| Title: | Azaindole Compounds, Synthesis Thereof, and Methods of Using the Same | ||
| Patent Application Number: | WO 2015/009525 Al | Publication date: | 22 January 2015 |
| Priority Application: | IN 3196/CHE/2013 | Priority date: | 17 July 2013 |
| IN 3196/CHE/2013 | 30 April 2014 | ||
| Inventors: | Naik, M. N.; Peer Mohamed, S. H.; Shandil, R. K.; Shinde, V. N.; Shirude, P. S.; Chatterji, M. | ||
| Assignee Company: | Global Alliance for TB Drug Development; 40 Wall Street, 24th Floor, New York, New York 10005, United States | ||
| Astrazeneca AB; S-151 85 Södertälje (SE) | |||
| Disease Area: | Tuberculosis (TB) | Biological Target: | Decaprenylphosphoryl-β-d-ribose 2′-epimerase 1 (DprE1) |
| Summary: | The invention in this patent application relates to 1H-pyrrolo[3,2-b]pyridine-3-carboxamide derivatives represented generally by formula (I). These compounds are inhibitors of DprE1 and may potentially be useful in treating Mycobacterium infections and tuberculosis. | ||
| Tuberculosis (TB) is an infectious disease that is caused by the bacterium Mycobacterium tuberculosis (Mtb). The disease has caused human suffering for centuries and remains a major health threat particularly in developed countries. The first successful treatment was introduced in 1946 with the use of the antibiotic streptomycin. Current treatment requires isolation and administration of multiple antibiotics for more than six months. However, there is an emerging problem with antibiotic resistance in multiple drug-resistant tuberculosis (MDR-TB) infections. Thus, there is a growing and urgent unmet need for new drugs with novel mechanisms of