Abstract
Rationale
Traditional protocols for inducing sensitization to psychostimulants use an intermittent or “binge”-like drug administration, and binge eating behavior is comorbid with drug abuse in humans. Food restriction increases the reinforcing properties and self-administration of many drugs of abuse.
Objective
The present study tested the hypotheses that (1) food restriction induces sensitization to the locomotor stimulation observed in response to methamphetamine and (2) a binge-like feeding schedule during food restriction produces increased sensitization compared to equally restricted mice fed in three daily meals.
Methods
Male DBA/2J mice were fed ad libitum or were food restricted to either an 8% or 16% loss of body weight. Additionally, the food-restricted mice were divided into two groups that were fed in either one meal (binge) or three equal-sized meals (meal). After the reduced body weight was stable, mice were tested for locomotor activity following saline and methamphetamine (1 mg/kg) injections.
Results
Both 16% body weight loss groups exhibited sensitization to methamphetamine. Opposite to our hypothesis, the 8% meal but not the 8% binge food-restricted group demonstrated locomotor sensitization. Serum corticosterone levels were significantly higher in the meal-fed groups when compared to the binge- and ad libitum-fed groups.
Conclusions
These results support a role for feeding schedule and plasma corticosterone levels in food restriction-induced enhancement of the effects of methamphetamine.
Keywords: Mouse, Corticosterone, Underfeeding, Psychostimulants, Binge
Introduction
Repeated exposure to a drug of abuse can produce a progressive increase in the behavioral effects of that drug, a process known as behavioral sensitization. Intermittent drug administration often produces behavioral sensitization while continuous administration produces behavioral tolerance (Post 1980), suggesting that the development of sensitization depends on the schedule of delivery. Sensitization is positively associated with the reinforcing effects of abused drugs, and development of sensitization causes a shift in the organism’s behavior towards a greater incentive salience or “wanting” (see Robinson and Berridge 2001). Thus, experiences that enhance an organism’s sensitivity to a drug of abuse are likely to contribute to the development of addiction- and substance-related disorders.
Either feeding state or stress can also increase behavioral sensitivity to the effects of many drugs even in drug-naive individuals. In rodent models of drug abuse, feeding state has direct and profound effects on drug-related behaviors. For example, food restriction increases opiate-induced stereotypies (Carroll et al. 1979), amphetamine-induced conditioned place preference (Stuber et al. 2002), and cocaine- and amphetamine-induced locomotion (Deroche et al. 1993; Macenski and Meisch 1999; Marinković et al. 2007; Stuber et al. 2002 but see Sevak et al. 2008). Food restriction not only enhances the behavioral consequences of drug use but also increases acquisition and self-administration of most, if not all, major classes of abused drugs (Bell et al. 1997; Carroll et al. 1979; Carroll and Meisch 1981; Carroll et al. 1981). Furthermore, human epidemiological data support a comorbidity between eating disorders and substance abuse (for a review, see Holderness et al. 1994), and binge eating itself may be an addictive disorder (Davis and Carter 2009). Animal research also suggests that cyclical access to food can produce a subtle but significant increase in sensitivity to amphetamine (Avena and Hoebel 2003). However, despite extensive behavioral evidence linking drug effects and eating disorders, the respective contributions of underfeeding and/or binge eating to these behaviors is unknown. This issue is particularly relevant to the preponderance of laboratories that study drug self-administration in rodents, as limited access to food is commonly used to enhance an animal’s motivational state and to increase the speed and reliability with which they learn to respond for drugs.
The present investigation probed the understudied relationship between dietary restriction and the effects of the commonly abused psychostimulant methamphetamine. Since most laboratories that conduct food restriction studies feed the animals in one large daily meal, we sought to determine the relative contribution of food restriction alone versus food restriction combined with binge-like feeding on psychostimulant sensitivity. Specifically, we varied the feeding schedule and level of food restriction in mice and then measured the acute locomotor activation produced by methamphetamine. We also assayed blood corticosterone in each group to determine whether it alone is responsible for feeding-induced changes or instead works in concert with other variables to affect methamphetamine sensitivity. We found that chronic moderate food restriction (resulting in 16% body weight loss) increased the locomotor activation produced by methamphetamine independent of feeding schedule. Surprisingly however, milder food restriction (resulting in 8% body weight loss) only produced methamphetamine sensitization when mice were fed in three meals and not in one binge-like meal each day. Furthermore, plasma corticosterone levels taken at the time of day that behavior was assessed reflected changes in feeding schedule rather than food restriction per se, suggesting that an acute rise in corticosterone may be sufficient but not necessary to enhance the locomotor response to methamphetamine.
