Abstract
The basal ganglia are a series of interconnected subcortical nuclei. The function and dysfunction of these nuclei has been studied intensively as it pertains to motor control, but more recently our knowledge of these functions has broadened to include prominent roles in cognition and affective control. This review will summarize historical models of basal ganglia function, findings which have supported or conflicted with these models, and emphasize recent work in animals and humans directly testing the hypotheses generated by these models.
Keywords: striatum, Parkinson's disease, dopamine
Introduction
The basal ganglia are a set of deep forebrain nuclei consisting of the striatum (caudate and putamen in primates), globus pallidus (internal and external segments), subthalamic nucleus, and substantia nigra (pars reticulata and pars compacta). Along with other brain regions, including the cerebral cortex, thalamus, and several brainstem nuclei, the basal ganglia form a network with both open and closed loop circuitry (see Figure 1). The basal ganglia are well conserved: their basic anatomy and connectivity is preserved across most vertebrates, from the lamprey to the human (Reiner et al 1998, Stephenson-Jones et al 2012). While the structure and basic units of neural computation are likely to be similar, the breadth of functions may differ across species.
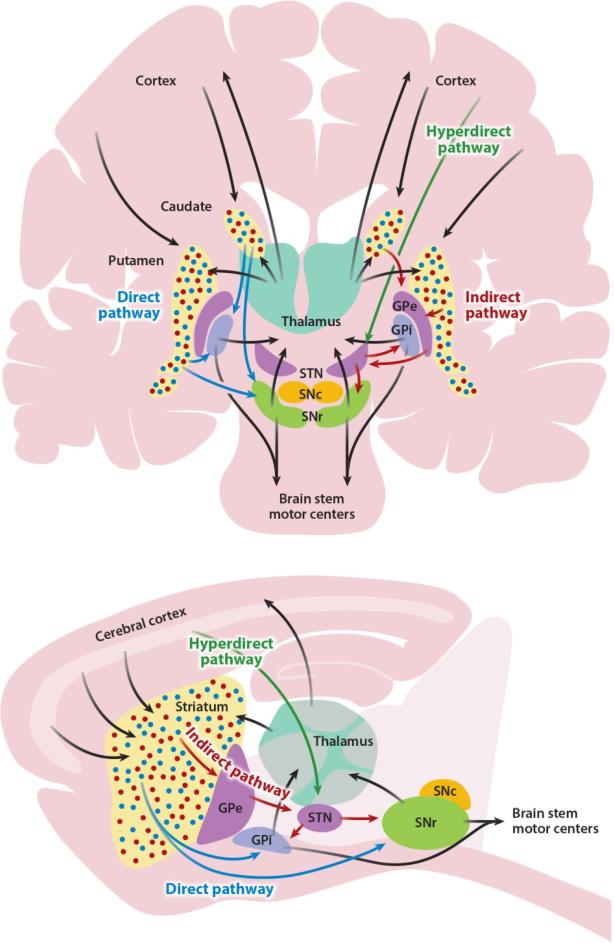
Figure 1.
Simplified basal ganglia circuit diagram. Basal ganglia nuclei and their major connections in primates (above), shown in coronal view, and rodents (below), shown in sagittal view. Many additional connections between nuclei are omitted for simplicity; see text for details. In both panels, the direct pathway is shown in blue, the indirect pathway in red, and the hyperdirect pathway in green. Black arrows represent connections shared by multiple pathways. Blue and red dots in the primate caudate/putamen and rodent striatum represent direct pathway–forming and indirect pathway–forming medium spiny neurons, respectively. Abbreviations: GPe, globus pallidus, pars externa; GPi, globus pallidus, pars interna; SNc, substantia nigra, pars compacta; SNr, substantia nigra, pars reticulata; STN, subthalamic nucleus.
Deciphering the basic neural computations that the basal ganglia perform, as well as the overall function this system serves, has been a longstanding area of intense research. Much of the ongoing interest in these nuclei derives from their role in human disease, and the striking symptoms humans experience with lesions of these structures. While inferring the normal function of a brain region or circuit from the disease state is challenging and in some cases perilous, examination of important human basal ganglia diseases gives a sense of the potential functions of the basal ganglia. Findings in humans with these diseases, combined with neuroanatomical and neurochemical studies in animals, led to the development of what we will call the Rate Model. Evolving over several years and critical papers, this model was formulated by several investigators, including Albin, Penney, Young, and DeLong (Albin et al 1989, DeLong 1983, DeLong 1990, Penney & Young 1983). The Rate Model described the basic anatomical and neurochemical connections between basal ganglia nuclei and postulated that human movement disorders are caused by imbalanced activity in basal ganglia nuclei. At the time of its description, the authors highlighted limitations and caveats of the Rate Model, but the simplicity of the model made it extraordinarily attractive for clinicians and basic scientists alike: it generated testable hypotheses regarding physiology and connectivity of basal ganglia nuclei in health and disease. We would argue that although current investigators continue to identify inconsistencies of the Rate Model, it has been an invaluable catalyst to research that has ultimately promoted greater knowledge of basal ganglia function as well as a more methodical approach to developing treatments.
In this review, we will begin with an overview of the functional neuroanatomy of the basal ganglia. We will describe the key components and predictions of the Rate Model and discuss findings which support, question, or refute the model, primarily in the context of motor control. Finally, we will examine some of the most recent experiments designed to test the rate model, and how these have revised our sense of how the basal ganglia circuit drives normal motor behavior as well as disease manifestations.
This review will not cover in detail the related role of the basal ganglia in reinforcement learning and habit formation, but the reader is directed to two excellent recent reviews of this topic (Graybiel 2008, Redgrave et al 2010). A complementary recent review examines the evidence for and against the rate model in animal models of Parkinson's disease (Ellens & Leventhal 2013).
