Abstract
Background
Peripheral neuropathy is the most common complications of diabetic patients and leads to loss of plantar cutaneous sensation, movement perception, and body balance. Thai foot massage is an alternative therapy to improve balance. Therefore, the purpose of this study was to investigate the effects of Thai foot massage on balance performance in diabetic patients with peripheral neuropathy.
Material/Methods
Sixty patients with type-2 diabetes were recruited and randomly assigned into either the Thai foot massage or control groups. The Thai foot massage group received a modified Thai traditional foot massage for 30 min, 3 days per week for 2 weeks. We measured timed up and go (TUG), one leg stance: OLS), the range of motion (ROM) of the foot, and foot sensation (SWMT) before treatment, after the first single session, and after the 2-week treatment.
Results
After the single treatment session, only the Thai foot massage group showed a significant improvement in TUG. After the 2-week treatment, both Thai foot massage and control groups showed a significant improvement of TUG and OLS (P<0.05); however, when comparing between 2 groups, the Thai foot massage group showed better improvement in TUG than the control group (p<0.05). The Thai foot massage group also showed significant improvements in ROM and SWMT after the 2-week treatment.
Conclusions
The results of this study suggest that Thai foot massage is a viable alternative treatment for balance performance, ROM of the foot, and the foot sensation in diabetic patients with peripheral neuropathy.
Keywords: Massage, Postural Balance, Sensation
Background
Peripheral neuropathy (PN) is the most common complication of diabetic patients and leads to loss of plantar cutaneous sensation, movement perception and body balance. Fifty percent of patients with diabetes over 60 years old are affected by PN [1]. The somatosensory deficit in diabetic PN can include loss of muscle spindle function in lower leg, loss of movement perception at the ankle joint and loss of plantar cutaneous sensation [2,3]. PN causes decreased proprioception and/or increased reflex reaction time [4–6]. Limited joint mobility is frequently observed in diabetic patients who have increased stiffness of articular capsule, ligaments and tendons [7–10]. These may lead to postural instability [11] and an increased risk of falls [12]. The sensory system is also important to control balance. Sensations from the bottom of the foot play an important role during dynamic postural response [13,14]. Also, ankle flexibility, foot sensation and strength of toe flexor are important predictors of balance and functional ability in older people [15,16].
Thai foot massage is a form of deep massage using thumb pressure applied along the meridian lines of the foot and the leg combining with toes distraction. Thai massage technique is similar to the acupressure massage [17]. Pressure is applied by using thumb, finger, palm or elbow of practitioner [18]. Each pressure is held for 5–10 seconds at the point when the patient starts to feel some pain and repeated 3–5 times for each point [19]. Plausible mechanism of deep massage pressure might improve of blood flow to enhance skin sensations from the bottom of the feet [20–22]. Combining with joint distraction which may increase joint mobility, Thai foot massage may be one of the alternative therapies to improve balance performance for diabetic patients as a result of increasing range of motion (ROM) and sensation of the foot. It may directly stimulate the nervous system to help the myelin sheath of the nerves [23]. Previous studies show that western foot massage combined with mobilization can stimulate cutaneous sensory and joint sense, improving standing balance in elderly people [24,25]. There is no studies to support the effectives of Thai foot massage on balance performance, ROM of the leg and the foot and the foot sensation in Type II diabetic patients with PN. Therefore, the purpose of this study was to investigate the immediate and short-term effects of Thai foot massage on balance performance, ROM of the leg and the foot and the foot sensation in Type II diabetic patients with PN.
Material and Methods
Subjects
Type II diabetic patients aged 40–70 years and with peripheral neuropathy were recruited and their demographic characteristics and health status were recorded, including duration of diabetes (Table 1). Patients were diagnosed as having an impaired level of diabetic foot [26] as per the following criteria: 1) Peripheral sensory deficit was assessed with the Semmes-Weinstein monofilaments test (SWMT) [27]. The third and fifth toes and the head of the first and third metatarsi can indicate sensory neuropathy [28]. One or more deficits of sensation were indicative of sensibility abnormality or peripheral neuropathy [26]. 2) Ability to walk 10 m without a walking aid. Patients with any of the following condition were excluded: 1) Parkinson’s disease and stroke, 2) severe cognitive disability, 3) acute illness, unstable hypertension, and angina, 4) myocardial infarction, 5) fracture of the lower limb within the 6 months before the study, 6) foot deformity and neuroarthropathy, 7) foot ulcer, due to the contraindication of Thai massage [17], 8) dependence on alcohol and/or drugs with known effects on the central nervous system, and 9) partial or complete blindness.
Table 1.
