Abstract
The evidence that neurovascular dysfunction is an integral part of Alzheimer’s disease (AD) pathogenesis has continued to emerge in the last decade. Changes in the brain vasculature have been shown to contribute to the onset and progression of the pathological processes associated with AD, such as microvascular reductions, blood brain barrier (BBB) breakdown and faulty clearance of amyloid β-peptide (Aβ) from the brain. Herein, we review the role of the neurovascular unit and molecular mechanisms in cerebral vascular cells behind the pathogenesis of AD. In particular, we focus on molecular pathways within cerebral vascular cells and the systemic circulation that contribute to BBB dysfunction, brain hypoperfusion and impaired clearance of Aβ from the brain. We aim to provide a summary of recent research findings implicated in neurovascular defects and faulty amyloid-β vasular clearance contributing to AD pathogenesis.
Keywords: Amyloid-β clearance, blood-brain barrier, low-density lipoprotein receptor-related protein 1, pericytes, receptor for advanced glycation end products
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disorder of the aging brain that results in progressive cognitive decline, and is associated with accumulation of amyloid β-peptide (Aβ) in the brain parenchyma and around cerebral blood vessels [1], intraneuronal tau-related lesions [2, 3] and neurovascular dysfunction and stress [4-7]. Recent studies have elucidated specific cellular and molecular mechanisms within the neurovascular unit and at the blood-brain barrier (BBB) mediating neurovascular defects in AD. In this review, we will discuss these cerebrovascular mechanisms and how they may contribute to disease onset and progression of AD. We will focus on recent studies that have identified molecular changes within the cerebrovascular system suggesting potentially novel cellular and molecular tragets in AD that might ultimately offer hope and provide new therapeutic opportunities for AD.
The neurovascular unit (NVU) consists of different cell types including (i) vascular cells such as brain endothelial cells lining the cerebral vascular tree, pericytes covering microvascular capillaries, and vascular smooth muscle cells (VSMCs) enwrapping cerebral arterioles and arteries, (ii) glial cells such as astrocytes, microglia and oliogodendrocytes, and (iii) neurons [8-10]. The BBB lies within the NVU. In contrast to leaky capillaries in most peripheral organs [11], the BBB restricts entry of polar molecules into the brain except for nutrients, energy metabolites, amino acids and vitamins that cross the BBB via specialized transport systems [12]. Peptides and proteins in general poorly cross the BBB [13, 14], but can be transported if specific peptide transporters and/or receptors are expressed at the BBB [15, 16].
The close proximity of non-neuronal neighboring cells with each other and with neurons allows for effective cell autonomous and non-autonomous communications that are critical for normal functions of the healthy central nervous system including regulation of cerebral blood flow (CBF), transport of oxygen and energy metabolites into the brain, clearance of waste and toxic products of metabolism including various potential neurotoxins from the brain, regulation of BBB permeability, control of inflammatory response, and stimulation of vascular repair and neuronal regeneration [17-20]. Alterations in the physiological signal transduction pathways between different cell types within the NVU has been increasingly recognized as an important factor which may contribute to disease initiation and progression in multiple neurological disorders [8, 9, 17, 18, 20-24].
Neurovascular stress mediates neuronal dysfunction
As described above, the functional integrity of the NVU is essential for normal neuronal and synaptic functioning. Here we will illustrate this concept with a few recent examples. For instance, recent studies have demonstrated that proper signaling between endothelial cells and pericytes mediated by brain endothelial platelet-derived growth factor B (PDGF-B) and platelet-derived growth factor receptor β (PDGFRβ) in pericytes importantly regulates functional and physical properties of the BBB, [25-27]. Studies using transgenic mouse models of pericyte deficiency generated by PDGF-B and/or PDGFRβ deficiency have shown that brain pericytes are essential safeguards of the BBB [25-27] contributing to BBB formation during development and BBB maintenance in the adult brain [20, 26]. Moreover, recent studies have shown that pericyte loss caused by faulty PDGF-B signal transduction from endothelium to PDGFRβ in pericytes leads to BBB breakdown, CBF reductions and hypoxia which in turn initiates age-dependent secondary neuronal and synaptic changes associated with neuronal and synaptic dysfunction [26]. Pericyte detachment from capillary endothelium has been shown to result in leakage of numerous toxic serum proteins, endothelial cell death, capillary regression and reductions in regional CBF [26]. It is also plausible that loss of brain pericytes may deprive the NVU from pericyte-derived growth factors including angioneurins [28], affecting health of the cerebrovascular system and neurons.
Another recent example relates vascular damage and BBB disruption mediated by apolipoprotein E4 (APOE4), a major genetic risk factor for AD, which has been shown to initiate vascular-mediated secondary neuronal dysfunction and neurodegenerative changes [29]. More specifically, using multiple transgenic apoE mouse lines with genetic ablation or pharmacological inhibition of cyclophilin A (CypA), a proinflammatory cytokine, we have identified that astrocyte-secreted human apoE2 and apoE3, but not apoE4, signal pericytes via the low density lipoprotein receptor related protein 1 (LRP1) to maintain low, physiological levels of CypA [29], previously shown that at increased concentrations disrupts the endothelial barrier damage in systemic vessels. In contrast to high affinity of apoE2 and apoE3 binding to LRP1 in vascular cells, poor interaction of astrocyte-derived apoE4 with LRP1 in pericytes activates a proinflammatory CypA pathway which in turn leads to activation of nuclear factor-κB (NF-κB)-matrix metalloproteinase-9 (MMP-9) pathway in pericytes causing degradation of the BBB endothelial tight junction and extracellular matrix proteins and BBB breakdown. This data has implicated CypA as a new target for treating neurovascular damage and the associated secondary neuronal dysfunction in neurological disorders affected by APOE4 genotype, such as AD.