The Basal Ganglia Circuit
Input Nuclei
The striatum and subthalamic nucleus (STN) are the primary input nuclei of the basal ganglia. The striatum receives input from nearly every cortical area, though anatomically this is organized into overlapping channels. As originally schematized (Alexander et al 1986), and more recently reviewed (Redgrave et al 2010), several cortical areas of related behavioral function send excitatory inputs to subregions of the striatum. These grouped inputs can be organized into channels subserving sensorimotor, cognitive, and affective functions (Redgrave et al 2010). While there is evidence that these channels have distinct functions, there is also evidence that they overlap at the level of the striatum and may serve an integrative function between cognitive, motor, and limbic signals deriving from cortical areas (Mailly et al 2013). The striatum also receives extensive excitatory input from the thalamus (reviewed in (Smith et al 2004)). The thalamostriatal projection is also topographically organized into parallel but overlapping motor, cognitive, and limbic circuits, based on their source and target subregions (Berendse & Groenewegen 1990, Elena Erro et al 2002, Ragsdale & Graybiel 1991, Smith & Parent 1986). Much of this input derives from a small region within the intralaminar nuclei of the thalamus, the centromedian and parafascicular (CM/Pf) complex (Jones & Leavitt 1974, Sadikot et al 1992), but input also derives from the mediodorsal (Parent 1976, Royce 1978, Sato et al 1979, Wall et al 2013) and ventrolateral nuclei of the thalamus (Elena Erro et al 2002, McFarland & Haber 2000, McFarland & Haber 2001). The striatum also receives a dense projection from midbrain dopaminergic neurons (SNc and ventral tegmental area).
The striatum is primarily composed of GABAergic spiny projection neurons, also called medium spiny neurons, for their characteristic medium size and numerous dendritic spines. These neurons receive excitatory inputs from cortex, thalamus, and in more ventral regions, amygdala and hippocampus, and are the sole striatal projection neurons. Approximately half of such neurons project to the globus pallidus, pars externa (GPe), and half project to basal ganglia output nuclei (SNr or GPi), which as described below, form the indirect and direct pathways, respectively. The striatum also contains cholinergic and GABAergic interneurons, whose role in overall circuit function is as yet unknown.
The other basal ganglia input nucleus, the STN, receives relatively restricted cortical input from primary motor, supplementary motor, and premotor cortices (Groenewegen & Berendse 1990, Kitai & Deniau 1981, Monakow et al 1978, Nambu et al 1996). The STN also receives input from the GPe through the indirect pathway (Carpenter et al 1981, Groenewegen & Berendse 1990). Composed primarily of glutamatergic neurons, the STN sends an excitatory projection to the basal ganglia output nuclei (Carpenter et al 1981, Parent & Hazrati 1995, Smith et al 1990). The cortex-STN-SNr/GPi projection, also known as the “hyperdirect” pathway, has been of growing interest (Nambu et al 2000, Nambu et al 2002). Recent papers have highlighted the involvement of this pathway in the development of action cancelling or “stop” signals for motor and cognitive programs (Sano et al 2013, Schmidt et al 2013). In addition to the canonical feedforward connection between STN and the output nuclei, anatomical studies have identified STN projections back to GPe (Carpenter et al 1981, Sato et al 2000b) and striatum (Kita & Kitai 1987, Smith et al 1990, Wall et al 2013). The function of these connections is unclear, but some have postulated that this loop forms an intrinsic pacemaker (Plenz & Kital 1999).
Output nuclei
The globus pallidus is composed of two regions, the GPe and GPi (an analogous structure in rodents is embedded in the internal capsule and is termed the entopeduncular nucleus). Both GPe and GPi make numerous connections within the basal ganglia (reviewed in (Jaeger & Kita 2011). GPe neurons receive GABAergic inputs from striatal projection neurons of the indirect pathway, as well as excitatory inputs from STN. GABAergic GPe neurons (Jessell et al 1978) send their axons to almost every basal ganglia nucleus, including the STN (forming the canonical indirect pathway), GPi and SNr, and also the striatum itself (Bevan et al 1998, Kita & Kita 2001, Mallet et al 2012, Sato et al 2000a). The GPi and SNr, the basal ganglia output nuclei, are also composed of GABAergic neurons. GPi receives inhibitory inputs from direct pathway striatal neurons, excitatory inputs from the STN (the canonical indirect pathway), thalamus (Deschenes et al 1996), pedunculopontine tegmental nucleus (PPTg) (Edley & Graybiel 1983), and from the GPe (Sato et al 2000a). The GPi projects to the thalamus, particularly ventral nuclei and the centromedian/parafascicular complex (CM/Pf) (Parent & Parent 2004). Basal ganglia output is also directed at several brainstem nuclei, including the superior colliculus and PPTg. Superior colliculus output pathways are important for regulation of eye movements and orienting behaviors (Hikosaka 2007). PPTg output pathways are believed to participate in both motor and attentional control mechanisms (Benarroch 2013). These basal ganglia-brainstem connections are an evolutionarily-conserved direct output pathway for regulation of motor behavior, as distinguished from the basal gangliathalamocortical pathway which requires looping back to the cortex to regulate behavior.
Dopaminergic neurons
The SNc and ventral tegmental area (VTA) are located in the midbrain and contain GABAergic and dopaminergic neurons. Recently, it has been shown that some midbrain dopamine neurons also release glutamate and GABA, though the behavioral role of co-release is unclear (Stuber et al 2010, Tritsch et al 2012). SNc dopaminergic neurons project primarily to the more dorsal/lateral (sensorimotor and cognitive) portions of the striatum, while VTA dopaminergic neurons project primarily to the more medial and ventral (cognitive/affective/limbic) portions of the striatum.
Basal Ganglia Diseases
As human basal ganglia diseases are a major motivation for ongoing research, as well as a source of much of the data for and against the Rate Model, we will briefly describe some of the major disease entities and how they relate to basal ganglia circuit function.
