Abstract

Conformational restriction of the pyrrolidine nitrogen in nicotine by the introduction of an ethylene bridge provided a potent and selective antagonist of the α4β2-subtype of the nicotinic acetylcholine receptors. Resolution by chiral SFC, pharmacological characterization of the two enantiomers, and determination of absolute configuration via enantioselective synthesis showed that the pharmacological activity resided almost exclusively in the (R)-enantiomer.
Keywords: Bridged-nicotine analogue, nAChRs, selective antagonist, Negishi cross-coupling reaction, oocyte
The neuronal nicotinic acetylcholine receptors (nAChRs) are members of the Cys-loop receptor family of ligand-gated ion channels. They are assembled by either α-subunits or a combination of α- and β-subunits and play an important role in the peripheral and central nervous systems where they mediate neurotransmission in response to acetylcholine (ACh).1,2 The involvement of nAChRs in a wide range of disease states as well as psychiatric and neurodegenerative disorders (such as depression, schizophrenia, attention deficit hyperactivity disorder, Alzheimer’s and Parkinson’s diseases, substance abuse, and pain) has made this class of receptors a highly pursued target for drug discovery.3−6
Nicotine is the principal psychoactive ingredient in tobacco and acts as a potent agonist of the nAChRs. Structural modifications of nicotine have been the starting point for many drug discovery programs, and the introduction of conformational restraint as in compounds 1,7,82,9 and 3(10,11) has previously been investigated to probe the different possible conformations of nicotine (see Figure 1). Ligands 1 and 2 lock the pyrrolidine nitrogen in nicotine in the “down” position, whereas compound 3 locks it in the “up” position.
Figure 1.
Structure of nicotine and selected conformational restricted analogues.
In continuation of that line of thinking we recently reported the synthesis of new bridged-nicotine analogues locked in the “up” position, i.e., compounds rac-4 and rac-5.12
Rigid nicotine analogue 1 was previously shown to be a selective partial agonist of rat α7 receptor, while 3 had no agonist activity at any nAChR of the investigated subtypes, whereas conformational restriction in 2 was reported to attenuate nicotinic activity.
Herein we report the pharmacological characterization of the racemic compounds rac-4 and rac-5 at several nAChR subtypes. Furthermore, we describe the resolution of rac-4 by chiral supercritical fluid chromatography (SFC on chiral phase) and determination of absolute configuration of the most potent enantiomer via enantioselective synthesis.
As depicted in Table 1, compound rac-5 exhibited low binding affinity at α4β2 nAChRs (Ki = 13 μM), while its affinity for other nAChR subtypes was negligible. In contrast, the direct analogue of nicotine, rac-4, was a potent and highly subtype selective ligand for α4β2 receptors in terms of affinity. Compound rac-4 displayed nanomolar affinity (Ki = 71 nM) toward the α4β2 nAChR with more than 100-fold selectivity over α4β4, α3β4, and α7 (Ki values of 7.2, ∼100, and ∼500 μM, respectively).
Table 1. Binding Affinities of Constrained Analogues, rac-4, (R)-4, (S)-4, and rac-5 at the nAChRsa.
|
Ki (μM) |
||||
|---|---|---|---|---|
| compds | α4β2 | α4β4 | α3β4 | α7/5-HT3A |
| (S)-nicotine | 0.011 | 0.051 | 0.23 | 15 |
| rac-4 | 0.071 | 7.2 | ∼100 | ∼500 |
| (R)-4 | 0.075 | 3.7 | ∼50 | ∼300 |
| (S)-4 | 3.4 | ∼100 | ∼100 | >300 |
| rac-5 | 13 | ∼100 | ∼100 | |
The Ki values for the compounds at the rat α4β2, α4β4, and α3β4 nAChR subtypes were obtained in a [3H]epibatidine binding assay, and the Ki values for the compounds at the α7/5-HT3A chimera were determined in a [3H]MLA binding assay. The Ki values are given in μM. The data are the means of 3–5 individual experiments performed in duplicate. The complete pharmacological data (including the SEM values) are given in the Supporting Information.
In the fluorescence-based FLIPR membrane potential assay rac-4 was found to be an antagonist at the α4β2 receptor (IC50 = 0.12 μM), whereas it acted as a weak agonist at the α3β4 nAChR. It is noteworthy that this simple manipulation of the structure of nicotine converts it from a full agonist to a competitive antagonist at the α4β2 receptor.
