap. Inhibiting Slit function in EphA2-deficient endothelium rescued VEGF-induced endothelial cell assembly and migration in culture, as well as subcutaneous vessel remodeling in vivo. Stable knockdown of Slit2 in EphA2-deficient endothelium rescued VEGF-mediated assembly and migration as well, whereas EphA2 overexpression reduced Slit2 expression. Lastly, inhibiting Slit function rescued VEGF-dependent tumor angiogenesis in vivo, and restored VEGF-induced activation of Src and Rac, both of which are required for VEGF-mediated angiogenesis. Thus, elevated Slit2 in the absence of EphA2 appears to be one mechanism that renders endothelium resistant to VEGF-induced vascular remodeling.
Materials and Methods
Ethics Statement
All animals were housed under pathogen-free conditions, and experiments were performed in accordance with AAALAC guidelines and with Vanderbilt University Institutional Animal Care and Use Committee approval. The laboratory animal care program of Vanderbilt University (PHS Assurance #A3227-01) has been accredited by AAALAC International since 1967 (File #000020). The AAALAC Council on Accreditation’s most recent review of VU’s program was done in 2011 and resulted in “Continued Full Accreditation.” EphA2-deficient Balb/C congenics were generated, genotyped, and maintained as described previously [39].
Reagents
Antibodies against the following proteins were used: Src, phospho-Src family (Tyr416; Cell Signaling Technology, Boston, MA); Rac (BD Biosciences, San Jose, CA); EphA2 (SC-924), Robo4 (SC-67057), phosphotyrosine (PY99, SC-7020; PY20, SC-508) and actin (SC-1616; Santa Cruz Biotechnology, Santa Cruz, CA); von Willebrand factor (vWF; Zymed Laboratories, South San Francisco, CA); tubulin (Sigma Aldrich, St. Louise, MO). Pak-PBD agarose Rac assay reagent was purchased from Millipore (Billerica, MA). Recombinant mouse VEGF 164, rat Robo1-Fc, human IgG, and recombinant Slit2 were purchased from R&D Systems (Minneapolis, MN). Gelfoam absorbable gelatin sponges (Pharmacia) were obtained from the Vanderbilt University Hospital Pharmacy. TRITC-dextran, FITC-dextran, and 4′,6-diamidino-2 phenylindole dihydrochloride (DAPI) were purchased from Sigma-Aldrich. Growth factor-reduced Matrigel was purchased from BD Biosciences. Arf6 activity assay kits were purchased from Cytoskeleton, Inc. (Denver, CO). Calbiochem SecinH3 Arf inhibitor was purchased from Millipore. Transwells were obtained from Corning, Inc. (Corning, NY). Mouse Slit2 ELISA kit was purchased from Novatein Biosciences (Woburn, MA). 4T1 tumor cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Gibco DMEM Media (Life Technologies, Carlsbad, CA) supplemented with penicillin-streptomycin (Cellgro/Mediatech) and 10% fetal bovine serum (Hyclone, Logan, UT). Adenoviruses harboring wild-type EphA2, kinase dead W42 mutant EphA2 [40], and control LacZ were generated as described previously [41].
Endothelial Cell Culture
Immortalized murine pulmonary microvascular endothelial cells (MPMEC) were isolated from three month old wild-type or EphA2-deficient H-2KB-tsA58 transgenic “Immorto-mice” [42, 43] as described previously [44]. Cells were maintained in EGM-2 medium (Lonza, Walkersville, MD) supplemented with penicillin-streptomycin and 10% fetal bovine serum. Cells were maintained at 33 °C in EGM-2 medium supplemented with interferon-γ (10 ng/mL; Millipore), a permissive condition that allows the expression of the temperature sensitive SV40 T-antigen (Tag) transgene. The cells were incubated at 37 °C for at least 3 days in the absence of interferon-γ to downregulate TAg expression and revert the cells to a non-immortalized state prior to experimental manipulation. Human primary retinal microvascular endothelial cells (HRMEC) were purchased from Cell Systems (Kirkland, WA) and maintained in EGM-2 medium as described above.
Quantitative Real Time PCR and ELISA
To generate conditioned medium (CM) from tumor cells and endothelial cells, 1×106cells were plated in 10 cm dishes and grown to approximately 75% confluence in normal growth medium, then incubated in 3 mL of serum-free Opti-MEM medium for 48-hours. CM was collected and filtered in (0.2 mm syringe filters, VWR International, Radnor, PA) prior to use as described previously [29].
For Real Time PCR analyses, total RNA from triplicate sets of endothelial cells was isolated using Trizol (Invitrogen) as per the manufacturer’s protocol. For some experiments, endothelial cells were incubated with CM from 4T1 tumor cells for 24-hours prior to RNA isolation. Expression of murine slit2 or robo1-4 mRNA in endothelial cells was validated by qRT-PCR analysis as described previously [29], using the following primers: Slit2 Fwd (20mer) 5′-agg gaa gat gag tgg cat tg-3′ (240>259; NM_178804.2); Slit2 Rev (20mer) 5′-gtg cct gag acc agc aaa at-3′ (486>467; NM_178804.2), and control 18S ribosomal RNA primers: Fwd (20mer) 5′-caa ctt tcg atg gta gtc gc-3′; Rev (21mer) 5′-cgc tat tgg agc tgg aat tac-3′. Primers for murine Robo1, 2, and 4 and endogenous control were purchased from Taqman (Mm00437762_m1 for B2m control; Mm00803879_m1 for Robo1; Mm00620713_m1 for Robo2; Mm00452963_m1 for Robo4). Expression of human slit2 mRNA in HRMEC and gapdh control was scored using the TaqMan Gene Expression Assay (Life technologies): SLIT2 - Hs00191193_m1, GAPDH – Hs02758991_m1. Real Time PCR was performed using a StepOnePlus Real-Time PCR System from Applied Biosciences (Foster City, CA) with iQ SYBR supermix from BioRad. We used a two-step amplification procedure (40 cycles of 95 C, 15 sec 60 C, 30 sec followed by melting temperature determination stage) and quantified relative changes in gene expression using the DDCt method as per manufacturer’s instructions.
Slit2 protein expression in undiluted endothelial CM was quantified by ELISA as per manufacturer’s protocol. Plates were read using a BioTek Synergy HT (Winooski, VT) plate reader and associated software and data exported to Microsoft Excel for quantification and statistical analyses.
Stable shRNA-mediated Slit2 and Robo1 knockdown in endothelial cells
pGIPZ based shRNA vectors to knockdown mouse Slit2 and Robo1 were purchased from Open Biosystems (Slit2 V2LMM_92930, V3LMM_471050; Robo1 V2LMM_195374, V2LMM_83507; Thermo Fisher Scientific, Pittsburgh, PA), and the viruses were produced in 293T cells for infection with Cell Biolabs 2nd generation lentivirus packaging system (San Diego, CA) as per supplier’s instructions. Infected EphA2-deficient MPMEC were selected in 2 μg/mL puromycin and pooled clones tested in assembly and migration assays as described below. We confirmed diminished Slit2 protein expression by ELISA analysis of CM from knockdown clones versus vector control, and diminished expression of Robo1 mRNA by Real-Time qRT-PCR, as described above.
Transient siRNA-mediated EphA2 knockdown in human endothelial cells
Human EphA2-targeting and control siRNAs were purchased from and transfected into HRMEC. EPHA2 ON-TARGETplus Human SMARTpool siRNA (L-003116-00-0005) and ON-TARGETplus Non-Targeting pool siRNA (D-001810-10-05) (Dharmacon/Thermo Scientific) were used at a concentration of 12.5 nM in conjunction with Lipofectamine RNAiMAX transfection reagent (Invitrogen) according to the manufacturer’s protocol as described previously [45]. Assembly assays were performed 48 hours post-transfection. Knockdown was confirmed by immunoblot analysis as described below.
In Vitro Angiogenesis Assays
In vitro vascular assembly assays were performed as described previously [41, 44]. Briefly, 12-wellplates were coated with 100 μL of growth factor reduced Matrigel (BD Biosciences). After 24 hour starvation in Opti-MEM, 25,000 MPMEC or HRMEC were plated in wells in the presence or absence of VEGF (50 ng/mL) plus or minus Slit2 (100 ng/mL), Robo1-Fc (1 μg/mL) or control IgG (1 μg/mL) and photographed after 24-hours. For some studies, assays were performed in the presence of SecinH3 Arf inhibitor (5 μM) or DMSO vehicle control. Images were acquired using an Olympus CK40 inverted microscope through an Optronics DEI-750C CCD video camera using CellSens capture software. Adjustments were applied to the entire image using Adobe Photoshop (CS6) software and were consistent between experimental and control images. The degree of assembly was quantified by measuring branch length, the distance from branching point to the tip of assembled cells. The branch length in assembled endothelial cell networks was expressed as arbitrary units per 10X field in four random fields from each well, with triplicate samples per condition, using Scion Image version 1.62c software. For some experiments, endothelial cells were transduced with recombinant adenoviruses (108 pfu/mL) or transfected with siRNAs 48 hours prior to assembly assay.