Huntington's Disease
Huntington's disease (HD) is a human neurodegenerative disease caused by autosomal dominant inheritance of an expanded trinucleotide repeat in the gene encoding huntingtin. Anatomically, patients with HD initially develop synaptic changes (Ferrante et al 1991) and later cell loss within the striatum (reviewed in (Reiner et al 2011)), with preferential early loss of indirect pathway striatal neurons (Albin et al 1990, Deng et al 2004, Reiner et al 1988). Later in the disease, neurodegeneration is observed in both striatum and cortex, with reduced striatal and cortical volume in living patients (Jernigan et al 1991, Rosas et al 2001) and at autopsy (Halliday et al 1998). Presymptomatic carriers show similar findings (Aylward et al 2004), and behavioral abnormalities are present during early symptomatic phases (Tabrizi et al 2013), suggesting an important role for the basal ganglia in affective control. At a microcircuit level, there is evidence that the psychiatric disease manifestations correlate with more prominent degeneration within the striosome compartment of the striatum (Tippett et al 2007). HD-related cognitive deficits include processing speed, set-switching, sequencing, and distractibility (reviewed in (Paulsen 2011), suggesting a role for the basal ganglia in many cognitive functions traditionally attributed to the frontal cortex.
Parkinson's Disease
Parkinson's disease (PD) is a neurodegenerative disease characterized by progressive cell loss in multiple brain regions, particularly brainstem nuclei, and most prominently as it pertains to motor symptoms, the SNc. In late stages of the disease, there can be more widespread neuropathology, including involvement of the cerebral cortex (Irwin et al 2012). PD, like HD, produces psychiatric, cognitive, and motor symptoms. It is difficult to tease apart the relationship of these symptoms to basal ganglia dysfunction, however, as there is neurodegeneration in other areas. Despite this difficulty, there is considerable evidence that basal ganglia dysfunction due to loss of striatal dopamine, does contribute to nonmotor symptoms, including mood and cognitive symptoms (Cools 2006).
Dystonia
Characterized by abnormal involuntary twisting movements and postures caused by co-activation of normally antagonistic muscle groups, dystonia has been historically characterized as a hyperkinetic movement disorder. As a symptom, it is a component of several different disease entities, including both Huntington's Disease and Parkinson's disease. However, in primary dystonias, little to no brain pathology is observed, suggesting circuit dysfunction, rather than neurodegeneration, may be the cause. These observations suggest that dystonia may not reflect a single pathophysiologic process, but rather a manifestation of convergent circuit mechanisms.
The Rate Model
Developed in the 1980s and early 1990s, the Rate Model incorporated a growing body of literature describing the connectivity, neurochemistry, and physiology of the basal ganglia (Albin et al 1989, DeLong 1990). In large part, it sprang from observations about deviations in basal ganglia structure and function in disease, particularly Huntington's disease, Parkinson's disease, and hemiballism, a movement disorder seen with acute lesions of the subthalamic nucleus. It postulated that the basal ganglia process cortical input through parallel pathways from striatum through to basal ganglia output nuclei, and feed it back to the cortex via a thalamic relay. Increases or decreases in firing rate of different basal ganglia nuclei are postulated to regulate basal ganglia output and behavior. Some of the key components and conclusions of the Rate Model are listed below:
The basal ganglia form an interconnected network
The basal ganglia are involved in not only motor function, but cognitive function
The cortex may generate motor or cognitive commands, but the execution and/or maintenance of these commands relies on the integrity of the basal ganglia as a positive feedback system to sustain activity
The network can be subdivided into two major pathways, the direct and indirect pathways
Dopamine differentially acts on the two major pathways at the level of the striatum
Among several possible actions, a subset are selected by striatum; competing actions are suppressed by lateral inhibition at several levels of the circuit
Network output is integrated at the level of the basal ganglia output nuclei, the GPi and SNr, which inhibit or disinhibit thalamocortical and/or brainstem areas to suppress or promote specific actions
These key concepts, in particular the idea that activity in the direct and indirect pathways has opposing effects on behavioral output, led to a number of physiological, pathological, and behavioral predictions, including:
Dopamine has opposing physiological effects on the activity of striatal neurons composing the direct and indirect pathways
Ablation or inactivation of indirect pathway neurons should lead to increased or excessive movement
Ablation or inactivation of direct pathway neurons should lead to decreased movement
Activation of indirect pathway neurons should lead to decreased movement
Activation of direct pathway neurons should lead to increased or excessive movement
Decreases in basal ganglia output (at the level of the GPi or SNr) should correlate with increased movement
Increases in basal ganglia output (at the level of the GPi or SNr) should correlate with decreased movement
The simplest formulation of the Rate Model treats the direct and indirect pathways as groups of neurons with uniform responses. However, given the somatotopic, functional and synaptic organization of the basal ganglia, a diversity of responses is more likely. Physiological recordings from normal nonhuman primates during motor and cognitive tasks show a wide variety of responses during a single task, even within the same anatomic region. This idea was articulated and advanced in a series of articles by Jonathan Mink (Mink 1996, Mink 2001, Mink & Thach 1993) and Okihide Hikosaka (Hikosaka 1991, Hikosaka 1998), often called the Action Selection Model. This model shares some of the key concepts of the Rate Model, but suggests that different neural ensembles within a basal ganglia pathway may activate or inhibit individual motor programs,
Support for the Rate Model
Humans
The surgical treatment of movement disorders, including deep brain stimulation (DBS), has allowed for physiological recordings of the human basal ganglia. One of the major caveats of such research is the lack of “control” subjects undergoing DBS: comparisons are often made to nonhuman primates, on or off therapy, or to patients with another disease. However, a number of key observations from human recordings support the Rate Model.
