Abstract
Alloimmune platelet refractoriness (alloPR) among actively bleeding surgical patients with thrombocytopenia represents a life-threatening problem. Here we present three cases in which surgical bleeding was complicated by life-threatening thrombocytopenia and alloPR. We demonstrate that the human leukocyte antigens (HLA) antibodies associated with alloPR are broadly reactive and in high concentration, are not removed by hemodilution, and are not absorbed by transfusion of multiple doses of platelet concentrates. HLA alloPR may be under-recognized among surgical patients. Research is needed to develop pre-operative screening methods that will identify patients in need of specialized platelet support using HLA compatible donor products.
Introduction
Alloimmunization to human leukocyte antigens (HLA) remains a major hurdle in achieving successful transplantation and is the primary cause of alloimmune platelet refractoriness (alloPR) [1-3]. Platelet transfusion support of HLA allosensitized patients presents distinct problems for the blood transfusion service. Whereas patients are routinely tested for red blood cell (RBC) alloantibodies prior to blood transfusion, no screening test is routinely performed for HLA alloantibodies prior to platelet transfusion. Moreover, there are no data available validating the use of any screening test for HLA alloantibodies as a reliable predictor of platelet refractoriness [4,5]. In the Trial to Reduce Alloimmunization to Platelets, the incidence of HLA alloimmunization exceeded that of clinical platelet refractoriness by twofold [4]. Thus, alloPR is currently recognized when inadequate platelet increments are documented following platelet transfusion [1-3]. Furthermore, while all blood banks have ready access to an inventory of antigen-typed RBC units that are compatible and available for transfusion to the majority of patients who are sensitized to red cell antigens, very few blood banks have an inventory of HLA-typed platelet components.
While alloPR due to HLA antibodies is well recognized among patients with chemotherapy-related thrombocytopenia [4,6], there are little data describing its frequency in other patient populations. The reported prevalence of detectable HLA antibodies in normal male and nulliparous female blood donors ranges from 1 to 17% and increases to 24-53% in females with prior pregnancy [7-10]. Pregnancy imparts a greater risk of HLA allosensitization compared with blood transfusion [8] and an increasing number of pregnancies is associated with higher HLA allosensitization rates [7,9]. In addition, there are data suggesting an increased prevalence of HLA alloantibodies among patients with antibodies to RBC antigens [11,12]. Given these data, it is reasonable to expect alloPR to occur outside of the setting of hematologic malignancies and that prior pregnancy and/or known RBC alloimmunization may identify at-risk patients.
Here, we present three surgical cases in which alloPR due to HLA antibodies compromised transfusion support for active bleeding and thrombocytopenia during major surgery and resulted in life-threatening hemorrhage. We show serologic evidence that HLA antibodies are found in much higher concentration than RBC antibodies and cannot be sufficiently “washed out” during massive transfusion or “adsorbed out” by transfusion of multiple doses of platelets. Given that alloPR is not typically considered in the context of intra-operative thrombocytopenia, it may play an under-recognized role in bleeding morbidity and mortality in the surgical patient population.
Patient 1
A male patient with Marfan's syndrome and an extensive history of prior cardiac surgery and multiple blood transfusions presented for a repeat aortic arch repair. His pre-surgical platelet count was 106,000/μL. A pre-operative specimen was noted to have anti-C and anti-Fya RBC antibodies. Intra-operatively, 10 units of compatible packed RBCs (C—, Fya —) were administered. Continued bleeding and depletion of all available Fya-negative RBCs necessitated switching to (C— Fya+) packed RBCs after an intra-operative specimen showed only weak reactivity with Fya+ cells. The patient also received numerous fresh frozen plasma (FFP) and platelet transfusions (see Table I). During surgery, the patient's platelet count dropped to 21,000/μL. In response to a 6 unit dose of platelets, the patient's platelet count increased to 30,000/μL within minutes of platelet transfusion, representing a corrected-count increment of 4950/μL. Transfusion of an additional 18 units of platelets increased the platelet count to only 46,000/μL and 45 min later, the patient's platelet count was only 30,000/μL despite transfusion of an additional 21 units of platelets. A presumptive diagnosis of alloPR was confirmed by detection of anti-HLA antibodies. Single antigen bead studies identified 68 different HLA Class I antigen specificities. The patient continued to bleed in the post-operative period despite administration of platelets, plasma, recombinant activated factor VII, and epsilon aminocaproic acid (EACA) and required surgical re-exploration. The highest platelet count obtained during surgery or the post-operative period was 98,000/μL with a nadir of 10,000/μL. He expired four days after the initial surgery after withdrawal of care.