Materials and methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and were performed in accordance with the National Institutes of Health guidelines for the care and use of mice. Eight-week-old male DBA/2J mice from Jackson Laboratory (Bar Harbor, ME, USA) were individually housed on a 14/10 reverse light–dark cycle with lights off at 1000 hours and on at 2000 hours. All mice were given free access to water throughout the experiment. Cotton nesting material (Shred-A-Bed, Novalek Inc., Hayward, CA, USA) was given to each mouse to decrease the stress of individual housing.
Food administration and drug treatment
At the start of the study, mice were allowed to acclimate to the reverse light–dark room for at least 5 days. Experiment 1 investigated the effects of repeated methamphetamine injections on methamphetamine-induced locomotion. Mice were separated into repeated methamphetamine and repeated saline (vehicle) groups and had standard mouse chow and water available ad libitum throughout the experiment. Experiment 2 investigated the effects of food restriction on methamphetamine-induced locomotor stimulation. Mice were individually housed for at least 4 days before food restriction was initiated. Control mice were fed ad libitum with free access to standard rodent chow. A preliminary study indicated that, under these conditions, DBA/2J mice given ad libitum access ate 4.1±0.1 g of chow per day (data not shown, n=7). Mice undergoing mild food restriction were fed approximately 3.0 g of standard rodent chow per day such that their weight loss plateaued at between 5% and 10% of their initial body weight (hereafter referred to as the 8% group). Mice undergoing moderate food restriction were fed approximately 2.4 g of standard rodent chow per day such that their weight loss plateaued at between 15% and 20% of their initial body weight (hereafter referred to as the 16% group). Two feeding schedules were also examined. Food-restricted mice were counterbalanced by initial body weight into binge and meal groups. Binge mice were fed their entire daily allotment of chow immediately after being weighed at 0900 hours and typically consumed the entire meal immediately. Meal mice were fed in three equally sized small meals at 0900, 1300, and 1800 hours. All mice in the ad libitum group increased in body weight while being monitored, except for one subject who lost weight for unknown reasons and was subsequently eliminated from the study. Food-restricted mice were weighed each morning and their daily food allotment adjusted for acquisition or maintenance of the appropriate (8% or 16%) level of weight loss. Restricted mice were subsequently maintained at their decreased weight for at least 5 days before behavioral experiments were initiated. The total duration of food restriction varied with each group but averaged 16.0 days.
Locomotor activity
Locomotor activity for experiments 1 and 2 was conducted during the dark cycle at 1200–1400 and 1500–1700 hours, respectively. For the food restriction study (experiment 2), this time was chosen to minimize the contributions of acute hunger and acute satiety. Mice were transported and tested for behavior in the dark. Locomotor behavior was measured in 16×16-in. Opto-Varimex automated activity monitors (Columbus Instruments, Columbus, OH, USA) that were connected to a personal computer running the Opto-Varimex AutoTracker Version 4.4 software. Injections and behavioral assessment always occurred together and in immediate succession. Mice were intraperitoneally (i.p.) injected with either 1 mg/kg methamphetamine or an equivalent amount of saline vehicle (0.08–0.11 ml), then immediately placed into the activity monitor for 30 min.
For experiment 1, mice were subjected to a 12-day repeated injection+locomotor activity protocol (Phillips et al. 1994; Kamens et al. 2005). As depicted in Fig. 1, all mice received saline injections and were subjected to 30-min locomotor activity sessions on days 1 and 2 to acclimate them to the locomotor activity chambers and again on day 12 to assess conditioned locomotor stimulation in the repeated methamphetamine group. Immediately prior to locomotor activity sessions on days 3, 5, 7, and 9, mice in the repeated methamphetamine group received methamphetamine injections, while mice in the repeated saline group received equal volume vehicle injections. On day 11, a methamphetamine injection was given to all mice from both the repeated methamphetamine and repeated saline groups before the locomotor activity session to assess sensitization and acute locomotor stimulation to methamphetamine, respectively. For experiment 2, mice were subjected to a 3-day injection+locomotor activity protocol. All groups received saline injections immediately preceding the locomotor activity session on days 1 and 2 and a methamphetamine challenge injection on day 3.