In HD, neuropathological studies have shown preferential degeneration of striatal indirect pathway neurons, as measured by immunoreactivity for enkephalin and other markers (Deng et al 2004, Reiner et al 1988), leading to the prediction that in HD, the GPe will be disinhibited, leading to decreased activity in the STN and output nuclei. A small number of human HD patients have undergone DBS implantation or lesion of the GPi (Cubo et al 2000, Kang et al 2011, Moro et al 2004). One study showed no significant change in the firing rate or pattern of GPi neurons in HD (Tang et al 2005), while two others found significant increases in GPe firing and significant decreases in GPi firing in HD as compared to PD, consistent with the rate model (Cubo et al 2000, Starr et al 2008).
Dystonia was not discussed at length in the original articulation of the Rate Model, but if dystonia is considered with chorea as a hyperkinetic movement disorder, the Rate Model would predict that an imbalance in direct and indirect pathway activity (with net loss of indirect pathway activity) should lead to disinhibition of the GPe and increased inhibition of the STN and GPi. Decreased STN and GPi firing rates would disinhibit thalamocortical and brainstem motor circuits, triggering aberrant co-activation of muscle groups. Several investigators have observed lowered firing rates in the GPi as measured in dystonia patients during intraoperative recordings, as compared to firing rates in control nonhuman primates or patients with PD (Starr et al 2005, Vitek et al 1999). In addition, some have observed lower STN firing rates in dystonia as compared to PD (Schrock et al 2009), though these rates were somewhat higher than those observed in essential tremor (Steigerwald et al 2008) and in normal nonhuman primates (Bergman et al 1994).
In Parkinson's disease, the Rate Model predicts that the loss of nigrostriatal dopamine should lead to an increase in indirect pathway activity (resulting in increased STN and GPi firing rates), and decreases in direct pathway activity (resulting in increased GPi firing rate). Indeed, the firing rate of STN and GPi neurons is increased in PD patients (Benazzouz et al 2002, Hutchison et al 1998). Intraoperative administration of a dopamine agonist reduces the firing rate of GPi neurons (Levy et al 2001a), and the development of levodopa-induced dyskinesias is associated with an even more profound decrease in GPi firing (Lozano et al 2000). A corollary is that decreasing STN or GPi firing rates should be therapeutic. In fact, local inactivation of STN neurons with muscimol or lidocaine produces short-latency antiparkinsonian effects in humans undergoing DBS surgery (Levy et al 2001b). Interestingly, in the same study, patients subsequently developed dyskinesias, suggesting that soon after infusion, a “normal” firing rate in the STN was achieved, relieving parkinsonian symptoms, but later firing rates were further depressed, producing hyperkinesias. In the case of dyskinesias associated with grafting of fetal midbrain neurons, lower firing rates, as well as bursting, were observed in the GPi (Richardson et al 2011), also supporting the Rate Model.
Animal Models
The MPTP primate model of parkinsonism facilitated a direct examination of basal ganglia nuclei in the context of parkinsonism. The Rate Model predicts that MPTP treatment would result in decreased striatal direct pathway activity and increased in indirect pathway activity, causing disinhibition of GPe, increased STN and basal ganglia output activity. GPe recordings show decreases in firing rate following MPTP treatment (Filion & Tremblay 1991), which reverse following dopamine agonist treatment (Filion et al 1991). STN firing rates increase following dopamine depletion with MPTP (Bergman et al 1994), and improvements in parkinsonism result from inhibition or lesion of the STN (Bergman et al 1990, Guridi et al 1994, Wichmann et al 1994). The output nuclei of the basal ganglia, GPi and SNr, showed overall higher rates of discharge (Filion & Tremblay 1991) as well as increased firing in response to movements. These firing abnormalities at the level of the output nuclei also reversed with dopamine agonist treatment (Filion et al 1991), resulting in profound decreases in firing rate in the context of levodopa-induced dyskinesias (Papa et al 1999).
In rodents, differential activity of the striatal direct and indirect pathway in parkinsonism has been reported, using a back-propagating action potential method for identifying striatal neurons of each pathway, a decrease in direct pathway and an increase in indirect pathway firing rate was observed (Mallet et al 2006), supporting the Rate Model. The advent of genetic techniques for selectively targeting direct and indirect pathway neurons has permitted more rigorous testing of Rate Model hypotheses. Direct and indirect pathway striatal neurons have several distinct, almost non-overlapping markers (Gerfen et al 1990), which can form the basis for cell-type specific manipulations. Direct pathway neurons express Substance P and the D1 dopamine receptor, while indirect pathway neurons express the D2 dopamine receptor, A2a adenosine receptor, and enkephalin (Schiffmann & Vanderhaeghen 1993). Ablation of indirect pathway neurons by selective expression of an immunotoxin under the D2 dopamine receptor promoter resulted in hyperlocomotor phenotypes (Sano et al 2003), much as would be expected in early Huntington's disease. Ablation of direct pathway neurons by selective expression of diphtheria toxin resulted in bradykinesia (Drago et al 1998), while ablation of indirect pathway neurons led to an increase in locomotor activity (Durieux et al 2009, Durieux et al 2012). Inducible and cell-type selective block of neurotransmission with tetanus toxin in direct or indirect pathway striatal neurons produced opposing effects on rotational behavior as well as psychostimulant-induced locomotion (Hikida et al 2010). Likewise, deletion of the key signaling protein DARPP-32 from either direct or indirect pathway neurons also resulted in divergent motor behaviors, including loss of hyperkinetic behaviors in the former and increased locomotion in the latter (Bateup et al 2010).
Neuronal ablation or blocking neurotransmission, even with rapid techniques, may lead to plasticity in basal ganglia microcircuits. To determine whether altering the balance of activity in the two pathways could achieve similar results, several groups have employed optogenetics, which allows millisecond timescale manipulations. Selective activation of striatal direct pathway neurons increases locomotor activity, while activation of indirect pathway neurons causes freezing (Kravitz et al 2010). Likewise, stimulation of the direct pathway or inactivation of the indirect pathway (via high frequency optogenetic stimulation of STN inputs) in parkinsonian animals ameliorates bradykinesia (Gradinaru et al 2009, Kravitz et al 2010). However, like electrical stimulation, optogenetic manipulations impose intense, probably supraphysiologic levels of activity on the basal ganglia circuit. They also show that that such imbalances in activity are sufficient to produce behavior, but not that they are necessary.