action; a task that requires the identification of new therapeutic targets. The FDA recently approved bedaquiline (Sirturo) to treat multidrug-resistant tuberculosis. However, the impact of this new drug as effective treatment for TB is still to be determined. | |||
| Decaprenylphosphoryl-β-d-ribose 2′-epimerase (DprE) is a heterodimeric enzyme that is composed of two proteins, DprE1 and DprE2. DprE1 and DprE2 are key enzymes in the biosynthesis of decaprenylphosphoryl-β-d-arabinofuranose (DPA). DPA is a precursor of mycobacterial cell wall arabinan, one of the essential Mtb cell wall components. The biosynthesis of DPA is performed via epimerization of decaprenylphosphoryl-β-d-ribose (DPR) in a 2-step oxidation–reduction reaction sequence (Scheme 1). The first step is oxidation of DPR to the intermediate ketone decaprenylphosphoryl-2-keto-β-d-erythro-pentofuranose (DPX), which is accomplished by the redox cofactor flavin adenine dinucleotide (FAD) together with DprE1 as a catalyst. The second step is a stereospecific reduction of the ketone DPX with nicotinamide adenine dinucleotide redox cofactor (reduced form; NADH) in the presence of DprE2 as a catalyst to provide DPA. | |||
Scheme 1: Biosynthesis of DPA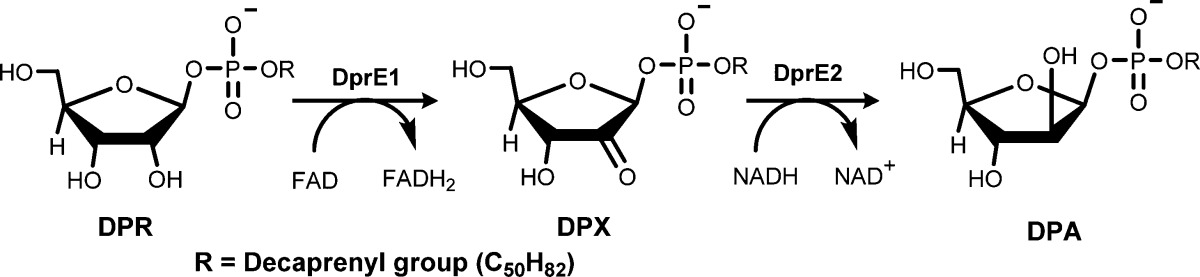
| |||
| A very significant recent development in the battle against TB revealed the structures of nitrobenzothiazinones (BTZs) and related derivatives that display good activities against Mycobacterium tuberculosis. Researchers have determined that these potential new drugs act by inhibiting DprE1 and consequently inhibiting the epimerization of DPR to DPA. The discovery of these