Naturally occurring (S)-nicotine is a considerably more potent nAChR agonist than (R)-nicotine,13,14 and we wanted to investigate if the two enantiomers of 4 would exhibit a similar tendency. Thus, the racemic mixture (rac-4) was resolved by SFC (see Supporting Information for details). The two enantiomers15 (R)-4 (corresponding to the first eluted compound, 97.8% ee) and (S)-4 (corresponding to the second eluted compound, 98.8% ee) were tested for binding affinity showing that the activity mainly resided in the first eluting enantiomer with Ki values of 0.075, 3.7, ∼50, and ∼300 μM at the α4β2, α4β4, α3β4, and α7 receptors, respectively. Thus, the pharmacological activity resides almost exclusively in the (R)-enantiomer, which in terms of absolute stereochemistry is opposite to nicotine for which the eutomer has (S)-configuration.
The absolute configuration of the two enantiomers of 4 was determined in the following way: several attempts to crystallize the compound as different salts for X-ray crystallographic analysis were made, albeit without success. Therefore, a synthetic pathway that would give enantio-enriched material with a known configuration was pursued. Several different approaches toward the enantioselective synthesis of 4 were initially explored. Eventually a strategy based on the enantioselective metalation of N-Boc-pyrrolidines16−22 (which has been demonstrated to proceed with a predictable stereo chemical outcome) proved to be fruitful. This required an enantioselective Negishi cross-coupling reaction with a 3-bromo-4-chloropyridine giving an enantioenriched version of known intermediate in our previous synthesis of rac-4.12
The synthesis of (R)-4 using this approach is depicted in Scheme 1. Stoichiometric lithiation of N-Boc-protected pyrrolidine 6 using (−)-sparteine/s-BuLi, subsequent transmetalation with ZnCl2, and palladium-catalyzed cross-coupling reaction with 3-bromo-4-chloropyridine at 65 °C gave α-arylpyrrolidine 7 in 58% yield. Suzuki–Miyaura cross-coupling between (E)-2-(2-ethoxyvinyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane and 7 in a CH3CN–water (3:2) mixture under microwave (MW) conditions at 85 °C for 3 h delivered 8 in 60% yield. Subsequent treatment in a TFA–CH2Cl2 (1:1) mixture led to a presumed iminium salt intermediate, which was reduced with an excess of NaBH4 in MeOH providing bridged-nicotine analogue (R)-4 in a 3-step synthesis in 10% overall yield from 6 with 70% ee. The HPLC chromatogram showed that (R)-4 had a retention time identical to that of the eutomer isolated by chromatographic separation of the racemic mixture (see Supporting Information for details).
Scheme 1. Enantioselective Synthesis of (R)-4.
Reaction conditions: (a) s-BuLi (1 equiv), (−)-sparteine (1 equiv), MTBE, −78 °C, 3 h then ZnCl2 (1 equiv), −78 °C, 30 min then to rt, 30 min; (b) 3-bromo-4-chloropyridine (0.73 equiv), Pd(OAc)2 (5 mol %), TTBP–HBF4 (5 mol %), 65 °C, 16 h, 58% (2 steps); (c) (E)-2-(2-ethoxyvinyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1.9 equiv), SPhos (6 mol %), Pd(OAc)2 (3 mol %), K3PO4 (2 equiv), CH3CN–water (3:2), MW, 85 °C, 3 h, 60%; (d) TFA–CH2Cl2 (1:1), 0 °C to rt, 3 h then NaBH4 (5 equiv), MeOH, 0 °C to rt, 15 h, 30%.
The reason for the moderate ee of 4 compared to those reported in the literature16 could be due to a possible racemization during the last reaction step, i.e., the imine intermediate could potentially isomerize to an achiral iminium intermediate affording rac-4 after reduction, as shown in Figure 2.
Figure 2.
Possible isomerization of the iminium intermediate causing racemization.
To investigate the pharmacological properties of (R)-4 at α4β2 nAChRs in more detail, we determined whole cell currents in Xenopus laevis oocytes23 expressing the two α4β2 nAChR stoichiometries upon application of (R)-4 alone and upon coapplication of (R)-4 with acetylcholine (see Figure 3). While there was no change in baseline current when 100 μM (R)-4 was applied, ACh (30 μM) induced current was inhibited when the same concentration of (R)-4 was coapplied (data not shown). This verified the findings from the FLIPR membrane potential assay: (R)-4 acts as an antagonist of α4β2 nAChR.