Inconsistencies in and Limitations of the Rate Model
A number of experimental observations do not seem to fit the Rate Model, which was highlighted both by the original papers and by subsequent studies.
Humans
Many clinicians and investigators have been puzzled by the observation that lesioning or stimulation of the GPi is therapeutic in both “hypokinetic” (Parkinson's Disease) and “hyperkinetic” movement disorders (dystonia, chorea). This paradox was included in initial articulation of the model, and the authors hinted that there might be some shared, rather than directly opposing, circuit mechanisms involved in diseases like Parkinson's Disease and dystonia. In some ways, this is not surprising, given the overlap of symptoms between classically hypokinetic and hyperkinetic disorders, such as the presence of dystonia and parkinsonism in both PD and HD. An interesting related observation is the fact that temporary inactivation of basal ganglia output in normal experimental animals does not cause marked behavioral dysfunction (Desmurget & Turner 2008). The mild parkinsonism observed in these primates mirrors what is observed in some dystonic patients with GPi DBS (Berman et al 2009). In other words, loss of basal ganglia output (as in pallidotomy or temporary inactivation) does not cause severe behavioral disturbances in normal animals, but gain of abnormal basal ganglia output (as in the disease state) can. Distinguishing how the basal ganglia circuit functions in normal versus pathological states remains an important question.
While firing rate changes have been observed in disease states, these are often small in magnitude, and there are more salient changes in patterning (bursting, oscillatory activity) and synchrony, both within and across nodes in the basal ganglia network. Abnormal activity patterns have been studied extensively in Parkinson's disease (Eusebio et al 2009, Litvak et al 2011) and dystonia (Chen et al 2006, Starr et al 2005, Weinberger et al 2012), and show correlations between such phenomena and disease manifestations. However, such observations cannot demonstrate causality (Eusebio & Brown 2009). Interestingly, the fact that similar oscillations are seen in Parkinson's disease and dystonia suggests that oscillations in and of themselves may not cause the motor phenotype. Additional disease-specific physiologic signatures are being identified in human recordings (de Hemptinne et al 2013, Shimamoto et al 2013), and these may help us develop hypotheses as to the causal role of neural activity in movement disorders. Less is known about how such bursting, synchrony, or oscillations contribute to normal basal ganglia function in humans, but this is an ongoing area of research, which can be most effectively carried out using animal models (Leventhal et al 2012).
Animal Models
Recordings from the striatum have been less supportive of the Rate Model, which predicts that loss of dopamine will produce reciprocal decreases and increases in the firing of direct and indirect pathway neurons. In chronically parkinsonian animals, the overall firing rate of striatal neurons increases markedly (Liang et al 2008, Rothblat & Schneider 1993). This observation may be due to oversampling of high firing rate indirect pathway neurons, but using rate responses to levodopa administration as a means for identifying neurons, it was observed that both direct and indirect pathway neurons have markedly elevated firing rates (Liang et al 2008).
As in humans, non-human primate studies have often shown minimal changes in overall firing rate but suggest patterned activity may be a driver of abnormal behavior. SNr, for example, shows essentially no firing rate change in MPTP monkeys, but an increase in bursting (Wichmann et al 1999). STN shows increases in both rate and bursting (Bergman et al 1994). GPe and GPi also show increased levels of synchrony and oscillations after MPTP treatment (Raz et al 2000, Wichmann et al 1994). Although definitive evidence that these oscillations are necessary and sufficient to produce parkinsonian motor behavior is still lacking, a variety of abnormalities in patterned activity do improve in MPTP monkeys with several different treatment modalities, including levodopa (Heimer et al 2006, Tachibana et al 2011), therapeutic pharmacologic inactivations of basal ganglia nuclei (Tachibana et al 2011) and DBS (Hahn et al 2008, McCairn & Turner 2009, Vitek et al 2012).
While optogenetic studies in rodents have shown that direct and indirect pathway activation is sufficient to cause opposing behaviors (Kravitz et al 2010, Tai et al 2012), they have not shown that such imbalances are necessary to produce behavior. Subsequent studies using observational (rather than interventional) methods, have told a more complicated story. Importantly, a minority of unidentified striatal neurons modulate firing during specific movements or tasks (Hikosaka et al 1989, Hollerman et al 1998, Kawagoe et al 1998, Kimchi & Laubach 2009). Combining cell-type-specific genetic methods with in vivo imaging, a recent paper demonstrated that both direct and indirect pathway striatal neurons are activated simultaneously during a motor task (Cui et al 2013). A similar study using single-unit recordings and juxtacellular labeling of striatal neurons also found cooperative activity of the direct and indirect pathways during voluntary movements (Isomura et al 2013). Using optogenetics and single-unit recordings, our laboratory has found co-activation of both direct and indirect pathway neurons during spontaneous locomotion (Kravitz et al, submitted). While these results seem at first glance to violate the the Rate Model, upon further examination, they support an action selection form of the model. If activation of the direct and indirect pathways is important for selecting certain actions, and suppressing others, it would stand to reason that for every direct pathway ensemble activated to choose a particular action, several indirect pathway ensembles would be activated to suppress competing actions. In fact, these conclusions are further supported by the observation that in rodents, SNr shows both excitatory and inhibitory responses during locomotion (Fan et al 2012, Gulley et al 2002, Gulley et al 1999, Jin & Costa 2010). Recent work in our laboratory (Freeze et al, in press) has also identified excitatory and inhibitory SNr responses to optogenetic activation of the direct pathway, but the Rate Model-predicted inhibitory responses had the strongest correlation with gating of movement. Excited neurons may suppress competing actions, supporting the Action Selection model.