Demographic characteristics and health status of study participants.
| Characteristics | Thai foot massage (FM) n=30 | Control group (CON) n=30 | Total n=60 |
|---|---|---|---|
| Female, n (%) | 20 (66.7) | 20 (66.7) | 40 (66.7) |
|
| |||
| Age (years) | 57.8±6.5 | 57.6±6.5 | 57.7±6.4 |
|
| |||
| Height (cm) | 157.7±7.4 | 158.9±8.2 | 158.3±7.8 |
|
| |||
| Body mass (kg) | 63.8±8.0 | 65.5±10.8 | 64.2±9.5 |
|
| |||
| BMI (kg/m2) | 25.3±2.7 | 25.9±3.7 | 25.6±3.3 |
|
| |||
| Occupation, n (%) | |||
| Government officer and employer | 9 (30.0) | 13 (43.3) | 22 (36.7) |
| Farmers | 21 (70.0) | 17 (56.7) | 38 (63.3) |
|
| |||
| Duration of diabetes (years) | 8.2±3.7 | 7.1±3.6 | 7.7±3.6 |
|
| |||
| Fasting blood sugar (mg/dl) | 126.0±33.4 | 132.4±29.2 | ±31.3 |
|
| |||
| Numbness; SWMT (points) | |||
| Left foot | 3.0±2.0 | 3.9±2.1 | 3.5±2.1 |
| Right foot | 3.8±2.1 | 3.9±1.8 | 3.8±1.9 |
All data are shown as mean ±SD.
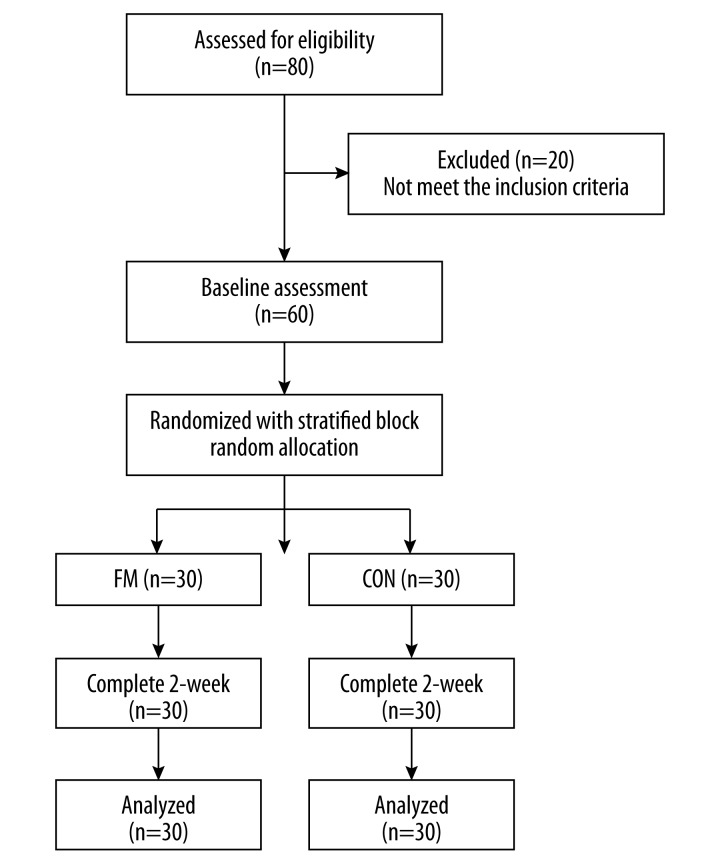
Patients who met the inclusion criteria were randomly allocated to either the Thai foot massage (FM) or control (CON) groups using stratified block random allocation with block sizes of 2, 4, and 6. The sex (male or female) and age groups (group 1=40–50 years old, group 2=51–60 years old and group 3=61–70 years old) were also used as stratification variables to achieve an approximate balance of age and sex of patient characteristics. A pre-generated random assignment scheme was made and enclosed in envelopes by the research assistant who was not involved in the process of treatment and outcome assessment. A 2-week prospective, parallel group, randomized, controlled clinical trial was conducted with physical therapists and a traditional Thai massage therapist. The local ethics committee approved the research protocol (HE562152). Written informed consent was obtained from all participants at the start of the study. A detailed summary of patient recruitment, participation, and attrition is shown in Figure 1.
Figure 1.
Participant flow and follow-up chart.