Vascular-specific genes implicated in AD
Mesenchyme homeobox 2
Impaired angiogenesis and ineffective vascular regeneration may lead to degeneration of brain endothelium in AD and AD models. Several studies have reported that focal vascular regression and diminished microvascular density occur in AD [18, 30-32] as well as in AD transgenic mouse models [33]. The anti-angiogenic activity of Aβ which accumulates in the brains of individuals with AD and in AD mouse models, may contribute to such vascular regression [33]. On the other hand, genome-wide transcriptional profiling of brain endothelial cells from patients with AD demonstrated reduced expression of the mesenchyme homeobox gene 2 (MEOX2) [31]. This homeodomain-containing transcription factor, whose expression in the adult brain is restricted to the vascular system, is a member of a subfamily of non-clustered, diverged, antennapedia-like homeobox-containing genes and has been identified as a master regulator of vascular differentiation and remodeling [34]. The low levels of MEOX2 specifically found in brain endothelial cells isolated from AD patients, but not neurologically normal age-matched controls or young individuals, were shown to mediate aberrant angiogenic responses of human brain endothelium to angiogenic factors such as vascular endothelial growth factor (VEGF) and hypoxia both in vivo and in vitro, leading to premature capillary pruning and death due to activation of the forehead transcription factor 4 which is transcriptionally regulated by MEOX2 [31]. Importantly, a recent genome-wide study examining rare copy number variations exclusive to extreme phenotypes of AD identified a rare rearrangement that targets MEOX2 [35]. Moreover, mice that lack one Meox2 allele have been shown to develop primary cerebral endothelial hypoplasia with an intact BBB but a pronounced reductions in vascular desnity and chronic brain hypoperfusion causing secondary neurodegenerative changes [26] (Figure 1). This data suggest that capillary degeneration in AD could be related to ineffective vascular repair and/or remodeling. Some studies have suggested presence of isolated areas of increased focal vascularity in AD brains [36] further supporting a concept that abnormalities in brain angiogenesis are implicated in AD pathogenesis.
Figure 1. Altered expression of vascular-specific genes in AD results in neurovascular dysfunction.
Hypoxia downregulates mesenchyme homeobox gene-2 (MEOX2) in brain endothelial cells (BEC) (Left). Reduced levels of MEOX2 lead to unsuccessful vascular remodeling and vascular regression resulting in a primary endothelial hypoplasia and brain hypoperfusion. On the other hand, reduced levels of MEOX2 stimulate proteosomal degradation of LRP1, a major Aβ clearance receptor, leading to a loss of LRP1 from BEC and reduced Aβ clearance from brain. Hypoxia increases expression of myocardin (MYOCD) in vascular smooth muscle cells (VSMCs) resulting in elevated levels of MYOCD and serum response factor (SRF) (Right). Elevated SRF/MYOCD levels lead to increased expression of several contractile proteins and calcium-regulated channels in VSMCs resulting in a hypercontractile phenotype of small cerebral arteries and brain hypoperfusion. On the other hand, increased SRF/MYOCD activity stimulates directed expression of the sterol binding protein-2 which is a major transcriptional suppressor of LRP1. Loss of LRP1 from VSMCs diminishes Aβ clearance from small cerebral arteries leading to deposition of Aβ and amyloid in the arterial wall known as CAA, cerebral amyloid angiopathy. Changes in the expression of vascular-restricted genes MEOX2 and MYCD can trigger both an Aβ-independent brain hypoperfusion and Aβ accumulation mediating neuronal dysfunction. Interestingly, hypoxia seems to be upstream to both, a diminished MEOX2 expression in BEC and an increased MYOCD expression in VSMCs. Adapted from [174].
Low levels of MEOX2 also promote proteasomal degradation of LRP1 in brain endothelium [31] which has been shown to diminish Aβ clearance at the BBB, as described below. Therefore, in this group of AD patients Aβ may act in concert with low MEOX2 levels at the BBB to focally reduce brain capillary density.
Myocardin and serum response factor
Previously, we identified that a vascular-specific transcriptional co-factor, myocardin (MYOCD) and a ubiquitously expressed transcription factor, serum response factor (SRF) are overexpressed in the cerebral VSMCs of AD patients and contribute to the development of a hypercontractile cerebral arterial phenotype resulting in brain hypoperfusion, diminished functional hyperemia and cerebral amyloid angiopathy (CAA) [37, 38]. The MYOCD-SRF transcriptional switch binds a cis element known as the CArG box to regulate gene transcription [39] and constitute a molecular switch for the VSMC differentiation program. A growing number of essential VSMC-restricted cytocontractile genes and genes regulting Ca2+ flux are downstream targets of SRFMYOCD such as taglin, smooth muscle alpha actin, calponin and myosin heavy chain, sacroplasmic/endoplasmic reticulum calcium ATPase2, calsequestrin 1, myosin light chain kinase, and the miRNA 143/145 cluster, respectively [38, 40-42]. The high levels of MYOCD and SRF in AD VSMCs lead to increased expression of these cytocontractile proteins and channels regulating Ca2+ fluxes ultimately inducing a VSMC “hypercontracile phenotype” and cerebral hypoperfusion [38, 43] (Figure 1). We further hypothesize that this hypoperfusion can decrease passive Aβ drainage due to reductions in arterial pulsatile blood flow, as previously proposed [43, 44].
In addition, it has been shown that increased levels of MYOCD and SRF in AD VSMCs also suppress Aβ clearance directly thus further exacerbating CAA [37]. Namely, elevated levels of MYOCD and SRF in VSMCs have been shown to lead to CArG box-dependent expression of sterol response element binding protein 2 which is a major LRP1 transcriptional suppressor [45] which results in LRP1 depletion and diminished LRP1-mediated Aβ clearance from the vessel wall [37]. Whether similar signaling mechanisms are present in brain pericytes has yet to be determined. Moreover, the exact mechanism leading to increased MYOCD and SRF in AD VSMCs is currently unclear, although it has been shown that hypoxia can increase MYOCD-SRF levels in cerebral VSMCs in vitro and in vivo [37, 38], thus implicating cerebral ischemia and micro or “silent strokes” as a plausible upstream mechanism.
Aβ clearance mechanisms
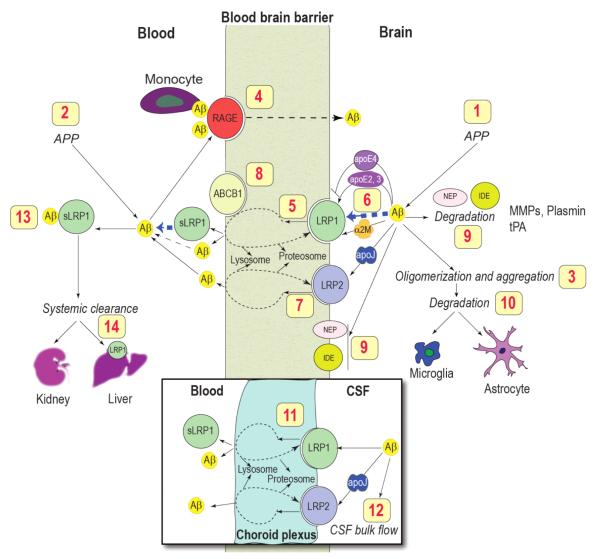
The major pathological hallmark of AD is the accumulation of neurotoxic Aβ, believed to be caused not by increased production [46] but due to faulty clearance from the brain [18]. The efficiency of Aβ clearance from brain interstitial fluid across the BBB is influenced by Aβ binding transport proteins such as apoE and apoJ (clusterin), and BBB receptors such as LRP1, LRP2 and receptor for advanced glycation end products (RAGE) which control Aβ efflux from brain and influx into the brain, respectively, and Aβ degrading enzymes [47-55] (Figure 2).