Conclusions
The Rate Model and subsequent elaborations have helped propel a large body of hypothesis-driven research on basal ganglia function. A number of its basic assumptions have been validated by both physiologic and behavioral studies, though these studies have suggested that the system is more complicated than initially assumed. The Rate Model suggested that firing rates might correlate with behavior. Subsequent studies in humans with basal ganglia disorders, as well as in animal models, suggest that different groups of neurons within a particular nucleus may be activated or suppressed in order to release certain motor programs, but not others, yielding a mixture of responses when physiological responses are measured. In addition, basal ganglia nuclei may use both the rate and timing codes contained in spike trains. Though the direct causal relationship of such patterned activity to behavior has not been explored in detail as yet, we hope this will be a focus of second-generation optogenetic, physiologic, and behavioral studies.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB, Handelin B, Balfour R, et al. Abnormalities of striatal projection neurons and N-methyl-D-aspartate receptors in presymptomatic Huntington's disease. The New England journal of medicine. 1990;322:1293–8. doi: 10.1056/NEJM199005033221807. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14845–50. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. 2013;80:1148–55. doi: 10.1212/WNL.0b013e3182886a76. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2002;17(Suppl 3):S145–9. doi: 10.1002/mds.10156. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. The Journal of comparative neurology. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–8. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. Journal of neurophysiology. 1994;72:507–20. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Berman BD, Starr PA, Marks WJ, Jr., Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotactic and functional neurosurgery. 2009;87:37–44. doi: 10.1159/000195718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:9438–52. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MB, Carleton SC, Keller JT, Conte P. Connections of the subthalamic nucleus in the monkey. Brain research. 1981;224:1–29. doi: 10.1016/0006-8993(81)91113-6. [DOI] [PubMed] [Google Scholar]
- Chen CC, Kuhn AA, Hoffmann KT, Kupsch A, Schneider GH, et al. Oscillatory pallidal local field potential activity correlates with involuntary EMG in dystonia. Neurology. 2006;66:418–20. doi: 10.1212/01.wnl.0000196470.00165.7d. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neuroscience and biobehavioral reviews. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cubo E, Shannon KM, Penn RD, Kroin JS. Internal globus pallidotomy in dystonia secondary to Huntington's disease. Movement disorders : official journal of the Movement Disorder Society. 2000;15:1248–51. doi: 10.1002/1531-8257(200011)15:6<1248::aid-mds1029>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–42. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4780–5. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. The neurophysiologic basis of abnormal movements in basal ganglia disorders. Neurobehavioral toxicology and teratology. 1983;5:611–6. [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in neurosciences. 1990;13:281–5. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington's disease: a quantitative immunohistochemical study. Journal of chemical neuroanatomy. 2004;27:143–64. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. The European journal of neuroscience. 1996;8:329–43. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Turner RS. Testing basal ganglia motor functions through reversible inactivations in the posterior internal globus pallidus. Journal of neurophysiology. 2008;99:1057–76. doi: 10.1152/jn.01010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Padungchaichot P, Wong JY, Lawrence AJ, McManus JF, et al. Targeted expression of a toxin gene to D1 dopamine receptor neurons by cre-mediated site-specific recombination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:9845–57. doi: 10.1523/JNEUROSCI.18-23-09845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nature neuroscience. 2009;12:393–5. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. The EMBO journal. 2012;31:640–53. doi: 10.1038/emboj.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edley SM, Graybiel AM. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. The Journal of comparative neurology. 1983;217:187–215. doi: 10.1002/cne.902170207. [DOI] [PubMed] [Google Scholar]
- Elena Erro M, Lanciego JL, Gimenez-Amaya JM. Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neuroscience research. 2002;42:45–55. doi: 10.1016/s0168-0102(01)00302-9. [DOI] [PubMed] [Google Scholar]
- Ellens DJ, Leventhal DK. Review: electrophysiology of Basal Ganglia and cortex in models of Parkinson disease. Journal of Parkinson's disease. 2013;3:241–54. doi: 10.3233/JPD-130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Brown P. Synchronisation in the beta frequency-band--the bad boy of parkinsonism or an innocent bystander? Experimental neurology. 2009;217:1–3. doi: 10.1016/j.expneurol.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Pogosyan A, Wang S, Averbeck B, Gaynor LD, et al. Resonance in subthalamo-cortical circuits in Parkinson's disease. Brain : a journal of neurology. 2009;132:2139–50. doi: 10.1093/brain/awp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Rossi MA, Yin HH. Mechanisms of action selection and timing in substantia nigra neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5534–48. doi: 10.1523/JNEUROSCI.5924-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Kowall NW, Richardson EP., Jr Proliferative and degenerative changes in striatal spiny neurons in Huntington's disease: a combined study using the section-Golgi method and calbindin D28k immunocytochemistry. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991;11:3877–87. doi: 10.1523/JNEUROSCI.11-12-03877.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain research. 1991;547:142–51. [PubMed] [Google Scholar]
- Filion M, Tremblay L, Bedard PJ. Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain research. 1991;547:152–61. [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–32. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–9. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annual review of neuroscience. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. The Journal of comparative neurology. 1990;294:607–22. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Kosobud AE, Rebec GV. Behavior-related modulation of substantia nigra pars reticulata neurons in rats performing a conditioned reinforcement task. Neuroscience. 2002;111:337–49. doi: 10.1016/s0306-4522(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Kuwajima M, Mayhill E, Rebec GV. Behavior-related changes in the activity of substantia nigra pars reticulata neurons in freely moving rats. Brain research. 1999;845:68–76. doi: 10.1016/s0006-8993(99)01932-0. [DOI] [PubMed] [Google Scholar]
- Guridi J, Herrero MT, Luquin R, Guillen J, Obeso JA. Subthalamotomy improves MPTP-induced parkinsonism in monkeys. Stereotactic and functional neurosurgery. 1994;62:98–102. doi: 10.1159/000098603. [DOI] [PubMed] [Google Scholar]
