Abstract
Background
Differentiated thyroid carcinomas (DTC) are the only tumors for which age is a determinant of stage in the American Joint Committee on Cancer’s (AJCC) staging protocol. In this study, we re-examined the relationship between age, extent of disease, and prognosis by using a large dataset with longer follow-up times.
Methods
We examined the Surveillance, Epidemiology, and End Results (SEER) registry data 1973 to 2005 for patients with DTC as their only known malignancy. We used Cox multivariate analyses to generate mortality hazard ratios, controlling for several variables, to evaluate the effects of age and disease extent.
Results
We identified 55,402 patients with DTC. Of these, 49,240 had sufficient data to generate a TNM stage on the basis of AJCC guidelines. Within stage II, younger patients (<45 years) have worse outcomes than older patients (P <.001). Younger patients had an 11-fold increase in mortality between stages I and II, whereas there was no difference for older patients. When we uniformly applied the 45-and-older staging protocol to all patients, we found that stages III–IVc had a significantly greater risk of mortality for all patients compared with stage I.
Conclusion
The presence of regional and metastatic thyroid cancer bears prognostic significance for all ages. Under current AJCC guidelines, young patients with metastatic thyroid cancer may be understaged.
Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) together comprise a generally indolent disease group known as differentiated thyroid carcinomas (DTCs) and are associated with overall excellent survival rates. Certain criteria have been identified that portend a worse outcome, including advanced age at diagnosis, the presence of distant metastases, and primary tumor size and extent. The effect of regional nodal status on survival, however, remains controversial.1,2 On the other hand, age has been shown to be such a strong prognostic factor that it features prominently in the American Joint Committee on Cancer’s (AJCC) staging protocol, where it is as a determinant of stage for DTC, a unique characteristic among all neoplasms.3
Currently, patients with DTC who are younger than 45 years of age are classified as either stage I, ie, in the absence of metastatic disease, or stage II, in the presence thereof, whereas those ages 45 years and older are divided into stages I through IVc (Table I). In this respect, it is thought that young age can, to some extent, dampen the deleterious effects generally associated with advanced disease. Although age is undeniably linked to prognosis, as has been demonstrated in all scoring systems developed for either or both components of DTCs, including the Mayo Clinic’s Metastases, Age, Complete resection, Invasion, Size (MACIS),4 the Lahey Clinic’s Age, Metastases, Extent, Size (AMES),5 and the European Organization for Research and Treatment of Cancer (EORTC) systems,6 a more thorough understanding of the relationship between age and disease extent and the impact this interaction has on DTC outcomes is needed.
Table I.
AJCC staging protocol for DTC, 6th edition
| Primary tumor (T) | |
| TX: | Primary tumor cannot be assessed |
| T0: | No evidence of primary tumor |
| T1: | ≤2 cm, limited to thyroid |
| T1a | ≤1 cm |
| T1b | >1 cm and ≤2 cm |
| T2: | >2 cm and ≤4 cm, limited to thyroid |
| T3: | >4 cm, limited to thyroid or minimal extrathyroid extension |
| T4a: | Tumor of any size that has invaded nearby tissues, such as the larynx, trachea, esophagus, or recurrent laryngeal nerve |
| T4b: | Tumor of any size that has grown either back toward the spine or into nearby large blood vessels |
| Regional nodes (N) | |
| NX: | Regional lymph nodes cannot be assessed |
| N0: | No regional node metastasis |
| N1: | Regional node involvement |
| N1a | Nodal metastasis to level VI (pretracheal, paratracheal, and prelaryngeal) |
| N1b | Nodal metastasis to cervical or superior mediastinal areas |
| Distant metastasis (M) | |
| MX: | Distant metastasis cannot be assessed |
| M0: | No distant metastasis |
| M1: | Distant metastasis |
| Staging grouping | |||
|---|---|---|---|
| For patients <45 years | |||
| Stage I | Any T | Any N | M0 |
| Stage II | Any T | Any N | M1 |
| For patients ≥45 years | |||
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1–3 | N1a | M0 | |
| Stage IVa | T4a | N0–1a | M0 |
| T1–4a | N1b | M0 | |
| Stage IVb | T4b | Any N | M0 |
| Stage IVc | Any T | Any N | M1 |
AJCC, American Joint Committee on Cancer; DTC, differentiated thyroid carcinoma.