Table I. Transfusion Data for the First Intra-operative Course.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| HLA type | A2, B7, B27 | A2, B27, B58 | A29, A68, B44 |
| Number of HLA Class I | A = 20 | A = 15 | A = 19 |
| allospecificitiesa | B = 40 | B = 26 | B = 42 |
| C = 8 | C=0 | C = 15 | |
| TBV, calculated | 5.6 L | 4.6 L | 3.8 L |
| Pre-surgery platelet count (× 103 per μL) | 106 | 351 | 206 |
| RBC (units) | 21 | 30 | 14 |
| FFP (units) | 14 | 16 | 11 |
| PLT (doses)b | 16 | 2 | 2 |
| OR time | 16 h | 9 h | 7 h |
| Blood product volume transfusedc | 16 L | 12.8 L | 7 L |
| Blood volume exchanged | 2.9 × | 2.8 × | 1.8 × |
| Ave Rate of transfusion (Liters/hour, L/hr) | 1 L/hr | 1.4 L/hr | 1 L/hr |
TBV: total blood volume, RBC: packed red blood cell, FFP: fresh frozen plasma, PLT: platelet concentrate, OR: operating room.
HLA Class I antigens specificities identified by a bead-based single antigen assay; the assay is designed to identify antibodies with specificity against 21 HLA-A, 43 HLA-B and 15 HLA-C antigens.
A dose of platelets is equal to one apheresis unit or 6-units of pooled platelet concentrates
Assumes the following volumes per blood product: RBC = 300 mL, FFP = 200 mL, and PLT = 300 mL. Patient 1 also received 1.5 L of blood collected by intraoperative blood recovery that was included in calculating the blood product volume given.
Patient 2
A female patient with localized pelvic osteosarcoma underwent neoadjuvant chemotherapy and radiation eight weeks prior to surgical resection of the tumor. The patient had two pregnancies at least 15 years prior to surgery and only one non-leukoreduced red cell transfusion 12 days prior to her surgery. Her pre-transfusion specimen did not have any red cell alloantibodies. Her pre-operative platelet count was 351,000/μL. Intra-operatively, the patient received massive transfusion support (see Table I). In response to an intra-operative platelet count of 49,000/μL, the patient was transfused 12 units of platelets, with no increment and a platelet count of 36,000/μL at the conclusion of the surgery ∼4 hr later. An additional 24 units of platelets were transfused over the following 7 hr in response to platelet counts below 20,000/μL and in the setting of ongoing hemorrhage. None of these platelet transfusions resulted in any documented increase in the platelet count. A presumptive diagnosis of alloPR was confirmed by the detection of anti-HLA antibodies. Single antigen bead studies identified 41 HLA Class I antigen specificities. The patient continued to bleed despite packing of the wound, administration of EACA, and continued blood product support. The highest platelet count obtained during surgery and the post-operative period was 36,000/μL with a nadir of 8,000/μL. She expired seven days following surgery from sepsis.
Patient 3
A female patient with recurrent chondrosarcoma underwent radiation therapy prior to surgery. The patient had at least four prior pregnancies but no previous transfusions. Her pre-operative platelet count was 206,000/μL. Intra-operatively, the patient required massive transfusion support (see Table I). During the operative procedure, the patient was transfused 12 units of platelets in response to a platelet count of 32,000/μL. A platelet count taken within 60 min of platelet transfusion was 31,000/μL. In the post-operative period, an additional 6 units of platelets were administered for a platelet count of 24,000/μL and a repeat platelet count taken within 60 min of transfusion was 23,000/μL. A presumptive diagnosis of alloPR was made and later confirmed by the detection of anti-HLA antibodies. Single antigen bead studies identified 76 HLA Class I antigen specificities. The highest platelet count obtained during surgery and the post-operative period was 39,000/μL with a nadir of 19,000/μL. Bleeding was subsequently controlled and transfusion support was minimal in the postoperative period. She was subsequently discharged to a rehabilitation center.