- Hahn PJ, Russo GS, Hashimoto T, Miocinovic S, Xu W, et al. Pallidal burst activity during therapeutic deep brain stimulation. Experimental neurology. 2008;211:243–51. doi: 10.1016/j.expneurol.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, McRitchie DA, Macdonald V, Double KL, Trent RJ, McCusker E. Regional specificity of brain atrophy in Huntington's disease. Experimental neurology. 1998;154:663–72. doi: 10.1006/exnr.1998.6919. [DOI] [PubMed] [Google Scholar]
- Heimer G, Rivlin-Etzion M, Bar-Gad I, Goldberg JA, Haber SN, Bergman H. Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine primate model of Parkinsonism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8101–14. doi: 10.1523/JNEUROSCI.5140-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia--possible role in motor coordination and learning. Current opinion in neurobiology. 1991;1:638–43. doi: 10.1016/s0959-4388(05)80042-x. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Neural systems for control of voluntary action--a hypothesis. Advances in biophysics. 1998;35:81–102. [PubMed] [Google Scholar]
- Hikosaka O. GABAergic output of the basal ganglia. Progress in brain research. 2007;160:209–26. doi: 10.1016/S0079-6123(06)60012-5. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. Journal of neurophysiology. 1989;61:814–32. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. Journal of neurophysiology. 1998;80:947–63. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Annals of neurology. 1998;44:622–8. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, et al. Neuropathologic substrates of Parkinson disease dementia. Annals of neurology. 2012;72:587–98. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Takekawa T, Harukuni R, Handa T, Aizawa H, et al. Reward-modulated motor information in identified striatum neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:10209–20. doi: 10.1523/JNEUROSCI.0381-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger D, Kita H. Functional connectivity and integrative properties of globus pallidus neurons. Neuroscience. 2011;198:44–53. doi: 10.1016/j.neuroscience.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Salmon DP, Butters N, Hesselink JR. Cerebral structure on MRI, Part II: Specific changes in Alzheimer's and Huntington's diseases. Biological psychiatry. 1991;29:68–81. doi: 10.1016/0006-3223(91)90211-4. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Emson PC, Paxinos G, Cuello AC. Topographic projections of substance P and GABA pathways in the striato- and pallido-nigral system: a biochemical and immunohistochemical study. Brain research. 1978;152:487–98. doi: 10.1016/0006-8993(78)91104-6. [DOI] [PubMed] [Google Scholar]
- Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–62. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Leavitt RY. Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. The Journal of comparative neurology. 1974;154:349–77. doi: 10.1002/cne.901540402. [DOI] [PubMed] [Google Scholar]
- Kang GA, Heath S, Rothlind J, Starr PA. Long-term follow-up of pallidal deep brain stimulation in two cases of Huntington's disease. Journal of neurology, neurosurgery, and psychiatry. 2011;82:272–7. doi: 10.1136/jnnp.2009.202903. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature neuroscience. 1998;1:411–6. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kimchi EY, Laubach M. Dynamic encoding of action selection by the medial striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3148–59. doi: 10.1523/JNEUROSCI.5206-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kita T. Number, origins, and chemical types of rat pallidostriatal projection neurons. The Journal of comparative neurology. 2001;437:438–48. doi: 10.1002/cne.1294. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. The Journal of comparative neurology. 1987;260:435–52. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Deniau JM. Cortical inputs to the subthalamus: intracellular analysis. Brain research. 1981;214:411–5. doi: 10.1016/0006-8993(81)91204-x. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–36. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Dostrovsky JO, Lang AE, Sime E, Hutchison WD, Lozano AM. Effects of apomorphine on subthalamic nucleus and globus pallidus internus neurons in patients with Parkinson's disease. Journal of neurophysiology. 2001a;86:249–60. doi: 10.1152/jn.2001.86.1.249. [DOI] [PubMed] [Google Scholar]
- Levy R, Lang AE, Dostrovsky JO, Pahapill P, Romas J, et al. Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain : a journal of neurology. 2001b;124:2105–18. doi: 10.1093/brain/124.10.2105. [DOI] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7537–47. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain : a journal of neurology. 2011;134:359–74. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lang AE, Levy R, Hutchison W, Dostrovsky J. Neuronal recordings in Parkinson's disease patients with dyskinesias induced by apomorphine. Annals of neurology. 2000;47:S141–6. [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau JM. The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5718–27. doi: 10.1523/JNEUROSCI.5248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3875–84. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, et al. Dichotomous organization of the external globus pallidus. Neuron. 2012;74:1075–86. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. Journal of neurophysiology. 2009;101:1941–60. doi: 10.1152/jn.91092.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3798–813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. The Journal of comparative neurology. 2001;429:321–36. doi: 10.1002/1096-9861(20000108)429:2<321::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Progress in neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. Neurobiology of basal ganglia circuits in Tourette syndrome: faulty inhibition of unwanted motor patterns? Advances in neurology. 2001;85:113–22. [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia intrinsic circuits and their role in behavior. Current opinion in neurobiology. 1993;3:950–7. doi: 10.1016/0959-4388(93)90167-w. [DOI] [PubMed] [Google Scholar]
- Monakow KH, Akert K, Kunzle H. Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1978;33:395–403. doi: 10.1007/BF00235561. [DOI] [PubMed] [Google Scholar]
- Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, et al. Bilateral globus pallidus stimulation for Huntington's disease. Annals of neurology. 2004;56:290–4. doi: 10.1002/ana.20183. [DOI] [PubMed] [Google Scholar]
- Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2671–83. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, et al. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. Journal of neurophysiology. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neuroscience research. 2002;43:111–7. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Papa SM, Desimone R, Fiorani M, Oldfield EH. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Annals of neurology. 1999;46:732–8. doi: 10.1002/1531-8249(199911)46:5<732::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Parent A. Striatal afferent connections in the turtle (Chrysemys picta) as revealed by retrograde axonal transport of horseradish peroxidase. Brain research. 1976;108:25–36. doi: 10.1016/0006-8993(76)90161-x. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain research. Brain research reviews. 1995;20:128–54. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. The pallidofugal motor fiber system in primates. Parkinsonism & related disorders. 2004;10:203–11. doi: 10.1016/j.parkreldis.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Paulsen JS. Cognitive impairment in Huntington disease: diagnosis and treatment. Current neurology and neuroscience reports. 2011;11:474–83. doi: 10.1007/s11910-011-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB, Jr., Young AB. Speculations on the functional anatomy of basal ganglia disorders. Annual review of neuroscience. 1983;6:73–94. doi: 10.1146/annurev.ne.06.030183.000445. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–82. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Ragsdale CW, Jr., Graybiel AM. Compartmental organization of the thalamostriatal connection in the cat. The Journal of comparative neurology. 1991;311:134–67. doi: 10.1002/cne.903110110. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8559–71. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nature reviews. Neuroscience. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5733–7. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Dragatsis I, Dietrich P. Genetics and neuropathology of Huntington's disease. International review of neurobiology. 2011;98:325–72. doi: 10.1016/B978-0-12-381328-2.00014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain research. Brain research reviews. 1998;28:235–85. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Freed CR, Shimamoto SA, Starr PA. Pallidal neuronal discharge in Parkinson's disease following intraputamenal fetal mesencephalic allograft. Journal of neurology, neurosurgery, and psychiatry. 2011;82:266–71. doi: 10.1136/jnnp.2009.201129. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Goodman J, Chen YI, Jenkins BG, Kennedy DN, et al. Striatal volume loss in HD as measured by MRI and the influence of CAG repeat. Neurology. 2001;57:1025–8. doi: 10.1212/wnl.57.6.1025. [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Schneider JS. Response of caudate neurons to stimulation of intrinsic and peripheral afferents in normal, symptomatic, and recovered MPTP-treated cats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:4372–8. doi: 10.1523/JNEUROSCI.13-10-04372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce GJ. Cells of origin of subcortical afferents to the caudate nucleus: a horseradish peroxidase study in the cat. Brain research. 1978;153:465–75. doi: 10.1016/0006-8993(78)90332-3. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Smith Y, Bolam JP. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. The Journal of comparative neurology. 1992;320:228–42. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- Sano H, Chiken S, Hikida T, Kobayashi K, Nambu A. Signals through the striatopallidal indirect pathway stop movements by phasic excitation in the substantia nigra. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7583–94. doi: 10.1523/JNEUROSCI.4932-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Yasoshima Y, Matsushita N, Kaneko T, Kohno K, et al. Conditional ablation of striatal neuronal types containing dopamine D2 receptor disturbs coordination of basal ganglia function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:9078–88. doi: 10.1523/JNEUROSCI.23-27-09078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Lavallee P, Levesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. The Journal of comparative neurology. 2000a;417:17–31. [PubMed] [Google Scholar]
- Sato F, Parent M, Levesque M, Parent A. Axonal branching pattern of neurons of the subthalamic nucleus in primates. The Journal of comparative neurology. 2000b;424:142–52. doi: 10.1002/1096-9861(20000814)424:1<142::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sato M, Itoh K, Mizuno N. Distribution of thalamo-caudate neurons in the cat as demonstrated by horseradish peroxidase. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1979;34:143–53. doi: 10.1007/BF00238347. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ. Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:1080–7. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nature neuroscience. 2013;16:1118–24. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrock LE, Ostrem JL, Turner RS, Shimamoto SA, Starr PA. The subthalamic nucleus in primary dystonia: single-unit discharge characteristics. Journal of neurophysiology. 2009;102:3740–52. doi: 10.1152/jn.00544.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto SA, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Miller KJ, Starr PA. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7220–33. doi: 10.1523/JNEUROSCI.4676-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Hazrati LN, Parent A. Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method. The Journal of comparative neurology. 1990;294:306–23. doi: 10.1002/cne.902940213. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience. 1986;18:347–71. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in neurosciences. 2004;27:520–7. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington's disease: support for selective loss of striatal cells originating the indirect pathway. Experimental neurology. 2008;211:227–33. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Rau GM, Davis V, Marks WJ, Jr., Ostrem JL, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. Journal of neurophysiology. 2005;93:3165–76. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Potter M, Herzog J, Pinsker M, Kopper F, et al. Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. Journal of neurophysiology. 2008;100:2515–24. doi: 10.1152/jn.90574.2008. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: dual-output pathways conserved throughout vertebrate phylogeny. The Journal of comparative neurology. 2012;520:2957–73. doi: 10.1002/cne.23087. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8229–33. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet neurology. 2013;12:637–49. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Iwamuro H, Kita H, Takada M, Nambu A. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. The European journal of neuroscience. 2011;34:1470–84. doi: 10.1111/j.1460-9568.2011.07865.x. [DOI] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nature neuroscience. 2012;15:1281–9. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JK, Moro E, Lozano AM, Lang AE, Hutchison WD, et al. Firing rates of pallidal neurons are similar in Huntington's and Parkinson's disease patients. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2005;166:230–6. doi: 10.1007/s00221-005-2359-x. [DOI] [PubMed] [Google Scholar]
- Tippett LJ, Waldvogel HJ, Thomas SJ, Hogg VM, van Roon-Mom W, et al. Striosomes and mood dysfunction in Huntington's disease. Brain : a journal of neurology. 2007;130:206–21. doi: 10.1093/brain/awl243. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–6. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Annals of neurology. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Zhang J, Hashimoto T, Russo GS, Baker KB. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Experimental neurology. 2012;233:581–6. doi: 10.1016/j.expneurol.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–60. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Alavi M, Hodaie M, Lozano AM, et al. Oscillatory activity in the globus pallidus internus: comparison between Parkinson's disease and dystonia. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2012;123:358–68. doi: 10.1016/j.clinph.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. Journal of neurophysiology. 1994;72:521–30. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]