Interventions
Thai foot massage (FM)
A modified FM [17] was applied on the area of the foot, ankle, lower leg, and knee in supine and side-lying positions of the participants (Figure 2). Thumb pressing was used in FM by the massage therapist by applying gentle and gradually increasing pressure along the 3 meridian lines on the feet, named in Thai as Ga-la-ta-ree, Sa-has-rang-see, and Ta-wa-ree. These 3 lines cover the sole and dorsal surface of the feet, tibialis anterior, and gastrocnemius muscles. According to the different threshold in each individual, each thumb pressure was applied until the participant started to feel some discomfort (below-pressure pain threshold) and maintain the pressure for 5–10 seconds at each massage point [29]. In addition; manipulation using distraction technique in each toe was applied after the massage. This sequence was repeated 3–5 times for each massage line or point, combined with a gentle pulls of all toes. The protocol was used combining massage for 25 min and stretching for 5 min according to the pattern of royal Thai massage [30]. All participants received 30 min per session, 3 sessions per week for the 2-week course with a traditional Thai massage therapist who had more than 5 years of experience.
Figure 2.
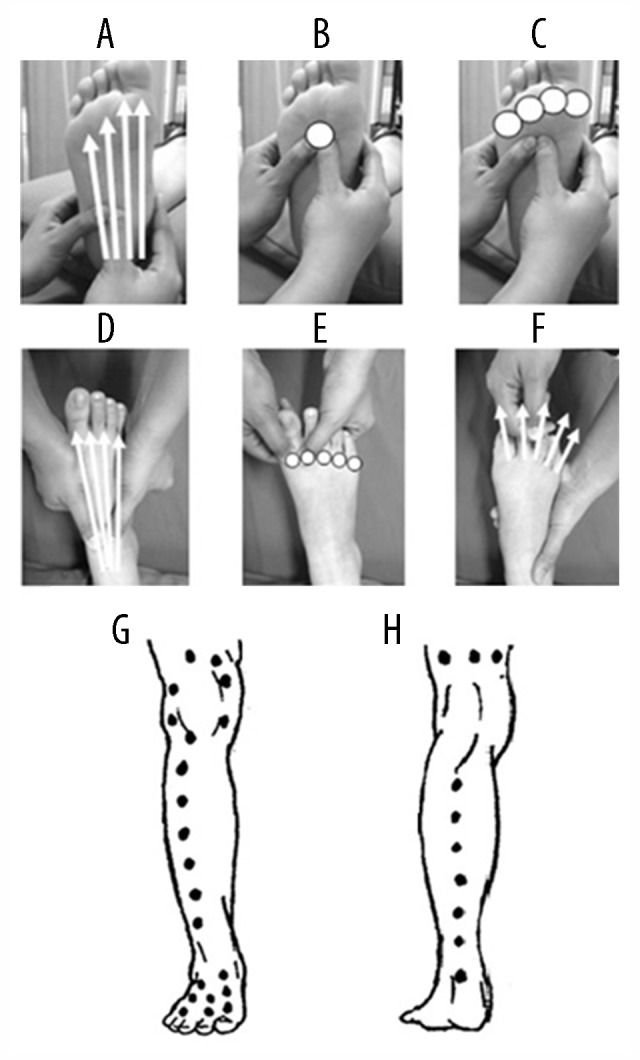
Thai foot and leg massage points and lines. At the sole of foot: 4-massage lines from the heel to the base of metatarsophalangeal joints (MTP) (A), 5-massage points; 1 point at the base of 3rd MTP (B) and 4 points at the head of MTP (C). At the dorsum of the foot: 4-massage lines from the ankle to the base of MTP (D), 5-massage points at the head of MTP (E), and distraction of all joints of toes (F). Massage lines at the anterior leg, posterior leg, and knee (G, H).
Control group (CON)
Each patient in the control group received the health education [31] using the 10 guidelines of foot self-care (e.g., washing and checking feet every day, applying skin lotion if skin is too dry, avoid wearing tight shoes, wearing shoes and socks at all times, and refraining from smoking). Patients were encouraged to practice active foot exercise in the direction of ankle dorsiflexion/plantarflexion for 5–10 min every day at home. All activities were done 30 min per session, 3 sessions per week for 2 weeks. These activities were performed at the hospital for the same period but in a different examination room for the intervention group.