Methods
The study protocol, including sample collection and medical record review, was approved by the Massachusetts General Hospital (MGH) Institutional Review Board. HLA Class I type was determined by either a serologic method (Lambda Monoclonal Typing Trays™ , One Lambda) or by molecular/DNA hybridization (Labtype™ SSO, One Lambda).
For antibody studies, discarded EDTA-anticoagulated blood specimens from the Massachusetts General Hospital Blood Bank were collected and frozen for later testing. Screening for the presence of RBC antibodies was performed using the anti-human IgG ID Microtyping System™ (Ortho Clinical Diagnostics) gel card system. RBC antibody identification was performed by tube testing using an extended red cell panel (Panocell, Immucor). RBC antibody reactivity was monitored using select heterozygous antigen-positive RBCs. Standard tube titration techniques for RBC antibodies were performed as described [13].
A solid-phase multiplex bead flow cytometric assay (Luminex™) using HLA Class I single antigen beads (One Lambda) was used to determine the specificity of HLA antibodies. A mean fluorescence intensity (MFI) of ≥1000 was used as a positive cut-off. For antigen specificities represented in multiple beads, the average MFI of the beads was used. Dilutions of plasma specimens were made using sterile phosphate buffered saline as diluent. The Luminex™ based C1qScreen (One Lambda) was used to assess complement fixation. The calculated panel reactive antibody (CPRA) was determined using the online “CPRA calculator” provided by the Organ Procurement and Transplantation Network (optn.transplant.hrsa.gov).
Adsorption studies were performed on patient plasma by using a pooled platelet concentrate (PPC) prepared from 35 donors. Briefly, sodium azide (final concentration 0.2%) was added within 24 hr to outdated platelet concentrates which were kept on a shaker at room temperature. Equal volume aliquots of 35 outdated platelet concentrates were combined to make the PPC (concentration of ∼1 × 106 platelets/μL). PPC volumes of 1 mL, 500 μL, and 50 μL were centrifuged at 200g for 5 min and the donor plasma carefully separated from the resulting PPC pellet. About 50 μL of patient plasma was added to the PPC pellet and mixed gently to create a slurry. The platelet-plasma slurry was incubated at room temperature for 30 min then centrifuged at maximum speed (∼17,000g) for 5 min. The adsorbed plasma was carefully removed and tested by Luminex™ single antigen bead.
Results
In this series, all three patients displayed clinical refractoriness to platelet transfusions in the intra-operative and post-operative period as evidenced by poor or absent platelet count increments following transfusion. Non-alloimmune causes of refractoriness to platelet transfusion were considered unlikely in these patients. All three were afebrile and clinically stable before surgery so platelet consumption related to sepsis was not a consideration. None were taking antimicrobial agents implicated in platelet destruction or refractoriness to platelet transfusion prior to surgery. Patients 2 and 3 did not have splenomegaly and had normal platelet counts before surgery. Patient 1 had mild thrombocytopenia attributable to mild splenomegaly and microangiopathic hemolytic anemia (MAHA) secondary to dysfunction of the prosthetic aortic valve. He was also being given unfractionated heparin prior to surgery. However, the clinical picture of rapid clearance of platelets despite multiple transfusions strongly favored an immune-mediated over these clinical factors. Similarly, although bleeding has been implicated in a mild reduction in post-transfusion platelet count increments [14], it was deemed an insufficient explanation for the severity of clinical refractoriness to platelet transfusion observed in these patients based on prior experience with similar surgical cases. Select transfusion data are provided for the first OR course (Table I) and in the perioperative period that required transfusion support (see Supporting Information Table II).
