Abstract
Youths at clinical high risk (CHR) for psychosis typically exhibit significant social dysfunction. However, the specific social behaviors associated with psychosis risk have not been well characterized. We administer the Social Responsiveness Scale (SRS), a measure of autistic traits that examines reciprocal social behavior, to the parents of 117 adolescents (61 CHR individuals, 20 age-matched adolescents with a psychotic disorder [AOP], and 36 healthy controls) participating in a longitudinal study of psychosis risk. AOP and CHR individuals have significantly elevated SRS scores relative to healthy controls, indicating more severe social deficits. Mean scores for AOP and CHR youths are typical of scores obtained in individuals with high functioning autism (Constantino & Gruber, 2005). SRS scores are significantly associated with concurrent real-world social functioning in both clinical groups. Finally, baseline SRS scores significantly predict social functioning at follow-up (an average of 7.2 months later) in CHR individuals, over and above baseline social functioning measures ( p < .009). These findings provide novel information regarding impairments in domains critical for adolescent social development, because CHR individuals and those with overt psychosis show marked deficits in reciprocal social behavior. Further, the SRS predicts subsequent real-world social functioning in CHR youth, suggesting that this measure may be useful for identifying targets of treatment in psychosocial interventions.
Social dysfunction is a hallmark feature of both schizophrenia and autism spectrum disorders (ASD), although the two disorders have a distinct developmental course. Rare copy number variants (insertions, deletions, and duplications of genomic sequence) within the same genomic loci have been linked to both schizophrenia and autism, suggesting shared biological pathways (da Silva Alves et al., 2011). In addition, both schizophrenia and autism have been conceptualized as disorders of neural “dysconnectivity,” referring to developmental disruption of widespread brain areas (Geschwind & Levitt, 2007; Karlsgodt et al., 2008). It is likely that this dysconnectivity contributes to social behavioral dysfunction, which is a relative deficit that may grow in comparison to typically developing peers, who show rapid maturation in social behavior in late childhood and adolescence. However, whether the social impairment observed in autism maps on to the same pathophysiological mechanisms as it does in schizophrenia and whether both groups could potentially respond to similar interventions depends on whether the same types of social impairments are present across both groups. While social deficits in the context of ASD have been described in fine-grained detail, the behaviors contributing to social impairment have not been well characterized in the early phases of psychotic illness.
Although youths at clinical high risk (CHR) for psychosis do not have full-blown psychotic symptoms, they show significant social dysfunction that is of similar magnitude to those with an established schizophrenia diagnosis (Addington, Penn, Woods, Addington, & Perkins, 2008). In schizophrenia, impairment in social functioning is more strongly associated with negative symptoms rather than positive symptoms (Rabinowitz et al., 2012) and is also related to subjective quality of life (Bengtsson-Tops & Hansson, 2001). This social dysfunction, which is characterized by a lack of involvement in social activities, difficulty communicating with others, and a reduced number of social supports (Bengtsson-Tops & Hansson, 2001; Couture, Penn, & Roberts, 2006), has been identified prior to illness onset, both prospectively and retrospectively (Cannon et al., 1997; Davidson et al., 1999; Done, Crow, Johnstone, & Sacker, 1994), and significant social deterioration has been identified between childhood and late adolescence in those who go on to develop psychosis (Strauss et al., 2012). In addition, Cannon et al. (2008) found that a more severe social functioning impairment at baseline contributes uniquely to the prediction of psychosis in clinically at-risk adolescents and young adults, suggesting that impaired social functioning marks elevated risk for psychosis conversion among CHR youth. However, little is known about the developmental course of social deficits in early psychosis, specifically whether the observed impairments in CHR and adolescents with a psychotic disorder (AOP) youths are similar in magnitude to those in other neurodevelopmental disorders (e.g., ASD), what social behaviors may predict social functioning, and whether these behaviors are stable over time in CHR youth.
Because a drop in social functioning often precedes the onset of psychosis and this disorder usually develops during late adolescence, it is important to examine these aims within a developmental framework. Adolescence is a stage in which the gap in functioning between youths exhibiting lower social competence and their more socially adept peers begins to rapidly increase (Monahan & Steinberg, 2011). Thus, less socially competent individuals face the increasingly difficult challenge of navigating a more complex social environment, while lacking the skills to do so. These rapid changes in the intensity and nature of social demands (e.g., increased time spent with peers, the development of romantic relationships, and handling conflict in interpersonal interactions; Roisman, Masten, Coatsworth, & Tellegen, 2004), coupled with lower baseline levels of social competence, have been cited as a potential psychosocial factors underlying the sharp increase in psychosis incidence during late adolescence (Paus, Keshavan, & Giedd, 2008). Finally, because social impairment persists when psychotic symptoms remit (Addington et al., 2011; Schlosser et al., 2012), it is essential to identify specific behaviors that contribute to impairments in social functioning in CHR youths and those diagnosed with adolescent-onset schizophrenia (AOP), and the capacity of these difficulties to predict future social dysfunction.
Furthermore, parsing the specific behaviors associated with social dysfunction in adolescents with psychosis and those at risk for the illness may aid in the development of more effective psychosocial interventions for improving functional outcomes for youths in the early phases of psychosis. Previous studies examining social dysfunction in CHR individuals have used very global measures of overall functioning (e.g., the Social Functioning Scale; Birchwood, Smith, Cochrane, Wetton, & Copestake, 1990) and have not looked at discrete behaviors that may contribute to social impairment (Addington et al., 2008). Finally, using well-validated measures commonly used to assess social deficits characteristic of ASD may be a unique approach for both understanding the phenotypic overlap between these groups and characterizing targets for early intervention in CHR individuals.
In individuals with autism, reciprocal social behavior (RSB), which is the ability to process social information, comprehend the message being conveyed, and appropriately respond in interpersonal interactions, is a hallmark characteristic of the disorder (Constantino, Przybeck, Friesen, & Todd, 2000). Skillful RSB is crucial for successfully navigating daily interpersonal interactions, such as making “small talk,” dealing with conflict, and maintaining social connections. Deficits in RSB likely lead to a decreased number of social interactions and contacts. Skills associated with RSB improve throughout childhood and adolescence (e.g., empathy; Dadds et al., 2009) and the ability to engage in specific social behaviors, such as initiating interactions, attending to others’ perspectives and needs, and providing social support, is associated with social competence (Durkin & Conti-Ramsden, 2007; Trentacosta & Fine, 2010).