compounds and their novel mechanism of action have underlined DprE1 as an attractive therapeutic target to address the urgent need for the introduction of new effective therapeutics to treat Mtb infections. While BTZs promise to be effective against TB, they are still in development, and there are no guarantees these new leads will become commercial drugs. Thus, there remains a need to continue to explore and discover additional compound classes of DprE1 inhibitors such as the compounds described in this patent application to reach the urgent goal of identifying new treatments for TB. | |||
| Important Compound Classes: | 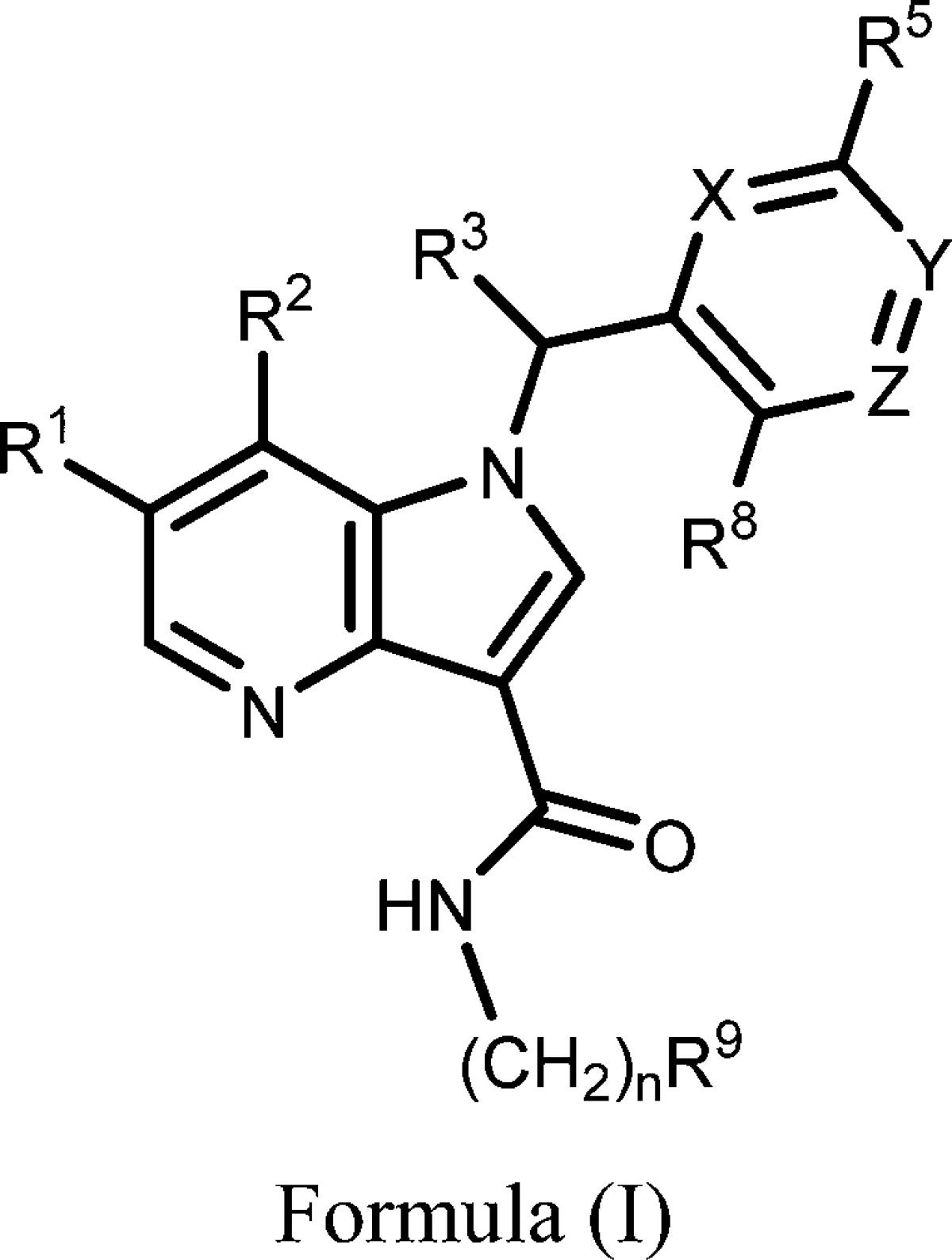 |
||
| Key Structures: | The inventors reported the structures of 46 examples of formula (I) including the following compounds: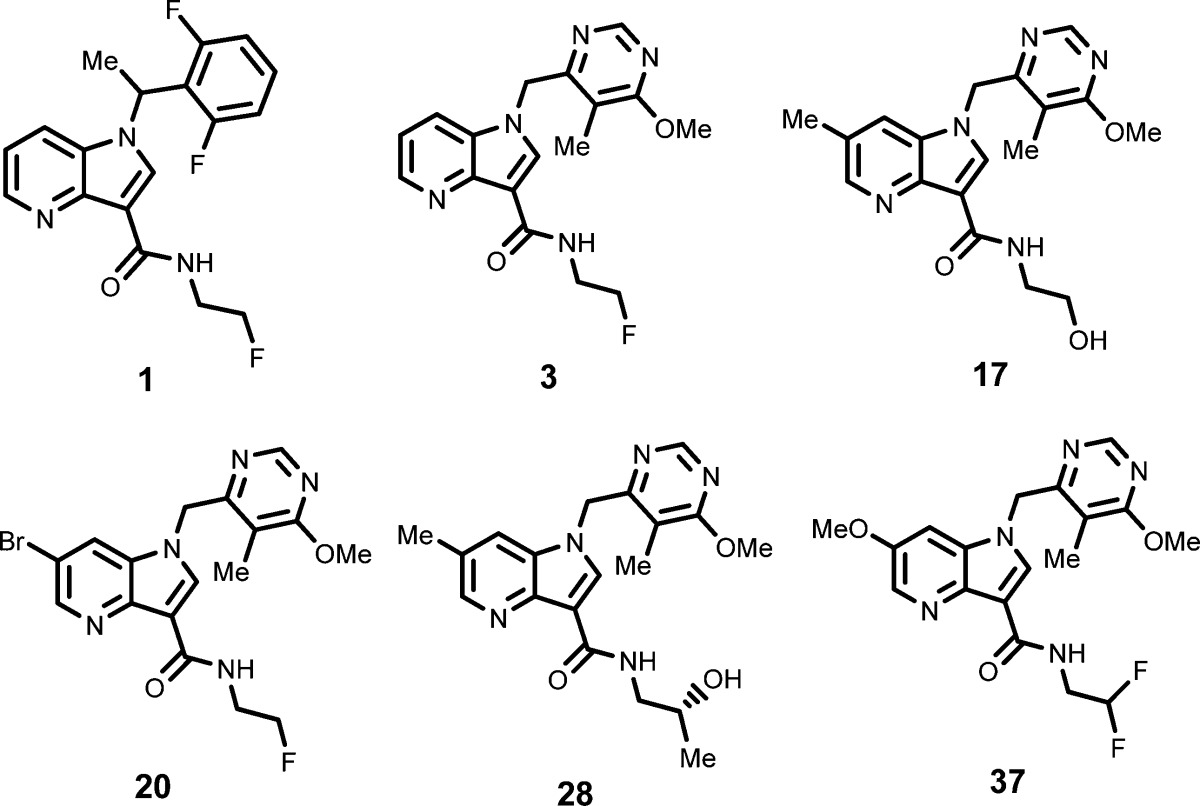
|
||
| Biological Assay: | The inventors used the following biological assays to test the compounds of the invention:
|
||
| Biological Data: | The minimal inhibitory concentration (MIC) data for Mtb were reported for the compounds of the invention. The following table shows the data obtained from testing the representative examples (structures above):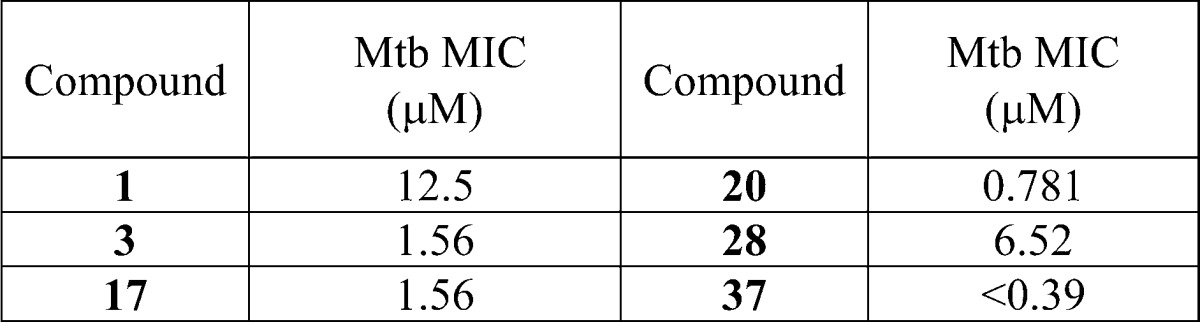
|
||
| Recent Review Articles: | 1. Mikusova K.; Makarov V.; Neres J.. Curr. Pharm. Des. 2014, 20 ( (27), ), 4379–4403. | ||
| 2. Riccardi G.; Pasca M. R.; Chiarelli L. R.; Manina G.; Mattevi A.; Binda C.. Appl. Microbiol. Biotechnol. 2013, 97 ( (20), ), 8841–8848. | |||
| 3. Cole S. T.; Riccardi G.. Curr. Opin. Microbiol. 2011, 14 ( (5), ), 570–576. | |||
The authors declare no competing financial interest.