Fig. 1.

Repeated injection+locomotor activity protocols. Mice were administered i.p. injections and immediately placed into activity monitors for 30-min sessions. For experiment 1 (top), mice were split into two groups, repeated methamphetamine (n=6) and repeated saline (n=7). Injections of saline (S) or methamphetamine (M) and locomotor activity measurements were performed on days 1, 2, 3, 5, 7, 9, 11, and 12. For experiment 2 (bottom), all mice were subjected to the same 3-day protocol. Food-restricted mice were maintained at their reduced body weight for at least 5 days before behavioral testing was initiated. Intraperitoneal injections of saline (S) were then administered on days 1 and 2, followed by a methamphetamine (M) challenge injection on day 3
Serum corticosterone
A combination of naive mice and mice from experiment 2 were used for corticosterone assessment with at least 4 days allowed to elapse between methamphetamine injection and blood collection. At the time of sacrifice, mice were rapidly removed from their home cage, taken to a separate room, deeply anesthetized with isoflurane, and decapitated for collection of trunk blood. Decapitation occurred within 45 s of moving the cage and within 35 s of lifting the cage top. The blood samples were stored on ice until centrifuged for serum extraction. Serum samples were subsequently stored at −80°C until corticosterone levels were determined with a corticosterone enzyme immunoassay kit (Immunodiagnostic Systems, Fountain Hills, AZ, USA).
Drugs
Methamphetamine hydrochloride was obtained from the National Institute on Drug Abuse Drug Supply Program (RTI International, Research Triangle Park, NC, USA) and was dissolved in 0.9% NaCl (saline) at a concentration of 0.25 mg/ml for i.p. injections.
Analyses
Data were subjected to t tests and one- and two-way analyses of variance (ANOVA) where applicable. Significant ANOVA results were followed by Dunnett’s or Bonferroni’s post hoc tests using GraphPad Prism 5. The threshold for statistical significance (α) was defined at 0.05. Graphs were made using GraphPad Prism 5 and Microsoft Office Excel software.
Results
Our first experiment determined if repeated i.p. injections of 1 mg/kg methamphetamine could produce locomotor sensitization in DBA mice when tested during their dark cycle. The mice that received repeated methamphetamine and repeated saline did not differ in locomotor activity following saline injections on days 1 or 2 (Fig. 2a). Both the repeated saline (D11) and the repeated methamphetamine (D3) groups showed locomotor stimulation to acute methamphetamine but did not differ from each other (t test, P=0.22). As expected, the repeated methamphetamine group exhibited substantial sensitization to the methamphetamine-induced locomotor stimulation compared to both their acute response to methamphetamine (D3) and the repeated saline group response to methamphetamine (D11) (one-way ANOVA; F2,18=44.8 and P<0.0001; Fig. 2b). As reported in previous literature, locomotor stimulation to methamphetamine progressively increased over each subsequent injection.
Fig. 2.
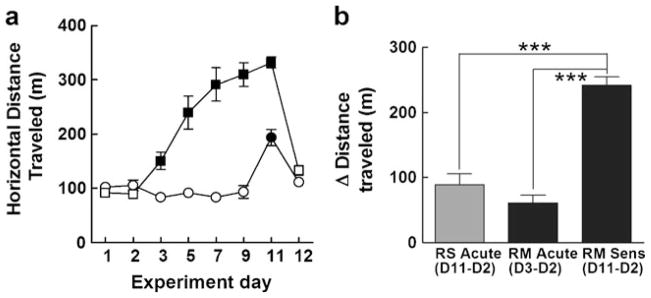
Repeated dark cycle injections of methamphetamine induce locomotor sensitization. Mice were injected on days 3, 5, 7, and 9 with either saline vehicle (a, open symbols) or 1 mg/kg methamphetamine (closed symbols). Both the repeated saline (circles) and repeated methamphetamine (squares) groups were given a methamphetamine challenge injection on day 11 and vehicle on day 12. The repeated methamphetamine mice exhibited significantly increased horizontal locomotion on day 11 when compared to their acute response to methamphetamine on day 3 and when compared to the acute response to methamphetamine for the repeated saline group on day 11 (b). Bonferroni’s multiple comparison test, ***P<0.001
Having established that mice tested in this manner are capable of substantial sensitization to the locomotor effects of 1 mg/kg methamphetamine, we next examined the effects of food restriction and meal schedule on methamphetamine-induced locomotor activity. Mice were placed into five groups (see the “Materials and methods” section): Ad Lib controls, 8% binge, 8% meal, 16% binge, and 16% meal. Initial and ending body weights are reported in Table 1. All four food-restricted groups exhibited slightly higher baseline locomotion than Ad Lib controls on the first chamber habituation day (day 1), but this was not statistically significant. This trend toward increased baseline locomotion was less pronounced on day 2 (data not shown).