The Social Responsiveness Scale (SRS; Constantino & Gruber, 2005) is a quantitative parent-report measure of RSB that has been extensively tested in both clinically ascertained and population-based samples (Constantino et al., 2000; Constantino & Todd, 2003). The measure represents the three criterion domains for autism, and multiple publications have shown that the SRS correlates strongly with a gold standard diagnosis of ASD, based on the Autism Diagnostic Interview (Constantino et al., 2003; Murray, Mayes, & Smith, 2011). RSB has been shown to be continuously distributed and moderately to highly heritable in the general population, with those in the autism spectrum representing the upper extreme of a constellation of quantitative traits (Constantino & Todd, 2003). In addition, RSB, as measured by the SRS, has been shown to be stable over time in typically developing youths and those with pervasive developmental disorders (range = 1–5 years; Constantino et al., 2009).
Although the SRS has been validated and widely used as a quantitative measure of autistic traits, to our knowledge it has never been investigated in studies of adolescents with psychotic disorder or those at risk for psychosis (CHR). Thus, the present study investigated the following questions:
Do AOP and CHR youths display deficits in RSB when compared with typically developing youths? Given that social dysfunction is a key feature of psychosis and this impairment is present prior to the onset of the disorder, we hypothesized that AOP and CHR individuals would have elevated SRS scores relative to age-matched, typically developing controls.
Is RSB related to clinical symptomatology, cognition, and psychosocial functioning in AOP and CHR individuals? In this exploratory aim, we wanted to examine how RSB is related to symptoms in these clinical populations, which may shed light on possible mechanisms of early intervention for social deficits in the early phases of psychosis. Given the well-established link between negative symptoms and social dysfunction in patients with schizophrenia (e.g., Gorna & Rybakowski, 1995), we hypothesized that RSB would be significantly associated with negative symptoms in both adolescents with psychosis and those at-risk for the illness. Because previous research has shown that social impairment continues to persist in individuals at CHR who do not convert to psychosis, including some individuals with attenuated positive symptoms that remitted entirely over the course of follow-up (Addington et al., 2011; Schlosser et al., 2012), and is not strongly associated with positive symptoms in those with an established diagnosis of a psychotic disorder (Rabinowitz et al., 2012), we did not expect to see a significant relationship between RSB and positive symptoms. Furthermore, because Constantino et al. (2003) have identified RSB as a factor separate from cognitive abilities, we also predicted that RSB would be associated with real-world social functioning in the two clinical samples, independent of any deficits in general intellectual function.
Is baseline RSB a significant predictor of social functioning over a 6- to 12-month follow-up period in CHR individuals? Because the SRS quantifies specific behaviors that are likely to be significantly related to global social functioning, we hypothesized that baseline SRS scores would uniquely predict social functioning 6–12 months later. Finally, as a secondary exploratory aim, we examined whether SRS scores represent a stable marker of RSB in CHR individuals. Based on prior evidence for stability of social dysfunction in youths at CHR for schizophrenia (Cornblatt et al., 2007), we hypothesized that RSB would show stability across a 6- to 12-month period in this group.
Methods
Participants
Study participants (ages 12–18 years old) were part of an ongoing longitudinal study at the Staglin Music Festival Center for the Assessment and Prevention of Prodromal States (CAPPS) and the Adolescent Brain and Behavior Research Center at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles. The current sample was drawn from a larger longitudinal study of individuals at high risk for psychosis and a healthy comparison group (North American Prodromal Longitudinal Study) who were assessed at baseline and at four separate points: 6, 12, 18, and 24 months. Cross-sectional baseline analyses are from data collected at the baseline time point. The longitudinal data was collected at either the 6- or the 12-month time point (as available).
The AOP and CHR sample consisted of help-seeking individuals who were referred to CAPPS or the Adolescent Brain and Behavior Research Center by community mental health professionals or who self-referred by responding to advertisements on the CAPPS website. Eligible CHR individuals met criteria at baseline for one of the three prodromal syndromes, as assessed by the Structured Interview for Prodromal Symptoms (SIPS; McGlashan, 2001): attenuated (subthreshold) psychotic symptoms; transient, recent-onset psychotic symptoms; or a substantial drop in social/role functioning in conjunction with schizotypal personality disorder diagnosis or a first-degree relative with a psychotic disorder. Axis I disorders were assessed via semistructured interviews: the Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., 1997) for participants aged 12–15, or the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I/P; First, 1997). Participants were classified within the AOP group if they met the criteria listed in DSM-IV for an Axis I schizophrenia spectrum diagnosis (i.e., schizophrenia, schizoaffective disorder, or schizophreniform disorder) based on Kiddie Schedule for Affective Disorders and Schizophrenia or a SCID diagnostic interview with the participant and his or her parent or legal guardian. Control participants were recruited from the community through advertisements posted in newspapers and on fliers. Inclusion criteria specified that control participants must not meet criteria for a major mental disorder or for a prodromal syndrome as determined by the diagnostic interview and must not have a first-degree family history of a psychotic disorder. Because the focus of the original longitudinal study was on risk factors for psychosis, our exclusion criteria did not include first-degree relatives with autism spectrum diagnoses. Participants were also excluded if they had a neurological disorder that might affect performance, insufficient fluency in English, an estimated IQ of <70, or if they endorsed substance or alcohol abuse and/or dependence within the past 6 months. All interviews were conducted by MA or PhD level psychologists who had participated in an in-depth “gold-standard” training program regarding the administration and scoring of the SIPS and the SCID-I/P and demonstrated excellent reliability with gold standard diagnoses (κs = 0.85–1.00); interrater reliability and case consensus procedures have been described in detail elsewhere (Meyer et al., 2005).
All study participants underwent a verbal and a written informed consent process. Subjects under the age of 18 years provided written assent, while parents/guardians provided written consent. The University of California, Los Angeles, Institutional Review Board approved all study procedures.
