| Title: | Pyrazolopyridine Derivatives for Use in the Treatment of Bladder Cancer | ||
| Patent Application Number: | WO 2014/198942 A1 | Publication date: | 18 December 2014 |
| Priority Application: | FR 1355578 | Priority date: | 14 June 2013 |
| Inventors: | Alcouffe, C. | ||
| Assignee Company: | Sanofi; 54 me La Boetie, F-75008 Paris, France | ||
| Disease Area: | Bladder Cancer | Biological Target: | Fibroblast Growth Factor Receptor 3 (FGF-R3) |
| Summary: | The invention in this patent application relates to pyrazolopyridine derivatives represented generally by formula (I), which are Fibroblast Growth Factor Receptor (FGF-R) inhibitors. These compounds may potentially provide a useful treatment for bladder cancer. | ||
Bladder cancer is the fourth most common cancer in the United States with more than 63 000 cases diagnosed every year mostly affecting individuals over the age of 50 and leading to more than 13 000 deaths. At least 300 000 cases are detected each year worldwide, and this number is growing. There are two main categories of bladder cancer:
| |||
| Superficial and noninvasive bladder cancer often presents multifocal carcinomas, which have about 70% recurrence rate and require repeated and invasive treatments that are long and expensive. The treatments are curative only for less than 30% of the cases and cause severe side-effects, such as pain during urination, fever, nausea, more frequent urinations, and bladder irritation. Therefore, there is a need for the development of new effective treatments for bladder cancer that are curative and cause fewer or no side-effects. | |||
| Fibroblast Growth Factors (FGFs) constitute a family of polypeptides synthesized in a large number of embryonic and adult tissue cells under various pathological conditions. Recently, superficial urothelial cancers (UCs) of the bladder were linked to the expression of a mutated form of FGF receptor 3 (FGF-R3) by a very strong correlation between the expression of mutated forms of FGF-R3 and low grade/stage bladder UCs. The mutations were also linked to urothelial papillomas, and they are