For migration assays, endothelial cells were serum-starved for 24-hours in Opti-MEM medium. Transwells were coated with growth factor reduced Matrigel (1:20 dilution with Opti-MEM) for 30 minutes and blocked with 1% bovine serum albumin solution for an additional 30 minutes. One hundred thousand cells were plated in the upper chamber of the transwells, and 600 μL of Opti-MEM medium containing VEGF (50 ng/mL) plus or minus Slit2 (100 ng/mL), Robo1-Fc (1 μg/mL) or control IgG was added to the lower chamber. After 5-hours, cells were fixed and stained with crystal violet to visualize endothelial cells. Cells that migrated to the lower surface of transwell filters were counted in four random fields from each well, with triplicate samples per condition as described previously [41, 44].
In Vivo Sponge Assays for Angiogenesis
Sponge assays for angiogenesis were performed as described previously [41, 46]. Briefly, gel foam sponges were cut into small pieces (2.5 to 3 mm wide by 5 mm long) and soaked with 100 μL of phosphate-buffered saline containing 100 ng of VEGFplus or minus Slit2 (100 ng), Robo1-Fc (2.5 μg) or control IgG. The sponges were then implanted into the subcutaneous dorsal flank of three-month-old female Balb/c wild-type or EphA2-deficient recipient female mice. Each recipient received one pro-angiogenic factor impregnated sponge and one relevant control factor impregnated sponge implanted in the opposite flank. After 7 days, the mice were injected with a 2% tetramethyl rhodamine isothiocyanate (TRITC)-dextran-phosphate-buffered saline solution or 2% fluorescein isothiocyanate (FITC)-dextran-phosphate-buffered saline solution to label host blood vessels [41, 46], and the sponges were collected and analyzed. Whole-mount images were acquired on an Olympus CK40 inverted microscope through an Optronics DEI-750C charge-coupled-device video camera using CellSens capture software. Density of blood vessels within the sponges was quantified by fluorescence intensity (10X magnification) of TRITC-dextran or FITC-dextran using Scion Image software, version 1.62c. Data are a representation of results from five independent sponges under each condition. Statistical significance was determined by a two-tailed, paired Student’s t test. Vessel identity was confirmed in paraffin sections prepared from sponges and counterstained with DAPI and/or co-stained with the endothelial cell marker von Willebrand Factor (vWF) as described previously [39, 47, 48].
Tumor-Endothelial Cell Co-culture Migration Assays
For co-culture experiments, transwells were coated with growth factor-reduced Matrigel (1:20 dilution) and 1×105 4T1-GFP cells were plated on the lower surface of the transwell filter. Wild-type or EphA2-deficient endothelial cells (1×105) labeled with CellTracker Orange CMTMR dye (Molecular Probes/Life Technologies)were added to upper transwell chambers in the presence or absence of Slit2 (100 ng/mL), Robo1-Fc (1 μg/mL), or control IgG (100 ng/mL to 1 μg/mL). After 5 h, cells were removed from the upper surface of the transwell filter using a cotton swab, and endothelial cells on the lower surface of the filter quantified. Similar experiments were performed comparing EphA2-deficient endothelial cells expressing control versus Slit2 shRNAs. Data are a representation of six to nine independent samples per condition with standard deviation, and statistical significance was assessed by two-tailed, paired Student’s t test.
Cell Line Statement
4T1-GFP cells generated in the laboratory of Mark Dewhirst (Duke University) were obtained from Dr. Charles Lin, where they were authenticated for tumor formation Balb/C mice in vivo [49], and were expanded and frozen upon receipt. Vials used in this study were passaged in our laboratory for fewer than six months after resuscitation.
Cutaneous Window Chamber Assay
Window assays were performed as described previously [50, 51]. Briefly, a 5 mm diameter flap of skin was dissected away from the dorsal skin flap of anesthetized recipient wild-type or EphA2-deficient three month old Balb/c female mice, leaving a fascial plane with associated vasculature. A gelfoam sponge (approximately 1 mm in diameter)impregnated with 1 μg of Slit2, Robo1-Fc, or control IgG in 50% Matrigel/PBS was implanted in the window chamber adjacent to a portion of 4T1 tumor (approximately 0.7 mm in diameter) isolated from a donor mouse. The chambers were sealed with glass coverslips and photographed on 1 day following implantation to measure initial tumor size and baseline vascular morphology. 7 days after implantation, FITC-conjugated dextran (2% in PBS, Sigma-Aldrich) was injected intravenously, and tumors in window chambers were photodocumented using an Olympus BX60 microscope and digital camera. Branches from host blood vessels within the window chambers were enumerated in at least three independent fields per mouse, and statistical significance was determined by two-tailed, paired Student’s t-test. Data are a representation of 6–8 independent samples per condition with standard error of the mean, and statistical significance was assessed by two-tailed, paired Student’s t-test.
Orthotopic Tumor Transplantation
4T1 tumor cells (1×105) were resuspended in growth-factor reduced Matrigel plus or minus IgG (1 μg), Slit2 (100 ng), or Robo1-Fc (1 μg) and orthotopically transplanted in the mammary glands of recipient wild-type (IgG or Slit2) or EphA2-deficient (IgG or Robo1-Fc) three month old Balb/c female mice as described previously [39]. Tumors were harvested after 7 days, measured by digital caliper, and volume was calculated [length × width2 × 0.52]. Tumor sections were stained for the endothelial marker vWF factor and microvascular density quantified based on pixel density as described previously [39, 47, 48].
Immunoblot Analyses
Endothelial cells were serum-starved for 24-hours in Opti-MEM + 2% FCS. For EphA2-deficient cells, 1 μg/mL Robo1-Fc or control IgG was added to the starvation medium. Rac activation in approximately 500 μg endothelial cell lysate was assessed by Pak-PBD agarose Rac assay reagent as described previously [41, 44]. Arf6 activation was assessed by GGA3-PBD effector pulldown assay as per supplier’s protocol (Cytoskeleton, Inc.). For some assays, cells were pre-treated with SecinH3 Arf inhibitor (5 μM) or DMSO vehicle for one hour prior to stimulation. For analysis of Src phosphorylation and expression, approximately 50 μg of endothelial cell lysates were collected and processed as per antibody supplier’s protocol (Cell Signaling Technologies). For all experiments, cells were stimulated with VEGF (50 ng/mL) plus or minus Slit2 (100 ng/mL), Robo1-Fc (1 μg/mL) or control IgG for 5 (Rac, Arf6) to 10 (Src) minutes, or for 16 hours with Robo1-Fc (1 μg/mL) to score rescue of basal Rac activity. The blots were stripped and re-probed with anti-actin or tubulin antibodies to confirm uniform loading and anti-EphA2 antibodies to confirm EphA2-deficiency in knockout cell lines. Data are a representation of three to five independent experiments.
Results
Slit2 expression is elevated in EphA2-deficient endothelial cells and affects signaling downstream of VEGF
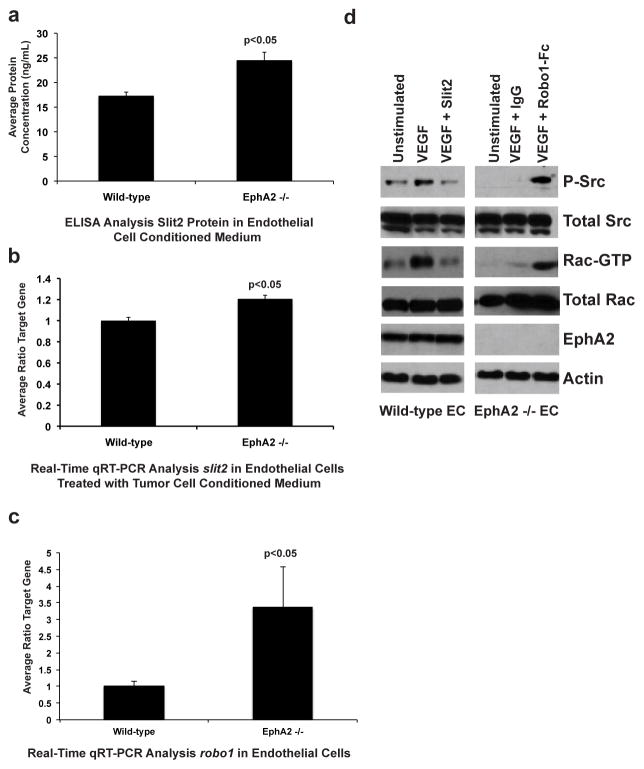
Recent studies demonstrated that slit2 mRNA was significantly increased in EphA2 deficient endothelium relative to wild-type controls [29]. Immunofluorescence staining suggested that protein levels were also higher in knockout cells, though these data were more qualitative. In this study, we quantified protein expression in endothelial cell conditioned medium by ELISA, which revealed significantly higher levels of Slit2 in EphA2-deficient endothelial cells relative to wild-type controls (Fig. 1a), consistent with our previous microarray and Real Time PCR studies [29].