Figure 2. The role of blood-brain barrier transport in homeostasis of brain Aβ.
Brain Aβ is regulated by multiple mechanisms including: (1) central and (2) systemic production from its precursor protein APP; (3) oligomerization and aggregation; (4) receptor-mediated re-entry across the BBB into the brain via the receptor for advanced glycation end products (RAGE) (5) receptor-mediated vascular clearance across the BBB via LRP1; (6) Aβ binding to apoE, α2-macroglobulin (α2M) and apoJ in brain interstitial fluid (ISF) which influences Aβ clearance and aggregation; (7) LRP2-mediated efflux of Aβ-apoJ complexes from brain; (8) ABCB1 at the luminal side may contribute to Aβ efflux from endothelium to blood; (9) enzymatic degradation by neprilysin (NEP), insulin-degrading enzyme (IDE), tissue plasminogen activator (tPA), matrix MMPs; (10) cellular degradation by astrocytes and microglia; (11) LRP1- and LRP2-mediated transport across the choroid plexus; (12) slow removal via the ISF–CSF bulk flow; (13) sequestration in plasma by soluble LRP1 (sLRP1), which is a major Aβ binding protein in plasma; (14) removal by the liver and kidneys. Modified from [8].
LRP1
LRP1, a member of the low-density lipoprotein (LDL) receptor family, is both a rapid cargo transporter and a cell signaling receptor [48]. Some of LRP1 functions include regulation of cholesterol and lipoprotein metabolism, regulation of coagulation, cell survival, synaptic transmission, APP trafficking and metabolism, and Aβ clearance [56-58]. LRP1 is essential for early embryonic development and deletion of Lrp1 gene in mice results in failure of embryo implantation into the uterus [59]. LRP1 is synthesized as a single ~600 kDa protein which is cleaved by furin in the trans-Golgi network to produce a 515 kDa entirely extracellular heavy α-chain and a non-covalently associated 85 kDa light β-chain [60]. During biosynthesis of LRP1, the receptor-associated protein (RAP) acts as a chaperone for its proper folding [61]. The α-chain of LRP1 has four ligand-binding domains (clusters I-IV) [62, 63]. Domains II and IV bind over forty structurally and functionally unrelated ligands such as apoE, activated α2-macroglobulin (α2M), tissue plasminogen activator (tPA), proteinase-inhibitors, blood coagulation factor VIII, RAP, prion protein, lactoferrin [48, 64, 65] and Aβ [66]. RAP blocks binding of ligands to LRP1 [67, 68]. The β-chain of LRP1 has an extracellular domain, a transmembrane region, and a short cytoplasmic tail which consists of two NPxY motifs, and one YxxL motif and two di-leucine motifs which are required for rapid endocytosis of LRP1 ligands [66, 69, 70]. The cytoplasmic tail phosphorylated on serine and/or tyrosine residues [71, 72] interacts with different adaptor proteins associated with cell signaling such as disabled-1 (Dab1), FE65 and postsynaptic density protein 95 (PSD-95) [58, 73, 74]. LRP1 expression is controlled at both transcriptional and translational levels [45, 75-77]. Cell surface levels and function of LRP1 are controlled by proteolytic shedding of its ectodomain [77-82]. Intact soluble LRP1 α-chain (sLRP1), consisting of the ligand binding domains of LRP1, is shed into plasma [78, 83-85]. A short membrane spanning and the intracellular fragment of LRP1 left after ectodomain shedding is further processed by the action of γ-secretase, a APP processing enzyme, resulting in release of LRP1 intracellular domain (LRP1-ICD), which can translocate to the nucleus and inhibit the transcription of the genes mediating inflammatory response [86].
Earlier genetic studies have suggested that LRP1 is linked to AD and CAA [87-90], but this has not been confirmed by later studies [91, 92]. It has been shown that LRP1 interacts with APP which influences Aβ generation [93, 94]. LRP1 also mediates Aβ neuronal uptake via α2M and apoE [70, 95-99]. The exact role of these findings for the development of Aβ pathology is not yet clear.
Within the NVU brain endothelial cells, cerebral VCMCs, pericytes, astrocytes and neurons, all express LRP1 [81, 100]. LRP1 internalizes its ligands and directs them to lysosomes for proteolytic degradation. LRP1 and/or another member of the LDL receptor family have been involved in apoE-dependent uptake of Aβ and degradation by astrocytes [101]. Several reports have shown that LRP1 also transports its ligands transcellularly across the BBB including Aβ [66, 102], RAP [103], tPA [104], lipid free and lipidated apoE2 and apoE3 including their complexes with Aβ [70] and a family of Kunitz domain-derived peptides [105].
As shown in Figure 2, numerous studies have demonstrated that LRP1 has a key role in a three-step serial clearance mechanism mediating Aβ elimination from brain and body [48]. In multiple animal models, we and others have shown that binding of Aβ to LRP1 at the abluminal side of the BBB results in its rapid clearance into the blood [66, 70, 102, 106-112]. A decreased expression of LRP1 in the choroid plexus epithelium [113] leads to Aβ accumulation in the choroid plexus [114, 115]. Studies using in vitro BBB models [116, 117] have confirmed the role of LRP1 in Aβ endothelial cellular uptake and endocytosis, respectively, resulting in clearance of Aβ.
LRP1 expression in brain endothelium decreases with normal aging in rodents, non-human primates and humans, as well as in AD models and AD patients [43, 66, 102, 118-120]. LRP1 reductions have been reported in cerebral VSMCs associated with Aβ accumulation in the wall of small pial and intracerebral arteries [37]. Reduced LRP1 levels in brain microvessels correlate with endogenous Aβ deposition in a chronic hydrocephalus model in rats [121] and Aβ cerebrovascular and brain accumulation in AD patients [102, 120]. Therefore, LRP1 downregulation at the BBB and in vascular cells may contribute to cerebrovascular and parenchymal Aβ deposition.