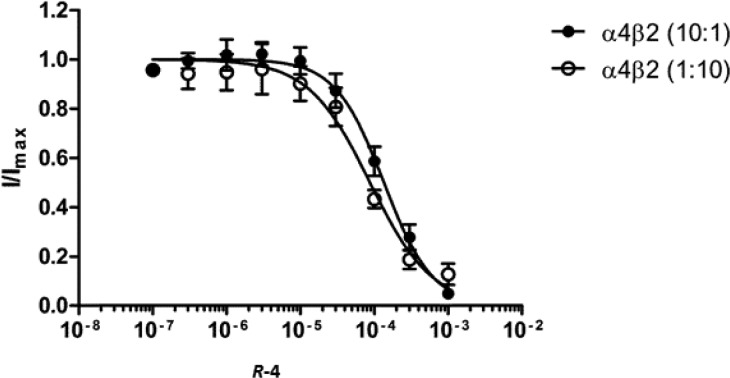
Figure 3.
Concentration–inhibition curves of (R)-4. Concentration–inhibition curve for (R)-4 in oocytes injected with α4β2 mRNA in 10:1 (●) and 1:10 (○) injection ratios to express (α4)3(β2)2 and (α4)2(β2)3, respectively. One millimolar ACh was used for 10:1 and 100 μM ACh was used for 1:10 injection ratios.
The antagonist potency of (R)-4 at the α4β2 nAChRs was subsequently determined in Xenopus oocytes measuring the peak responses to a fixed ACh concentration in the presence of increasing concentrations of (R)-4. These recordings were performed on both receptor stoichiometries (α4)3(β2)2 and (α4)2(β2)3 that are controlled by maintaining subunit mRNA injection ratios of α4:β2 at 10:1 and 1:10, respectively. (R)-4 inhibited maximal ACh (1 mM) current in (α4)3(β2)2 nAChR (α4/β2, 10:1) with an IC50 value of 135 μM (Ki = 15 μM). On (α4)2(β2)3 receptors (α4/β2, 1:10), (R)-4 inhibited the max current elicited by ACh (100 μM), with an IC50 of 87 μM (Ki = 0.34 μM) (see Figure 3). Although, there is marginal difference in the inhibition curve (1.5-fold difference in IC50) between two stoichiometries, the inhibition constants (Ki) of (R)-4 are significantly different (45-fold) between (α4)3(β2)2 and (α4)2(β2)3. As the (α4)2(β2)3 receptor contains only α4(+)β2(−) binding sites whereas (α4)3(β2)2 contains both α4(+)β2(−) and α4(+)α4(−) sites,24 the results suggest that (R)-4 exhibits higher affinity to the α4(+)β2(−) binding site.
Given the high in vitro potency of (R)-4 we wanted to conduct a preliminary evaluation of the compounds’ in vivo potential. Antidepressant effects of both nAChR agonists and antagonists have been reported,25 and we previously evaluated another α4β2-selective antagonist in the mouse forced swim test and found it to have antidepressant-like activity.26 Thus, (R)-4 was tested in the mouse forced swim test (1, 3, and 10 mg/kg, subcutaneous administration), but no effects on swimming was found at the tested doses (see Supporting Information for details).
In conclusion, we have shown that 4 is a new potent antagonist of the α4β2 nAChR. The pharmacological activity resides almost exclusively in the (R)-enantiomer, which in terms of absolute stereochemistry is opposite to nicotine for which the eutomer has the (S)-configuration. (R)-4 holds significant selectivity for α4β2 nAChRs over the other nAChR subtypes tested in this study and is an interesting scaffold for development of new nAChR antagonists. In comparison, 3 (another conformationally restrained ligand with the pyrrolidine nitrogen fixed in the “up” position, see Figure 1) showed no affinity toward the nAChRs, highlighting the profound effect on the pharmacological profile, depending on the placement of the conformational restraint in the ligand. Furthermore, we have shown that it is possible to convert nicotine from a full agonist to a competitive antagonist with a very minor structural modification, and further studies with (R)-4 may pave the way for a better understanding of the structural requirement for agonism versus competitive antagonism at the nAChRs.
Acknowledgments
Birgitte Nielsen and Peter Brøsen are gratefully acknowledged for their helpful contribution.