Fig. 1. Slit2 expression is elevated in EphA2-deficient endothelial cells and affects signaling downstream of VEGF.
(a) Endothelial cells were grown in EGM-2 for 24-hours. Growth medium was replaced with 3 mL of serum-free media and cells incubated for 48-hours to generate conditioned medium. Conditioned medium was harvested and protein expression of secreted murine Slit2 quantified by ELISA. Levels of secreted Slit2 were significantly higher in EphA2-deficient endothelial cells relative to wild-type endothelial cells. (b) Endothelial cells were grown in EGM-2 for 24-hours. Growth medium was replaced with 3 mL of conditioned medium from 4T1 mouse mammary tumor cells and cells incubated for 48-hours. RNA was harvested and subjected to Real-Time PCR to quantify slit2 mRNA expression. Expression of slit2 mRNA was significantly elevated in EphA2-deficient endothelial cells relative to wild-type endothelial cells. (c) Real-Time PCR analysis confirmed significantly elevated levels of robo1 mRNA in EphA2-deficient endothelial cells relative to wild-type controls. (d) Slit2 inhibited VEGF-induced activation of the intracellular serine/threonine kinase Src, as measured by phosphorylation, as well as activation of Rac-GTPase, as measured by detection of GTP-bound (active) Rac, in wild-type endothelial cells. Addition of soluble Robo1-Fc receptor, a Slit ligand trap, partially rescued VEGF-induced Src and Rac activity in EphA2-deficient endothelial cells. Data are a representation of three independent wild-type versus three independent EphA2-deficient immortalized endothelial cell lines/genotype from two independent experiments, with average +/− standard deviation. Statistical significance was assessed by two-tailed, paired Student’s t-test.
Our previous studies compared Slit2 expression in normal endothelial cells harvested from wild-type and EphA2-deficient animals and cultured in endothelial cell growth medium. To determine if overexpression persisted in knockout cells in the context of tumor angiogenesis, we repeated our analyses in endothelial cells treated with conditioned medium from 4T1 mouse mammary tumor cells. Treatment with tumor conditioned medium reduced levels of Slit2 relative to what we previously observed in normal endothelial cells, as expected based on the reported tumor suppressive role for Slit2[ 29]. Still, elevated expression of slit2 persisted in EphA2 -deficient endothelial cells relative to wild-type control endothelial cells upon treatment with tumor conditioned medium (Fig. 1b). These data suggest that Slit2 overexpression in the absence of EphA2 may affect both normal physiologic and tumor angiogenesis. While we did not detect high expression levels of robo2 or robo4 in our lung microvascular endothelial cell lines (data not shown), robo1 levels were significantly elevated in EphA2-null endothelial cells relative to wild-type cells (Fig. 1c).
We next assessed activation of signaling pathways downstream of Slit-Robo in wild-type versus EphA2-deficient endothelium. Previous studies reported that Slit2 co-stimulation impairs VEGF-induced activation of Src and Rac, two major downstream signaling mediators through which VEGF stimulates angiogenic remodeling [24, 26]. As EphA2-deficient endothelial cells are resistant to VEGF-induced angiogenesis [10–15], we compared Src phosphorylation and levels of active, GTP-bound Rac in wild-type cells treated with VEGF plus exogenous Slit2 to levels in EphA2-deficient endothelial cells treated with VEGF in the presence of Robo1-Fc, a ligand trap for available Slit proteins. We confirmed inhibition of Src and Rac activities in wild -type endothelial cells stimulated with VEGF in the presence of recombinant Slit2 (Fig. 1d). VEGF-induced activation of Src and Rac were markedly reduced in EphA2-deficient cells, consistent with elevated expression of Slit2 in these cells. Pre-treatment with Robo1-Fc rescued activation of Src and Rac in response to VEGF in EphA2-deficient endothelial cells (Fig. 1d). These data suggest that elevated Slit2 levels in EphA2-deficient endothelium may promote resistance to VEGF-mediated angiogenesis through its angiostatic function.
Inhibiting Slit activity rescues VEGF-induced vascular assembly and migration in EphA2-deficient endothelial cells
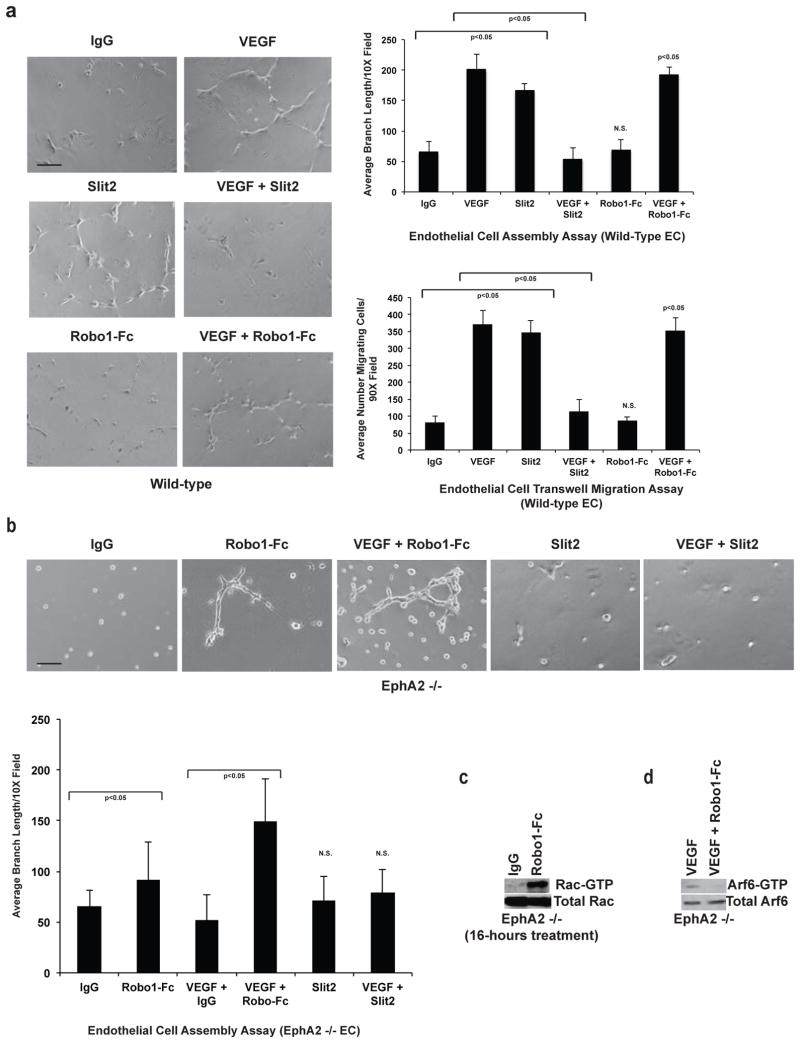
EphA2-deficiency not only impairs angiogenic remodeling in response to ephrins, but also in response to VEGF (Fig. 2; [10–15]). As Slit2 has also been reported to inhibit angiogenesis induced by VEGF [23–26], we hypothesized that Slit2 overexpression in EphA2-deficient endothelium might account for this defect. To test this hypothesis, we scored endothelial assembly on Matrigel and migration through transwells, comparing wild-type cells treated with VEGF to EphA2-deficient cells treated with VEGF in the presence or absence ofRobo1 -Fc ligand trap. Wild-type endothelial cells assembled into interconnected structures resembling a primitive capillary plexus when plated on a thin layer of Matrigel in the presence of VEGF (Fig. 2a). While VEGF stimulation in the presence of control IgG failed to induce a robust assembly response in EphA2-deficient cells, pre-treatment with soluble Robo1-Fc partially rescued assembly (Fig. 2a). Robo1-Fc treatment also partially rescued VEGF-induced migration of EphA2-deficient endothelial cells (Fig. 2b).
Fig. 2. Inhibiting Slit activity rescues VEGF-induced vascular assembly and migration in EphA2-deficient endothelial cells.