SRS
The SRS is a 65-item parent questionnaire asking about the child’s social behavior in the past 6 months, particularly focusing on the parent’s perception of the child’s ability to process social information and respond appropriately in interpersonal interactions (Constantino & Gruber, 2005). Items representing all three criterion domains for autism (i.e., deficits in reciprocal communication, social deficits, and restricted/ stereotypic behaviors or interests) are included in this measure, as are additional items asking about other types of social behaviors. A 4-point Likert scale (0 = not true, 1 = sometimes true, 2 = often true, 3 = almost always true) is used to rate how often the child engages in the behavior. Based on a previous study that identified five separate factors through principal components analysis of the SRS (Constantino et al., 2009), the total score is often broken down into five subscales: receptive awareness, cognition, expressive communication, motivation, and autistic mannerisms (see Table 1 for examples of subscale items). Raw scores for each scale are converted to a gender-specific T score representing the individual’s social behavioral impairment in each of the five domains (Constantino & Gruber, 2005). The five subscales (raw scores) are summed and converted into a T score, resulting in an overall composite SRS score. Using population norms to calculate the overall score, we are able to identify where an individual falls along the continuum of RSB deficits. A higher score indicates greater RSB impairment. The T scores of each subscale and the overall score are calculated using a normative sample as a reference. Reliability and validity of this measure has been previously established (Constantino et al., 2003, 2009). Elevated SRS scores have been found in other clinical populations, such as youths with attention-deficit/hyperactivity disorder (Reiersen, Constantino, Volk, & Todd, 2007). Despite evidence for prominent social impairment in early psychosis, specific aspects of RSB have not previously been investigated in this population.
Table 1.
Example items from the Social Reciprocity Scale (SRS)
| SRS Subscale | SRS Example Items |
|---|---|
| Motivation | Would rather be alone than with others Avoids starting social interactions with peers or adults Seems self-confident when interacting with others |
| Receptive awareness | Expressions on his/her face do not match what he/she is saying Focuses his/her attention to where others are looking or listening Does not seem to mind being “out of step” or not on the “same wavelength” with others |
| Cognition | Takes things too literally and does not “get” the real meaning of a conversation Has a sense of humor, understands jokes Does not recognize when others are trying to take advantage of him/her |
| Expressive communication | Avoids eye contact, or has unusual eye contact Gets frustrated trying to get ideas across in conversations Offers comfort to others when they are sad |
| Autistic mannerisms | Has repetitive, odd behaviors such as hand flapping or rocking Has more difficulty than other children with changes in his/her routine Cannot get his/her mind off something once he/she starts thinking about it |
Clinical, psychosocial outcome, and cognitive measures
A trained clinician assessed participants with the SIPS in its entirety, including the positive, negative, disorganized, and general symptom subscales. Clinicians also assessed and rated social and role functioning at the time of the clinical assessment. Outcome scores were obtained using the Global Functioning Social Scale (GFS) and the Global Functioning Role Scale (GFR), which were developed specifically to assess psychosocial functioning in younger individuals (Cornblatt et al., 2007). The scores on these two measures range from 1 to 10, with higher numbers corresponding to better levels of functioning. The GFS evaluates how much time one spends with friends and family, how one deals with peer-related conflicts, and whether or not one seeks out interactions with others. The GFR assesses the level at which an individual is functioning in an academic or a work environment. Both scales have shown adequate construct validity and strong interrater reliability and were sensitive to social and role impairment in a CHR sample (Cornblatt et al., 2007). All raters of the current sample demonstrated interrater reliability, with values of κ = 0.95 for GFS and κ = 0.91 for the GFR, based on eight randomly selected cases. For all clinician-administered measures, information about symptoms and functioning is obtained from both the participants and the parent/guardians. All clinical measures were administered at both the baseline and the follow-up time point (when available). Due to attrition and/or failure to collect these particular measures at follow-up time points, longitudinal data were only available for a subset of the CHR participants. At the baseline assessment, an estimate of general intellectual functioning was obtained from the two-subtests (vocabulary and matrix reasoning) version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
Statistical analyses
All statistical analyses were performed using Statistical Package for the Social Sciences software version 18 (SPSS Inc.). We compared baseline demographic characteristics among the three groups using univariate analyses of variance (ANOVAs) for continuous variables and chi-squared tests for categorical variables. To determine whether overall RSB differed between CHR individuals, AOP individuals, and controls, we conducted a one-way univariate ANOVA with total SRS T score as the dependent variable. To determine whether different components of RSB differed between CHR individuals, AOP individuals, and controls, we conducted separate univariate ANOVAs with SRS subscales as the dependent variables. Because age and gender are taken into account when the SRS T scores are calculated, we did not include these variables as covariates in our analyses. To examine whether subscales showed differential levels of impairment, we conducted follow-up t tests comparing the three groups on the five SRS subscales (receptive awareness, cognition, expressive communication, motivation, and autistic mannerisms). Pearson correlations were conducted separately within each group to examine the relationship among baseline RSB (as indexed by baseline overall SRS T score), clinical, IQ, and psychosocial measures. A paired t test examining SRS scores at Time 1 and Time 2 was also used to examine the stability of RSB within the CHR group.
Finally, we performed longitudinal analyses in the CHR group alone, in order to examine whether baseline RSB was a significant predictor of functioning (social and role) over time. Two separate hierarchical regression analyses were conducted, with role and social functioning at follow-up assessment (Time 2) as the dependent variables. For social functioning, the GFS score at the initial assessment (Time 1) was entered as a predictor in the first block and the baseline total SRS score (Time 1) was then entered as a predictor in the second block. For role functioning, the GFR score at the initial assessment (Time 1) was entered as a predictor in the first block, and the baseline total SRS score (Time 1) was then entered as a predictor in the second block. Within each respective analysis, the magnitude of R2 change was tested for significance.
Results
The baseline sample consisted of 61 CHR individuals (40 males, 21 females), 20 AOP individuals (11 males, 9 females), and 36 healthy control participants (18 males, 18 females). Three of the CHR individuals met criteria for an ASD; therefore, these individuals were excluded from all analyses and the remaining CHR sample consisted of 58 participants. As shown in Table 2, participants in the three groups did not differ significantly in age, participant education level, parental education level, race, ethnicity, IQ level, or gender distribution.
Table 2.