Glossary
ABBREVIATIONS
- nAChR
nicotinic acetylcholine receptor
- ACh
acetylcholine
- SFC
supercritical fluid chromatography
- mRNA
messenger ribonucleic acid
- CNS
central nervous system
- MTBE
methyl tert-butyl ether
- MW
microwave
- TFA
trifluoroacetic acid
- TTBP–HBF4
tri-tert-butylphosphonium tetrafluoroborate
- ee
enantiomeric excess
Supporting Information Available
Full experimental details and pharmacological characterization of the compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
I.N.P., F.C., and J.L.K. conceived the experiments. I.N.P. and F.C. synthesized the compounds. H.P. performed the chiral separation. A.A.J., D.C.I., and T.B. tested the compounds in vitro. J.T.A. performed the in vivo experiments. I.N.P., F.C., A.A.J., T.B., and J.L.K. wrote the manuscript.
The authors thank the Lundbeck Foundation, the Novo Nordisk Foundation, and the Danish Council for Independent Research–Medical Sciences for financial support.
The authors declare no competing financial interest.
Supplementary Material
References
- Dougherty D. A. Cys-Loop Neuroreceptors: Structure to the Rescue?. Chem. Rev. 2008, 108, 1642–1653. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X.; Perpeira E. F. R.; Alkondon M.; Rogers W. R. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev. 2009, 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. A.; Frolund B.; Liljefors T.; Krogsgaard-Larsen P. Neuronal Nicotinic Acetylcholine Receptors: Structural Revelations, Target Identifications, and Therapeutic Inspirations. J. Med. Chem. 2005, 48, 4705–4745. [DOI] [PubMed] [Google Scholar]
- Arneric S. P.; Holladay M.; Williams M. Neuronal Nicotinic Receptors: A Perspective on Two Decades of Drug Discovery Research. Bio. Pharmacol. 2007, 74, 1092–1101. [DOI] [PubMed] [Google Scholar]
- Steinlein O. K. Ion Channel Mutations in Neuronal Diseases: A Genetics Perspective. Chem. Rev. 2012, 112, 6334–6352. [DOI] [PubMed] [Google Scholar]
- Lemoine D.; Jiang R.; Taly A.; Chataigneau T.; Specht A.; Grutter T. Ligand-Gated Ion Channels: New Insights into Neurological Disorders and Ligand Recognition. Chem. Rev. 2012, 112, 6285–6318. [DOI] [PubMed] [Google Scholar]
- Glassco W.; Suchocki J.; George C.; Martin B. R.; May E. L. Synthesis, Optical Resolution, Absolute Configuration, and Preliminary Pharmacology of (+)- and (−)-cis-2,3,3a,4,5,9b-Hexahydro-1-methyl-1H-pyrrolo[3,2-h]isoquinoline, a Structural Analog of Nicotine. J. Med. Chem. 1993, 36, 3381–3385. [DOI] [PubMed] [Google Scholar]
- Damaj M. I.; Glassco W.; Marks M. J.; Slobe B.; James J. R.; May E. L.; Rosecrans J. A.; Collins A. C.; Martin B. R. Pharmacological Investigation of (+)- and (−)-cis-2,3,3a,4,5,9b-Hexahydro-1-methyl-1H-pyrrolo[3,2-h]isoquinoline, a Bridged-Nicotine Analog. J. Pharmacol. Exp. Ther. 1997, 282, 1425–1434. [PubMed] [Google Scholar]
- Kachur J. F.; May E. L.; Awaya H.; Egle J. L. Jr.; Aceto M. D.; Martin B. R. Pharmacological Effects of 1,2,3,5,6,10b-Hexahydropyrido[2,3-g]indolizine, a Bridged-Nicotine Analog. Life Sci. 1986, 38, 323–330. [DOI] [PubMed] [Google Scholar]
- Xu R.; Dwoskin L. P.; Grinevich V.; Sumithran S. P.; Crooks P. A. Synthesis and Evaluation of Conformationally Restricted Pyridino N-Alkylated Nicotine Analogs as Nicotinic Acetylcholine Receptor Antagonists. Drug Dev. Res. 2002, 55, 173–186. [Google Scholar]
- Papke R. L.; Zheng G.; Horenstein N. A.; Dwoskin L. P.; Crooks P. A. The Characterization of a Novel Rigid Nicotine Analog with α7-selective nAChR Agonist Activity and Modulation of Agonist Properties by Boron Inclusion. Bioorg. Med. Chem. Lett. 2005, 15, 3874–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestey F.; Hooyberghs G.; Kristensen J. L. Concise Synthesis of New Bridged-Nicotine Analogues. Tetrahedron 2012, 68, 1417–1421. [Google Scholar]
- Copeland J. R.; Adem A.; Jacob P. III; Nordberg A. A Comparison of the Binding of Nicotine and Nornicotine Stereoisomers to Nicotinic Binding Sites in Rat Brain Cortex. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1991, 343, 123–127. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Nordberg A. The Competition of (−)-[3H]Nicotine Binding by the Enantiomers of Nicotine, Nornicotine and Anatoxin-a in Membranes and Solubilized Preparations of Different Brain Regions of Rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993, 348, 28–34. [DOI] [PubMed] [Google Scholar]
- The absolute configuration of two enantiomers (R)-4 and (S)-4 has been assigned accordingly throughout the Letter even though at the time of the study the absolute configuration was not yet established. The ee of (R)-4 (the first compound to elute) after chiral SFC separation was 97.8% while the ee of (S)-4 (the second compound to elute) was 98.8%. See Supporting Information for more details.