(a) Endothelial cells were plated on a thin layer of growth factor-reduced Matrigel to score assembly into interconnected vascular networks in response to VEGF. EphA2-deficiency impaired VEGF-induced assembly relative to wild-type controls. Addition of soluble Robo1-Fc receptor partially rescued VEGF-induced assembly of EphA2-deficient endothelial cells relative to IgG control plus VEGF. Scale bar = 20 μm. (b) We observed a similar trend in endothelial cell motility as scored by transwell assay. Relative to wild-type endothelial cells, EphA2-deficienct cells displayed impaired migration in response to VEGF, though addition of Robo1-Fc partially rescued VEGF-induced migration. (c) Co-stimulation with VEGF and Slit2 impairs endothelial cell assembly relative to VEGF alone. We observed that co-stimulation induced activation of Arf6-GTPase, as measured by detection of GTP-bound (active) Arf6, in wild-type endothelial cells relative to untreated controls or cells treated with VEGF or Slit2 as single agents. (d) Treatment with an Arf inhibitor significantly rescued assembly in cells co-stimulated with VEGF and Slit2 relative to DMSO vehicle control. The inhibitor had no effect on assembly induced by VEGF or Slit2 as a single agent in wild-type endothelial cells. (e) We confirmed Arf6 inhibitor reduced levels of active, GTP-bound Arft6 in wild-type endothelial cells co-stimulated with VEGF and Slit2 by effector pull-down followed by western analysis. Data are a representation of three to five independent experiments, with replicate samples analyzed in each experiment, with average +/− standard deviation. Statistical significance was assessed by two-tailed, paired Student’s t-test.
Co-stimulation of wild-type endothelial cells with Slit2 and VEGF inhibits angiogenic remodeling ([24, 26]; Fig. 2c). Previous studies have linked Slit2 and VEGF to inhibitory modulation of ADP ribosylation factor 6 (Arf6) GTPase [24, 26], an upstream regulator of Rac activity [52]. Therefore, we assessed levels of active, GTP-bound Arf6 in wild-type endothelial cells treated with VEGF versus VEGF plus Slit2. Surprisingly, co-stimulation resulted in activation of Arf6 relative to untreated cells or cells stimulated with VEGF or Slit2 alone in our model system (Fig. 2c), though VEGF was reported to activate Arf6 in HUVEC [53]. As high levels of Arf6 activity upon VEGF and Slit2 co-stimulation correlate with angiostasis, we wish to determine if blocking Arf6 restored angiogenesis in co-stimulated cells. Treatment with SecinH3 Arf inhibitor significantly rescued assembly in wild-type endothelial cells co-stimulated with VEGF and Slit2 relative to vehicle control (Fig. 2d). Consistent with our activity assays, the Arf inhibitor did not affect angiogenesis induced by either VEGF or Slit2 alone (Fig. 2d). We confirmed Arf6 inhibition of upon treatment with SecinH3 in wild-type endothelial cells co-stimulated with VEGF and Slit2 (Fig. 2e).
Consistent with our previous studies [38], treatment of wild-type endothelial cells with Slit2 as a single agent induced endothelial assembly and migration (Fig. 3a). Slit2 and VEGF co-stimulation inhibited assembly and migration of wild-type endothelial cells (Fig. 3a) as previously reported [24, 26], and at a dose range consistent with levels observed in our ELISA analyses (Supplemental Fig. S1). Robo1-Fc did not induce assembly or migration in wild-type endothelium, nor did it enhance VEGF-induced assembly or migration (Fig. 3a). Robo1-Fc treatment, however, partially rescued basal assembly in EphA2-deficient endothelial cells, though to a lesser extent relative to co-stimulation with VEGF (Fig. 3b). Prolonged treatment with Robo1-Fc alone (16-hours) induced Rac activity (Fig. 3c) in EphA2-deficient endothelial cells, consistent with rescue of assembly by Robo1-Fc (24-hours; Fig. 3b). Activated, GTP-bound Arf6 was detected in EphA2-deficient endothelial cells stimulated with VEGF (Fig. 3d). As expression of Slit2 is elevated in EphA2-deficient endothelial cells, these data are consistent with activation of Arf6 in the context ofSlit2 and VEGF co-stimulation (Fig. 2c) and support an angiostatic function for Arf6 in our cell model. In addition, Arf6-GTP levels were reduced in EphA2-deficient endothelial cells treated with VEGF in the presence of Robo1-Fc ligand trap (Fig. 3d), supporting the hypothesis that Arf6 activation inhibits angiogenesis in Slit2/VEGF-stimulated microvascular endothelial cells. Addition of exogenous Slit2 did not induce assembly in EphA2-deficient endothelial cells, nor did it affect assembly in the presence of VEGF (Fig. 3b).
Fig. 3. Slit2 inhibits VEGF-induced assembly and migration in wild-type cells, and inhibiting Slit activity in EphA2-deficient cells rescues basal vascular assembly.
(a) Individually, Slit2 or VEGF stimulated assembly and transwell migration in wild-type endothelial cells. The combination of VEGF and Slit2, however, significantly inhibited both assembly and migration. Robo1-Fc alone or in combination with VEGF did not significantly alter wild-type endothelial cell assembly or migration. (b) Addition of soluble Robo1-Fc receptor partially rescued basal assembly of EphA2-deficient endothelial cells in serum-free media after 24-hours, though to a lesser extent than in the presence of VEGF. Addition of Slit2 to EphA2-deficient endothelial cells did not affect basal or VEGF-induced angiogenesis. (c) Consistent with these data, EphA2-deficient endothelial cells treated with Robo1-Fc for 16-hours elevated basal Rac activity. (d) Levels of active, GTP -bound Arf6 were significantly reduced in VEGF-treated EphA2-deficient endothelial cells upon co-treatment with Robo1-Fc, as observed using effector pull-down followed by western analysis. Data are a representation of two to three independent experiments, with replicate samples analyzed in each experiment, with average +/− standard deviation. Statistical significance was assessed by two-tailed, paired Student’s t-test. N.S. = not significant (relative to IgG control).
To confirm our results from EphA2-deficient murine microvascular endothelial cells, we tested the impact of EphA2 siRNA-mediated knockdown in human endothelial cells. EphA2 knockdown in primary human retinal microvascular endothelial cells (HRMEC) significantly reduced assembly in response to VEGF relative to control siRNA expressing cells (Supplemental Fig. S2a). Addition of Robo1-Fc partially rescued VEGF-mediated assembly in EphA2 knockdown lines, as did prolonged treatment of Robo1-Fc alone, albeit to a lesser extent. Exogenous Slit2 promoted assembly in control siRNA lines, whereas co-stimulation with Slit2 and VEGF impaired assembly (Supplemental Fig. S2a), consistent with our findings in murine cell lines and in previous studies [23–26]. We confirmed EphA2 knockdown in lysates from HRMEC via immunoblot (Supplemental Fig. S2b) and upregulation of Slit2 expression by Real-Time qRT-PCR analysis (Supplemental Fig. S2c).
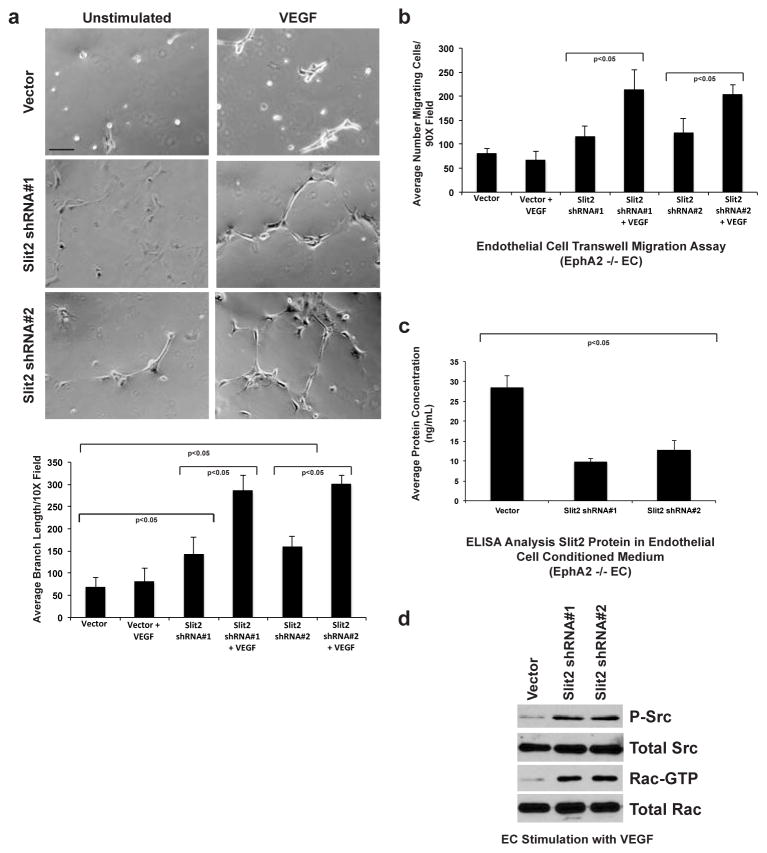
To confirm our results and to determine if blocking Slit2 expression specifically rescues VEGF-induced angiogenic remodeling, we generated two independent EphA2-deficient cell lines stably expressing slit2 shRNA sequences. Relative to vector control, VEGF induced a significant assembly response in EphA2-deficient Slit2 knockdown lines (Fig. 4a). In addition, VEGF-induced migration was also rescued in Slit2 knockdown clones (Fig. 4b). Analysis and quantification of Slit2 protein in endothelial cell conditioned medium confirmed knockdown in shRNA clones relative to vector control (Fig. 4c). Consistent with data derived from Robo1-Fc treatment, stable Slit2 shRNA knockdown in EphA2-deficient endothelial cells rescued Src and Rac activity upon VEGF stimulation (Fig. 4d).