To determine whether anti-HLA antibodies were affected in vivo by massive transfusion, we compared antibody levels using a single antigen bead assay in pre-transfusion and post-operative blood samples in the two patients who received the largest volume of blood resuscitation. For each patient, the MFI of representative antibody specificities against HLA Class I antigens are presented. A single Class II specificity is also included (HLA-DR17). These specificities were selected because they represent commonly found HLA-A and -B antigens in the donor population and both patients made strong antibodies against these antigens (except HLA-B44). In the case of Patient 1 (Fig. 1 A), pre-operative MFI values were lower on post-operative day 1 following massive transfusion. Interestingly, there was a suggestion of a rebound in antibody levels (as determined by MFI) from POD1 to POD3. In contrast, the preoperative HLA antibodies in Patient 2 appeared to increase over time, despite massive transfusion (Fig. 1B). The combined antibody trends for Patient 1 and 2 are shown (Fig. 1C). Since, the MFI of the HLA alloanti-bodies approached the saturation range for the bead-based assay in both the pre-operative and post-operative blood samples, assays were repeated and findings confirmed using plasma diluted 100-fold (data not shown). Additionally, the CPRA was determined for pre-operative and post-operative blood samples and was found to be largely unchanged despite massive volume resuscitation (see Supporting Information Table III). Thus, in both these patients, HLA alloantibodies remained at high levels and demonstrated broad alloreactivity despite massive volume resuscitation and blood replacement. Any declines in MFI due to massive transfusion appeared to be transient.
Figure 1.
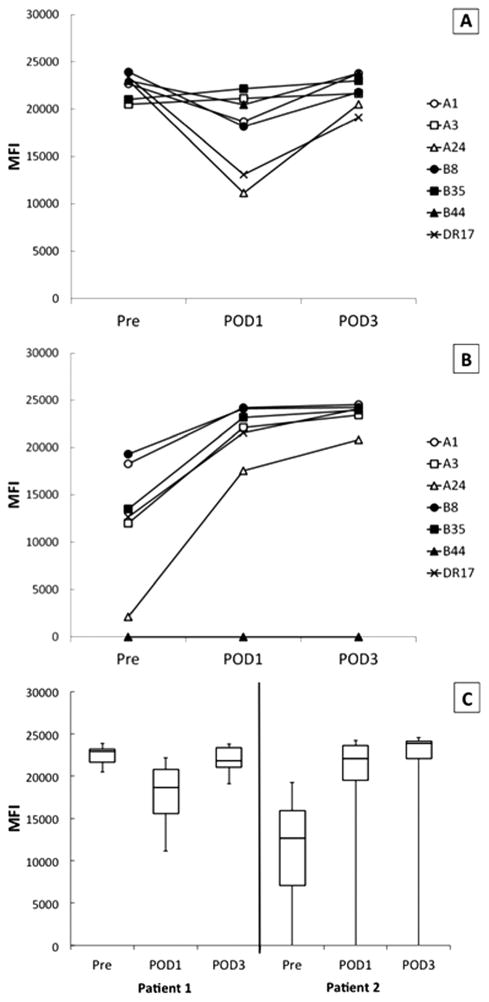
Effect of massive transfusion and bleeding on anti-HLA antibodies. Antibodies against common HLA Class I antigens are shown as representative examples of the effect of massive transfusion on specific HLA allo-specificities; DR17 (an HLA Class II antigen not present on platelets) is shown as a control for nonspecific changes in MFI reactivity. (A) Patient 1 and (B) Patient 2 antibodies over time. (C) The combined MFI of the selected antibodies are shown depicted in a box and whisker graph where the box denotes the interquartile range (IQR), the line inside the box represents the median and the whiskers indicate the maximum and minimum. MFI: mean fluorescence intensity units; Pre: blood sample prior to massive transfusion; POD1: post-operative day 1; and POD3: post-operative day 3.
Patient 1 also had RBC alloantibodies against the red cell antigens C and Fya that were identified in the pre-operative specimen. We therefore monitored the strength of this patient's RBC alloantibodies over the same time course as that used to measure HLA allo-antibodies. Preoperative antibody reactivity against Fya+ and C+ red cells was 2 + and weakly reactive, respectively. Neither red cell antibody was reactive in specimens from POD1 and POD3. We observed that, in contrast to his HLA alloantibodies, the RBC alloantibodies became undetectable. These data suggest that the HLA alloantibodies in this patient were at a significantly higher concentration than his RBC alloantibodies.