Table 1.
Weights and baseline locomotion of food-restricted mice and ad libitum-fed controls
| Starting BW (g) | End BW (g) | ΔBW (%) | D1 30 min LA (m) | |
|---|---|---|---|---|
| Ad lib (n=10) | 21.3±1.28 | 23.4±1.02 | +10.7±2.15 | 94.9±5.64 |
| 8% binge (n=10) | 24.5±0.69 | 22.3±0.46 | −8.5±1.05 | 110.7±4.69 |
| 8% meal (n=13) | 24.6±0.64 | 22.6±0.58 | −8.0±0.40 | 110.4±4.98 |
| 16% binge (n=8) | 24.0±0.32 | 19.9±0.30 | −17.1±0.60 | 106.7±3.72 |
| 16% meal (n=9) | 24.1±0.52 | 19.9±0.37 | −17.4±0.94 | 114.5±5.75 |
Data are depicted describing starting and ending body weights (BW, in grams), percent change in body weight (ΔBW), and day 1 locomotor activity (LA, 30-min horizontal activity) for each group. Mice were maintained at a stable reduced body weight for at least 5 days before behavioral studies were initiated. A one-way ANOVA comparing horizontal distance traveled in 30 min on the habituation day was not significantly different between groups (P=0.22)
Chronic moderate food restriction to approximately 16% weight loss produced substantial enhancement of methamphetamine-induced horizontal locomotion at both 15 min (one-way ANOVA, F4,49=5.95, P=0.0006) and 30 min (one-way ANOVA, F4,49=6.00, P=0.0006) when compared to Ad Lib controls. This was true whether mice were fed once per day or in three meals (30 min data; Dunnett’s multiple comparison test; P<0.05 for binge, P< 0.001 for meal; Fig. 3). However, when mice were subjected to milder food restriction (8% weight loss), only the mice that were fed in three meals exhibited a statistically significant enhancement of horizontal locomotion when compared to Ad Lib controls (30 min data; Dunnett’s multiple comparison test; not significant for binge, P<0.05 for meal; Fig. 3). When the Ad Lib group was removed, analysis of the food-restricted mice revealed significant main effects of both meal schedule and restriction intensity (two-way ANOVA; main effect of schedule, F1,36=4.6, P=0.04; main effect of restriction intensity, F1,36=6.0, P=0.02). Data measuring total vertical beam breaks and total resting time were also analyzed over the first 15 min of the session from the same days (Fig. 4). A similar pattern was observed for each dependent variable, with all food-restricted groups except for the 8% binge group significantly different than Ad Lib controls (vertical beam breaks; one-way ANOVA; F4,48 =4.1, P=0.007; resting time; one-way ANOVA; F4,49=6.4, P=0.004).
Fig. 3.
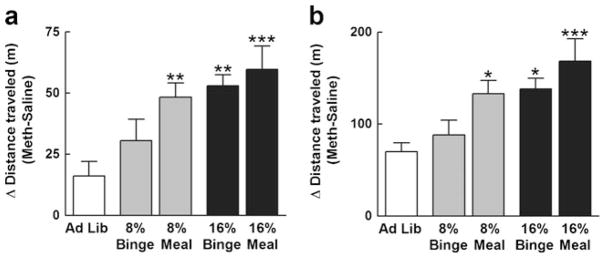
Food restriction increases methamphetamine-induced locomotor activity. On day 3 of the protocol, Ad Lib and food-restricted mice were given an i.p. challenge injection of methamphetamine (1 mg/kg). Data are presented as the difference in forward locomotion subsequent to injection of methamphetamine (day 3) versus saline (day 2). At both 15- and 30-min time points (a and b, respectively), all restricted groups except for the 8% binge group exhibited significantly higher locomotion than Ad Lib controls. Dunnett’s multiple comparison post hoc, *P<0.05; **P<0.01; ***P<0.001
Fig. 4.