Measurements
The outcome measurements were assessed before and after the first single session and re-assessed again after the 2-week treatment (2–3 day after the last treatment). The primary outcome measure was the time up and go test (TUG). The secondary outcome measures included one-leg standing tests (OLS), range of motion (ROM) of first metatarsophalangeal (MTP), ankle and knee joints, and sensation of the foot measured by Semmes-Weinstein Monofilament Test (SWMT). All outcome measures were assessed and the assessor was blinded to treatment of each participant. TUG is an outcome measure to assess functional dynamic balance. Dynamic balance is the ability to maintain total body equilibrium while moving the body and static balance is the ability to maintain total body equilibrium while standing in one spot [32–33]. The intra-rater reliability of TUG is excellent and intraclass correlation coefficient (ICC) is 0.98 (95% CI 0.96 to 0.99) [25]. During measurement, subjects stood up from a standard chair with a command “Go”, walked 3 meters, turned at the marked cone, and walked back to the chair as fast as possible. Timing started when the command was given and stopped when the subject was back to the chair again. Each participant performed 3 trials, and the best result of the 3 trials was recorded as the time in seconds [34]. OLS was measured on left and right legs with both eyes open and closed condition. Subjects stood with hands on the hips. The non-stance leg was standardized in a position of 30° of hip flexion and 90° of knee flexion with the ankle relaxed. The test was finished when the stance foot moved or shifted in any way or the non-stance foot touched the ground [35]. Each participant performed 3 trials, and the best result of the 3 trials was recorded. The active ROM for the 1st MTP, ankle, and knee joints in both legs were measured with a goniometer. Measurements were performed with 3 trials, and the average value of the trials was used for further analysis [36]. SWMT was used to apply a consistent 10-g force on 10 different sites on the plantar surface of the foot. A filament was placed perpendicular to the skin surface avoiding callosity, and the filament was pushed with sufficient force until it bent or twisted. Subjects were asked if they felt anything touching the skin. The examination was repeated 3 times for each site, and included at least 1 sham examination in which the filament was not actually placed on the skin. If the patient gave incorrect answers 2 or more times out of the 3 examinations per site, the site was considered as positive. If an incorrect answer occurred once or less, the site was considered as negative. The examination was conducted at all 10 sites, with random orders. The number of positive points was recorded for each side [37].
Statistical analysis
All data are presented as means ± standard deviations (SD). Estimation of the sample size was based on a previous study [18] done in the healthy elderly. The effect size (0.7) of timed up and go (TUG) in minutes after foot massage of treated and untreated groups was used to calculate the sample size for a statistical power of 80% at a 5% significance level. A drop-out rate of 10% was added for estimating the final sample size of 30 participants per group [38]. This study aimed to analyze each outcome separately at different points of time over the period of treatment to detect the immediate effect (after the first treatment) and short-term effect (after 2-week treatment). Since the randomization method did not guarantee that baseline characteristics would be the same between groups, analysis of co-variance (ANCOVA) was performed to compare post-test data between groups using the pre-test as a covariant. Shapiro-Wilk W test for testing the normality of all variables was performed before the treatment. This analysis was used to compare differences in outcome measures between the 2 treatment groups and to estimate the adjusted mean differences with 95% confidence intervals for each outcome measure in each group. Pearson’s correlation coefficient (r) and its 95% confidence interval were used to explore the correlations among parameters. The level of significance was set at p<0.05.
Results
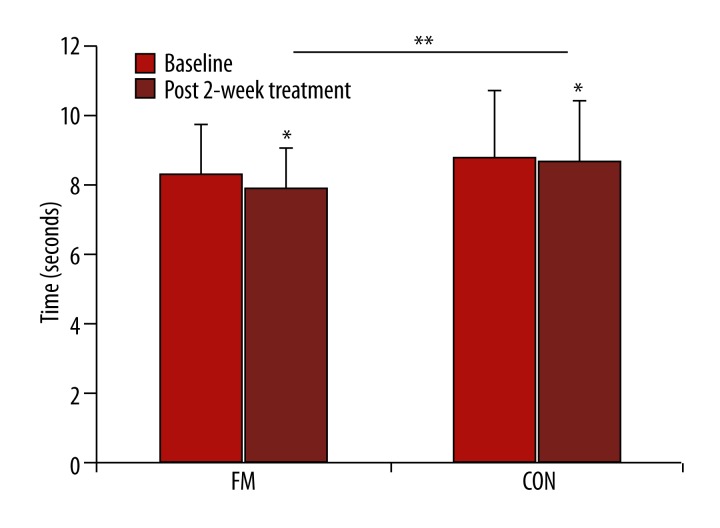
Table 1 shows the details of the demographic characteristics of the subjects in the 2 groups. These variables were not significantly different between groups. For immediate effects of the first single-session treatment, TUG for FM group was significantly improved from the baseline, while the CON did not change. However, when comparing the 2 groups after adjustment for baseline levels, TUG was not significantly different between the groups (p>0.05) (Table 2). Similar findings were found in OLS on the left leg with closed eyes. ROM in both groups significantly improved from baseline (p<0.01); however, only ROM of 1st MTP in FM was significantly larger than CON (p<0.05). In contrast, SWMT was not significantly different either within or between groups. After the 2-week treatment, both groups significantly improved from baseline in all parameters. When comparing between the 2 groups after adjustment for baseline levels, TUG for the FM group was faster than for CON as 1.13 s (95% CI of 0.76 to 1.50 s, p<0.001) (Table 2 and Figure 3). In terms of the effect size (ES or Cohen’s d) [39], this effect was evaluated as a large degree (ES=1.00). Similar findings were found for SWMT and ROM, except for ROM in right ankle plantarflexion and knee extension. In contrast, OLS was not significantly different between the 2 groups. Sex differences were not found in any outcome measurements. In addition, TUG was correlated with pre-test variables of both left and right 1st MTP in extension (left; r=0.39, p=0.0023 and right; r=0.35, p=0.0064). OLS with eyes open condition also correlated with SWMT (left; r=0.31, p=0.0116 and right; r=0.32, p=0.0085).