Because blood volume exchanged in vivo was determined by the degree of hemorrhage and the consequent volume resuscitation and blood transfusion, we sought to quantify the degree of hemodilution required to decrease the MFI of detectable anti-HLA alloantibodies below 1000. We therefore performed in vitro dilutions of the preoperative specimens from all three patients and tested the plasmas using the single-antigen bead-based assay. Results are presented as the CPRA as this metric takes into account the frequency of a given HLA antigen in the donor population and therefore better estimates the likelihood of finding an antigen negative donor [15]. All three of these patients had clinically significant CPRAs in pre-operative samples even when diluted 100-fold (see Supporting Information Table III). Indeed, for Patients 1 and 3, the pre-operative CPRAs remained at 100% despite 1,000-fold dilution. Although the pre-operative and POD1 CPRAs were decreased by dilution in Patient 2, over her clinical course, the MFI of HLA alloantibodies increased significantly (Fig. 1C). Thus, by POD3 the CPRA remained at 100% despite 1,000-fold dilution (see Supporting Information Table III). These data corroborate our earlier observations that HLA alloantibodies can occur in extraordinarily high titer, and support the notion that such antibodies could not be effectively removed by massive transfusion, exchange transfusion, or pre-operative plasmapheresis.
HLA antibodies can be removed from plasma in vitro using platelet adsorption [16], a technique utilized in the clinical laboratory to reduce interference by certain HLA antibodies during RBC alloanti-body identification. Using a similar approach, we determined whether allogeneic platelets used for transfusion are able to adsorb out anti-HLA antibodies in the plasma from platelet refractory patients. Incubation of patient plasma with increasing numbers of platelets reduced the MFI of HLA Class I alloantibodies (Fig. 2). Because platelets do not express HLA Class II (HLA-DR) molecules [17], adsorption of anti-HLA-DR17 was used as a control for non-specific adsorption. Not all Class I HLA antibodies are partially adsorbed out by PPC equally (e.g., B44 antibody). While HLA-B44 antigen has a frequency (∼20%) that would predict representation in the 35-donor platelet pool used for adsorption, the observed lack of adsorption may be due to differences in the affinities of the HLA antibodies, variable HLA-B44 antigen expression on platelets [18], or a lack of B44 donors in the platelet pool used for adsorption. The equivalent of 200 therapeutic doses of platelets (1,200 units of platelets) decreased the MFI reactivity to only one of the five representative HLA Class I antigens below 1,000 (i.e., anti-B8 antibody decreased from MFI 15,891 down to 565). These data strongly suggest that high-titer anti-HLA alloantibodies cannot be overwhelmed in patients by transfusion of multiple doses of platelets.
Figure 2.
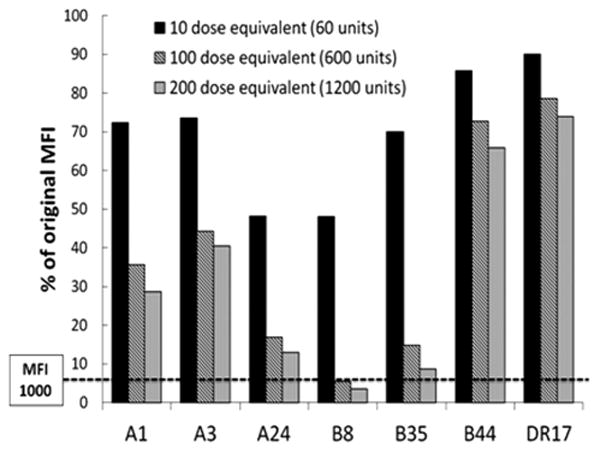
Effect of in vitro adsorption of anti-HLA antibodies using platelet pool concentrate (PPC). Postoperative day 1 plasma from Patient 1 was mixed with varying amounts of PPC. The amount of PPC used to adsorb is quantified as dose equivalents, which is the equivalent number of platelets given in a therapeutic dose (6 units) of platelets when transfused to a 75 kg man with a presumed hematocrit of 45% (plasma volume ∼3 L). A dose of platelets (6 units) introduces ∼3 × 1011 platelets into ∼3L of plasma, resulting in a ratio of 1 × 105 platelets/μL plasma. For instance, a 10-dose equivalent is achieved by adding 5 × 107 platelets to 50 uL of patient plasma, resulting in 10 × 105 platelets/μL plasma. Data are shown as the percent MFI remaining after in vitro adsorption of HLA Class I antibodies compared with unadsorbed plasma (100%).