In blood, circulating form of LRP1 (i.e., soluble LRP1, sLRP1) provides a key endogenous peripheral ‘sink’ activity for Aβ as shown in a mouse model of AD [84]. In neurologically healthy humans and mice, sLRP1 binds over 70% of circulating Aβ preventing free Aβ access to the brain [84] (Figure 2). In AD patients and AD transgenic mice, Aβ binding to sLRP1 is compromised by oxidation resulting in increased levels of oxidized sLRP1 which does not bind Aβ [84]. This is associated with elevated levels of free Aβ40 and Aβ42 in plasma [85] which can re-enter the brain via RAGE-mediated transport across the BBB [49, 84, 122-124]. An increased RAGE expression in brain endothelium has been shown in advanced AD compared to early stage AD and/or individuals with mild cognitive impairment (MCI) [125]. This might further contribute to Aβ accumulation in brain via accelerated Aβ influx from blood. We have recently reported that a diminished sLRP1-Aβ peripheral binding precede an increase in the tau/Aβ42 CSF ratio and a drop in global cognitive decline in individuals with MCI converting into AD [85]. Replacement of damaged sLRP1 with recombinant LRP1 ligand binding domains can sequester free Aβ in plasma in AD patients and AD transgenic mice and reduce Aβ-related pathology in brain [84].
LRP1 in the liver mediates rapid peripheral clearance of Aβ [126, 127]. Reduced hepatic LRP levels have been shown to be associated with decreased peripheral Aβ clearance in the aged rats [126, 127]. Regulation of Aβ brain levels by the liver has been recently demonstrated by an independent study [128, 129]. Enhancing expression of LRP1 in brain microvessels and liver and circulating sLRP1 in plasma using plant-derived pharmacologically active compounds from Withania somnifera resulted in rapid reversal of Aβ pathology and improved cognitive function in APP/PS1 transgenic mouse model of AD [130, 131].
RAGE
RAGE is a multiligand receptor of the immunoglobulin (Ig) superfamily [132]. In brain, RAGE is expressed on the cell surface of vascular endothelial cells, pericytes, smooth muscle cells, neurons, and glial cells and acts as a receptor for Aβ [133-135]. RAGE contains one V-type and two C-type immunoglobulin domains which bind to several ligands, and a short cytoplasmic tail that is required for RAGE-mediated cell signaling [134]. The extracellular V domain of RAGE binds to ligands such as AGE proteins, S100/calgranulins, monomeric and oligomeric Aβ, amphoterin, and the family of crossed β-sheet macromolecules [136]. Interaction of ligands with RAGE activates signal transduction pathways leading to increased cellular stress as seen in chronic diseases such as diabetes, inflammation and AD [137-139]. RAGE mediates Aβ-induced neurotoxicity directly by causing oxidant stress and indirectly by activating microglia [133]. In addition, intraneuronal Aβ transport via RAGE leads to mitochondrial dysfunction [140]. RAGE amplifies Aβ-mediated inflammatory response in microglia [133]. Targeted expression of RAGE in neurons in APP transgenic mice accelerated cognitive decline and Aβ-induced neuronal perturbation [141]. Expression of RAGE is increased in cerebrovascular endothelial cells under pathological conditions including those seen in AD models and AD [49, 133, 142].
At the BBB, RAGE mediates transport of circulating Aβ into the brain [49, 143] which results in NF-κB-dependent endothelial cell activation and neuroinflammatory response, from one hand, and generation of endothelin-1 suppressing the CBF, from the other [49]. In addition, expression of RAGE in brain endothelium initiates cellular signaling leading to monocyte trafficking across the BBB [144]. RAGE expression is increased in both neurons and endothelium in an Aβ-rich or AGE-rich environment as in AD [142], which amplifies Aβ-mediated pathogenic responses. The cellular events triggered by RAGE at the BBB, neurons, microglia and VSMCs may be associated with the onset and progression of AD. Therefore, RAGE is a potential therapeutic target in AD and blocking RAGE might contribute to control of Aβ-mediated brain disorder [49, 55, 145].
ApoE
The human apoE is a 34 kDa glycoprotein of 299 amino acids [146]. It plays an important role in lipoprotein transport and cholesterol homeostasis [147]. In humans there are three common isoforms of apoE: apoE2 (cys112, cys158), apoE3 (cys112, arg158) and apoE4 (arg112, arg158), differing from each other by one or two amino acids at position 112 and 158 [148]. The ε4 allele of the APOE gene encoding apoE4 is a major risk factor for early-onset AD [149, 150]. How apoE4 influences onset and progression of AD is not completely understood [51, 53, 151].
In addition to earlier described Aβ-independent pathway of apoE4-mediated vascular defects causing neuronal dysfunction and neurodegeneration [29], several studies have suggested that interaction of apoE with Aβ plays an important role in the pathogenesis of AD [70, 97, 106, 152-155]. Studies by our group showed that binding of Aβ to lipidated or lipid-poor apoE can influence Aβ clearance from the brain [70, 106, 156] or its transport from the circulation across the BBB [156]. Binding of Aβ to apoE reduced Aβ efflux across the BBB compared to Aβ alone [70, 106], and this process was dependent on apoE isoform and the status of lipidation. Namely, apoE4 had a greater disruptive effect on BBB clearance of Aβ than either apoE3 or apoE2 [70]. We have also shown that clearance of Aβ40 and Aβ42 via apoE2 and apoE3 involves mainly LRP1 at the BBB, whereas apoE4 complexes with Aβ40 and Aβ42 have affinity for very low density lipoprotein receptor (VLDLR) which mediates slow internalization of its ligands into the endothelium of the BBB compared to rapid internalization and transcytosis provided by LRP1 [70, 106].
ApoJ
ApoJ, also known as clusterin, is a 75 kDa chaperone protein. It is highly conserved and ubiquitously expressed sulfated glycoprotein [50, 157, 158]. ApoJ is involved in apoptosis, inflammation, cancer and many neurodegenerative disorders including AD [50]. ApoJ is expressed at high levels in the brain and found associated with senile plaques in AD [159]. Recent genome-wide association studies (GWAS) identified clusterin gene (CLU) as one of the risk factor for Alzheimer’s disease [91, 92, 160-166]. How variations in CLU gene influence transcription or loss or gain of protein function, especially how it affects Aβ metabolism in the brain is not completely understood and needs further investigation [166].
ApoJ forms a stable complex with Aβ [106, 167-169]. Our earlier studies have shown that soluble Aβ40-apoJ complexes are taken up at the BBB in vivo [47, 170] by a receptor-mediated transport mechanism involving megalin/gp330 receptor [169]. However, megalin at the BBB is saturated under physiological levels of plasma apoJ which prevents influx of circulating Aβ-apoJ complex into the brain via megalin [169]. In the CNS, megalin is expressed in brain vascular endothelial cells and the epithelial cells of the choroid plexus [171, 172]. In a recent study we found that apoJ facilitates clearance of soluble Aβ across the BBB [106]. ApoJ and apoJ-Aβ42 complexes are rapidly cleared across the BBB via LRP2 and our data suggest that clusterin/megalin pathway at the BBB plays a crucial role in removal of soluble Aβ particularly Aβ42 from the mouse brain [106].