Baseline characteristics of study participants
| Healthy Comparison Participants (n = 36) |
CHR Participants (n = 58)a |
AOP Participants (n = 20) |
p | |
|---|---|---|---|---|
| Age years (±SD) | 15.0 (1.5) | 15.5 (1.9) | 15.7 (1.6) | .37 |
| Participant education years (±SD) | 8.9 (2.2) | 8.9 (2.2) | 9.4 (1.8) | .33 |
| Parental education years (+SD) | 16.6 (2.8) | 16.7 (2.8) | 16.5 (1.9) | .94 |
| Gender N (% female) | 18 (50%) | 19 (33%) | 9 (55%) | .11 |
| Race (Native American/Asian/African | ||||
| American/Caucasian/other) | 1/4/5/17/9 | 1/7/4/37/9 | 0/3/2/7/8 | .41 |
| Ethnicity N (% Latino) | 7 (19%) | 14 (24%) | 3 (15%) | .66 |
| WASI IQ, two subtests (±SD) | 106.9 (15.9) | 103.8 (15.8) | 96.7 (13.4) | .10 |
| SRS overall T score | 47.8 (11.5) | 67.2 (14.9) | 70.7 (12.2) | <.001 |
| Global functioning | ||||
| Social | 8.6 (0.7) | 6.3 (1.6) | 5.5 (1.8) | <.001 |
| Role | 8.4 (0.9) | 5.9 (2.1) | 5.2 (1.7) | <.001 |
| SIPS | ||||
| Positive symptoms | 1.9 (2.1) | 11.6 (8.4) | 20.6 (4.7) | <.001 |
| Negative symptoms | 1.8 (1.9) | 11.2 (5.6) | 18.2 (6.0) | <.001 |
| Disorganized symptoms | 0.7 (0.9) | 5.6 (4.5) | 8.0 (4.2) | <.001 |
| General symptoms | 1.0 (1.3) | 8.2 (4.7) | 9.6 (4.1) | <.001 |
Note: CHR, clinical high risk; AOP, adolescent-onset psychosis; WASI, Wechsler Abbreviated Scale of Intelligence; SRS, Social Reciprocity Scale; SIPS, Structured Interview for Prodromal Symptoms.
Excluding three CHR subjects with autism spectrum diagnoses.
Comparison of RSB in CHR individuals, AOP individuals, and controls
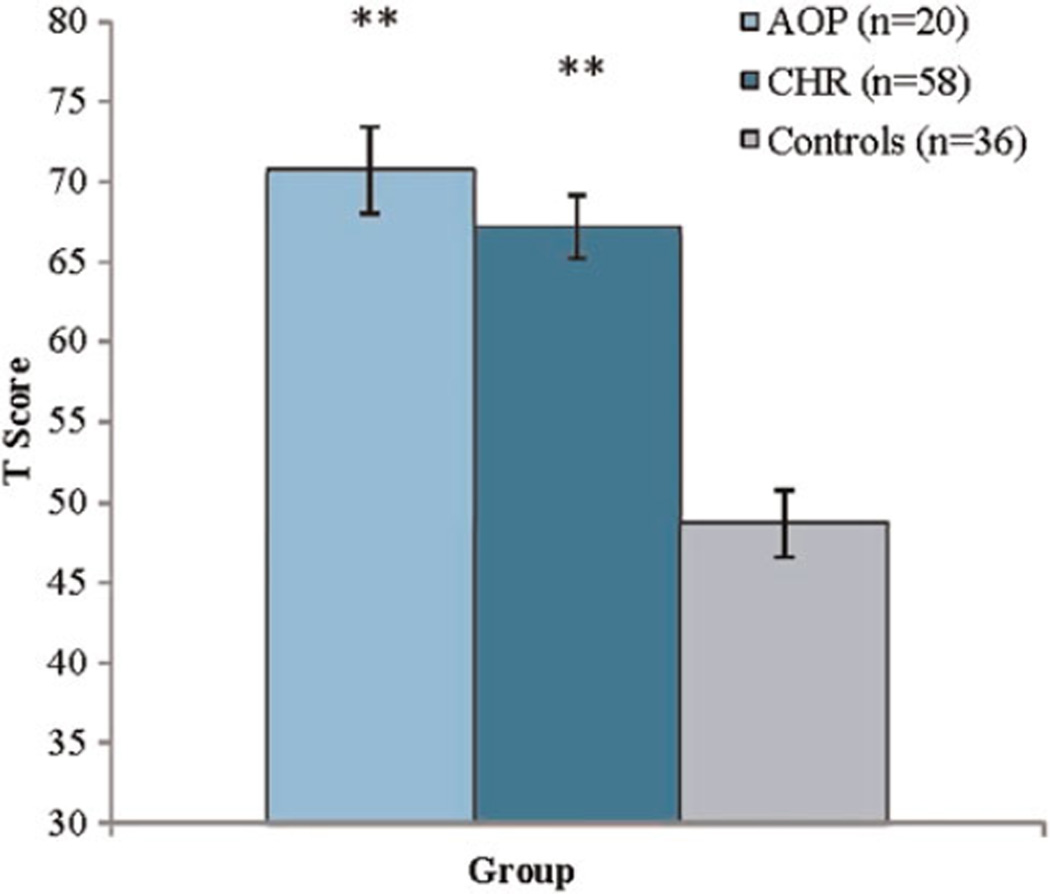
An ANOVA for the overall composite SRS score revealed a significant main effect of group, F (2, 114) = 28.3, p < .001, partial η2 = 0.34. CHR (M = 67.2, SD = 15.0) and AOP (M = 70.7, SD = 12.2) individuals both showed RSB impairment relative to controls (M = 47.8, SD = 11.4, see Figure 1).
Figure 1.
(Color online) Social Responsiveness Scale (SRS) T scores in adolescents with recent-onset psychotic disorder (AOP), clinical high-risk (CHR) youths, and typically developing controls. In comparison to controls, **p < .001.