Finally, it has been suggested that in patients with HLA-mediated alloPR, complement fixing anti-HLA antibodies result in poorer platelet count increments compared with non-complement fixing antibodies [19]. Patient 1's antibodies were complement fixing as determined by the C1q assay (Fig. 3). Interestingly, complement fixation appeared to correlate with antibody titer. After 100-fold dilution, Patient 1's antibodies still had MFIs exceeding 10,000 (Fig. 3A), but the resultant C1q binding to most of the antigens was disproportionally decreased (Fig. 3B).
Figure 3.
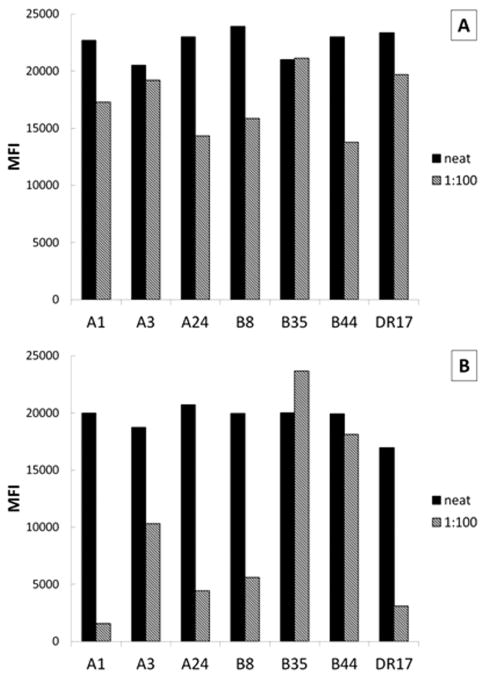
Complement fixation of anti-HLA antibodies in platelet refractoriness. (A) Preoperative plasma from Patient 1 was tested using the single antigen bead assay and compared with (B) the C1q binding assay. Antibodies against common HLA Class I antigens are shown as representatives; DR17 is shown as an example of HLA Class II antigen. MFI - mean fluorescence intensity units.
Discussion
AlloPR due to HLA antibodies in an actively bleeding surgical patient with thrombocytopenia is a serious medical problem. Two of our three patients died in the early post-operative period despite very aggressive transfusion support. There is no specific treatment for bleeding in the setting of alloPR. The only effective intervention currently available for such patients is the provision of platelets from HLA-matched or HLA-compatible donors. Here, we show that anti-HLA alloantibodies implicated in three clinical cases of serious alloPR are found in high concentration, are associated with broad reactivity (high CPRA), are not depleted by massive blood transfusion, and cannot be absorbed by transfusion of multiple therapeutic doses of platelets. Indeed, in Patient 2, HLA alloantibody levels were observed to increase following repeated transfusion of incompatible platelets, demonstrating that such transfusions may only worsen immune refractoriness by stimulating further allosensitization.
In addition to the morbidity and mortality associated with inadequate platelet support of the actively bleeding surgical patient, alloPR is a costly medical problem. According to the National Blood Utilization Survey 2011 [20], hospitals paid an average of $535.17 for an apheresis platelet unit. The average number of platelet doses for the three cases shown here was 21 (median = 27), which translates into $11,238 per patient without clinical benefit, and without accounting for the other expenditures related to the care of the patient.
Critical laboratory tests need to be performed to maximize successful platelet transfusion support in patients who are suspected to have alloPR. These include determining the HLA type (Class I) of the patient and the anti-HLA antibody specificities present in the patient's plasma. Compatible allogeneic platelets may be HLA matched to the patient or lack the antigens to which the patient has demonstrable alloantibodies. HLA alloimmunized patients with high-titer and broadly reactive antibodies or those whose HLA haplotypes are under-represented in the blood donor population pose the greatest challenge to providing compatible/matched platelets for transfusion. Additionally, while HLA matching for platelet transfusion primarily focuses on HLA-A and HLA-B antigens only, alloantibodies against HLA-C resulting in platelet refractoriness have also been reported [21]. Had the HLA antibody specificities of our patients been known prior to surgery, HLA compatible platelets would likely have been found for all three patients as each had at least one common HLA haplotype (frequencies of 4% A2-B7, 7.8% A2-B27, 2.2% A2-B58, and 3% A29-B44; see Table I for patient HLA type) in the USA donor population [22]. Transfusion of compatible platelets with better increments may have translated into the use of fewer transfusions and potentially better outcomes. While selection of HLA compatible platelet products is possible prior to an elective major surgical procedure, obtaining HLA compatible platelets in an emergency is not currently a practical reality.