Food restriction increases methamphetamine-induced vertical movements and decreases resting time. Data from the first 15 min of the session are presented as the difference in either vertical beam breaks (a) or resting time (b) induced by methamphetamine versus saline on the previous day. For both dependent variables, all restricted groups except for the 8% binge group were significantly different from Ad Lib controls (one-way ANOVA followed by Dunnett’s post hoc test). *P<0.05; **P<0.01; ***P<0.001
Since corticosterone is implicated in food restriction-induced locomotor sensitization to drugs of abuse, serum corticosterone was sampled in mice from all groups at the same time of day as the behavioral testing (but at least 4 days removed from methamphetamine test day). A two-way ANOVA (excluding the Ad Lib group) revealed significant main effects of intensity of food restriction (P=0.0002) and schedule of feeding (P<0.0001) and a significant interaction between the factors (P=0.007). Only the meal-fed groups showed significant increases above Ad Lib controls when compared in a one-way ANOVA with Dunnett’s post hoc analysis (Fig. 5a). Further analysis examining the relationship between serum corticosterone and change in body weight (on the day of the blood sampling) showed a significant correlation between the two factors only in meal mice (P=0.004, R2=0.395), with nonsignificant relationships in the Ad Lib (P=0.65) and binge (P=0.27) groups (Fig. 5b).
Fig. 5.

Corticosterone is increased in meal-fed groups, but not binge-fed groups, when compared to ad libitum-fed mice. Meal-fed mice at both a mild (8% weight loss) and a moderate (16% weight loss) degree of food restriction had significantly higher levels of corticosterone than the Ad Lib-fed mice. The corticosterone level of binge-fed mice did not significantly differ from the Ad Lib mice at either degree of food restriction (a, n=8–12/group). Similarly, only the meal-fed mice demonstrated a significant correlation between body weight loss on the day of blood sampling and corticosterone levels (b, correlation line, n=12–20/group, R2=0.395, P=0.004)
Further analysis of the correlation between serum corticosterone and locomotor behavior did not demonstrate a significant relationship between the variables (P=0.10, data not shown). This should be interpreted cautiously because the two measures were not recorded on the same day. However, both the meal (P=0.042) and binge (P=0.019) groups (Fig. 6) did exhibit significant correlations between locomotor stimulation to methamphetamine and body weight loss recorded on that same day. Thus, taken together, these data support the notion that the locomotor stimulation to methamphetamine is related to the corticosterone levels.
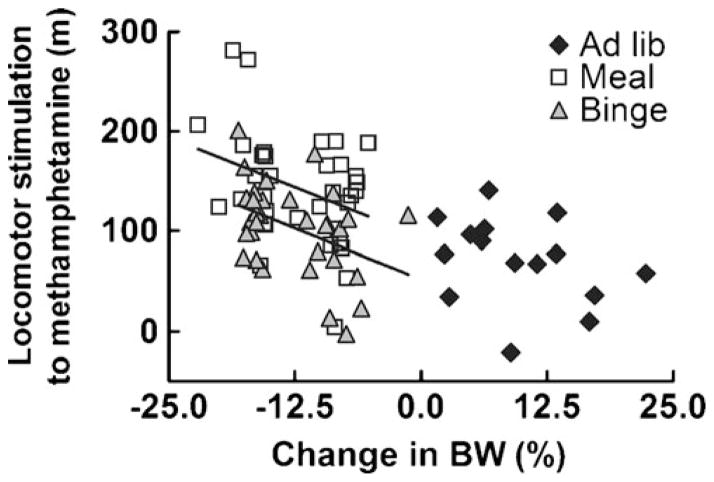
Fig. 6.
Food-restricted mice show a significant correlation between body weight loss and stimulation to methamphetamine. Both meal-and binge-fed food-restricted mice showed a significant correlation between change in body weight and locomotor stimulant effects of methamphetamine (R2=0.12 and 0.17, P=0.042 and 0.019, respectively). The regression lines for meal- and binge-fed mice exhibited similar slopes but different y-intercepts (n=15–35/group)
Discussion
This is the first detailed investigation into the effects of feeding schedule and food restriction intensity on methamphetamine-induced locomotion. Food restriction produced a robust enhancement of methamphetamine-induced horizontal and vertical locomotion, a finding that is consistent with effects observed using other psychostimulants. In mice that received chronic moderate food restriction (to 16% body weight loss), the enhancement of methamphetamine-related effects was due to the food restriction per se and was independent of the number of meals per day. However, milder food restriction (to 8% body weight loss) produced enhancement of methamphetamine-induced locomotion only in mice that were fed three meals per day. Interestingly, serum corticosterone levels were elevated over Ad Lib controls only in the meal-fed mice. This association suggests that the mechanism responsible for food restriction-induced enhancement of methamphetamine sensitivity could be more complicated than an acute elevation of corticosterone at the time of behavioral analysis.