Conclusions
Recent clinical observations provide strong evidence for the link between cerebrovascular disease and AD and the role of vascular risk factors in AD [18]. In this review, we have briefly reviewed literature on dysregulated and diminished CBF, BBB dysfunction and impaired vascular clearance of Aβ from brain supporting an essential role of the neurovascular and BBB mechanisms in AD pathogenesis. Several studies in animal models of AD and more recently in AD patients [46] have demonstrated a diminished Aβ clearance from brain. The recognition of Aβ clearance pathways opens exciting new therapeutic opportunities for AD. It is now established that faulty clearance from brain and across the BBB leads to elevated Aβ levels in brain which in turn have been shown to contribute to formation of neurotoxic Aβ oligomers [173] and the development of Aβ-mediated brain storage disorder and cerebral β-amyloidosis [4].
The activation of neurovascular pathogenic pathways has been shown to compromise synaptic and neuronal functions prior to and/or in parallel with Aβ accumulation and development of intraneuronal tangles, neuronal loss and dementia. Some early molecular targets within the neurovascular pathway include receptors RAGE and LRP1 at the BBB, Aβ chaperone proteins such as apoE and apoJ, and possibly vascular-specific genes MEOX2 and MYOCD. ApoE4, the major genetic risk factor for AD, which affects AD pathogenesis by both Aβ-independent and Aβ-dependent pathways, involves in either case effects on the BBB and the neurovascular mechanisms of disease.
Focusing on comorbidity, vascular risk factors associated with AD such as hypoperfusion, hypertension, mini strokes and/or diabetes might generate useful new models of human dementia according to the vascular two-hit hypothesis of AD [18]. The proposed neurovascular model of AD raises a set of new important questions that require further study, as recently discussed [18]. For example, the molecular basis of the neurovascular link with neurodegenerative disorders is still poorly understood as well as the molecular cues underlying the cross-talks between different cell types of the NVU including vascular and glia cells, and how these cellular interactions influence neuronal activity. Addressing these questions will lead to better understanding of the neurovascular link with neurodegeneration process which will lead to the development of novel neurovascular-based approaches for AD [18].
Acknowledgement
B.V.Z. wants to thank the United States National Institutes of Health grants R37 AG023084, R37 NS34467 and RO1 AG039452 and the Zilkha family for supporting his research.
References
- [1].Querfurth HW, LaFerla FM. Alzheimer’s disease. The New England journal of medicine. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- [2].Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature reviews. Neuroscience. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- [3].Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nature reviews. Neuroscience. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- [4].Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [5].Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- [6].de la Torre JC. Vascular risk factor detection and control may prevent Alzheimer’s disease. Ageing research reviews. 2010;9:218–225. doi: 10.1016/j.arr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- [7].Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- [8].Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- [9].Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke; a journal of cerebral circulation. 2009;40:S4–7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta. 1985;819:241–248. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- [12].Zlokovic BV, Apuzzo ML. Cellular and molecular neurosurgery: pathways from concept to reality--part I: target disorders and concept approaches to gene therapy of the central nervous system. Neurosurgery. 1997;40:789–803. doi: 10.1097/00006123-199704000-00027. [DOI] [PubMed] [Google Scholar]
- [13].Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucineenkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- [14].Zlokovic BV, Segal MB, Begley DJ, Davson H, Rakic L. Permeability of the blood-cerebrospinal fluid and blood-brain barriers to thyrotropin-releasing hormone. Brain Res. 1985;358:191–199. doi: 10.1016/0006-8993(85)90963-1. [DOI] [PubMed] [Google Scholar]
- [15].Zlokovic BV, Mackic JB, Djuricic B, Davson H. Kinetic analysis of leucine-enkephalin cellular uptake at the luminal side of the blood-brain barrier of an in situ perfused guinea-pig brain. J Neurochem. 1989;53:1333–1340. doi: 10.1111/j.1471-4159.1989.tb08522.x. [DOI] [PubMed] [Google Scholar]
- [16].Zlokovic BV, Hyman S, McComb JG, Lipovac MN, Tang G, Davson H. Kinetics of argininevasopressin uptake at the blood-brain barrier. Biochim Biophys Acta. 1990;1025:191–198. doi: 10.1016/0005-2736(90)90097-8. [DOI] [PubMed] [Google Scholar]
- [17].Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vangilder RL, Rosen CL, Barr TL, Huber JD. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacology & therapeutics. 2011;130:239–247. doi: 10.1016/j.pharmthera.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40:S4–7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vangilder RL, Rosen CL, Barr TL, Huber JD. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol Ther. 2011;130:239–247. doi: 10.1016/j.pharmthera.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- [25].Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- [26].Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Quaegebeur A, Segura I, Carmeliet P. Pericytes: blood-brain barrier safeguards against neurodegeneration? Neuron. 2010;68:321–323. doi: 10.1016/j.neuron.2010.10.024. [DOI] [PubMed] [Google Scholar]
- [28].Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- [29].Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- [32].Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. Journal of neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett. 2004;366:80–85. doi: 10.1016/j.neulet.2004.05.017. [DOI] [PubMed] [Google Scholar]
- [34].Gorski DH, Walsh K. Control of vascular cell differentiation by homeobox transcription factors. Trends in cardiovascular medicine. 2003;13:213–220. doi: 10.1016/s1050-1738(03)00081-1. [DOI] [PubMed] [Google Scholar]
- [35].Rovelet-Lecrux A, Legallic S, Wallon D, Flaman JM, Martinaud O, Bombois S, Rollin-Sillaire A, Michon A, Le Ber I, Pariente J, Puel M, Paquet C, Croisile B, Thomas-Anterion C, Vercelletto M, Levy R, Frebourg T, Hannequin D, Campion D. A genome-wide study reveals rare CNVs exclusive to extreme phenotypes of Alzheimer disease. Eur J Hum Genet. 2011 doi: 10.1038/ejhg.2011.225. doi: 10.1038/ejhg.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Desai BS, Schneider JA, Li JL, Carvey PM, Hendey B. Evidence of angiogenic vessels in Alzheimer’s disease. J Neural Transm. 2009;116:587–597. doi: 10.1007/s00702-009-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sun Q, Chen G, Streb JW, Long X, Yang Y, Stoeckert CJ, Jr., Miano JM. Defining the mammalian CArGome. Genome Res. 2006;16:197–207. doi: 10.1101/gr.4108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- [41].Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain pathology. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Llorente-Cortes V, Costales P, Bernues J, Camino-Lopez S, Badimon L. Sterol regulatory element-binding protein-2 negatively regulates low density lipoprotein receptor-related protein transcription. J Mol Biol. 2006;359:950–960. doi: 10.1016/j.jmb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- [46].Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zlokovic BV. Cerebrovascular transport of Alzheimer’s amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996;59:1483–1497. doi: 10.1016/0024-3205(96)00310-4. [DOI] [PubMed] [Google Scholar]