Patients at risk for ineffective intra-operative platelet support due to HLA-medicated alloPR are not routinely identified pre-operatively Unlike the detection of RBC alloantibodies, there are no processes in place that might help identify pre-operatively which patients harbor high titer HLA antibodies. Research is needed to develop algorithms that will identify surgical patients at risk for alloPR. Unlike the routine “type and screen” for detecting red cell alloantibodies, screening all patients for HLA alloantibodies using current methods is not likely to be a cost-effective strategy. It may be necessary to identify risk factors, in addition to pregnancy and blood transfusions, which increase the pre-test probability of identifying clinically relevant HLA antibodies in patients at risk for platelet refractoriness. One promising risk factor is the concurrent presence of red cell alloantibodies in patients [11,12]. As evidenced in this case series, the number of prior pregnancies may also identify patients at risk for HLA-mediated alloPR.
Pharmacologic agents such as rituximab [23] or bortezomib [24] have been used in patients with alloPR and concomitant hematologic diseases to reduce HLA antibody levels and thereby improve the ease of platelet transfusion support. Similarly, immunosuppressive drugs have been combined with therapeutic plasma exchange to reduce donor-specific HLA alloantibodies and treat or prevent acute humoral rejection of renal allografts [25,26]. However, these approaches require prior recognition of alloPR and investment of substantial time before any efficacy is likely to be observed. Furthermore, they are associated with substantial morbidity in comparison with transfusion of compatible platelets. Therefore, their use cannot be advocated to treat alloPR in the surgical patient population.
In summary, we present three cases that highlight the problem of HLA alloantibody-mediated refractoriness to platelet transfusion in actively bleeding surgical patients undergoing massive transfusion support. In each case, intra-operative thrombocytopenia could not be effectively managed despite very large doses of routine platelet transfusions. Two of three patients expired in the early post-operative period. Our studies demonstrate that these antibodies cannot be overcome by massive transfusion, dilution, or adsorption. Understanding the pathogenesis of HLA antibodies in platelet refractoriness may aid in identifying potential therapeutic approaches for platelet refractory patients, especially those undergoing major surgery. Wider recognition of the importance of HLA alloantibodies in surgical patients may facilitate development of cost-effective screening methods to identify patients with alloimmune platelet refractoriness.
Supplementary Material
Acknowledgments
Contract grant sponsor: Luick Fund, a gift of the Luick family to the Blood Transfusion Service, Department of Pathology, MGH.
Contract grant sponsor: NIH/NHLBI; Contract grant number: K12HL087164-07.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Both J.A.P. and S.L.S. received reagent support from One Lambda, Inc. for other studies. R.S.M. received research support from Alexion Pharmaceuticals for other studies. All other authors declare no competing financial interests.