Effects of food restriction versus binge feeding on drug-related behaviors
Food restriction increases both the acute behavioral effects and self-administration of most, if not all, major classes of abused drugs (Bell et al. 1997; Carroll et al. 1979, 1981). Many laboratories that study drug self-administration use food restriction to enhance the animal’s motivational state and to increase the speed and reliability with which they learn to respond for the drug. This is typically done by feeding the subjects in one large meal per day. Our results suggest that this binge-like food restriction is no more effective at producing sensitization than feeding the subjects in three restricted meals, although the mechanisms that produce an increase in locomotion may not entirely overlap with those responsible for drug self-administration. The intensity of food restriction and the relationship between the time of feeding and the behavior may, however, be critically important determinants of the increased sensitivity. As discussed below, our results suggest that behavioral sensitivity to drugs of abuse may be enhanced near the time of corticosterone peak associated with feeding. Thus, we hypothesize that behavioral sensitivity to methamphetamine is lower after feeding (when corticosterone is decreasing) than if it were tested before feeding (when corticosterone is high; see Donny et al. 1998).
Given that binge eating and bulimia are associated with substance abuse in human populations, we were surprised to observe that binge-like behavior itself did not produce an increase in methamphetamine sensitivity. Avena and Hoebel (2003) previously reported that cyclic chow access (12 h/day only) produced a small but significant increase in locomotor stimulation to a low dose of amphetamine, an effect that was more pronounced in rats that had cyclic consumption of sugar and chow. They hypothesized that cyclic feeding may have intermittently activated the dopaminergic system sufficiently to produce sensitization. In that study, no difference in body weight was observed between the cyclic- and ad libitum-fed groups and the behavioral response to amphetamine was assessed 9 days after the cyclical feeding procedure was discontinued. Given these results, we would have expected methamphetamine sensitization in both the 8% meal and 8% binge groups. Avena and Hoebel did not investigate corticosterone levels, and thus, it is not known if this may have played a role in the differences between our findings.
The role of stress in food restriction-induced sensitization
Previous work suggests that chronic stress may be largely responsible for the behavioral effects produced by underfeeding. In rodents, food restriction increases circulating corticosterone levels several fold (Broocks et al. 1990; Klebanov et al. 1995). Changes in drug-induced locomotion can be blocked by adrenalectomy (Deroche et al. 1993), and improved acquisition of cocaine self-administration can be blocked by inhibiting corticosterone synthesis (Campbell and Carroll 2001). The results presented here suggest that moderate food restriction (similar to levels previously shown to increase 24-h corticosterone) enhances sensitivity to methamphetamine regardless of feeding schedule. However, corticosterone measures at the time of behavioral testing indicate that the 16% binge group did not have elevated levels at that time. This suggests that, at this level of food restriction, acute basal increases in corticosterone are not necessary to observe increased sensitivity to methamphetamine.
At the more modest food restriction level (8% loss of body weight), increased locomotor sensitization to methamphetamine was only seen in the group of mice fed multiple meals. Serum corticosterone levels for these groups suggest that only the meal group displayed a significant increase compared to ad libitum-fed mice at the time of the behavioral assay. These data agree with previous findings that corticosterone acutely peaks prior to scheduled meals, but would be lower in binge-fed mice that had already received their daily meal (Gallo and Weinberg 1981; Honma et al. 1983, 1984). It is possible that, in the 8% meal group, acute increases in corticosterone might have contributed to the enhanced locomotor activation in response to methamphetamine. Corticosterone itself can sustain self-administration and also facilitates the positive reinforcing effects of drugs of abuse (for a review, see Piazza and Le Moal 1997). Indeed, acute administration of the corticosterone synthesis inhibitor metyrapone reverses the food restriction-induced increase in locomotion produced by cocaine (at 10% body weight loss; Rougé-Pont et al. 1995; Marinelli et al. 1996). Thus, an acute rise in corticosterone may be sufficient (as in the 8% meal group), but not necessary (as in the 16% binge group) for the increased sensitivity to the locomotor stimulatory effects of methamphetamine. Future studies determining the effects of food restriction on a full methamphetamine dose–response curve (instead of just one dose) may help to clarify the precise contribution of acute corticosterone to locomotor stimulation.