In an effort to answer this question and to elucidate the prognostic values of both age and disease extent under the current AJCC staging protocol, we sought to examine the survival trends of patients with DTC as reported by the Surveillance, Epidemiology, and End Results (SEER) Program from 1973 to 2005. Specifically, we compared the survival of patients younger than 45 years of age with metastatic disease (stage II) with that of patients 45 years of age and older who had tumors limited to the thyroid gland that measured between 2 and 4 cm (T2N0M0, also stage II). We wanted to determine whether young age alone could offset metastatic disease enough to warrant the joint classification in stage II of patients younger than 45 years of age with metastases with older patients with large localized tumors. Furthermore, we assessed whether all patients younger than 45 years of age without metastatic disease (stage I) had uniform outcomes, thus evaluating the effect of regional nodal involvement on survival. Overall, the goal of our study was to determine whether the risk stratification performed under the current AJCC staging guidelines accurately reflected the outcomes for DTC in young patients.
METHODS
SEER registry and study population
The SEER project is a United States population-based cancer registry that began in 1973 and is supported by the National Cancer Institute and Centers for Disease Control and Prevention. SEER contains data across multiple geographic regions on incidence, prevalence, mortality, and population-based variables and currently represents approximately 28% of the U.S. population. The SEER data set also contains information on the primary characteristics of the tumor, including site, spread, and histology when available, as well as limited information regarding treatment, excluding chemotherapy. Histological diagnoses in the SEER database use the International Classification of Disease for Oncology (ie, ICD-O) coding system and may overlap (eg, different codes for papillary carcinoma and papillary adenocarcinoma).
Data collection and analysis
This study was reviewed and approved by the Institutional Review Board at the University of California, San Diego. We examined SEER data between 1973 and 2005 and selected patients with a diagnosis of well-differentiated thyroid carcinoma as their only known malignancy, as defined by a combination of ICD-O site code of C73.9 (ie, thyroid), papillary and/or follicular histology, and tumor sequence number equal to zero. To capture all patients with DTC, the following codes were included in the study: “papillary carcinoma,” “papillary adenocarcinoma,” “follicular adenocarcinoma,” “papillary & follicular adenocarcinoma,” and “papillary cyst-adenocarcinoma.” Patients with less than 1 month of follow-up were excluded from the study.
The current AJCC TNM staging criteria for DTC depend on whether patients are 45 years of age or older (hereafter referred to as older patients) or younger than 45 years of age (hereafter referred to as younger patients) at the time of diagnosis (Table I). For parts of this study, the current AJCC TNM staging criteria for older patients were applied uniformly to restage tumors for all patients, regardless of their age. As the result of coding overlap, tumor categories T4a and T4b could not be discerned, so that stages IVa and IVb were combined as stage IVa/b. We used Cox multivariate proportional hazard models to generate relative risk of death by any cause with 95% confidence intervals, controlling for sex, race, marital status, histology, surgical and radiation treatment, and when appropriate, age at diagnosis, and stage. Subset analyses explored the influence and interaction of other variables, including sex, ethnicity, marital status, stage at diagnosis, age at diagnosis, and treatment modality. Age at diagnosis was categorized into patients diagnosed at 30 years of age or younger, and then in 10-year intervals beginning at age 31. Ethnic categories as defined by SEER included white, black, Asian, Hispanic, American Indian/Alaskan Native, and other/unknown. Marital status included the categories: single, married, separated, divorced, and widowed.
Statistics
Analyses were performed with the STATA 10 (Stata Corp., College Station, TX) software package. Statistical significance was defined as a Type I error probability of <.05; all confidence intervals (CI) are reported as 95% CI.