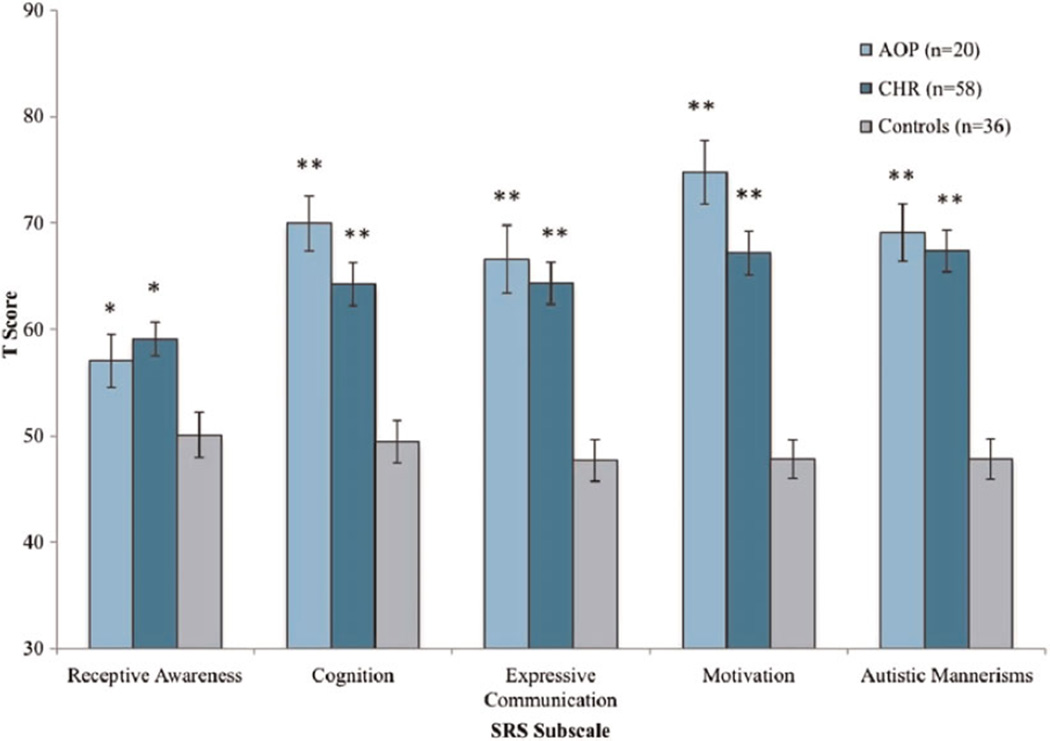
Analyses of group differences (CHR vs. AOP vs. controls) in SRS subscales score revealed a main effect of group for all subscales: receptive awareness, F (2, 114) = 6.3, p < .005, partial η2 = 0.10; cognition, F (2, 114) = 18.0, p < .001, partial η2 = 0.25; expressive communication, F (2, 114) = 18.8, p < .001, partial η2 = 0.25; motivational awareness, F (2, 114) = 30.9, p < .001, partial η2 = 0.36; and autistic mannerisms, F (2, 114) = 30.1, p < .001, partial η2 = 0.35. Post hoc contrasts revealed that both the CHR and AOP groups showed significant impairments on all five RSB subscales relative to controls: cognition, expressive communication, motivation, receptive awareness, and autistic mannerisms (all p < .05 or greater; see Figure 2). The largest magnitude of difference between CHR individuals versus controls was seen in the autistic mannerisms subscale, with an effect size (d) of 1.58 ( p < .001). For AOP individuals versus healthy controls, the largest magnitude of difference was seen in the motivation subscale (d < 2.2, p < .001). The autistic mannerisms subscale was also substantially elevated in AOP individuals relative to healthy controls (d = 1.96; p < .001). The difference between scores on the motivation subscale in AOP and CHR participants approached significance ( p = .06), with AOP individuals (M = 74.5, SD = 13.3) showing a trend toward greater impairment on this scale in comparison to CHR individuals (M = 67.2, SD = 15.6). However, there were no other significant differences between the two clinical groups on the other subscales (all ps > .10).
Figure 2.
(Color online) Social Responsiveness Scale (SRS) subscale T scores in adolescents with recent-onset psychotic disorder (AOP), clinical high risk (CHR) youths, and typically developing controls. In comparison to controls, *p≤ .05, **p< .001.
Relationships among baseline RSB, clinical symptomatology, cognition, and psychosocial measures
The associations among baseline RSB and concurrent clinical symptomatology, IQ score, and psychosocial measures were also examined within each group. In both the CHR and the AOP groups, higher overall SRS scores (indicating greater RSB impairment) were associated with significantly lower social functioning scores (CHR: r = −.37, p < .005; AOP: r = −.52, p < .05, see Table 3). In CHR individuals, the relationship between RSB and negative symptoms (r = −.25, p = .06) approached significance at a trend level. CHR individuals did not show any relationships among total SRS score and IQ, global role functioning, positive, disorganized, or general symptoms, as measured by the SIPs (all ps ≥ .10). In AOP individuals, RSB was not correlated with global role functioning, IQ, positive, negative, disorganized, and/or general symptoms (all ps ≥ .11). Controls did not show significant relationships between SRS scores and any of the clinical, cognitive, or psychosocial measures (all ps ≥ .08). Because of the trend-level relationship between SRS scores and negative symptoms, we redid our primary analyses with negative symptoms as acovariate and all significant differences remained.
Table 3.
Correlation values of SRS T score with clinical, psychosocial, and cognitive measures in healthy controls, CHR youths, and those with AOP
| Global Functioning Symptoms |
||||||||
|---|---|---|---|---|---|---|---|---|
| Total SRS | T Score | Social | Role | Positive | Negative | Disorganized | General | WASI IQ |
| Healthy controls | r | −.30 | −.24 | .28 | .12 | −.22 | .12 | −.32 |
| p | .08 | .19 | .12 | .50 | .25 | .51 | .08 | |
| CHR individuals | r | −.37 | −.08 | .16 | .25 | .17 | .15 | −.22 |
| p | .004 | .54 | .22 | .06 | .21 | .28 | .10 | |
| AOP individuals | r | −.52 | −.38 | −.01 | .37 | .27 | .17 | −.20 |
| p | .02 | .11 | .99 | .20 | .35 | .55 | .44 | |
Note: SRS, Social Reciprocity Scale; CHR, clinical high risk; AOP, adolescent-onset psychosis; WASI, Wechsler Abbreviated Scale of Intelligence.
RSB as a predictor of later social functioning in CHR individuals
We then explored the ability of RSB to predict later social functioning in CHR individuals. Independent samples t tests comparing individuals without follow-up data (N = 30) to those with follow-up data (N = 28) revealed that these two groups did not differ at baseline in total SRS scores, t (56) = 0.39, p = .70, or demographic variables (all ps > .18). In addition, these two groups did not differ with regard to baseline SIPS positive, t (56) = 0.83, p = .41; negative, t (56) = 0.32, p = .75; disorganized, t (56) = 0.13, p = .90; or general, t (56) = 1.4, p = .13, symptoms. There were also no significant differences between the two groups on baseline GFS, t (56) − 0.44, p = .66, or GFR scores, t (56) = 0.01, p = .99. The average time between the two data collection points was 7.2 months (range = 6–12 months).
In the regression model, when baseline GFS and baseline total SRS score (Time 1) were entered as predictors of follow-up GFS (Time 2), the two combined predictors accounted for 27% of the variance in social functioning, and this model was statistically significant F (1, 27) = 5.9, p < .009. In this model, the b-value for baseline SRS score was significant (b = –0.04, p < .05), although baseline GFS was not itself a significant predictor of follow-up social functioning, with baseline SRS included in the model (b = 0.28, p = .15). These findings suggest that baseline SRS measures are better predictors of global social functioning at follow-up, over and above baseline measures of GFS.