- [48].Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- [50].Calero M, Rostagno A, Frangione B, Ghiso J. Clusterin and Alzheimer’s disease. Subcell Biochem. 2005;38:273–298. [PubMed] [Google Scholar]
- [51].Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Marzolo MP, Farfan P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res. 2011;44:89–105. doi: 10.4067/S0716-97602011000100012. [DOI] [PubMed] [Google Scholar]
- [53].Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Saido TLM. Proteolytic degradation of amyloid-beta protein. Cold Spring Harb Perspect Med. 2012 doi: 10.1101/cshperspect.a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Deane R, Singh I, Sagare AP, Bell RD, Ross NT, Larue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29:571–581. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- [57].Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. The Journal of clinical investigation. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Herz J, Chen Y, Masiulis I, Zhou L. Expanding functions of lipoprotein receptors. Journal of lipid research. 2009;50(Suppl, S2):87–292. doi: 10.1194/jlr.R800077-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- [60].Herz J, Kowal RC, Goldstein JL, Brown MS. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990;9:1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Willnow TE, Rohlmann A, Horton J, Otani H, Braun JR, Hammer RE, Herz J. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 1996;15:2632–2639. [PMC free article] [PubMed] [Google Scholar]
- [62].Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. Journal of cell science. 2001;114:899–908. doi: 10.1242/jcs.114.5.899. [DOI] [PubMed] [Google Scholar]
- [63].Meijer AB, Rohlena J, van der Zwaan C, van Zonneveld AJ, Boertjes RC, Lenting PJ, Mertens K. Functional duplication of ligand-binding domains within low-density lipoprotein receptor-related protein for interaction with receptor associated protein, alpha2-macroglobulin, factor IXa and factor VIII. Biochimica et biophysica acta. 2007;1774:714–722. doi: 10.1016/j.bbapap.2007.04.003. [DOI] [PubMed] [Google Scholar]
- [64].Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Herz J, Chen Y, Masiulis I, Zhou L. Expanding functions of lipoprotein receptors. J Lipid Res. 2009;50(Suppl):S287–292. doi: 10.1194/jlr.R800077-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- [67].Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992;267:9035–9040. [PubMed] [Google Scholar]
- [68].Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- [69].Li Y, Lu W, Marzolo MP, Bu G. Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. The Journal of biological chemistry. 2001;276:18000–18006. doi: 10.1074/jbc.M101589200. [DOI] [PubMed] [Google Scholar]
- [70].Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bu G, Sun Y, Schwartz AL, Holtzman DM. Nerve growth factor induces rapid increases in functional cell surface low density lipoprotein receptor-related protein. The Journal of biological chemistry. 1998;273:13359–13365. doi: 10.1074/jbc.273.21.13359. [DOI] [PubMed] [Google Scholar]
- [72].van der Geer P. Phosphorylation of LRP1: regulation of transport and signal transduction. Trends in cardiovascular medicine. 2002;12:160–165. doi: 10.1016/s1050-1738(02)00154-8. [DOI] [PubMed] [Google Scholar]
- [73].Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. The Journal of biological chemistry. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- [74].Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. The Journal of biological chemistry. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- [75].Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid beta-peptide (1 40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Mol Pharmacol. 2007;72:850–855. doi: 10.1124/mol.107.036913. [DOI] [PubMed] [Google Scholar]
- [76].Ceschin DG, Sanchez MC, Chiabrando GA. Insulin induces the low density lipoprotein receptor-related protein 1 (LRP1) degradation by the proteasomal system in J774 macrophage-derived cells. J Cell Biochem. 2009;106:372–380. doi: 10.1002/jcb.22014. [DOI] [PubMed] [Google Scholar]
- [77].Selvais C, D’Auria L, Tyteca D, Perrot G, Lemoine P, Troeberg L, Dedieu S, Noel A, Nagase H, Henriet P, Courtoy PJ, Marbaix E, Emonard H. Cell cholesterol modulates metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 (LRP-1) and clearance function. FASEB J. 2011;25:2770–2781. doi: 10.1096/fj.10-169508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA. Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997;272:23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- [79].May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–18743. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- [80].von Arnim CA, Kinoshita A, Peltan ID, Tangredi MM, Herl L, Lee BM, Spoelgen R, Hshieh TT, Ranganathan S, Battey FD, Liu CX, Bacskai BJ, Sever S, Irizarry MC, Strickland DK, Hyman BT. The low density lipoprotein receptor-related protein (LRP) is a novel beta-secretase (BACE1) substrate. J Biol Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- [81].Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Liu Q, Zhang J, Tran H, Verbeek MM, Reiss K, Estus S, Bu G. LRP1 shedding in human brain: roles of ADAM10 and ADAM17. Mol Neurodegener. 2009;4:17. doi: 10.1186/1750-1326-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Grimsley PG, Quinn KA, Chesterman CN, Owensby DA. Evolutionary conservation of circulating soluble low density lipoprotein receptor-related protein-like ("LRP-like") molecules. Thromb Res. 1999;94:153–164. doi: 10.1016/s0049-3848(98)00208-4. [DOI] [PubMed] [Google Scholar]
- [84].Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sagare AP, Deane R, Zetterberg H, Wallin A, Blennow K, Zlokovic BV. Impaired lipoprotein receptor-mediated peripheral binding of plasma amyloid-beta is an early biomarker for mild cognitive impairment preceding Alzheimer’s disease. J Alzheimers Dis. 2011;24:25–34. doi: 10.3233/JAD-2010-101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Science signaling. 2008;1:ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- [88].Lambert JC, Wavrant-De Vrieze F, Amouyel P, Chartier-Harlin MC. Association at LRP gene locus with sporadic late-onset Alzheimer’s disease. Lancet. 1998;351:1787–1788. doi: 10.1016/s0140-6736(05)78749-3. [DOI] [PubMed] [Google Scholar]
- [89].Wavrant-DeVrieze F, Lambert JC, Stas L, Crook R, Cottel D, Pasquier F, Frigard B, Lambrechts M, Thiry E, Amouyel P, Tur JP, Chartier-Harlin MC, Hardy J, Van Leuven F. Association between coding variability in the LRP gene and the risk of late-onset Alzheimer’s disease. Hum Genet. 1999;104:432–434. doi: 10.1007/s004390050980. [DOI] [PubMed] [Google Scholar]