References
- 1.Rebulla P. Refractoriness to platelet transfusion. Curr Opin Hematol. 2002;9:516–520. doi: 10.1097/00062752-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Pavenski K, Freedman J, Semple JW. HLA alloimmunization against platelet transfusions: Pathophysiology, significance, prevention and management. Tissue Antigens. 2012;79:237–245. doi: 10.1111/j.1399-0039.2012.01852.x. [DOI] [PubMed] [Google Scholar]
- 3.Vassallo RR., Jr New paradigms in the management of alloimmune refractoriness to platelet transfusions. Curr Opin Hematol. 2007;14:655–663. doi: 10.1097/MOH.0b013e3282eec526. [DOI] [PubMed] [Google Scholar]
- 4.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 5.Jackman RP, Deng X, Bolgiano D, et al. Low-level HLA antibodies do not predict platelet transfusion failure in TRAP study participants. Blood. 2013;121:3261–3266. doi: 10.1182/blood-2012-12-472779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedberg RC, Donnelly SF, Boyd JC, et al. Clinical and blood bank factors in the management of platelet refractoriness and alloimmunization. Blood. 1993;81:3428–3434. [PubMed] [Google Scholar]
- 7.Powers A, Stowell CP, Dzik WH, et al. Testing only donors with a prior history of pregnancy or transfusion is a logical and cost-effective transfusion-related acute lug injury prevention strategy. Transfusion. 2008;48:2549–2558. doi: 10.1111/j.1537-2995.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 8.Kakaiya RM, Triulzi DJ, Wright DJ, et al. National Heart L, Blood Institute Retrovirus Epidemiology Donor S. II. Prevalence of HLA antibodies in remotely transfused or alloexposed volunteer blood donors. Transfusion. 2010;50:1328–1334. doi: 10.1111/j.1537-2995.2009.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quillen K, Medrano C, Adams S, et al. Screening plateletpheresis donors for HLA antibodies on two high-throughput platforms and correlation with recipient outcome. Transfusion. 2011;51:504–510. doi: 10.1111/j.1537-2995.2010.02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McPherson ME, Anderson AR, Castillejo MI, et al. HLA alloimmunization is associated with RBC antibodies in multiply transfused patients with sickle cell disease. Pediatric Blood Cancer. 2010;54:552–558. doi: 10.1002/pbc.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buetens O, Shirey RS, Goble-Lee M, et al. Prevalence of HLA antibodies in transfused patients with and without red cell antibodies. Transfusion. 2006;46:754–756. doi: 10.1111/j.1537-2995.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 13.Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical Manual. Bethesda, Maryland: AABB; 2011. Antibody detection, identification and compatibility testing; pp. 897–915. [Google Scholar]
- 14.Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cecka JM. Calculated PRA (CPRA): The new measure of sensitization for transplant candidates. Am J Transplantat. 2010;10:26–29. doi: 10.1111/j.1600-6143.2009.02927.x. [DOI] [PubMed] [Google Scholar]
- 16.Aster RH, Miskovich BH, Rodey GE. Histocompatibility antigens of human plasma. Localization to the HLD-3 lipoprotein fraction. Transplantation. 1973;16:205–210. doi: 10.1097/00007890-197309000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Boshkov LK, Kelton JG, Halloran PF. HLA-DR expression by platelets in acute idiopathic thrombocytopenic purpura. Br J Haematol. 1992;81:552–557. doi: 10.1111/j.1365-2141.1992.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 18.Schiffer CA, O'Connell B, Lee EJ. Platelet transfusion therapy for alloimmunized patients: selective mismatching for HLA B12, an antigen with variable expression on platelets. Blood. 1989;74:1172–1176. [PubMed] [Google Scholar]
- 19.Fontaine MJ, Kuo J, Chen G, et al. Complement (C1q) fixing solid-phase screening for HLA antibodies increases the availability of compatible platelet components for refractory patients. Transfusion. 2011;51:2611–2618. doi: 10.1111/j.1537-2995.2011.03194.x. [DOI] [PubMed] [Google Scholar]
- 20.Services UDoHaH, editor. Washington DC: Office of the Assistant Secretary for Health; 2011. The 2011 National Blood Collection and Utilization Survey Report. [Google Scholar]
- 21.Saito S, Ota S, Seshimo H, et al. Platelet transfusion refractoriness caused by a mismatch in HLA-C antigens. Transfusion. 2002;42:302–308. doi: 10.1046/j.1537-2995.2002.00051.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: A database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39:D913–D919. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu QH, Shen YP, Ye BD, Zhou YH. Successful use of rituximab in platelet transfusion refractoriness in a multi-transfused patient with myelo-dysplastic syndrome. Platelets. doi: 10.3109/09537104.2013.789842. [DOI] [PubMed] [Google Scholar]
- 24.Miki H, Ozaki S, Tanaka O, et al. Marked improvement of platelet transfusion refractoriness after bortezomib therapy in multiple myeloma. Int J Hematol. 2009;89:223–226. doi: 10.1007/s12185-009-0255-z. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery RA, Zachary AA, Racusen LC, et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- 26.Schweitzer EJ, Wilson JS, Fernandez-Vina M, et al. A high panel-reactive antibody rescue protocol for cross-match-positive live donor kidney transplants. Transplantation. 2000;70:1531–1536. doi: 10.1097/00007890-200011270-00023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