probably responsible for the lesions that appear to be a warning sign of papillary UCs. The most common mutations are the replacement of Ser249 or Tyr375 in the extracellular domain of FGF-R3 with cysteine. The cysteine mutation causes the formation of an interchain disulfide bridge leading to dimerization of the receptor and resulting in its permanent activation and the activation of the underlying intracellular signaling pathways. These activation mutations are believed to contribute to the proliferation of tumor cells to grow beyond confluence and to resist apoptosis. Furthermore, the expression of mutated FGF-R3 appears to increase in the majority of superficial tumors but is not detected in healthy urothelium. | |||
| Therefore, the inhibition of mutated FGF-R3 is a promising therapeutic target for the development of new treatments of superficial and noninvasive bladder cancers. FGF-R3 antagonists such as the compounds described in this patent application may potentially provide effective therapeutics for the treatment of bladder cancer through counteracting the effects of the pro-tumor Ser249Cys mutated FGF-R3. | |||
| Important Compound Classes: | 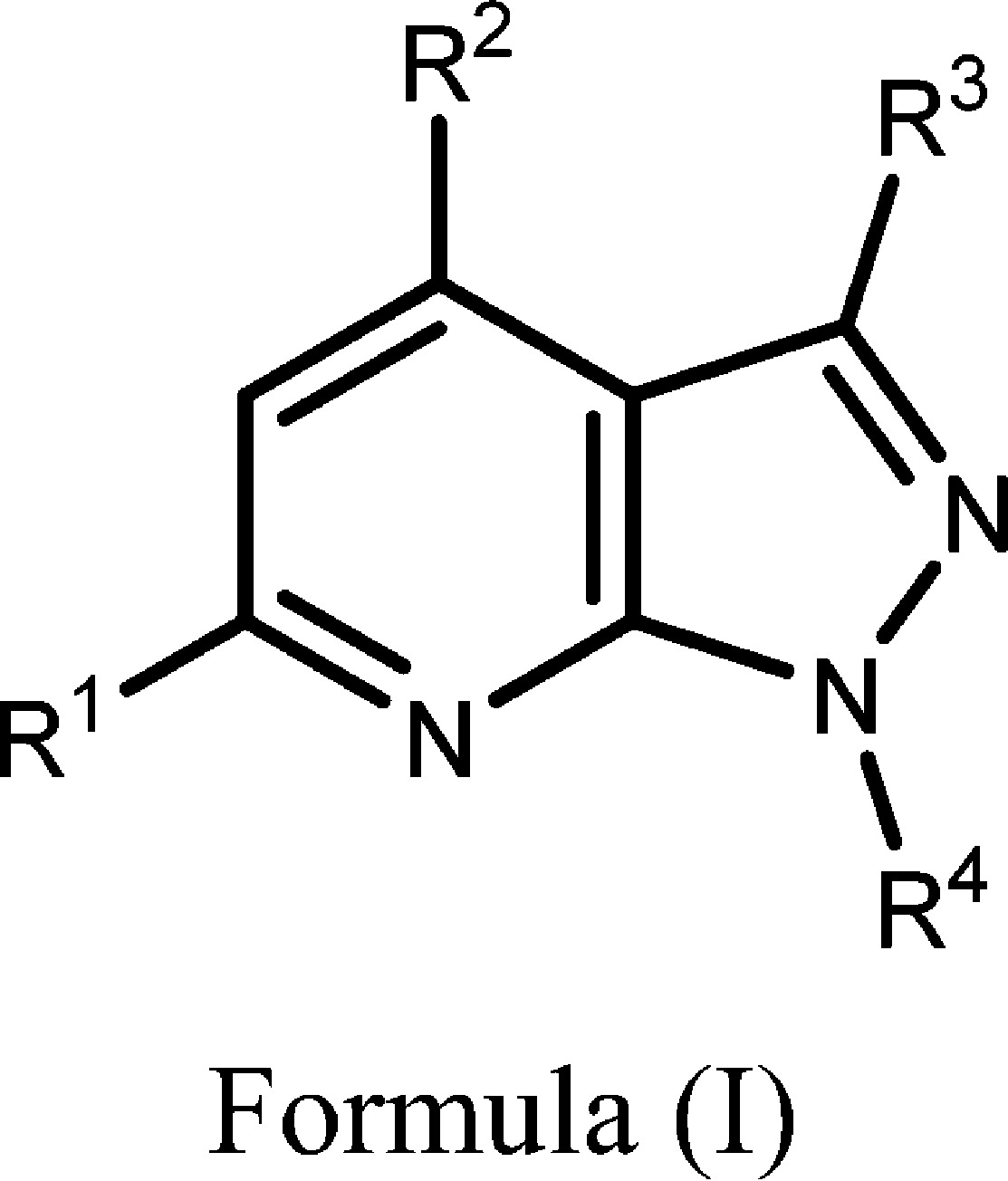 |
||
| Key Structures: | The inventors described the synthesis and structures of 24 examples of formula (I) including the following four compounds:
|
||
| Biological Assay: | The inventors used the following assays to evaluate the compounds of the invention:
|
||
| Biological Data: | The biological data obtained from testing the above representative examples are listed in the following table:
|
||
| Recent Review Articles: | 1. Ghosh M.; Brancato S. J.; Agarwal P. K.; Apolo A. B.. Curr. Opin. Oncol. 2014, 26 ( (3), ), 305–320. | ||
| 2. Iyer G.; Milowsky M. I.. Urol. Oncol.: Semin. Orig. Invest. 2013, 31 ( (3), ), 303–311. | |||
| 3. Martinez-Frias M. L.; de Frutos C. A.; Bermejo E.; Nieto M. A.; Gomar J. L.; Nieto C.; Aparicio P.; Centeno F.; Felix V.; Rodriguez F.; et al. Am. J. Med. Genet., Part A 2010, 152A ( (1), ), 245–255. | |||
The authors declare no competing financial interest.