- Kopach M. E.; Meyers A. I. Metalation–Alkylation of N-Activated Pyrrolidines. Rigorous Proof of Retention for Both Steps. J. Org. Chem. 1996, 61, 6764–6765. [DOI] [PubMed] [Google Scholar]
- Gross K. M. B.; Beak P. Complex-Induced Proximity Effects: The Effect of Varying Directing-Group Orientation on Carbamate-Directed Lithiation Reactions. J. Am. Chem. Soc. 2001, 123, 315–321. and references cited therein. [DOI] [PubMed] [Google Scholar]
- Campos K. R.; Klapars A.; Waldman J. H.; Dormer P. G.; Chen C.-Y. Enantioselective, Palladium-Catalyzed α-Arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2006, 128, 3538–3539. [DOI] [PubMed] [Google Scholar]
- Bilke J. L.; O’Brien P. On the Two-Ligand Catalytic Asymmetric Deprotonation of N-Boc Pyrrolidine: Probing the Effect of the Stoichiometric Ligand. J. Org. Chem. 2008, 73, 6452–6454. [DOI] [PubMed] [Google Scholar]
- Stead D.; O’Brien P.; Sanderson A. A New Sparteine Surrogate for Asymmetric Deprotonation of N-Boc Pyrrolidine. Org. Lett. 2008, 10, 1409–14012. [DOI] [PubMed] [Google Scholar]
- Barker G.; McGrath J. L.; Klapars A.; Stead D.; Zhou G.; Campos K. R.; O-Brien P. Enantioselective, Palladium-Catalyzed α-Arylation of N-Boc Pyrrolidine: In Situ React IR Spectroscopic Monitoring, Scope, and Synthetic Applications. J. Org. Chem. 2011, 76, 5936–5953. [DOI] [PubMed] [Google Scholar]
- Cordier C. J.; Lundgren R. J.; Fu G. C. Enantioconvergent Cross-Couplings of Racemic Alkylmetal Reagents with Unactivated Secondary Alkyl Electrophiles: Catalytic Asymmetric Negishi α-Alkylations of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2013, 135, 10946–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absalom N. L.; Quek G.; Lewis T. M.; Qudah T.; von Arenstorff I.; Ambrus J. I.; Harpsøe K.; Karim N.; Balle T.; McLeod M. D.; Chebib M. Covalent Trapping of Methyllycaconitine at the α4-α4 Interface of the α4β2 Nicotinic Acetylcholine Receptor. J. Biol. Chem. 2013, 288, 26521–26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpsøe K.; Ahring P. K.; Christensen J. K.; Jensen M. L.; Peters D.; Balle T. Unraveling the high and low sensitivity agonist responses of nicotinic acetylcholine receptors. J. Neurosci. 2011, 31, 10759–10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N. S.; Carpenter L. L.; Tyrka A. R.; Price L. H. Nicotinic Acetylcholine Receptors and Depression: A Review of the Preclinical and Clinical Literature. Psychopharmacology 2010, 212, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestey F.; Jensen A. A.; Borch M.; Andreasen J. T.; Andersen J.; Balle T.; Kristensen J. L. Design, Synthesis, and Biological Evaluation of Erythrina Alkaloid Analogues as Neuronal Nicotinic Acetylcholine Receptor Antagonists. J. Med. Chem. 2013, 56, 9673–9682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.