Table 2.
Comparison between group of the adjusted immediate post after the first treatment and adjusted post after 2-weeks treatment of all parameters.
| Outcome | Group | Baseline (Mean ±SD) | Immediate post after 1st treatment (Mean ±SD) | Post after 2st week of treatment Mean ±SD) |
|---|---|---|---|---|
| Timed up and go test; TUG (seconds) | FM | 8.31±1.42 | 7.87±1.18* | 7.06±1.14** |
| CON | 8.80±1.91 | 8.67±1.75 | 8.56±1.67* | |
|
| ||||
| One leg stance test; OLS opened eyes(seconds): Left | FM | 27.4±29.52 | 28.18±32.74 | 56.18±52.90* |
| CON | 25.58±26.97 | 22.4±22.98 | 39.98±44.98* | |
|
| ||||
| One leg stance test; OLS opened eyes (seconds) Right | FM | 33.62±39.45 | 32.48±37.80 | 69.3±69.66* |
| CON | 32.8±39.30 | 33.46±41.51 | 48.32±57.25* | |
|
| ||||
| One leg stance test; OLS closed eyes(seconds): Left | FM | 2.9±2.99 | 4.68±2.98* | 12.24±12.04* |
| CON | 3.82±4.88 | 4.7±4.21 | 8.3±7.33* | |
|
| ||||
| One leg stance test; OLS closed eyes (seconds): Right | FM | 2.74±1.91 | 2.88±2.12 | 9.68±12.73* |
| CON | 3.44±2.58 | 3.4±3.15 | 5.9±3.61* | |
|
| ||||
| 1st MTP flexion (degree, °): Left | FM | 24.97±8.88 | 26.00±8.65** | 28.20±8.03** |
| CON | 21.63±5.07 | 22.40±4.93* | 24.03±5.35* | |
|
| ||||
| 1st MTP flexion (degree, °): Right | FM | 26.77±8.29 | 28.33±8.06** | 31.50±7.77** |
| CON | 24.10±4.58 | 24.93±4.56* | 26.90±4.80* | |
|
| ||||
| 1st MTP extension (degree, °): Left | FM | 62.00±12.01 | 63.60±11.83** | 67.17±11.32** |
| CON | 73.70±7.74 | 74.40±7.85* | 76.23±7.97* | |
|
| ||||
| 1st MTP extension (degree, °): Right | FM | 65.37±11.82 | 66.70±11.62** | 69.63±11.24** |
| CON | 74.57±7.89 | 75.23±8.06* | 76.93±7.75* | |
|
| ||||
| Ankle dorsiflexion (degree, °): Left | FM | 10.13±4.28 | 12.13±3.95* | 15.30±4.04** |
| CON | 10.00±3.71 | 11.57±3.56* | 14.17±3.65* | |
|
| ||||
| Ankle dorsiflexion (degree,0): Right | FM | 12.63±4.92 | 14.37±4.90* | 16.60±4.55** |
| CON | 13.23±3.70 | 14.50±3.56* | 16.17±3.92* | |
|
| ||||
| Ankle plantarflexion (degree, °): Left | FM | 32.50±5.98 | 34.33±5.70* | 38.27±5.25** |
| CON | 34.17±8.62 | 35.80±8.21* | 38.23±7.93* | |
|
| ||||
| Ankle plantarflexion (degree, °): Right | FM | 34.53±4.83 | 35.67±4.61* | 38.60±4.80* |
| CON | 37.07±8.60 | 37.87±8.19* | 40.20±8.01* | |
|
| ||||
| Knee flexion (degree, °): Left | FM | 119.83±7.37 | 121.60±7.45* | 124.77±7.66** |
| CON | 122.57±10.99 | 123.33±10.65* | 124.30±10.07* | |
|
| ||||
| Knee flexion (degree, °): Right | FM | 120.13±6.99 | 121.40±6.67* | 123.93±6.48** |
| CON | 124.33±10.49 | 125.20±10.19* | 126.83±10.16* | |
|
| ||||
| Knee extension (degree, °): Left | FM | −6.17±2.26 | −5.37±2.11* | −3.60±2.18** |
| CON | −7.43±3.16 | −6.83±3.21* | −5.47±2.96* | |
|
| ||||
| Knee extension (degree, °): Right | FM | −7.77±1.81 | −6.77±1.98* | −4.67±2.09* |
| CON | −8.93±2.52 | −8.10±2.34* | −6.33±2.40* | |
|
| ||||
| Positive of SWMT (number): Left | FM | 3.03±2.04 | 3.03±2.04 | 1.43±1.52** |
| CON | 3.93±2.07 | 3.90±2.02 | 3.70±2.02* | |
|
| ||||
| Positive of SWMT (number): Right | FM | 3.77±2.06 | 3.77±2.06 | 1.83±1.64** |
| CON | 3.90±1.81 | 3.90±1.81 | 3.60±1.71* | |
Denotes statistically different (p<0.05) from baseline;
indicates statistically different (p<0.05) from baseline and between groups using analysis of covariance (ANCOVA) with adjusted for baselines, FM – Thai foot massage; CON – control; 95%CI – 95 percent confidence interval; statistically significant different defined as a p-value of <0.05.