Fig. 4. Slit2 knockdown rescues VEGF-induced vascular assembly and migration in EphA2-deficient endothelial cells.
(a) Relative to vector controls, EphA2-deficient endothelial cells stably expressing two independent slit2 shRNA constructs displayed increased basal and VEGF-induced assembly. (b) Stable slit2 knockdown also rescued VEGF-induced migration of EphA2-deficient endothelial cells relative to vector controls in transwell assays. (c) We confirmed decreased expression of Slit2 protein in knockdown clones relative to vector control lines by ELISA analysis. Data are a representation of two independent experiments, with replicate samples analyzed in each experiment, with average +/− standard deviation. Statistical significance was assessed by two-tailed, paired Student’s t-test.(d) Consistent with data from treatment with Robo1-Fc, slit2 knockdown rescued VEGF -induced Src and Rac activity.
EphA2 gain-of-function significantly diminishes slit2 expression in endothelium
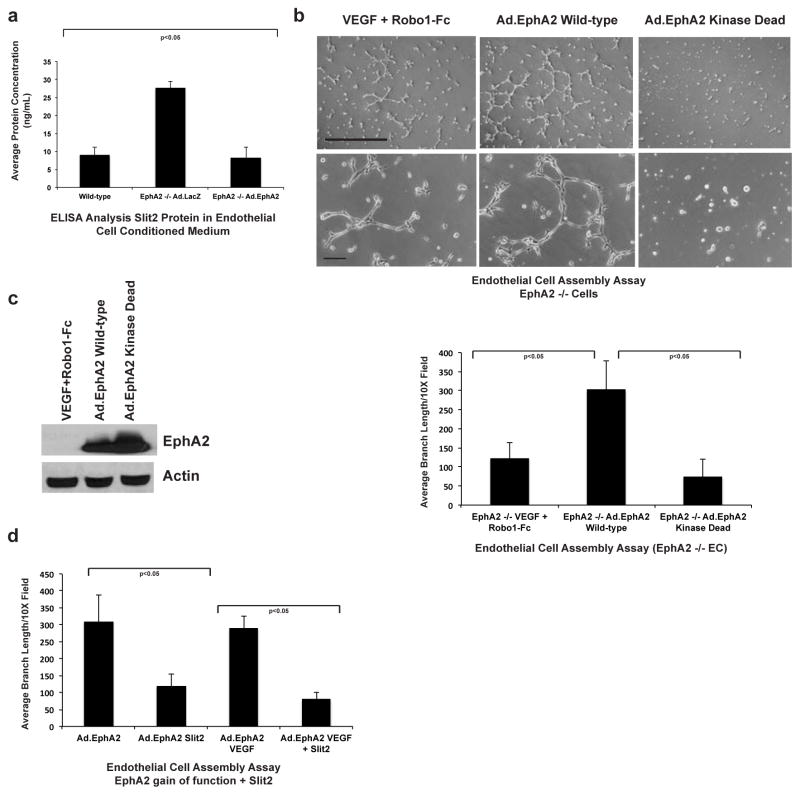
To determine if EphA2 gain-of-function modulates Slit2 expression, we analyzed Slit2 protein levels in conditioned medium from EphA2-deficient cells transduced with adenoviruses harboring control LacZ (Ad.LacZ) or wild-type EphA2 (Ad.EphA2) transgenes. Relative to Ad.LacZ controls, Ad.EphA2-expressing cells secreted significantly lower levels of Slit2 protein into conditioned medium, comparable to levels detected in wild-type cells (Fig. 5a). EphA2-deficiency or knockdown results in elevated expression of slit2, which is at least partially responsible for EphA2-mediated resistance to VEGF-induced angiogenesis. Overexpression of wild-type, but not kinase dead EphA2 in EphA2-deficient endothelial cells was sufficient to induce spontaneous assembly, with branch lengths significantly greater compared to partial rescue by Robo1-Fc plus VEGF (Fig. 5b). Immunblot analysis confirmed overexpression of Ad.EphA2 transgenes in transduced endothelial cells (Fig. 5c). Consistent with data derived from wild-type endothelial cells, addition of exogenous Slit2 significantly inhibited assembly in Ad.EphA2 overexpressing cells, both alone and in the presence of VEGF (Fig. 5d). These data suggest that suppression of Slit2 expression by EphA2 facilitates VEGF-induced angiogenesis.
Fig. 5. EphA2 overexpression reduces Slit2 expression, and exogenous Slit2 inhibits endothelial cell assembly induced by EphA2 gain-of-function.
(a) EphA2-deficient endothelial cells were transduced with adenoviruses harboring control LacZ (Ad.LacZ) or wild-type EphA2 (Ad.EphA2). Conditioned medium was harvested and protein expression of secreted murine Slit2 quantified by ELISA. Levels of secreted Slit2 were significantly higher in EphA2-deficient endothelial cells expressing control Ad.LacZ relative to wild-type endothelial cells, and overexpression of Ad.EphA2 reduced Slit2 protein levels back to the level observed in wild-type controls. (b) Overexpression of wild-type, but not kinase dead, EphA2 rescued assembly in EphA2-deficient cells to a greater extent than VEGF + Robo1-Fc, highlighting the importance of EphA2 receptor function as an additional regulator of angiogenesis. Scale bar = 100 μm (upper panels) and 20 μm (lower panels). (c) Expression of adenoviral gene products was confirmed by immunoblot. (d) Consistent with data from wild-type endothelial cells, assembly induced by overexpression of Ad.EphA2 was significantly reduced by treatment with exogenous Slit2 in the presence or absence of VEGF. Data are a representation of two independent experiments, with replicate samples analyzed in each experiment, with average +/− standard deviation. Statistical significance was assessed by two-tailed, paired Student’s t-test.