RESULTS
General findings
We identified 55,402 patients with PTC or FTC as their only known malignancy. Demographic characteristics of this sample are noted in Table II. Of these, 49,240 had sufficient data to generate a TNM stage on the basis of the AJCC Cancer Staging Manual, 6th edition. Consistent with the existing body of literature, most tumors were diagnosed in women (77.4%), and they predominantly affected white subjects (78.3%) relative to other ethnic groups. Under current staging guidelines, most patients were diagnosed in stage I (77.1%). Although complete treatment data were not available for all patients, approximately 99% of those patients who had data regarding surgical treatment did have surgery (n = 38,895). Approximately one-half of patients recorded had radiation treatment of any kind (n = 25,181; 46.2%). Age at diagnosis ranged from 2 to 100 years of age, with a mean of 44.6 (SD: 15.5) and a median of 43 years of age.
Table II.
Summary of SEER data on DTC
| Patient Characteristics | N (%) |
|---|---|
| Sex | |
| Female | 42,871 (77.4) |
| Male | 12,531 (22.6) |
| Ethnicity | |
| White | 39,111 (78.3) |
| Asian | 2,932 (5.87) |
| Black | 5,850 (11.7) |
| Hispanic | 1,551 (3.11) |
| American Indian/Alaskan Native | 333 (0.67) |
| Other/unknown | 160 (0.32) |
| AJCC stage at diagnosis (N = 49,240) | |
| Stage I | 37,950 (77.1) |
| Stage II | 3,058 (6.21) |
| Stage III | 5,091 (10.3) |
| Stage IVa/b | 2,358 (4.79) |
| Stage IVc | 783 (1.59) |
| Treatment | |
| Surgery (n = 39,287) | 38,895 (99.0) |
| Radiation (n = 54,496) | 25,181 (46.2) |
| Both | 17,075 |
AJCC, American Joint Committee on Cancer; DTC, differentiated thyroid carcinomas; SEER, Surveillance, Epidemiology, and End Results.
Overall, surgery but not radiation demonstrated significant improvement in mortality hazard ratios (HRs) irrespective of stage, with ratios of 0.37 (95% CI 0.30–0.47) and 1.03 (95% CI 0.93–1.14) respectively. Protective demographic factors include female sex (HR 0.61, 95% CI 0.55–0.68) and being married versus single (HR 0.65, 95% CI 0.57–0.75). Of the nonwhite ethnic groups, only black subjects had a statistically significant increased mortality risk when compared with whites (HR 1.53, 95% CI 1.29–1.81).
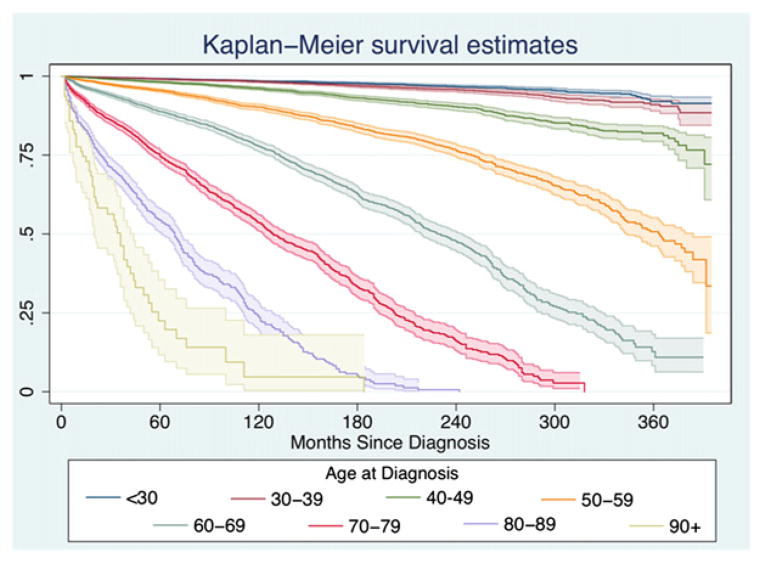
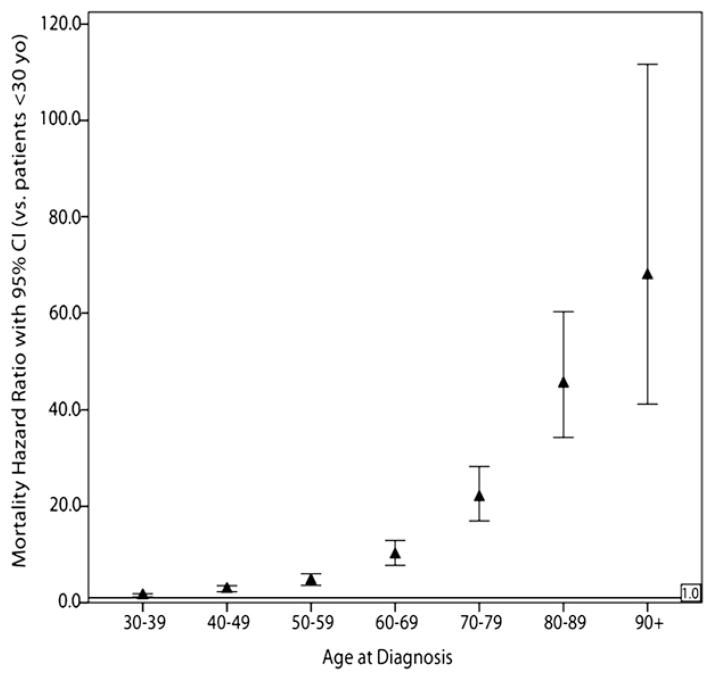