Figure 3.
Time up and go (TUG) after the 2-week treatment. * Denotes statistically different (p<0.05) from baseline and ** indicates statistically different (p<0.05) between groups.
Discussion
This study is the first reported study to show that Thai foot massage using deep pressure and stretching improved TUG performance, ROM of the foot, and foot sensation in type II diabetic patients with PN after a 2-week treatment. TUG after the 2-week treatment was 1.13 s (95% CI of 0.76 to 1.50 s) faster in FM than CON. This was similar to the minimal detectable change of 1–2 s considered as clinically significant and was found in previous studies [40,41]. Vaillant et al. [25] show significant improvement in TUG after 20-min massage and mobilization of the foot and ankle joints in elderly adults, with the different mean change about 0.7 s when compared with the placebo protocol group. Cho et al. [42] also show significant improvement in TUG after ankle joint mobilization for 4 weeks, 3 times a week with 2 min per sessions, with different mean change 2.54 s when compared with the control group. In contrast, Pertille et al. [43] reported non-significant improvement in the TUG after 3 sets of 30-s ankle joint mobilization in elderly women. Although there has been controversy about effects of massage and mobilization on TUG in elderly adults, the combined treatment of massage and joint mobilization of the foot could have better effects for improvement in dynamic balance performance in type II diabetic patients with PN.
The mechanism underling the effects of Thai foot massage on the dynamic balance in diabetic patients with PN remains unclear; however, it may be explained by improvements in ROM and skin sensation of the foot after Thai foot massage using deep pressure and stretching of the foot and the leg. We showed that improvements of ROM and skin sensation of the foot were associated with improved dynamic balance performance. Using direct-deep pressure combined with gentle distraction on the muscle and joints of the foot and lower leg increases local blood circulation and stimulates the somatosensory system, including multiple receptors [25]. These effects may reverse neuropathy by changing pressure distribution, proprioceptive systems, muscle tension, joint angle, and muscle length. These sensory and segmental adjustments play an important role in postural control [14]. Direct-deep pressure technique could increase the extensibility of the non-contractile capsular and ligamentous tissues [13,14] and stimulating the joint mechanoreceptors may enhance the neuromuscular function of joint stabilizing muscles [44]. Joint mobilizations also increase flexibility [45] and may enhance postural control [37]. This was supported by positive relationships between TUG and ROM of 1st MTP. In line with this result, Mecagni et al. [11] showed a correlation between ankle ROM and balance. Therefore, it can be suggested that adequate ROM of the ankle and MTP joints plays an important role in better dynamic balance [15].
The limitation of the study is lack of inter- and intra-reliability of the TUG in diabetic patients. This might be a reason for an improvement of TUG in an active control group, although this result might be explained by the effect of active exercise as a part of the health education program. Also, it is not clear what duration of treatments is long enough to achieve the maximum effects and nor is it clear how long this positive effect can last. Therefore, a further study is needed to understand the long-term effect of improvement in mobility and balance to confirm the effect of Thai foot massage in diabetic patients with PN.
Conclusions
This study revealed that Thai foot massage using the pressure applied along the Thai meridian lines of the foot and the leg significantly improved dynamic balance performance, ROM of the foot, and foot sensation in diabetic patients with PN. Because Thai foot massage technique is not complex and is easy to perform, it may be a viable alternative treatment for home health care and in clinical settings for diabetic patients.
Acknowledgments
We gratefully acknowledge the Faculty of Associated Medical Sciences Khon Kaen University, Thailand for supporting the research. Furthermore, we are indebted to the Research Center in Back, Neck, Other Joint Pain and Human Performance (BNOJPH), Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand for their valuable research funding. Finally, we would like to express our sincere gratitude and appreciation to all patients for their generosity and willingness to participate in this study.