Stability of RSB in CHR individuals
Of the 58 CHR individuals, a subset (N = 18) had two assessment points in which the SRS was administered. These 18 individuals did not significantly differ from those who did not have a second SRS (N = 40), with regard to age (p = .18), participant education level (p = .09), parental education (p = .78), gender (p = .67), race (p = .42), ethnicity (p = .89), clinical symptomatology (positive: p = .60; negative: p = .59; disorganized: p = .61; and general: p = .17), or total SRS T score (p = .78). Paired samples t test between the two time points in these individuals revealed a highly significant relationship between SRS score at Time 1 and Time 2 (r = .56, p < .05). Furthermore, there were no significant differences between SRS score at Time 1 and Time 2, t (18) = 1.3, p = .20. These preliminary findings indicate that RSB is a relatively stable construct in CHR individuals. Because we did not have sufficient follow-up SRS data in AOP and control participants, the stability of RSB in these two groups was not examined.
Discussion
To our knowledge, this is the first study to examine RSB in youths at CHR for psychosis and adolescents with a recent-onset psychotic disorder. Several novel findings emerged. First, both CHR and AOP youths had significantly elevated overall scores on the SRS in comparison to typically developing controls, but they did not differ from each other, indicating greater RSB impairment in both clinical groups. Second, SRS scores in CHR and AOP patients were significantly related to real-world social functioning, and those with greater RSB impairment also had lower levels of social functioning, as measured by the GFS (Cornblatt et al., 2007). However, positive, disorganized, and general symptoms and cognitive abilities were not significantly correlated with SRS scores in either clinical group, suggesting that these findings were not attributable to acute symptomatology, nor general intellectual function. Third, in a subset of CHR youths with longitudinal follow-up data, baseline SRS score was a significant predictor of real-world social functioning at a follow-up time point. Fourth, in the CHR sample, SRS scores appeared to be relatively stable over an average of 7.2 months, providing support for the notion that the SRS may index a traitlike characteristic in youths at risk for psychosis.
The current findings build upon the established literature regarding social functioning in CHR individuals. It has been established that those at CHR for developing psychosis show similar levels of social impairment to those with a psychotic disorder diagnosis (Addington et al., 2008). Our findings extend upon these observations by providing a finer-grained analysis of the specific social behaviors that CHR and AOP individuals engage in and appear to contribute to the overall social dysfunction typically seen in this population. In particular, this study highlights unique information about the kind of social impairments these individuals have. For instance, CHR and AOP individuals may struggle in interpersonal interactions for a number of reasons, and this quantitative measure identifies specific struggles that these individuals may have, such as not understanding jokes or getting the “real” meaning of some conversations, or going on “auto-pilot” when feeling stressed out in a social situation (autistic mannerisms). Considering that researchers who have examined CHR individuals’ subjective complaints found that distress about ability to handle social situations (59%) and/or ability to make or maintain social contacts (36%) were two of the primary disturbances that CHR individuals reported experiencing (Hambrecht, Lammertink, Klosterkotter, Matuschek, & Pukrop, 2002), this study is an important step in better characterizing the social behavior deficits seen in this clinical population.
These results also provide further evidence of the established phenotypic overlap that has been documented in autism and schizophrenia (Couture et al., 2010; Rapoport, Chavez, Greenstein, Addington, & Gogtay, 2009). Both the CHR individuals and the AOP youths showed RSB deficits of a similar magnitude to one another and to those diagnosed with an autistic spectrum disorder. An overall T score over 60 on the SRS is typically associated with a diagnosis of a high functioning ASD (i.e., pervasive developmental disorder or Asperger syndrome; Constantino & Gruber, 2005), and the average overall SRS T scores in our AOP and CHR samples were 67.2 and 70.7, respectively. Furthermore, in comparison to healthy controls, both CHR and AOP individuals showed significant elevations on the autistic mannerisms subscale of the SRS (see Figure 2). This subscale includes items such as “Thinks or talks about the same thing over and over” and “Has repetitive, odd behaviors such as hand flapping or rocking.” These findings suggest that RSB, as a quantitative trait, has clinical relevance for both autism spectrum, and CHR and AOP populations alike.
The current findings contrast with the initial assertion by Constantino et al. (2000) that RSB impairment is specific to individuals with autism, based on a small sample of 10 youths with a psychotic disorder in which no RSB impairments were observed. However, since then, elevation of clinical significant RSB impairments (measured quantitatively by the SRS) have been identified in other studies of childhood psychiatric disorders, such as attention-deficit/hyperactivity disorder (Reiersen et al., 2007), providing evidence that RSB impairments may not be specific to autism spectrum diagnoses.
In the second portion of our study, we found a significant relationship between RSB and social functioning in both individuals with CHR and individuals with AOP. Although the SRS assesses specific behaviors that have been identified from a theoretical understanding of social behavior in autism, this measure was still strongly related to measures of real-world functioning (i.e., GFS) in CHR and AOP youth. Although the latter construct can be affected by many factors, the significant statistical relationship between RSB and the GFS suggests that RSB contributes strongly to day-to-day social functioning. However, it should be noted that there was a trend-level relationship between the SRS and negative symptoms in the CHR group. This is not surprising, given that previous research has identified a relationship between negative symptoms and social functioning in adult schizophrenia (Bowie, Reichenberg, Patterson, Heaton, & Harvey, 2006). Negative symptoms, such reduced ability to experience pleasure or express emotions, likely interfere with one’s interpersonal interactions. With a larger sample, it is likely that a statistically significant relationship between RSB and negative symptoms may emerge. In contrast, other factors that contribute to RSB (e.g., repetitive behaviors or stereotyped interests) may decrease the emerging relationship between these two variables. It is interestingly that RSB in CHR and AOP individuals was not correlated with IQ. This result is consistent with the prior findings of Constantino et al. (2003), who did not find a relationship with IQ and RSB in youths with ASD. Although specific domains of cognition have been consistently found to be mildly impaired in CHR individuals relative to healthy controls (Jahshan, Heaton, Golshan, & Cadenhead, 2010; Niendam et al., 2006), it does not appear that cognitive difficulties are related to RSB deficits in our population.
The longitudinal portion of our study also yielded important results. Similar to published findings on global social functioning in a CHR sample (Schlosser et al., 2012), our preliminary evidence suggests that RSB impairments are relatively stable over time. We also identified baseline RSB as a significant predictor of social functioning in CHR youth. Others have shown that in typically developing youths and those with ASD, RSB, as measured by the SRS, remains stable over time (Constantino et al., 2009). However, we wanted to examine this construct within CHR youth, where it has not been examined before. These findings are unique, considering that a parent-rated questionnaire, as opposed to using only a clinician-rated global scale, was a better predictor of functioning at follow-up. These findings also highlight the importance of obtaining information from multiple sources, particularly in younger individuals.