We found that increasing age significantly correlated with increased mortality in our multivariate analysis. Kaplan-Meier survival functions were calculated for these age groups (see Fig 1), and they were compared by the use of the log-rank method. Both the direct comparisons and the trend were statistically significant (P <.001 for both). Furthermore, on multivariable-adjusted analysis, compared with patients younger than 30 years of age, we found that there was a steep and significant increase in mortality risk as age increases in 10-year increments, with an approximately 50% increase in mortality risk for patients ages 30 to 40 (HR 1.49, 95% CI 1.20–1.84) and a doubling of HRs for every 10-year increase in age until patients are 90 and older (Fig 2). In subset analyses, we found that this effect of increasing age was significant in both women and men but that the magnitude of the effect was much larger in women: comparing age >90 patients to age 30 to 40 patients, the mortality HR in women was 95.1 (95% CI 52.8–171.4) but only 26 in men (95% CI 9.84–73.2).
Fig 1.
Kaplan-Meier survival curves (95% CIs are shaded) divided across age groups depict the overall good outcomes of differentiated thyroid cancers but also illustrate the increasingly poor prognosis associated with each 10-year increase in age.
Fig 2.
Mortality HRs for death by any cause in all patients, divided into 10-year increments and compared with patients younger than 30. All HRs are significant when compared with the patients younger than 30, and the magnitude roughly doubles for every 10-year increment until age 90. In this study, age is a strong and independent predictor of outcome, and one that appears to increase linearly without a step function increase in risk at any particular age.
Outcomes of current AJCC staging
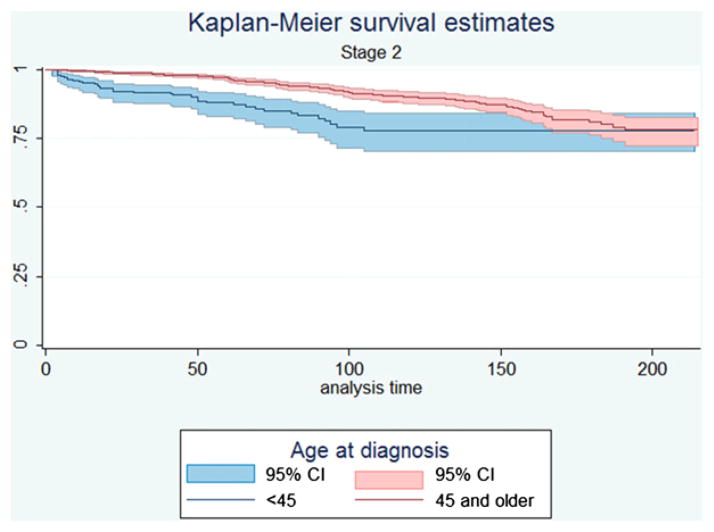
Under current AJCC guidelines, patients younger than the age of 45 were either stage I or stage II; patients older than 45 were staged differently and could have stages 1 through stage 4c disease. We examined survival data for patients under these guidelines. When we compared the Kaplan-Meier survival functions between the age groups with stage 2 disease (Fig 3) by using the log-rank test, we found that survival was significantly worse for patients younger than 45 who had metastatic disease than their older counterparts who only have tumors limited to the thyroid gland (P < .001). When we examined multivariable-adjusted survival, we found that in patients younger than 45 years of age, there was a greater than 11-fold increase in mortality between stages I and II (HR 11.5, 95% CI 7.65–17.2). In patients 45 and older, there was no significant increase in risk between stages I and II (HR 1.06, 95% CI 0.85–1.32).