Footnotes
Source of support: Faculty of Associated Medical Sciences Khon Kaen University, Thailand
References
- 1.van Schie CH. Neuropathy: mobility and quality of life. Diabetes Metab Res Rev. 2008;24(Suppl 1):S45–51. doi: 10.1002/dmrr.856. [DOI] [PubMed] [Google Scholar]
- 2.van Deursen RW, Sanchez MM, Ulbrecht JS, et al. The role of muscle spindles in ankle movement perception in human subjects with diabetic neuropathy. Exp Brain Res. 1998;120(1):1–8. doi: 10.1007/s002210050371. [DOI] [PubMed] [Google Scholar]
- 3.van Deursen RW, Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther. 1999;29(12):718–26. doi: 10.2519/jospt.1999.29.12.718. [DOI] [PubMed] [Google Scholar]
- 4.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82(2):205–9. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25(10):1749–54. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 6.Shimada H, Uchiyama Y, Kakurai S. Specific effects of balance and gait exercises on physical function among the frail elderly. Clin Rehabil. 2003;17(5):472–79. doi: 10.1191/0269215503cr638oa. [DOI] [PubMed] [Google Scholar]
- 7.Schulte L, Roberts MS, Zimmerman C, et al. A quantitative assessment of limited joint mobility in patients with diabetes. Goniometric analysis of upper extremity passive range of motion. Arthritis Rheum. 1993;36(10):1429–43. doi: 10.1002/art.1780361016. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay JR, Kennedy L, Atkinson AB, et al. Reduced prevalence of limited joint mobility in type 1 diabetes in a U.K. clinic population over a 20-year period. Diabetes Care. 2005;28(3):658–61. doi: 10.2337/diacare.28.3.658. [DOI] [PubMed] [Google Scholar]
- 9.Petrulewicz-Salamon I. The influence of diabetes mellitus on joint mobility. Ortop Traumatol Rehabil. 2006;8(5):555–65. [PubMed] [Google Scholar]
- 10.Savas S, Koroglu BK, Koyuncuoglu HR, et al. The effects of the diabetes related soft tissue hand lesions and the reduced hand strength on functional disability of hand in type 2 diabetic patients. Diabetes Res Clin Pract. 2007;77(1):77–83. doi: 10.1016/j.diabres.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Mecagni C, Smith JP, Roberts KE, et al. Balance and ankle range of motion in community-dwelling women aged 64 to 87 years: a correlational study. Phys Ther. 2000;80(10):1004–11. [PubMed] [Google Scholar]
- 12.van Deursen R. Footwear for the neuropathic patient: offloading and stability. Diabetes Metab Res Rev. 2008;24(Suppl 1):S96–100. doi: 10.1002/dmrr.827. [DOI] [PubMed] [Google Scholar]
- 13.Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 2000;877(2):401–6. doi: 10.1016/s0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]
- 14.Perry SD. Evaluation of age-related plantar-surface insensitivity and onset age of advanced insensitivity in older adults using vibratory and touch sensation tests. Neurosci Lett. 2006;392(1–2):62–67. doi: 10.1016/j.neulet.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Lung MW, Hartsell HD, Vandervoort AA. Effects of aging on joint stiffness: implications for exercise. Physiotherapy Canada. 1996;48(2):96–106. [Google Scholar]
- 16.Menz HB, Morris ME, Lord SR. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sci Med Sci. 2005;60(12):1546–52. doi: 10.1093/gerona/60.12.1546. [DOI] [PubMed] [Google Scholar]
- 17.Tuntipidok Y. Therapeutic Thai massage. 3rd ed. Bangkok: U-Sa Printing; 2007. pp. 240–99. [Google Scholar]
- 18.Subcharoen P, Ananthigo P, Kaengketkit B, et al. Manual of the Rachsamnak style therapeutic massage. Bangkok: Samchareounpanich; 2007. [Google Scholar]
- 19.Prateepavanich P, Leewanun C. Thai traditional massage. Siriraj Med J. 2003;55:668–89. [Google Scholar]
- 20.Castro-Sánchez AM, Moreno-Lorenzo C, Matarán-Peñarrocha GA, et al. Connective tissue reflex massage for type 2 diabetic patients with peripheral arterial disease: randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:1–12. doi: 10.1093/ecam/nep171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori H, Ohsawa H, Tanaka TH, et al. Effect of massage on blood flow and muscle fatigue following isometric lumbar exercise. Med Sci Monit. 2004;10(5):CR173–78. [PubMed] [Google Scholar]
- 22.Field T, Diego M, Hernandez-Reif M. Massage therapy research. Dev Rev. 2007;27(1):75–89. [Google Scholar]
- 23.Watson S, Voner V. Practical reflexology: Interpretation and techniques. Boston: McGraw-Hill; 2009. [Google Scholar]