- [90].Christoforidis M, Schober R, Krohn K. Genetic-morphologic association study: association between the low density lipoprotein-receptor related protein (LRP) and cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2005;31:11–19. doi: 10.1111/j.1365-2990.2004.00614.x. [DOI] [PubMed] [Google Scholar]
- [91].Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature genetics. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature genetics. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- [93].Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:4259–4265. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiology of disease. 2008;31:188–197. doi: 10.1016/j.nbd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- [95].Narita M, Holtzman DM, Schwartz AL, Bu G. Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. Journal of neurochemistry. 1997;69:1904–1911. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- [96].Qiu Z, Strickland DK, Hyman BT, Rebeck GW. Alpha2-macroglobulin enhances the clearance of endogenous soluble beta-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. Journal of neurochemistry. 1999;73:1393–1398. doi: 10.1046/j.1471-4159.1999.0731393.x. [DOI] [PubMed] [Google Scholar]
- [97].DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- [98].Zerbinatti CV, Wozniak DF, Cirrito J, Cam JA, Osaka H, Bales KR, Zhuo M, Paul SM, Holtzman DM, Bu G. Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1075–1080. doi: 10.1073/pnas.0305803101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zerbinatti CV, Bu G. LRP and Alzheimer’s disease. Reviews in the neurosciences. 2005;16:123–135. doi: 10.1515/revneuro.2005.16.2.123. [DOI] [PubMed] [Google Scholar]
- [100].Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annual Review of Biochemistry. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- [101].Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nature medicine. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- [102].Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pan W, Kastin AJ, Zankel TC, van Kerkhof P, Terasaki T, Bu G. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. Journal of cell science. 2004;117:5071–5078. doi: 10.1242/jcs.01381. [DOI] [PubMed] [Google Scholar]
- [104].Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- [105].Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, Gabathuler R, Castaigne JP, Beliveau R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. Journal of neurochemistry. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- [106].Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ito S, Ohtsuki S, Terasaki T. Functional characterization of the brain-to-blood efflux clearance of human amyloid-beta peptide (1-40) across the rat blood-brain barrier. Neuroscience research. 2006;56:246–252. doi: 10.1016/j.neures.2006.07.006. [DOI] [PubMed] [Google Scholar]
- [109].Shiiki T, Ohtsuki S, Kurihara A, Naganuma H, Nishimura K, Tachikawa M, Hosoya K, Terasaki T. Brain insulin impairs amyloid-beta(1-40) clearance from the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9632–9637. doi: 10.1523/JNEUROSCI.2236-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, Lynch JL, Robinson SM, Niehoff ML, Johnson SN, Kumar VB, Banks WA. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. Journal of Alzheimer’s disease : JAD. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Shinohara M, Sato N, Kurinami H, Takeuchi D, Takeda S, Shimamura M, Yamashita T, Uchiyama Y, Rakugi H, Morishita R. Reduction of brain beta-amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. The Journal of biological chemistry. 2010;285:22091–22102. doi: 10.1074/jbc.M110.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Qosa H, Abuznait AH, Hill RA, Kaddoumi A. Enhanced Brain Amyloid-beta Clearance by Rifampicin and Caffeine as a Possible Protective Mechanism against Alzheimer’s Disease. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-120319. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Johanson C, Flaherty S, Messier A, Duncan JI, Silverberg G. Expression of the beta-amyloid transporter, LRP1, in aging choroid plexus: implications for the CSF-brain systemin NPH and Alzheimer’s disease. Cerebrospinal Fluid Research. 2006;3:S29. [Google Scholar]
- [114].Behl M, Zhang Y, Shi Y, Cheng J, Du Y, Zheng W. Lead-induced accumulation of beta-amyloid in the choroid plexus: role of low density lipoprotein receptor protein-1 and protein kinase C. Neurotoxicology. 2010;31:524–532. doi: 10.1016/j.neuro.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Behl M, Zhang Y, Monnot AD, Jiang W, Zheng W. Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicology and applied pharmacology. 2009;240:245–254. doi: 10.1016/j.taap.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, Ohtsuki S, Terasaki T, Iwatsubo T. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. The Journal of biological chemistry. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiology of disease. 2008;30:94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. The Journal of clinical investigation. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, Ghiso J, Frangione B, Zlokovic BV. Brain clearance of Alzheimer’s amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. Journal of drug targeting. 2002;10:359–368. doi: 10.1080/10611860290031831. [DOI] [PubMed] [Google Scholar]
- [120].Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- [121].Klinge PM, Samii A, Niescken S, Brinker T, Silverberg GD. Brain amyloid accumulates in aged rats with kaolin-induced hydrocephalus. Neuroreport. 2006;17:657–660. doi: 10.1097/00001756-200604240-00020. [DOI] [PubMed] [Google Scholar]
- [122].Donahue J, Flaherty S, Johanson C, Duncan J, Silverberg G, Miller M, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa E. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- [123].Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- [124].Mackic JB, Bading J, Ghiso J, Walker L, Wisniewski T, Frangione B, Zlokovic BV. Circulating amyloid-beta peptide crosses the blood-brain barrier in aged monkeys and contributes to Alzheimer’s disease lesions. Vascul Pharmacol. 2002;38:303–313. doi: 10.1016/s1537-1891(02)00198-2. [DOI] [PubMed] [Google Scholar]
- [125].Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain research. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tamaki C, Ohtsuki S, Iwatsubo T, Hashimoto T, Yamada K, Yabuki C, Terasaki T. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharmaceutical research. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- [127].Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid beta-peptide (1 40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Molecular pharmacology. 2007;72:850–855. doi: 10.1124/mol.107.036913. [DOI] [PubMed] [Google Scholar]
- [128].Sutcliffe JG, Hedlund PB, Thomas EA, Bloom FE, Hilbush BS. Peripheral reduction of beta-amyloid is sufficient to reduce brain beta-amyloid: implications for Alzheimer’s disease. Journal of neuroscience research. 2011;89:808–814. doi: 10.1002/jnr.22603. [DOI] [PubMed] [Google Scholar]
- [129].Sagare AP, Winkler EA, Bell RD, Deane R, Zlokovic BV. From the liver to the blood-brain barrier: an interconnected system regulating brain amyloid-beta levels. Journal of neuroscience research. 2011;89:967–968. doi: 10.1002/jnr.22670. [DOI] [PubMed] [Google Scholar]