The comparability of RSB deficits in CHR and AOP patients with the nature and severity of the deficits observed in high-functioning ASD raises interesting questions about the developmental course of social dysfunction. Because abnormal social behavior is a hallmark of ASD diagnosis, which typically occurs very early in life, the initial observation of social dysfunction, the “onset” of illness, and the development of earliest forms of typical social behavior are usually confounded. However, the present findings suggest that emergence of social dysfunction associated with psychosis risk follows a course virtually independent of illness onset and exacerbation, despite similarities between the sets of behavioral abnormalities in CHR/AOP and ASD samples. Future investigations will be necessary to map the developmental trajectory of RSB, both typical and impaired. A critical question for this work will be, does RSB impairment emerge over time during late childhood and adolescence or does a longstanding social deficit simply become more obvious in the context of increasing social demands of adolescence? If the latter is found to be the case, and profound RSB impairment does precede psychosis and its prodrome even in early childhood, a second question might be, does RSB impairment in CHR, AOP, and ASD samples reflect the same underlying pathophysiological process? Similarly, could RSB impairment represent a phenotypic expression of the genetic liability shared between these disorders?
Certain limitations of this study must be noted. Although we were able to show that RSB is a relatively stable construct over time, we are not able to draw conclusions about premorbid RSB in this sample. With the limited follow-up data that we obtained on CHR youth, these findings suggest stability of RSB, but these results are far from conclusive. In addition, we found that AOP youths did not have significant impairments on the receptive awareness subscale; however, it is likely that this null finding is due to the small sample size of our AOP group, resulting in a lack of power. Furthermore, we did not have sufficient data to examine the stability of RSB or its ability to predict social functioning in typically developing controls or those with AOP. It should also be kept in mind that this is a parental measure, so it is useful in understanding how the parents of these clinical samples view their child’s RSB, which may be different from how CHR and AOP youths view their own RSB behavior. However, other measures in our study, specifically the GFS/GFR and SIPs, rely mainly upon participant report, while including corroborating information from parents. Finally, although we did exclude three individuals who presented with a historical diagnosis of an ASD, a formal diagnostic evaluation of ASD (e.g., Autism Diagnostic Interview—Revised) was not used in conjunction with this parental questionnaire. Therefore, it is difficult to determine whether elevated SRS scores indicate “true” autistic features versus general impairment in social functioning. Recent research found a significant relationship between early autistic traits and psychotic experiences in adolescence (Jones, Thapar, Lewis, & Zammit, 2012). This intriguing finding suggests that there may be shared etiology between the two disorders or that autistic traits may serve as early indicators of psychotic symptoms in adolescence. Nevertheless, both clinical groups showed significant elevations on multiple subscales, indicating that our findings are not an artifact of overlapping symptoms in one specific social domain.
These findings may influence the future development of interventions to address the specific impairments in social behaviors (e.g., does the individual make eye contact when having a conversation). Addressing these behavioral impairments may ultimately help remediate the social dysfunction previously identified in these groups (Addington et al., 2008). Social skills training interventions have been shown to be an effective intervention for patients with schizophrenia (Kurtz & Mueser, 2008); however, such treatments may need to be modified in a developmentally appropriate fashion to be used effectively with younger individuals in the early stages of psychosis. Thus, interventions that have been successfully used in treating youths with ASD diagnoses may be more readily applied to this population. For example, teens with developmental disorders and ASD show significant improvement in social skills in 14 weeks, after participating in an evidence-based social skills intervention (Laugeson, Frankel, Mogil, & Dillon, 2009). In both treatments, a critical component of the intervention is practicing behavioral skills and receiving corrective feedback. However, in the aforementioned intervention, while the individuals with an ASD diagnosis were partaking in the intervention, a parent group met separately to provide them with ways to help their children with the targeted skills. This unique feature may be useful to apply in psychosocial interventions with CHR or AOP youth. Finally, earlier identification of RSB impairment in these individuals may be informative for clinical staging, which takes into account the individual’s current level of dysfunction in multiple domains when identifying the most effective path of treatment (McGorry, Hickie, Yung, Pantelis, & Jackson, 2006).
These findings provide a foundation for future studies of RSB in CHR and AOP youth. Future studies with larger sample sizes are warranted in order to determine whether the SRS is a significant predictor of conversion to psychosis, given that poor global social functioning has been shown to predict conversion to a psychotic disorder in CHR adolescents and young adults (Cannon, Cadenhead, et al., 2008). Other researchers have also used the SRS to examine the neural basis of autistic traits in healthy adults and found that increased connectivity between the anterior midinsula and pregenual portions of the anterior cingulate, a brain area that has been implicated in social processing (Di Martino, Ross, et al., 2009), was related to lower levels of autistic traits (Di Martino, Shehzad, et al., 2009). Identifying whether a similar pattern is observed in CHR and/or AOP individuals may provide us with information about how neural mechanisms may contribute to the social dysfunction in these clinical groups.
Given that there are overlapping characteristic in social cognition deficits in those with ASD and psychosis (Couture et al., 2010), another logical next step would be to look at RSB in relation to social cognition in both clinical samples. In addition, comparing directly RSB in age-matched individuals with autism to CHR and/or AOP youth, while focusing on the individual items endorsed on the SRS, may help us parse out different mechanisms underlying these social behavioral deficits in the three groups. Finally, like work by Sporn et al. (2004), who found that 25% of individuals with early-onset schizophrenia also had comorbid diagnoses of ASD and provided evidence that autistic symptoms may serve as a nonspecific marker of this disorder, this study provides further evidence for etiological overlap between ASD and psychosis. These findings give us further reason to examine how the same genetic structural variants may give rise to abnormal neurodevelopmental processes and potentially manifest in similar behavioral disturbances, like social functioning, that are common to heterogeneous, but separate, disorders like schizophrenia and/or autism.
These findings offer novel information about social impairments in domains critical for adolescent social development and provide a basis for further examination of social dysfunction in AOP and CHR individuals. In this study, we have shown that RSB is significantly impaired in CHR and AOP youths and that this deficit is a significant predictor of subsequent real-world social functioning. Ultimately, the findings from this study not only have practical implications for clinical assessment and treatment in the early phases of psychosis but also provide key information for better understanding points of diagnostic overlap between schizophrenia and autism.
Acknowledgments
Funding for this study was provided by a Maxine and Jack Zarrow Investigator Award (NARSAD Young Investigator Award to C.E.B.); National Institute of Mental Health Grants K23MH74644 (to C.E.B.), MH65079 (to T.D.C.), and NIMH P50 MH066286 (to T.D.C. and C.E.B.); the UCLA Graduate Summer Research Mentorship Program (to M.J.); and donations from the International Mental Health Research Organization and the Staglin Music Festival for Mental Health to the University of California, Los Angeles, Foundation.