Fig 3.
Kaplan-Meier survival curves (95% CIs are shaded) illustrating the difference in outcomes in stage II disease between patients 45 and older, in whom that stage is defined by tumor extent, and patients younger than 45 years of age, in whom that stage is defined by metastatic disease.
Outcomes of age-independent staging
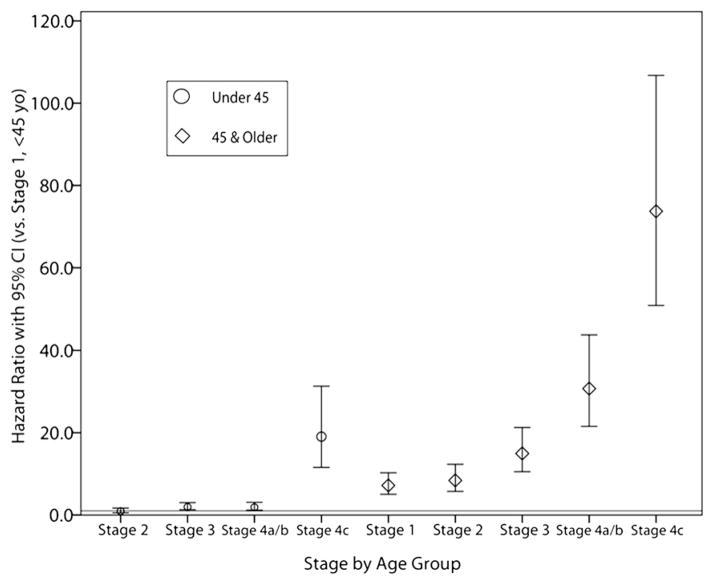
When we uniformly applied the 45 years-and-older staging protocol to all patients irrespective of age, we found that compared with stage I, patients younger than 45 years of age have increasing mortality HRs as stage increases, but that this is only significant for stage III disease and greater (Table III). This trend was similar in patients older than the age of 45 years, where again HRs increase with increasing stage but are only statistically significant in stage III and greater (Table IV). To compare the stages between 2 age groups directly, all stage/ age group combinations were analyzed such that all HRs would be versus stage I in patients younger than 45. Those results are plotted in Fig 4 and confirm that old age is a significant prognostic factor for worse outcomes.
Table III.
Mortality hazard ratios by stage for patients <45 years at diagnosis
| Hazard ratio (vs Stage 1) | 95% CI | P | |
|---|---|---|---|
| Stage 2 | 0.89 | 0.50–1.63 | .725 |
| Stage 3 | 2.09 | 1.36–3.24 | .001 |
| Stages 4a/4b | 2.39 | 1.44–3.94 | .001 |
| Stage 4c | 20.2 | 11.7–34.7 | <.001 |
Table IV.
Mortality hazard ratios by stage for patients ≥45 years of age at diagnosis
| Hazard ratio (vs Stage 1) | 95% CI | P | |
|---|---|---|---|
| Stage 2 | 1.05 | 0.84–1.33 | .62 |
| Stage 3 | 1.74 | 1.47–2.07 | <.001 |
| Stages 4a/4b | 3.36 | 2.81–4.01 | <.001 |
| Stage 4c | 8.48 | 6.91–10.4 | <.001 |
Fig 4.
Mortality HRs and 95% CIs for patients staged irrespective of age. Ratios are all compared with stage I disease in patients younger than 45 years of age; the horizontal line represents the corresponding HR of 1.0. Younger patients with disseminated disease fare worse than their age-matched counterparts with local disease and are at lower risk than older patients with equivalent disease stage. Young women with extranodal metastases in particular are at very high risk compared with other women of the same age.