- [130].Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E, Khanna P, Jain SC, Thakur SS, Ravindranath V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Dries DR, Yu G, Herz J. Extracting beta-amyloid from Alzheimer’s disease. Proc Natl Acad Sci U S A. 2012;109:3199–3200. doi: 10.1073/pnas.1121560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. The Journal of biological chemistry. 1992;267:14998–15004. [PubMed] [Google Scholar]
- [133].Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- [134].Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sturchler E, Galichet A, Weibel M, Leclerc E, Heizmann CW. Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J Neurosci. 2008;28:5149–5158. doi: 10.1523/JNEUROSCI.4878-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circulation research. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- [138].Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. Journal of molecular medicine. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- [139].Schmidt AM, Sahagan B, Nelson RB, Selmer J, Rothlein R, Bell JM. The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer’s disease. Current opinion in investigational drugs. 2009;10:672–680. [PubMed] [Google Scholar]
- [140].Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. The EMBO journal. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, et al. Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nature medicine. 1995;1:693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- [143].Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, Zlokovic BV. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1-40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279:C1772–1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- [145].Webster SJ, Mruthinti S, Hill WD, Buccafusco JJ, Terry AV., Jr. An Aqueous Orally Active Vaccine Targeted Against a RAGE/AB Complex as a Novel Therapeutic for Alzheimer’s Disease. Neuromolecular Med. 2012 doi: 10.1007/s12017-012-8176-z. [DOI] [PubMed] [Google Scholar]
- [146].Mahley RW, Rall SC., Jr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- [147].Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- [148].Zannis VI, Breslow JL. Apolipoprotein E. Mol Cell Biochem. 1982;42:3–20. doi: 10.1007/BF00223534. [DOI] [PubMed] [Google Scholar]
- [149].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- [150].Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- [151].Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- [153].Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, McComb JG, Frangione B, Ghiso J, Zlokovic BV. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood-brain barrier transport of circulating Alzheimer’s amyloid beta. J Neurochem. 1997;69:1995–2004. doi: 10.1046/j.1471-4159.1997.69051995.x. [DOI] [PubMed] [Google Scholar]
- [157].May PC, Finch CE. Sulfated glycoprotein 2: new relationships of this multifunctional protein to neurodegeneration. Trends Neurosci. 1992;15:391–396. doi: 10.1016/0166-2236(92)90190-j. [DOI] [PubMed] [Google Scholar]
- [158].Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev. 2009;61:89–104. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]
- [159].McGeer PL, Kawamata T, Walker DG. Distribution of clusterin in Alzheimer brain tissue. Brain Res. 1992;579:337–341. doi: 10.1016/0006-8993(92)90071-g. [DOI] [PubMed] [Google Scholar]
- [160].Yu JT, Li L, Zhu QX, Zhang Q, Zhang W, Wu ZC, Guan J, Tan L. Implication of CLU gene polymorphisms in Chinese patients with Alzheimer’s disease. Clin Chim Acta. 2010;411:1516–1519. doi: 10.1016/j.cca.2010.06.013. [DOI] [PubMed] [Google Scholar]
- [161].Carrasquillo MM, Belbin O, Hunter TA, Ma L, Bisceglio GD, Zou F, Crook JE, Pankratz VS, Dickson DW, Graff-Radford NR, Petersen RC, Morgan K, Younkin SG. Replication of CLU, CR1, and PICALM associations with alzheimer disease. Arch Neurol. 2010;67:961–964. doi: 10.1001/archneurol.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Corneveaux JJ, Myers AJ, Allen AN, Pruzin JJ, Ramirez M, Engel A, Nalls MA, Chen K, Lee W, Chewning K, Villa SE, Meechoovet HB, Gerber JD, Frost D, Benson HL, O’Reilly S, Chibnik LB, Shulman JM, Singleton AB, Craig DW, Van Keuren-Jensen KR, Dunckley T, Bennett DA, De Jager PL, Heward C, Hardy J, Reiman EM, Huentelman MJ. Association of CR1, CLU and PICALM with Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. Hum Mol Genet. 2010;19:3295–3301. doi: 10.1093/hmg/ddq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EM, Ramirez-Lorca R, Debette S, Longstreth WT, Jr., Janssens AC, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson PV, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MM. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lee JH, Cheng R, Barral S, Reitz C, Medrano M, Lantigua R, Jimenez-Velazquez IZ, Rogaeva E, St George-Hyslop PH, Mayeux R. Identification of novel loci for Alzheimer disease and replication of CLU, PICALM, and BIN1 in Caribbean Hispanic individuals. Arch Neurol. 2011;68:320–328. doi: 10.1001/archneurol.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Szymanski M, Wang R, Bassett SS, Avramopoulos D. Alzheimer’s risk variants in the Clusterin gene are associated with alternative splicing. Transl Psychiatry. 2011:1. doi: 10.1038/tp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Bettens K, Brouwers N, Engelborghs S, Lambert JC, Rogaeva E, Vandenberghe R, Le Bastard N, Pasquier F, Vermeulen S, Van Dongen J, Mattheijssens M, Peeters K, Mayeux R, St George-Hyslop P, Amouyel P, De Deyn PP, Sleegers K, Van Broeckhoven C. Both common variations and rare non-synonymous substitutions and small insertion/deletions in CLU are associated with increased Alzheimer risk. Mol Neurodegener. 2012;7:3. doi: 10.1186/1750-1326-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Narayan P, Orte A, Clarke RW, Bolognesi B, Hook S, Ganzinger KA, Meehan S, Wilson MR, Dobson CM, Klenerman D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-beta(1-40) peptide. Nat Struct Mol Biol. 2012;19:79–83. doi: 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Matsubara E, Frangione B, Ghiso J. Characterization of apolipoprotein J-Alzheimer’s A beta interaction. J Biol Chem. 1995;270:7563–7567. doi: 10.1074/jbc.270.13.7563. [DOI] [PubMed] [Google Scholar]
- [169].Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci U S A. 1996;93:4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Zlokovic BV, Martel CL, Mackic JB, Matsubara E, Wisniewski T, McComb JG, Frangione B, Ghiso J. Brain uptake of circulating apolipoproteins J and E complexed to Alzheimer’s amyloid beta. Biochem Biophys Res Commun. 1994;205:1431–1437. doi: 10.1006/bbrc.1994.2825. [DOI] [PubMed] [Google Scholar]
- [171].Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Chun JT, Wang L, Pasinetti GM, Finch CE, Zlokovic BV. Glycoprotein 330/megalin (LRP-2) has low prevalence as mRNA and protein in brain microvessels and choroid plexus. Exp Neurol. 1999;157:194–201. doi: 10.1006/exnr.1999.7052. [DOI] [PubMed] [Google Scholar]
- [173].Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- [174].Sagare AP, Bell RD, Zlokovic BV. Neurovascular dysfunction and faulty amyloid beta-peptide clearance in Alzheimer’s disease. In: Selkoe DJ, Mandelkow E, Holtzman DM, editors. The Biology of Alzheimer’s Disease. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2012. pp. 405–422. doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]