Inhibiting Slit activity rescues VEGF-induced angiogenesis in EphA2-deficient animals in vivo
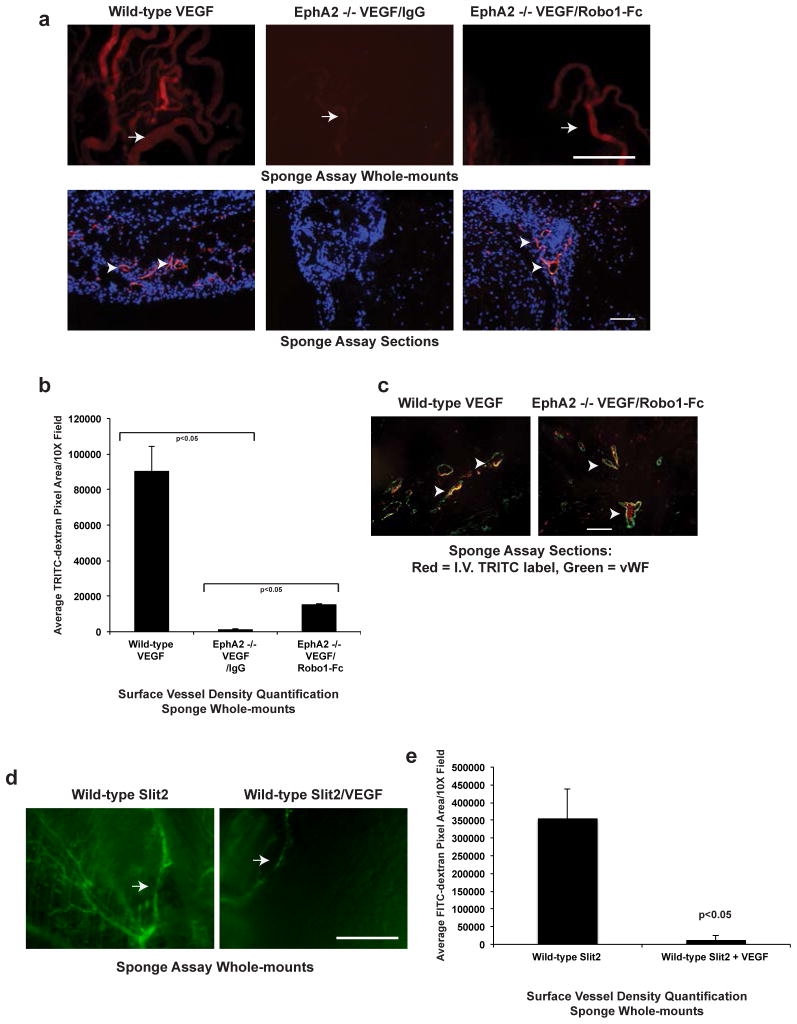
To determine if modulating Slit function affects vascular remodeling from intact vessels in vivo, we implanted sponges seeded with VEGF plus or minus IgG control versus recombinant Robo-1-Fc subcutaneously into the dorsal flank of recipient wild-type or EphA2-deficient mice. One week following implantation, we injected mice intravenously with TRITC-dextran to visualize and quantify blood vessel infiltration into the sponge. Sponges harboring VEGF stimulated a robust angiogenic response in wild-type mice, with a significant increase in TRITC+ surface blood vessels (upper panels) and TRITC+ blood vessels infiltrating sponges in tissue sections (lower panels), relative to sponges containing IgG control (Fig. 6a, b; data not shown). VEGF failed to induce angiogenesis in EphA2-deficient mice in the presence of control IgG. In the presence of Robo1-Fc, however, we observed a partial rescue of subcutaneous angiogenesis (Fig. 6a, b). The TRITC+ structures in sponge sections co-stained with von Willebrand Factor (vWF, green; Fig. 6c), a marker for vascular endothelium, confirming that the TRITC+ structures observed were functional blood vessels. Consistent with our previous studies [38], single agent Slit2 induced angiogenesis in sponge assays (Fig. 6d), whereas Slit2 impaired VEGF-induced subcutaneous vascular remodeling (Fig. 6d, e; [23–26]), supporting the pro-angiogenic function of Slit2 as a single agent and its angiostatic function in the presence of VEGF co-stimulation. Taken together, these data support the hypothesis that elevated Slit2 in EphA2 -deficient endothelium, at least in part, mediates resistance to VEGF-mediated angiogenesis.
Fig. 6. Inhibiting Slit activity rescues VEGF-induced angiogenesis in EphA2-deficient animals in vivo.
(a) Gelfoam sponges were loaded with VEGF plus or minus control IgG or Robo1-Fc and subcutaneously implanted the sponges into the dorsal flank of recipient mice. After 7 days, the mice were injected intravenously with TRITC-dextran to label vasculature and the sponges were excised for analysis. While wild-type recipients displayed a robust angiogenic remodeling response to sponges harboring VEGF, with numerous TRITC-positive surface vessels (arrows in upper panels, whole mounts; arrowheads, sections), EphA2-deficient recipients failed to respond to VEGF. Addition of Robo1-Fc partially rescued VEGF-induced vascular remodeling in EphA2-deficient host animals. Scale bar = 5 mm (upper panels). Scale bar = 100 μm (lower panels). (b) We quantified surface vessel density in whole-mounts based TRITC+ pixel area using NIH Image J software analysis. (c) We confirmed that TRITC+ structures also expressed the endothelial marker von Willebrand factor (vWF, green staining; arrowheads indicate TRITC+/vWF+ blood vessels). (d) Slit2 alone induced subcutaneous angiogenic remodeling in wild-type animals, with numerous FITC-positive vessels observed on the surface of sponges (arrows), whereas Slit2 inhibited subcutaneous angiogenesis in the presence of VEGF. Scale bar = 5 mm. (e) We quantified surface vessel density in whole-mounts based FITC+ pixel area using NIH Image J software analysis. Data are a representation of 10 independent animals total/condition in analyzed in 2 independent experiments (5 animals/condition/experiment), with average +/− standard deviation. Statistical significance was assessed by two-tailed, paired Student’s t-test.
Inhibiting Slit activity rescues tumor-induced angiogenesis and growth in EphA2-deficient animals in vivo
Given (i) the role of VEGF and EphA2 in tumor angiogenesis, (ii) the observation that loss of EphA2 impairs VEGF-induced angiogenesis, (iii) the known role of Slit2 in suppression of VEGF-induced angiogenesis and (iv) our data demonstrating that Slit2 is elevated in EphA2-deficient endothelium, we wished to determine if Slit2 upregulation was, at least in part, responsible for defective tumor neovascularization in EphA2-deficient animals. Indeed, EphA2-deficiency and inhibition impairs angiogenesis and growth of VEGF-expressing tumors in vivo [10–12, 39, 47, 50]. To determine if Slit function mediates this phenotype, we first assessed endothelial cell migration in response to tumor cells in modified transwell chamber co-culture assays [Supplemental Fig. S3a; [50]]. 4T1-GFP tumor cells were plated on the lower surface of Matrigel-coated transwells. Endothelial cells were labeled with CellTracker dye and added to the upper chamber, and migration and intercalation of endothelial cells was quantified by counting the number of red fluorescent cells on the lower surface(Supplmental Fig. S3b, c). Consistent with previous studies, migration of EphA2-deficient endothelial cells was significantly lower than wild-type endothelial cell migration in response to tumor cells [39]. Addition of Slit2 inhibited migration of wild-type endothelial cells in response to 4T1 cells, whereas addition of Robo1-Fc partially rescued tumor-induced migration of EphA2-deficient endothelial cells (Supplemental Fig. S3b). These data suggest that elevated Slit2 levels in EphA2-deficient endothelium affects the ability of endothelial cell recruitment by VEGF-expressing tumor cells.
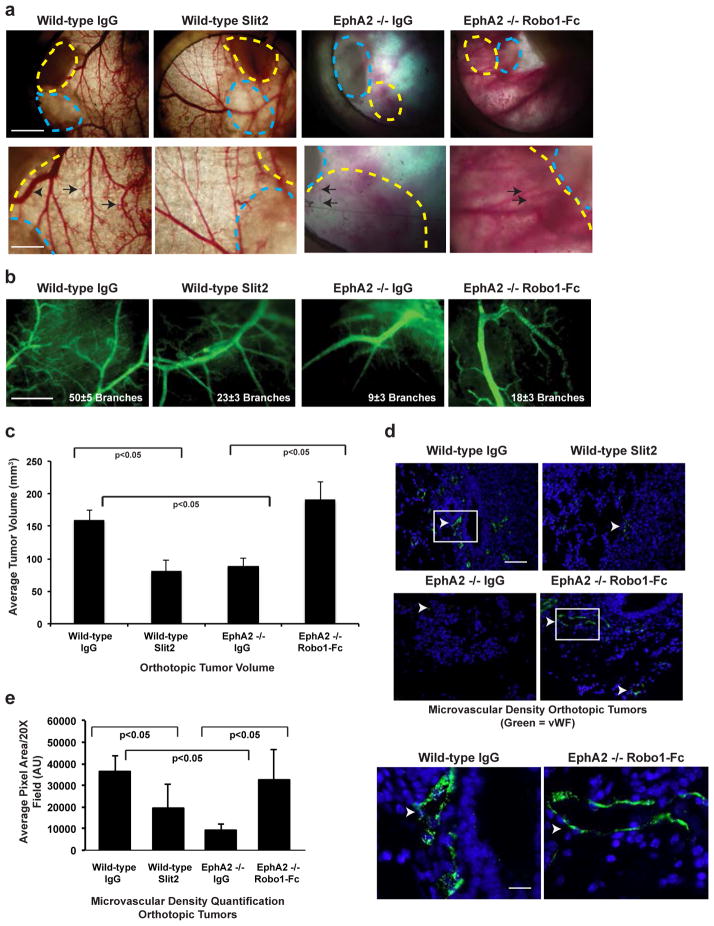
To confirm these findings in vivo, we scored changes in tumor angiogenesis and growth in wild-type versus EphA2-deficient animals using cutaneous window chamber assays. VEGF-dependent 4T1 tumors [49, 54, 55] were co-transplanted into cutaneous window chambers with sponges impregnated with control IgG or Slit2 (wild-type hosts) or control IgG or Robo1-Fc (EphA2-deficient hosts). Treatment with Slit2 significantly reduced tumor neovascularization in wild-type hosts, whereas Robo1-Fc partially rescued 4T1-induced tumor angiogenesis in EphA2-deficient hosts as scored by branching from primary vessels (Fig. 7a, b). We also tested the impact of modulating Slit activity in orthotopically-transplanted tumors. EphA2-deficient hosts displayed a significant reduction in tumor volume relative to wild-type hosts, consistent with previous studies [39, 47]. Addition of Slit2 reduced tumor volume in wild-type hosts relative to IgG controls, whereas addition of Robo1-Fc rescued tumor volume in EphA2-deficient hosts cells (Fig. 7c). Consistent with our window assay data, Slit2 reduced, whereas Robo1-Fc partially rescued, microvascular density in orthotopic tumors implanted in wild-type or EphA2-deficient hosts, respectively (Fig. 7d, e).