Is food restriction-induced sensitization produced by changes in dopamine?
Sensitization to repeated injections of methamphetamine (Fig. 2) has been previously reported (e.g., Phillips et al. 1994), although, to our knowledge, never before during the animals’ dark cycle. The large magnitude of sensitization produced by repeated methamphetamine injections gave us confidence that monitoring locomotor activity for 30 min would be a sensitive indicator of the behavior produced by an acute challenge of methamphetamine. Moderate food restriction also produced a robust sensitization to a challenge injection of methamphetamine, an effect that was approximately the same amplitude as that produced by repeated drug injections. Drug-induced sensitization has been studied more extensively than food restriction- or stress-induced sensitization. Initiation of psychostimulant sensitization occurs at the dopamine neuron cell bodies in the ventral tegmental area and is thought to involve D1 receptor signaling, glutamate receptors, and L-type calcium channels (Bjijou et al. 1996; Pierce et al. 1996, 1998; Vezina 1996). The mechanisms responsible for feeding- or stress-induced sensitization are largely unknown but appear to be at least partially different than those responsible for the effects produced by repeated administration of psychos-timulants (Carr et al. 2003). For instance, food restriction lowers the threshold of drug reward in intracranial self-stimulation paradigms (Cabeza de Vaca and Carr 1998) while repeated drug administration does not do so (Bauco and Wise 1997). Both stress and noncontingent injections of psychostimulants do produce an increase in the AMPA receptor/NMDA receptor signaling ratio in dopamine neurons (Ungless et al. 2001; Borgland et al. 2004; Saal et al. 2003). So while some overlapping cellular mechanisms could be shared by drug- and food restriction-induced sensitization, the extent has not been determined.
Although never previously demonstrated, the observation that food restriction produces an enhancement of methamphetamine-induced locomotion was not surprising given the established literature concerning food restriction and other psychostimulants (Deroche et al. 1993; Macenski and Meisch 1999; Marinković et al. 2007; Stuber et al. 2002). Cocaine, D-amphetamine, and methamphetamine all inhibit the uptake of extracellular dopamine through inhibition of the dopamine transporter, and considerable evidence suggests that central dopaminergic mechanisms are involved in the interaction between food restriction and drug effects. For instance, dopamine D1 receptor antagonists block underfeeding-induced augmentation of amphetamine-induced locomotion (Carr et al. 2001). Food restriction decreases dopamine uptake (Sevak et al. 2008; Zhen et al. 2006) and increases dopamine receptor protein and mRNA levels in the striatum (Thanos et al. 2008; Lindblom et al. 2006), suggesting that dopamine function in brain terminal regions is upregulated. Consistent with this notion, food restriction increases the G protein coupling of D2 receptors in dopamine terminal regions and augments the effects of dopamine receptor agonists on locomotion (Carr et al. 2001, 2003). Finally, food restriction decreases D2 receptor agonist-induced yawning and D2 receptor antagonist-induced catalepsy (Baladi and France 2009; Sevak et al. 2008; Collins et al. 2008), further suggesting that feeding state affects central dopaminergic mechanisms. Despite behavioral and neurochemical evidence implicating dopamine, the physiological consequences of food restriction and the precise cellular and synaptic mechanisms responsible for them have not been identified.
Studies are underway to identify specific brain regions and neurotransmitters responsible for the effects of food restriction and determine if food restriction-induced sensitization is produced by similar mechanisms as sensitization produced by repeated drug administration. Elucidation of the cellular and synaptic processes that occur in response to chronic food restriction could improve our understanding of the neurophysiological underpinnings of drug intake and suggest treatment strategies for drug abusers and patients with chronic eating disorders. Moreover, the determination of the precise effects of feeding schedule could be used to develop better strategies for caloric restrictors to minimize the risk of drug intake or relapse.
Acknowledgments
This work was funded by a Research Enhancement Fund through the San Antonio Life Sciences Institute, a K01 award through the National Institutes of Health (DA21699) to MJB, and an American Heart Association grant (SDG4350066) to ALS. The authors acknowledge that there are no conflicts of interest, either real or perceived.