Subset analyses using this staging system reveal that radiation therapy improves mortality risk in patients with stage I disease (HR 0.60, 95% CI 0.44–0.82) in patients under 45 (HR 0.69, 95% CI 0.51–0.95) but not in patients 45 and older (HR 1.01, 95% CI 0.90–1.13). Furthermore, the overall increased mortality risk as the result of being black loses statistical significance in the younger-than 45 population (HR 1.51, 95% CI 0.89–2.55; P = .13) and in men (HR 1.38, 95% CI 0.97–1.94; P = .071).
DISCUSSION
In oncology, staging systems exist to simplify the complex elements that comprise the cancer path-ophysiology into categories that can ideally both predict prognosis and direct therapy. In this regard, and for most if not all cancers, the AJCC staging system is the most widely accepted and is considered the gold standard. It relies on 3 main determinants: the anatomic extent of the primary tumor (T), the involvement of lymph nodes (N), and the presence of metastatic disease (M), to stratify cancer patients into different risk groups or stages. In the unique case of DTC, however, the AJCC system also considers a fourth component, age, in its staging definitions (Table I). One of the early staging systems to advocate age as a critical element in risk stratification for DTC was proposed by Cady et al7 at the Lahey Clinic, who used it as the sole prognostic criterion in their allocation of patients into either a low-risk group (men ≤40 and women ≤50) or a high-risk group (all older patients). The investigators have since revised their staging system to include other key parameters, but age remains a central component of their and all other staging systems developed for either or both FTC and PTC.
The AJCC staging system for DTC downgrades the impact of advanced disease extent in the setting of young age, such that currently, younger patients with metastatic disease are co-staged with older patients with large tumors limited to the thyroid gland, whereas local or regional nodal status is completely discounted in younger patients without metastases, who all share the same stage I designation. Our study sought to evaluate the validity of these aspects of the AJCC nomenclature by analyzing the SEER registry data collected from 1973 to 2005, which offers the advantages of covering a large and diverse population and avoiding potential selection, referral, and other biases inherent to single institution studies. This database contains information that permitted us to perform a dual set of analyses, 1 assessing risk on the basis of a combination of age and stage under the current AJCC guidelines, and the other reevaluating this interaction under a new age-independent staging system whereby we simply applied the current staging criteria for older patients to all patients, regardless of age. Within these parameters, we were able to effectively evaluate the prognostic value of nodal status in the younger population.
It is interesting to note that the mean age at diagnosis of DTC in our study was 44 (±15 years), which means that a large number of patients would be stratified into the current staging groups by a matter of months 1 way or the other. In fact, 24% of patients with newly diagnosed DTC were between the ages of 40 and 50 years. Given these findings, a clear-cut threshold age appears less likely to be a definitive staging determinant. This echoes the results of a previous review of the SEER program 1973–1991 by Gilliland et al,8 in which they found no age threshold for decreased survival, but rather a more complex, multiplicative relationship between age and risk. Similarly, we observed here a near doubling of the mortality HR with each 10-year age increment, starting at age 30 (Fig 2). Still, under the current AJCC guidelines, we did find a clear, nearly 4-fold increase in mortality for patients ≥45 compared with their younger peers.
When we examined the effect of AJCC cancer stage on mortality, our analysis indicated that all stages were associated with worse outcomes relative to stage I. Of particular interest, stage II had a slightly greater, but statistically significant, risk of mortality (HR 1.38, 95% CI 1.13–1.69, P = .02). However, this risk disappeared in the older group while becoming more pronounced in the younger group (HR 11.48, 95% CI 7.65–17.22, P < .001) upon subset analysis. This finding indicates that the worse survival observed for stage II is solely caused by the effect of metastatic disease in the younger group. In fact, our data showed a statistically worse outcome for younger patients compared with their older counterparts within stage II, possibly reflecting an underestimation of the effect of metastatic disease in young patients under the current AJCC staging guidelines. Although a previous nested case-control study based on the Swedish Cancer Registry failed to find a difference in survival between the 2 age groups in stage II,9 their study population differed markedly from ours, with mean age at time of diagnosis of 64 compared to 44 in our study.