References
- Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlas-han TH, Perkins DO, et al. At clinical high risk for psychosis: Outcome for nonconverters. American Journal of Psychiatry. 2011;168:800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophrenia Research. 2008;99:119–124. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson-Tops A, Hansson L. Quantitative and qualitative aspects of the social network in schizophrenic patients living in the community: Relationship to sociodemographic characteristics and clinical factors and subjective quality of life. International Journal of Social Psychiatry. 2001;47:67–77. doi: 10.1177/002076400104700307. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale: The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: Correlations with cognition, functional capacity, and symptoms. American Journal of Psychiatry. 2006;163:418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, et al. Premorbid social functioning in schizophrenia and bipolar disorder: Similarities and differences. American Journal of Psychiatry. 1997;154:1544–1550. doi: 10.1176/ajp.154.11.1544. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Abbacchi AM, Lavesser PD, Reed H, Givens L, Chiang L, et al. Developmental course of autistic social impairment in males. Development and Psychopathology. 2009;21:127–138. doi: 10.1017/S095457940900008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview—Revised. Journal of Autism and Developmental Disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Developmental & Behavioral Pediatrics. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Archives of General Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: More convergence than divergence. Psychological Medicine. 2010;40:569–579. doi: 10.1017/S003329170999078X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophrenia Bulletin. 2006;32(Suppl. 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, Hawes DJ, Frost AD, Vassallo S, Bunn P, Hunter K, et al. Learning to “talk the talk”: The relationship of psychopathic traits to deficits in empathy across childhood. Journal of Child Psychology and Psychiatry. 2009;50:599–606. doi: 10.1111/j.1469-7610.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- da Silva Alves F, Schmitz N, Bloemen O, van der Meer J, Meijer J, Boot E, et al. White matter abnormalities in adults with 22q11 deletion syndrome with and without schizophrenia. Schizophrenia Research. 2011;132:75–83. doi: 10.1016/j.schres.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. American Journal of Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biological Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. American Journal of Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done DJ, Crow TJ, Johnstone EC, Sacker A. Childhood antecedents of schizophrenia and affective illness: Social adjustment at ages 7 and 11. British Medical Journal. 1994;309:699–703. doi: 10.1136/bmj.309.6956.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin K, Conti-Ramsden G. Language, social behavior, and the quality of friendships in adolescents with and without a history of specific language impairment. Child Development. 2007;78:1441–1457. doi: 10.1111/j.1467-8624.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders: Patient edition. New York: New York State Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gorna K, Rybakowski J. Social functioning of patients with schizophrenia: A follow-up study. Psychiatria Polska. 1995;29:619–629. [PubMed] [Google Scholar]
- Hambrecht M, Lammertink M, Klosterkotter J, Matuschek E, Pukrop R. Subjective and objective neuropsychological abnormalities in a psychosis prodrome clinic. British Journal of Psychiatry. 2002;43:s30–s37. doi: 10.1192/bjp.181.43.s30. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology. 2010;24:109–120. doi: 10.1037/a0016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BR, Thapar A, Lewis G, Zammit S. The association between early autistic traits and psychotic experiences in adolescence. Schizophrenia Research. 2012;135:164–169. doi: 10.1016/j.schres.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Development and Psycho-pathology. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Mueser KT. A meta-analysis of controlled research on social skills training for schizophrenia. Journal of Consulting and Clinical Psychology. 2008;76:491–504. doi: 10.1037/0022-006X.76.3.491. [DOI] [PubMed] [Google Scholar]
- Laugeson EA, Frankel F, Mogil C, Dillon AR. Parent-assisted social skills training to improve friendships in teens with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:596–606. doi: 10.1007/s10803-008-0664-5. [DOI] [PubMed] [Google Scholar]
- McGlashan TH. Structured Interview for Prodromal Syndromes (SIPS) New Haven, CT: Yale University; 2001. [Google Scholar]
- McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging of psychiatric disorders: A heuristic framework for choosing earlier, safer and more effective interventions. Australian and New Zealand Journal of Psychiatry. 2006;40:616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- Meyer SE, Bearden CE, Lux SR, Gordon JL, Johnson JK, O’Brien MP, et al. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. Journal of Child and Adolescent Psychopharmacology. 2005;15:434–451. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- Monahan KC, Steinberg L. Accentuation of individual differences in social competence during the transition to adolescence. Journal of Research on Adolescence. 2011;21:576–585. doi: 10.1111/j.1532-7795.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Mayes SD, Smith LA. Brief report: Excellent agreement between two brief autism scales (Checklist for Autism Spectrum Disorder and Social Responsiveness Scale) completed independently by parents and the Autism Diagnostic Interview—Revised. Journal of Autism and Developmental Disorders. 2011;41:1586–1590. doi: 10.1007/s10803-011-1178-0. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, McKinley M, Loewy R, O’Brien M, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophrenia Research. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S. Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: Analysis of CATIE data. Schizophrenia Research. 2012;137:147–150. doi: 10.1016/j.schres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: Clinical and biological contributions to a relation revisited. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:10–18. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry. 2007;48:464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Masten AS, Coatsworth JD, Tellegen A. Salient and emerging developmental tasks in the transition to adulthood. Child Development. 2004;75:123–133. doi: 10.1111/j.1467-8624.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Schlosser DA, Jacobson S, Chen Q, Sugar CA, Niendam TA, Li G, et al. Recovery from an at-risk state: Clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophrenia Bulletin. 2012;38:1225–1233. doi: 10.1093/schbul/sbr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn AL, Addington AM, Gogtay N, Ordonez AE, Gornick M, Clasen L, et al. Pervasive developmental disorder and childhood-onset schizophrenia: Comorbid disorder or a phenotypic variant of a very early onset illness? Biological Psychiatry. 2004;55:989–994. doi: 10.1016/j.biopsych.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Miski P, Buchanan RW, Kirkpatrick B, Carpenter WT., Jr Differential patterns of premorbid social and academic deterioration in deficit and nondeficit schizophrenia. Schizophrenia Research. 2012;135:134–138. doi: 10.1016/j.schres.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentacosta CJ, Fine SE. Emotion knowledge, social competence, and behavior problems in childhood and adolescence: A meta-analytic review. Social Development. 2010;19:1–29. doi: 10.1111/j.1467-9507.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Intelligence Scale (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]