We would like to thank Sarah Y. Branch, Calais S. Williams, and Dr. Carlos A. Paladini for their helpful contributions. All experiments performed comply with the state, local, and federal laws.
Contributor Information
Amanda L. Sharpe, Department of Physiology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, MSC7756, San Antonio, TX 78229, USA
Joshua D. Klaus, Department of Physiology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, MSC7756, San Antonio, TX 78229, USA
Michael J. Beckstead, Email: beckstead@uthscsa.edu, Department of Physiology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, MSC7756, San Antonio, TX 78229, USA. Center for Biomedical Neuroscience, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, MSC7756, San Antonio, TX 78229, USA
References
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Baladi MG, France CP. High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol. 2009;610:55–60. doi: 10.1016/j.ejphar.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Synergistic effects of cocaine with lateral hypothalamic brain stimulation reward: lack of tolerance or sensitization. J Pharmacol Exp Ther. 1997;283:1160–1167. [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Bjijou Y, Stinus L, Le Moal M, Cador M. Evidence for selective involvement of dopamine D1 receptors of the ventral tegmental area in the behavioral sensitization induced by intra-ventral tegmental area injections of D-amphetamine. J Pharmacol Exp Ther. 1996;277:1177–1187. [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broocks A, Schweiger U, Pirke KM. Hyperactivity aggravates semistarvation-induced changes in corticosterone and triiodothyronine concentrations in plasma but not luteinizing hormone and testosterone levels. Physiol Behav. 1990;48:567–569. doi: 10.1016/0031-9384(90)90301-j. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacology (Berl) 2001;154:311–318. doi: 10.1007/s002130000627. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacology (Berl) 2001;154:420–428. doi: 10.1007/s002130000674. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology (Berl) 1981;74:197–200. doi: 10.1007/BF00427092. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–247. [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Le Moal M, Simon H. Sensitization to the psychomotor effects of amphetamine and morphine induced by food restriction depends on corticosterone secretion. Brain Res. 1993;611:352–356. doi: 10.1016/0006-8993(93)90526-s. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Holderness CC, Brooks-Gunn J, Warren MP. Co-morbidity of eating disorders and substance abuse review of the literature. Int J Eat Disord. 1994;16:1–34. doi: 10.1002/1098-108x(199407)16:1<1::aid-eat2260160102>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Honma KI, Honma S, Hiroshige T. Critical role of food amount for prefeeding corticosterone peak in rats. Am J Physiol. 1983;245:R339–R344. doi: 10.1152/ajpregu.1983.245.3.R339. [DOI] [PubMed] [Google Scholar]
- Honma KI, Honma S, Hiroshige T. Feeding-associated corticosterone peak in rats under various feeding cycles. Am J Physiol. 1984;246:R721–R726. doi: 10.1152/ajpregu.1984.246.5.R721. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Klebanov S, Diais S, Stavinoha WB, Suh Y, Nelson JF. Hyperadrenocorticism, attenuated inflammation, and the life-prolonging action of food restriction in mice. J Gerontol A Biol Sci Med Sci. 1995;50:B78–B82. doi: 10.1093/gerona/50a.2.b78. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Johansson A, Holmgren A, Grandin E, Nedergård C, Fredriksson R, Schiöth HB. Increased mRNA levels of tyrosine hydroxylase and dopamine transporter in the VTA of male rats after chronic food restriction. Eur J Neurosci. 2006;23:180–186. doi: 10.1111/j.1460-9568.2005.04531.x. [DOI] [PubMed] [Google Scholar]
- Macenski MJ, Meisch RA. Cocaine self-administration under conditions of restricted and unrestricted food access. Exp Clin Psychopharmacol. 1999;7:324–337. doi: 10.1037//1064-1297.7.4.324. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res. 1996;724:251–255. doi: 10.1016/0006-8993(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Marinković P, Pesić V, Loncarević N, Smiljanić K, Kanazir S, Ruzdijić S. Behavioral and biochemical effects of various food-restriction regimens in the rats. Physiol Behav. 2007;92:492–499. doi: 10.1016/j.physbeh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Born B, Adams M, Kalivas PW. Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. J Pharmacol Exp Ther. 1996;278:384–392. [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol. 2008;592:109–115. doi: 10.1016/j.ejphar.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse. 2008;62:637–642. doi: 10.1002/syn.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Reith ME, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]