- 24.Vaillant J, Vuillerme N, Janvey A, et al. Effect of manipulation of the feet and ankles on postural control in elderly adults. Brain Res Bull. 2008;75(1):18–22. doi: 10.1016/j.brainresbull.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Vaillant J, Rouland A, Martigne P, et al. Massage and mobilization of the feet and ankles in elderly adults: effect on clinical balance performance. Man Ther. 2009;14(6):661–64. doi: 10.1016/j.math.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Forouzandeh F, Aziz Ahari A, Abolhasani F, et al. Comparison of different screening tests for detecting diabetic foot neuropathy. Acta Neurologica Scandinavica. 2005;112(6):409–13. doi: 10.1111/j.1600-0404.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- 27.Kamei N, Yamane K, Nakanishi S, et al. Effectiveness of Semmes-Weinstein monofilament examination for diabetic peripheral neuropathy screening. J Diabetes Complications. 2005;19(1):47–53. doi: 10.1016/j.jdiacomp.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Smieja M, Hunt DL, Edelman D, et al. Clinical examination for the detection of protective sensation in the feet of diabetic patients. International Cooperative Group for Clinical Examination Research. J Gen Intern Med. 1999;14(7):418–24. doi: 10.1046/j.1525-1497.1999.05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatchawan U, Thinkhamrop B, Kharmwan S, et al. Effectiveness of traditional Thai massage versus Swedish massage among patients with back pain associated with myofascial trigger points. J Bodywork Mov Ther. 2005;9(4):298–309. doi: 10.1016/j.jbmt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Chatchawan U, Eungpinichpong W, Sooktho S, et al. Effects of thai traditional massage on pressure pain threshold and headache intensity in patients with chronic tension-type and migraine headaches. J Altern Complement Med. 2014;20(6):486–92. doi: 10.1089/acm.2013.0176. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association. Standards of medical care in diabetes 2014. Diabetes Care. 2014;37(Suppl 1):S15–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 32.Shumway-Cook A, Woollacott MH. Control of postural and balance In: Motor Control: Theory and Practical Applications. Baltimore: Williams & Wilkins; 1995. pp. 119–42. [Google Scholar]
- 33.Morrow JR, Jackson AW, Disch JG, Mood DP. Measurement and Evaluation in Human Performance. 4th ed. Illinois: Human Kinetics; 2011. Assessment of sport skill and Motor abilities; pp. 304–13. [Google Scholar]
- 34.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–48. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 35.Jonsson E, Seiger A, Hirschfeld H. One-leg stance in healthy young and elderly adults: a measure of postural steadiness? Clin Biomech (Bristol, Avon) 2004;19(7):688–94. doi: 10.1016/j.clinbiomech.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Clarkson HM. Ankle and foot. In: Clarkson HM, editor. Musculoskeletal assessment: joint Range of Motion and Muscle testing. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 318–99. [Google Scholar]
- 37.Nather A, Neo SH, Chionh SB, et al. Assessment of sensory neuropathy in diabetic patients without diabetic foot problems. J Diabetes Complications. 2008;22(2):126–31. doi: 10.1016/j.jdiacomp.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60(12):1234–38. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 40.Piva SR, Fitzgerald GK, Irrgang JJ, et al. Get up and go test in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2004;85(2):284–89. doi: 10.1016/j.apmr.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Lim LI, van Wegen EE, de Goede CJ, et al. Measuring gait and gait-related activities in Parkinson’s patients’ own home environment: a reliability, responsiveness, and feasibility study. Parkinsonism Relat Disord. 2005;11(1):19–24. doi: 10.1016/j.parkreldis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Cho B, Ko T, Lee D. Effect of ankle joint mobilization on range of motion and functional balance of elderly adults. J Phys Ther Sci. 2012;24(4):331–33. [Google Scholar]
- 43.Pertille A, Macedo AB, Dibai Filho AV, et al. Immediate effects of bilateral grade III mobilization of the talocrural joint on the balance of elderly women. JManipulative Physiol Ther. 2012;35(7):549–55. doi: 10.1016/j.jmpt.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Bernard-Demanze L, Burdet C, Berger L, Rougier P. Recalibration of somesthetic plantar information in the control of undisturbed upright stance maintenance. J Integr Neurosci. 2004;3(4):433–51. doi: 10.1142/s0219635204000580. [DOI] [PubMed] [Google Scholar]
- 45.Hoch MC, McKeon PO. Joint mobilization improves spatiotemporal postural control and range of motion in those with chronic ankle instability. J Orthop Res. 2011;29(3):326–32. doi: 10.1002/jor.21256. [DOI] [PubMed] [Google Scholar]