Fig. 7. Inhibiting Slit activity rescues tumor-induced angiogenesis and growth in EphA2-deficient animals in vivo.
(a) 4T1 tumors (outlined with yellow dashed lines) were co-transplanted with gelfoam sponges (outlined with blue dashed lines) plus or minus control IgG, Slit2, or Robo1-Fc and subcutaneously implanted into window chambers mounted on the dorsal flank of recipient mice. After 7 days, FITC-conjugated dextran (2% in PBS, Sigma-Aldrich) was injected intravenously, and tumors vessels (arrows) in window chambers were photodocumented. Scale bar = 2 mm (upper panels) and 5 mm (lower panels). (b) Branches from host blood vessels within the window chambers were enumerated in at least three independent fields per mouse, and statistical significance was determined by two-tailed, paired Student’s t-test (p<0.05 wild-type IgG versus EphA2 −/− IgG; wild-type IgG versus Slit2; EphA2 −/− IgG versus Robo1-Fc). Scale bar = 5 mm. Slit2 reduced tumor volume (c) and microvascular density (MVD; d, e) in orthotopically transplanted 4T1 tumors in wild-type hosts. By contrast, addition of Robo1-Fc partially rescued tumor volume and MVD in EphA2 −/− hosts. Arrowheads indicate vWF+ blood vessels (green). Scale bar = 100 μm (upper panels) and 20 μm (lower panels). Lower panels are high magnification images of areas indicated by white boxes in upper panels. Data are a representation of 6–8 independent samples per condition with standard error of the mean, and statistical significance was assessed by two-tailed, paired Student’s t-test.
Discussion
Emerging evidence suggests the effects of VEGF on vascular remodeling can be modulated by the activity of other signaling pathways. For example, VEGF-induced motility in tip cells versus proliferation in stalk cells is mediated by lateral inhibition upon Notch1 receptor activation by delta-like 4 (Dll4) ligand. VEGF migration versus proliferation in Notch-expressing stalk cells is further modulated by Jagged-1 ligand, which antagonizes Dll4 by competing for Notch receptor (Reviewed in [56]). In this study, we found that EphA2 regulation of Slit2 also regulates endothelial cell response to VEGF. Slit2 mRNA and protein levels were significantly elevated in EphA2-deficient endothelium, including endothelium exposed to tumor-cell conditioned medium, as was Robo1 mRNA. EphA2-deficient endothelium is resistant to VEGF-induced angiogenesis, and inhibition of Slit activity by soluble Robo receptors rescued EphA2-deficient endothelial cell assembly, migration, subcutaneous vascular remodeling, and tumor angiogenesis mediated by VEGF.
Slit2 can either promote or inhibit angiogenesis, depending upon molecular context [18–22] [23–27]. Indeed, we recently reported that single agent Slit2 induces angiogenesis in vitro and in vivo, whereas co-stimulation with ephrin-A1 inhibits the pro-angiogenic function of both factors [38]. Others observed similar inhibitory effects of Slit2 upon co-stimulation with VEGF [23–26]. These data are consistent with our observed alleviation of angiostasis in EphA2-deficient endothelium upon inhibition of Slit function, though blocking this receptor likely affects a host of other molecular signaling pathways that culminate to inhibit angiogenesis. At least one mechanism through that appears to regulate the pro- versus anti-angiogenic functions of Slit2 is activation of Arf6 GTPase, an upstream regulator of Rac. While several previous studies showed that active Arf6 is necessary and sufficient for Rac1 activation [26, 57–59], Arf6 has also been reported to inhibit Rac activity by others [52, 60, 61], as we observed in our cell model systems. Activation of Arf6 by co-stimulation with Slit2 and VEGF, though not VEGF alone or Slit2 alone, may inhibit Rac-dependent angiogenesis, as supported by data from our model systems. Indeed, an Arf inhibitor rescued assembly in co-stimulated cells, supporting this model. Moreover, blocking Slit2 activity in EphA2-deficient endothelial cells with Robo1-Fc significantly reduced Arf6 activation, which was elevated in the presence of VEGF. Thus, reduced Arf6 activity correlates with rescue of VEGF-induced Rac activity and angiogenic remodeling in EphA2-deficient endothelial cells treated with Robo1-Fc.
It should be noted that other studies reported Slit2 inhibits Arf6 activity and that VEGF activates Arf6 as a single agent [26, 53]. Slit2-mediated inhibition of Arf6 was reportedly dependent on Robo4 activity [26], which may account for differential effects in our model systems that express relatively low levels of robo4 and appear to primarily depend on Robo1 receptor function. In addition, most of the data from Jones et al. were derived from bovine aortic and human umbilical vein endothelial cells [26], large vessel sources, whereas our data were derived from microvascular endothelial cells from murine lung and human retina. Similarly, Hashimoto et al. reported VEGF-induced Arf6 activation in large-vessel-derived HUVEC [53]. Moreover, differences in stimulation conditions (e.g. culturing endothelial cells on plates co-coated with Slit2 and fibronectin in Jones et al. versus acute stimulation of serum-starved endothelial cells with soluble Slit2 in our study) may also lead to differential signaling. Taken together, these differences in model systems, especially Robo receptor expression profiles, and experimental designs could account for differential regulation of Arf6/Rac by VEGF in the presence or absence of Slit2.
Our data support a model in which EphA2 receptor suppresses Slit2 in microvascular endothelial cells, which enables microvascular endothelium to respond to VEGF and ephrins to promote angiogenesis. Indeed, our gain-of-function studies in which overexpression of wild-type EphA2 reduced Slit2 expression support this model. As we observed elevated Robo1 levels in our EphA2-deficient cells, it is possible that the angiostatic function of Slit2 in the absence of EphA2 are enhanced by signaling through this receptor, a hypothesis that we are actively exploring.
In addition, EphA2 regulation of Slit2 angiocrine function may alleviate tumor suppressive effects. Indeed, blocking Slit function in EphA2-defcient endothelial cell conditioned media restored angiocrine-mediated tumor growth and motility in culture and in vivo [29]. Moreover, we observed an inverse correlation between EphA2 and Slit2 expression in human breast tumor vasculature, supporting the clinical relevance of this observation [29]. Together, these data suggest that elevated Slit2 expression in the absence of EphA2 is one mechanism of resistance to VEGF-induced angiogenesis, including tumor neovascularization.
Supplementary Material
Implications.
Modulation of angiostatic factor Slit2 by EphA2 receptor regulates endothelial responses to VEGF-mediated angiogenesis and tumor neovascularization.