To ascertain the effect of nodal involvement in younger patients, we restaged all patients in the study with the staging criteria currently applied to older patients under the AJCC protocol. Under this system, our study demonstrated a statistically significant prognostic value to nodal involvement in both age groups, as patients with nodal disease (stages III and IVa/b) fared worse than those without (stages I and II). Although the authors of some studies have shown that nodal status bears no effect on prognosis,5,6 others have demonstrated a statistical association between nodal disease and survival.8–10 Here, the results of our review of the SEER registry supported the latter. The worse outcomes associated with nodal involvement are likely related to greater recurrence risk, although we were unable to assess recurrence here.
It is important to point out that most clinicians currently do not base therapeutic decisions on staging systems for DTC for a few reasons.11 Because DTC, and especially PTC, have overall good prognosis for patients, who have a long duration of survival, a staging system that predicts tumor recurrence and relapse-free survival would be preferable. Moreover, age is generally not considered in the choice of therapy for patients with DTC.
It must also be noted that databases such as the SEER registry have inherent limitations that must be taken into consideration in the interpretation of our results. First, we were unable to discern between stages IVa and IVb because of coding overlaps. Furthermore, information such as family history, vascular invasion, or other histologic findings were not evaluated nor included in our dataset. Treatment, which influences survival, was not controlled. However, large population-based registries offer a more accurate reflection of actual practice and outcomes than individual institutional studies and are a valuable resource for outcome-directed research.
Overall, it would appear that under the current AJCC staging guidelines for DTC, the protective effects of age may be overestimated, especially in the setting of metastatic disease, resulting in an understaging of young patients. This may explain the findings by Lang et al12,13 in their review and comparison of current staging systems for both FTC and PTC that they all fail to account for a small proportion of cancer-related death in the low-risk younger group. Although there is no doubt that age should remain a key variable in the staging of DTC, our findings suggest that young and old patients with DTC may best be staged with 2 separate, but similar, systems akin to the 1 currently used by the AJCC to stage older patients. This revised staging system may be of greater prognostic value for young patients, particularly those with metastatic disease, a group that deserves more future in-depth staging review.
Footnotes
Presented at the 6th Annual Academic Surgical Congress, February 1–3, 2011, Huntington Beach, CA.
References
- 1.Sato N, Oyamatsu M, Koyama Y, Emura I, Tamiya Y, Hatakeyama K. Do the level of nodal disease according to the TNM classification and the number of involved cervical nodes reflect prognosis in patients with differentiated carcinoma of the thyroid gland? J Surg Oncol. 1998;69:151–5. doi: 10.1002/(sici)1096-9098(199811)69:3<151::aid-jso6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 3.Wada N, Nakayama H, Suganuma N, et al. Prognostic value of the sixth edition AJCC/UICC TNM classification for differentiated thyroid carcinoma with extrathyroid extension. J Clin Endocrinol Metab. 2007;92:215–8. doi: 10.1210/jc.2006-1443. [DOI] [PubMed] [Google Scholar]
- 4.Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–95. [PubMed] [Google Scholar]
- 5.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 6.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–41. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 7.Cady B, Rossi R, Silverman M, Wool M. Further evidence of the validity of risk group definition in differentiated thyroid carcinoma. Surgery. 1985;98:1171–8. [PubMed] [Google Scholar]
- 8.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79:564–73. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524–31. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- 10.Onitilo AA, Engel JM, Lundgren CI, Hall P, Thalib L, Doi SA. Simplifying the TNM system for clinical use in differentiated thyroid cancer. J Clin Oncol. 2009;27:1872–8. doi: 10.1200/JCO.2008.20.2382. [DOI] [PubMed] [Google Scholar]
- 11.Amdur RJ, Mazzaferri EL. The American Joing Committee on Cancer system of staging thyroid cancer. In: Amdur RJ, Mazzaferri EL, editors. Essentials of Thyroid Cancer Management. New York: Springer-Verlag Inc; 2005. pp. 33–6. [Google Scholar]
- 12.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for papillary thyroid carcinoma: a review and comparison. Ann Surg. 2007;245:366–78. doi: 10.1097/01.sla.0000250445.92336.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for follicular thyroid carcinoma: application to 171 consecutive patients treated in a tertiary referral centre. Endocr Relat Cancer. 2007;14:29–42. doi: 10.1677/erc.1.01284. [DOI] [PubMed] [Google Scholar]