Acknowledgments
Financial Support:
NIH/NCI Grant CA148934 (WS, DW, DBS, YH, SW)
Department of Veterans Affairs through a VA Merit Award (JC)
NIH/NCI Grant CA95004 (JC, WS, YH)
NIH/National Heart, Lung, and Blood Institute training grant T32HL007551 (VY)
This work was supported by National Institutes of Health/National Cancer Institute grant CA148934 (D. Brantley-Sieders), the Department of Veterans Affairs through a VA Merit Award (Jin Chen) and NIH/NCI grant CA95004 (J. Chen) and NIH/National Heart, Lung, and Blood Institute training grant T32HL007551 (V. Youngblood). The work was also supported in part by the NCI Cancer Center Support Grant #P30 CA068485 utilizing the Translational Pathology Shared Resource. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Potential Conflicts of Interest: None
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Tie J, Desai J. Antiangiogenic therapies targeting the vascular endothelia growth factor signaling system. Crit Rev Oncog. 2012;17:51–67. doi: 10.1615/critrevoncog.v17.i1.50. [DOI] [PubMed] [Google Scholar]
- 3.Waldner MJ, Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:5–13. doi: 10.1517/14728222.2011.641951. [DOI] [PubMed] [Google Scholar]
- 4.Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- 5.Brantley-Sieders D, Schmidt S, Parker M, Chen J. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr Pharm Des. 2004;10:3431–3442. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- 6.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed Z, Bicknell R. Angiogenic signalling pathways. Methods Mol Biol. 2009;467:3–24. doi: 10.1007/978-1-59745-241-0_1. [DOI] [PubMed] [Google Scholar]
- 8.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 9.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 10.Cheng N, Brantley DM, Liu H, Lin Q, Enriquez M, et al. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res. 2002;1:2–11. [PubMed] [Google Scholar]
- 11.Cheng N, Brantley D, Fang WB, Liu H, Fanslow W, et al. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia. 2003;5:445–456. doi: 10.1016/s1476-5586(03)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrzanski P, Hunter K, Jones-Bolin S, Chang H, Robinson C, et al. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910–919. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- 13.Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–10324. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Hicks D, Brantley-Sieders D, Cheng N, McCollum GW, et al. Inhibition of retinal neovascularization by soluble EphA2 receptor. Exp Eye Res. 2006;82:664–673. doi: 10.1016/j.exer.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 16.Legg JA, Herbert JM, Clissold P, Bicknell R. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11:13–21. doi: 10.1007/s10456-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, et al. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood. 2009;114:4300–4309. doi: 10.1182/blood-2008-12-193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur S, Castellone MD, Bedell VM, Konar M, Gutkind JS, et al. Robo4 signaling in endothelial cells implies attraction guidance mechanisms. J Biol Chem. 2006;281:11347–11356. doi: 10.1074/jbc.M508853200. [DOI] [PubMed] [Google Scholar]
- 19.Kaur S, Samant GV, Pramanik K, Loscombe PW, Pendrak ML, et al. Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC Cell Biol. 2008;9:61. doi: 10.1186/1471-2121-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheldon H, Andre M, Legg JA, Heal P, Herbert JM, et al. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. Faseb J. 2009;23:513–522. doi: 10.1096/fj.07-098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbich C, Rossig L, Kaluza D, Potente M, Boeckel JN, et al. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood. 2009;113:5669–5679. doi: 10.1182/blood-2009-01-196485. [DOI] [PubMed] [Google Scholar]
- 22.Yang XM, Han HX, Sui F, Dai YM, Chen M, et al. Slit-Robo signaling mediates lymphangiogenesis and promotes tumor lymphatic metastasis. Biochem Biophys Res Commun. 2010;396:571–577. doi: 10.1016/j.bbrc.2010.04.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, et al. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 24.Jones CA, London NR, Chen H, Park KW, Sauvaget D, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Hou J, Hu X, Wang X, Xiao Y, et al. Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell migration by suppressing small GTPase Rac1 activation. Circ Res. 2006;98:480–489. doi: 10.1161/01.RES.0000205764.85931.4b. [DOI] [PubMed] [Google Scholar]
- 26.Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X, Zhang MC. Potential anti-angiogenic role of Slit2 in corneal neovascularization. Exp Eye Res. 2010;90:742–749. doi: 10.1016/j.exer.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Zhang X, Kuzontkoski PM, Jiang S, Zhu W, et al. Slit2N and Robo4 regulate lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun Signal. 2014;12:25. doi: 10.1186/1478-811X-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brantley-Sieders DM, Dunaway CM, Rao M, Short S, Hwang Y, et al. Angiocrine factors modulate tumor proliferation and motility through EphA2 repression of Slit2 tumor suppressor function in endothelium. Cancer Res. 2011;71:976–987. doi: 10.1158/0008-5472.CAN-10-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad A, Fernandis AZ, Rao Y, Ganju RK. Slit protein-mediated inhibition of CXCR4-induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. J Biol Chem. 2004;279:9115–9124. doi: 10.1074/jbc.M308083200. [DOI] [PubMed] [Google Scholar]
- 31.Dallol A, Da Silva NF, Viacava P, Minna JD, Bieche I, et al. SLIT2, a human homologue of the Drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Res. 2002;62:5874–5880. [PubMed] [Google Scholar]
- 32.Schmid BC, Rezniczek GA, Fabjani G, Yoneda T, Leodolter S, et al. The neuronal guidance cue Slit2 induces targeted migration and may play a role in brain metastasis of breast cancer cells. Breast Cancer Res Treat. 2007;106:333–342. doi: 10.1007/s10549-007-9504-0. [DOI] [PubMed] [Google Scholar]
- 33.Marlow R, Strickland P, Lee JS, Wu X, Pebenito M, et al. SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res. 2008;68:7819–7827. doi: 10.1158/0008-5472.CAN-08-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prasad A, Paruchuri V, Preet A, Latif F, Ganju RK. Slit-2 induces a tumor-suppressive effect by regulating beta-catenin in breast cancer cells. J Biol Chem. 2008;283:26624–26633. doi: 10.1074/jbc.M800679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci U S A. 2009;106:14530–14535. doi: 10.1073/pnas.0801262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macias H, Moran A, Samara Y, Moreno M, Compton JE, et al. SLIT/ROBO1 signaling suppresses mammary branching morphogenesis by limiting basal cell number. Dev Cell. 2011;20:827–840. doi: 10.1016/j.devcel.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang PH, Hwang-Verslues WW, Chang YC, Chen CC, Hsiao M, et al. Activation of Robo1 signaling of breast cancer cells by Slit2 from stromal fibroblast restrains tumorigenesis via blocking PI3K/Akt/beta-catenin pathway. Cancer Res. 2012;72:4652–4661. doi: 10.1158/0008-5472.CAN-12-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunaway CM, Hwang Y, Lindsley CW, Cook RS, Wu JY, et al. Cooperative signaling between Slit2 and Ephrin-A1 regulates a balance between angiogenesis and angiostasis. Mol Cell Biol. 2011;31:404–416. doi: 10.1128/MCB.00667-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, et al. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 2005;19:1884–1886. doi: 10.1096/fj.05-4038fje. [DOI] [PubMed] [Google Scholar]
- 40.Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- 41.Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, et al. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037–2049. doi: 10.1242/jcs.01061. [DOI] [PubMed] [Google Scholar]
- 42.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, et al. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971–2976. [PubMed] [Google Scholar]
- 44.Fang WB, Brantley-Sieders DM, Hwang Y, Ham AJ, Chen J. Identification and functional analysis of phosphorylated tyrosine residues within EphA2 receptor tyrosine kinase. J Biol Chem. 2008;283:16017–16026. doi: 10.1074/jbc.M709934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amato KR, Wang S, Hastings AK, Youngblood VM, Santapuram PR, et al. Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J Clin Invest. 2014;124:2037–2049. doi: 10.1172/JCI72522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunter SG, Zhuang G, Brantley-Sieders D, Swat W, Cowan CW, et al. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol Cell Biol. 2006;26:4830–4842. doi: 10.1128/MCB.02215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brantley-Sieders DM, Zhuang G, Vaught D, Freeman T, Hwang Y, et al. Host deficiency in Vav2/3 guanine nucleotide exchange factors impairs tumor growth, survival, and angiogenesis in vivo. Mol Cancer Res. 2009;7:615–623. doi: 10.1158/1541-7786.MCR-08-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li CY, Shan S, Huang Q, Braun RD, Lanzen J, et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 50.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 51.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 53.Hashimoto A, Hashimoto S, Ando R, Noda K, Ogawa E, et al. GEP100-Arf6-AMAP1-cortactin pathway frequently used in cancer invasion is activated by VEGFR2 to promote angiogenesis. PLoS One. 2011;6:e23359. doi: 10.1371/journal.pone.0023359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, et al. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 55.Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, et al. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One. 2009;4:e7669. doi: 10.1371/journal.pone.0007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas JL, Baker K, Han J, Calvo C, Nurmi H, et al. Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cell Mol Life Sci. 2013;70:1779–1792. doi: 10.1007/s00018-013-1312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tushir JS, D’Souza-Schorey C. ARF6-dependent activation of ERK and Rac1 modulates epithelial tubule development. EMBO J. 2007;26:1806–1819. doi: 10.1038/sj.emboj.7601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu B, Shi B, Jarzynka MJ, Yiin JJ, D’Souza-Schorey C, et al. ADP-ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway. Cancer Res. 2009;69:794–801. doi: 10.1158/0008-5472.CAN-08-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muralidharan-Chari V, Hoover H, Clancy J, Schweitzer J, Suckow MA, et al. ADP-ribosylation factor 6 regulates tumorigenic and invasive properties in vivo. Cancer Res. 2009;69:2201–2209. doi: 10.1158/0008-5472.CAN-08-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhanot H, Young AM, Overmeyer JH, Maltese WA. Induction of nonapoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6. Mol Cancer Res. 2010;8:1358–1374. doi: 10.1158/1541-7786.MCR-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawaguchi K, Saito K, Asami H, Ohta Y. ADP ribosylation factor 6 (Arf6) acts through FilGAP protein to down-regulate Rac protein and regulates plasma membrane blebbing. J Biol Chem. 2014;289:9675–9682. doi: 10.1074/jbc.M113.546051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.